Activation of Cannabinoid Type 2 Receptor in Microglia Reduces Neuroinflammation through Inhibiting Aerobic Glycolysis to Relieve Hypertension
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Drugs
2.2. Animal Methods
2.2.1. Animals
2.2.2. Brain Stereotaxic Injection
2.2.3. Implant Osmotic Pump and Intraperitoneal Injection
2.2.4. Blood Pressure Measurement
2.2.5. Measurement of Plasma Norepinephrine
2.2.6. Frozen Section and Immunofluorescence Staining
2.3. Cell Culture Methods
2.3.1. Cell Culture
2.3.2. Cell Proliferation Activity and Cell Morphology
2.3.3. Lactic Acid Detection
2.3.4. Cell Aerobic Glycolysis Rate
2.4. Western Blot
2.5. Total RNA Extraction and RT-qPCR
2.6. Statistical Analyses
3. Results
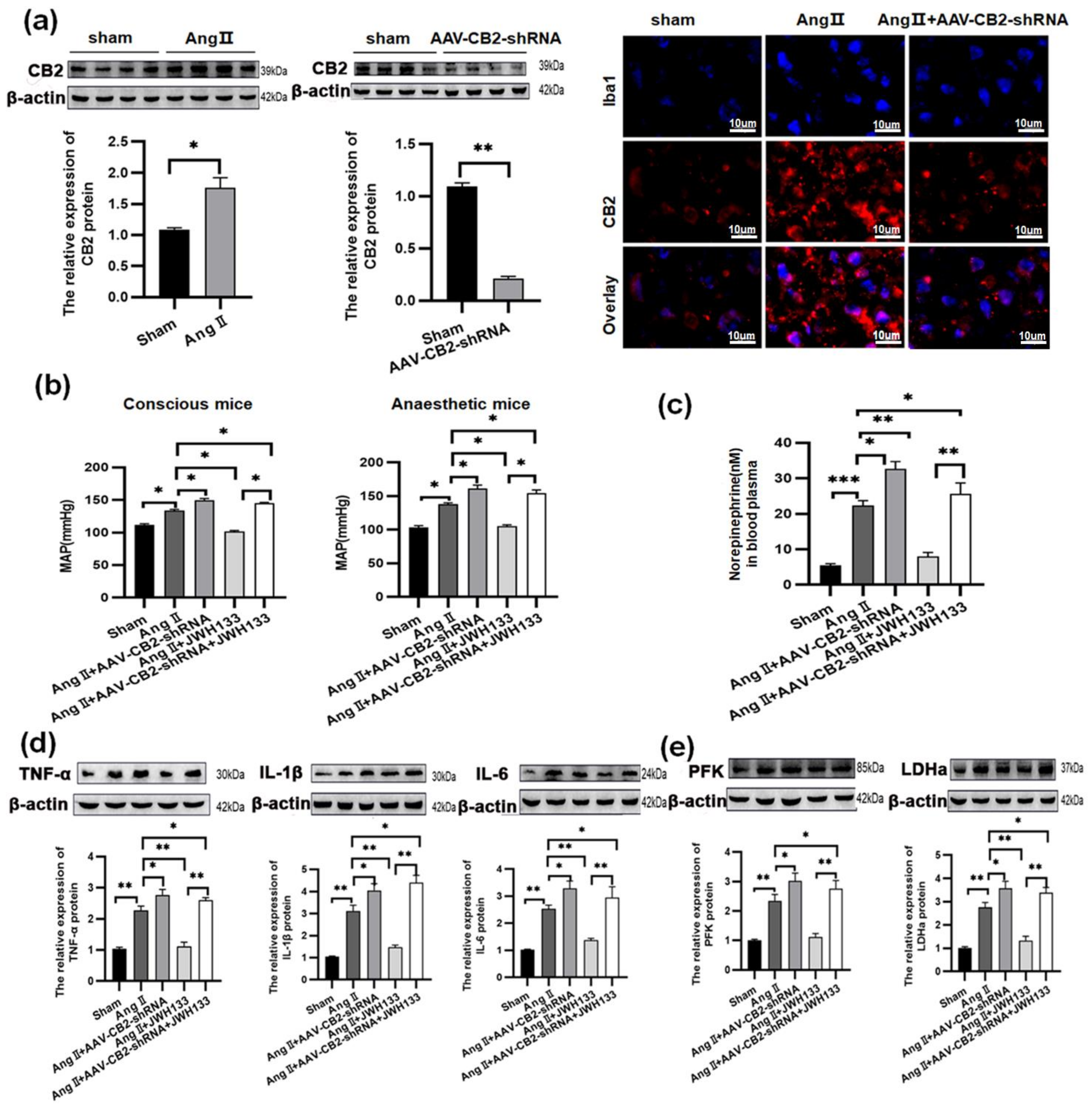
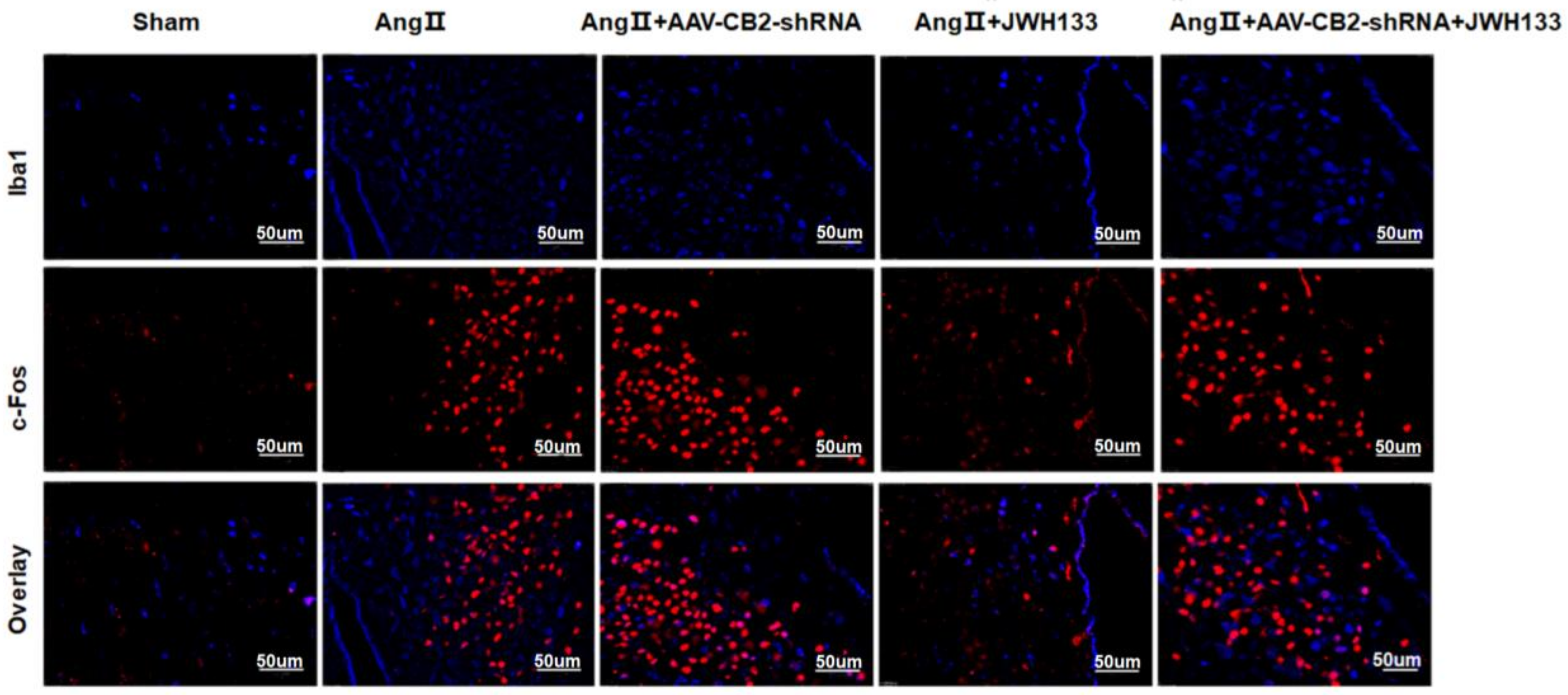
3.1. In AngII-treated Mice, Activation of CB2 Receptors by JWH133 Decreased the Blood Pressure, Plasma Norepinephrinein, c-Fos Expression, Proinflammatory Cytokine, and Enzyme Associated with Glycolysis in the PVN
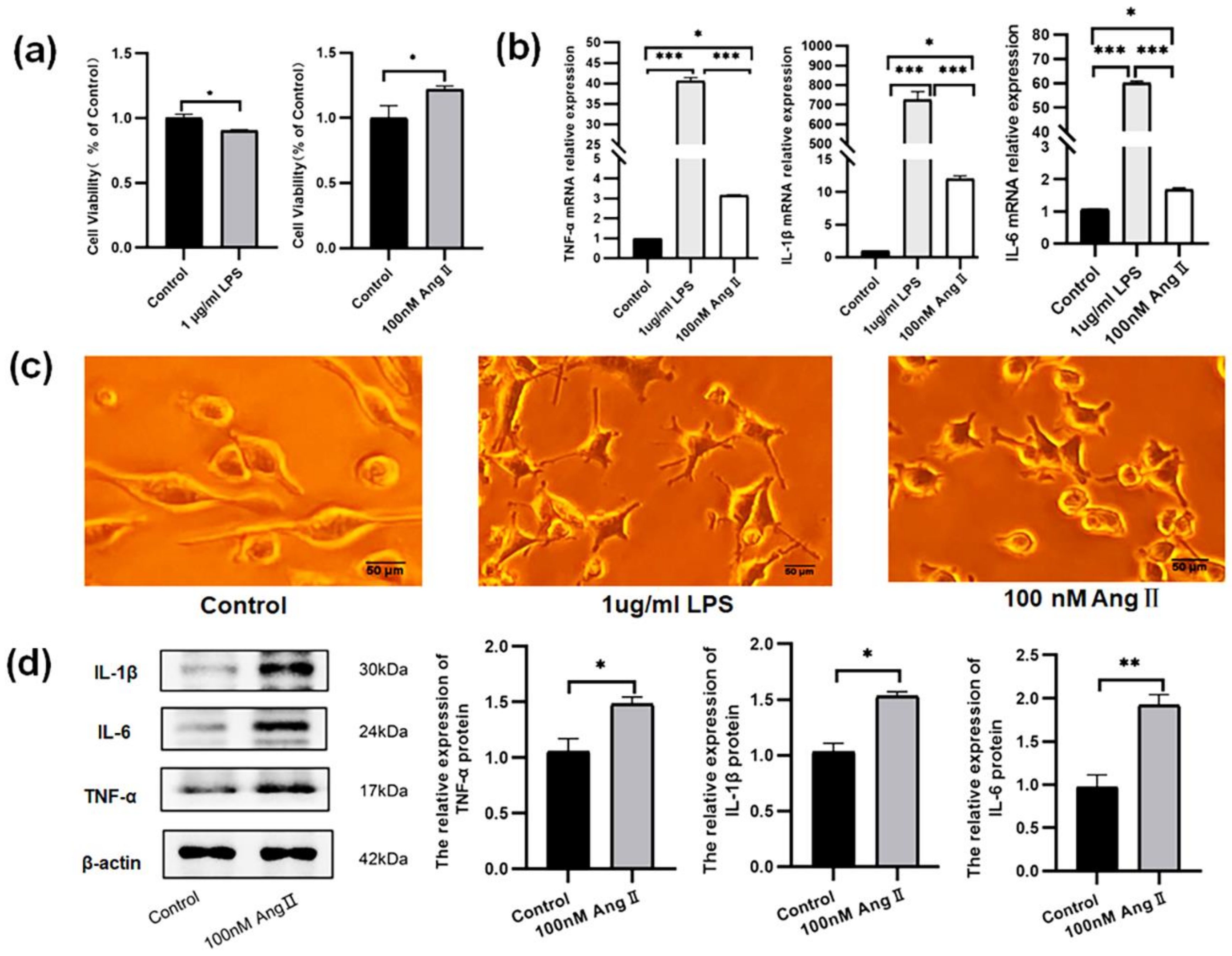
3.2. The BV2 Cells Treated with AngII Exhibited an Increase in Proinflammatory Cytokines
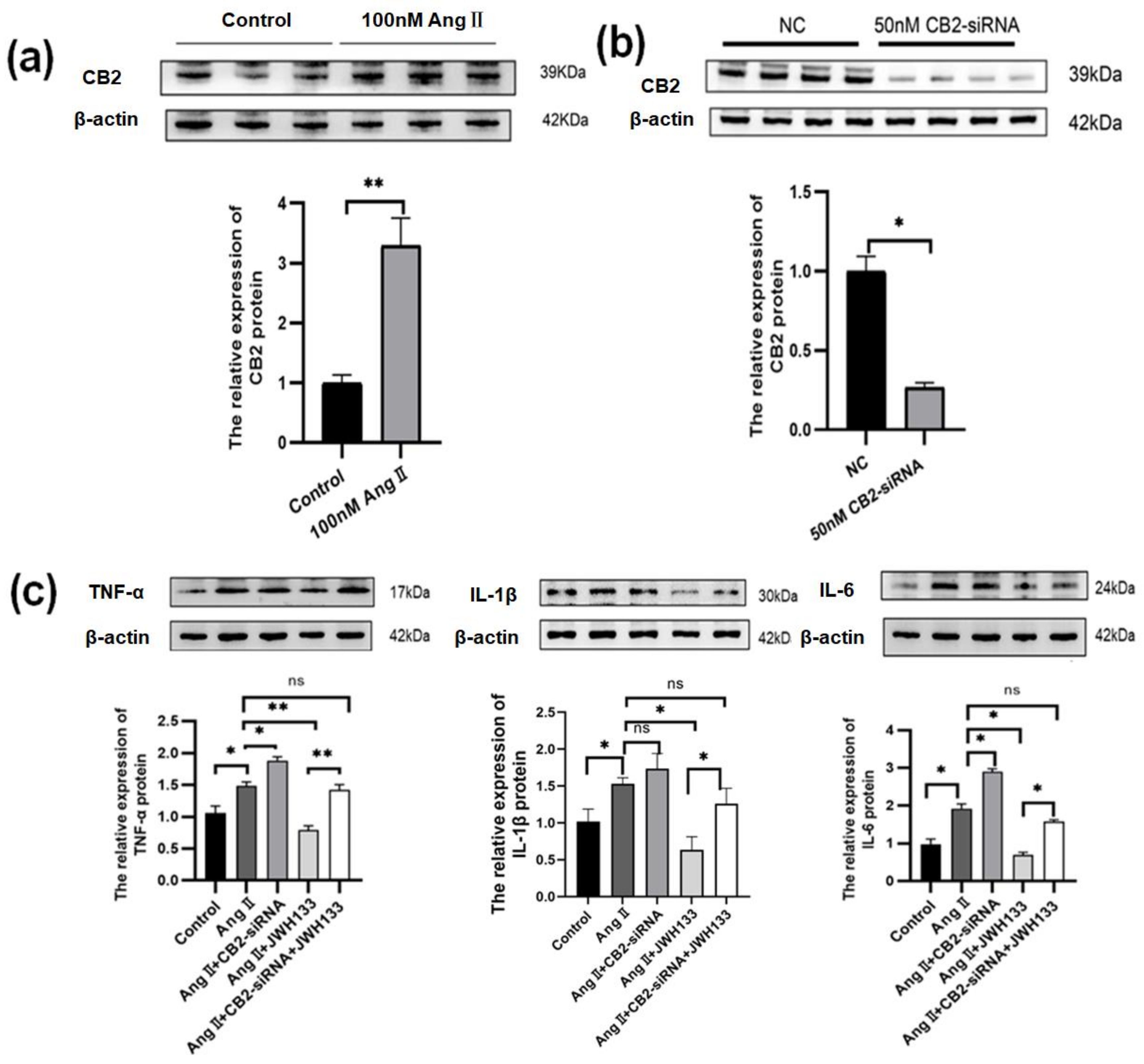
3.3. An Increase in Proinflammatory Cytokines Caused by AngII in BV2 Cells Was Reduced by Activating CB2 Receptors with the Agonist JWH133
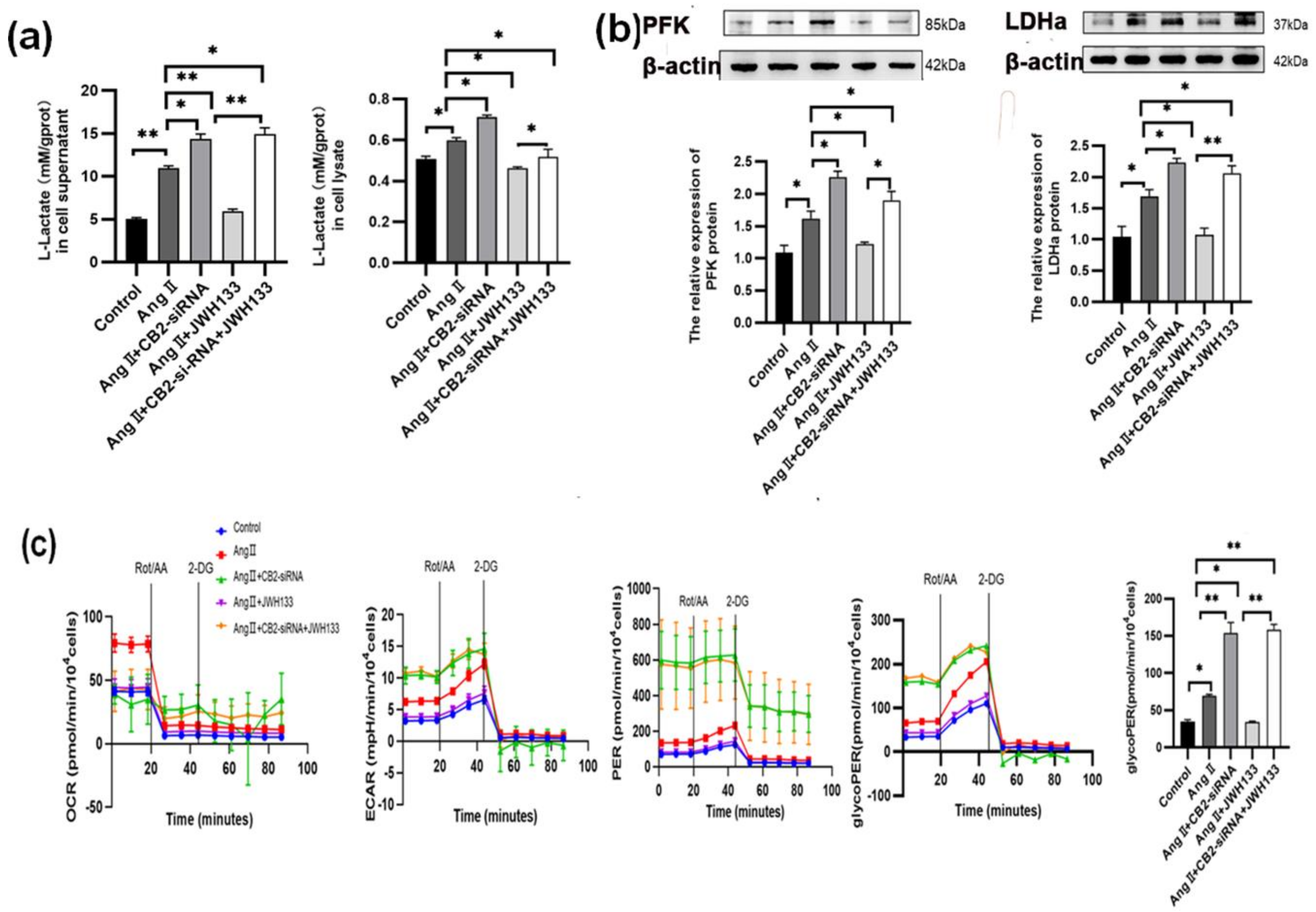
3.4. The Increase in Aerobic Glycolysis Produced by AngII in BV2 Cells Was Reduced by Activating the CB2 Receptor with the Agonist JWH133
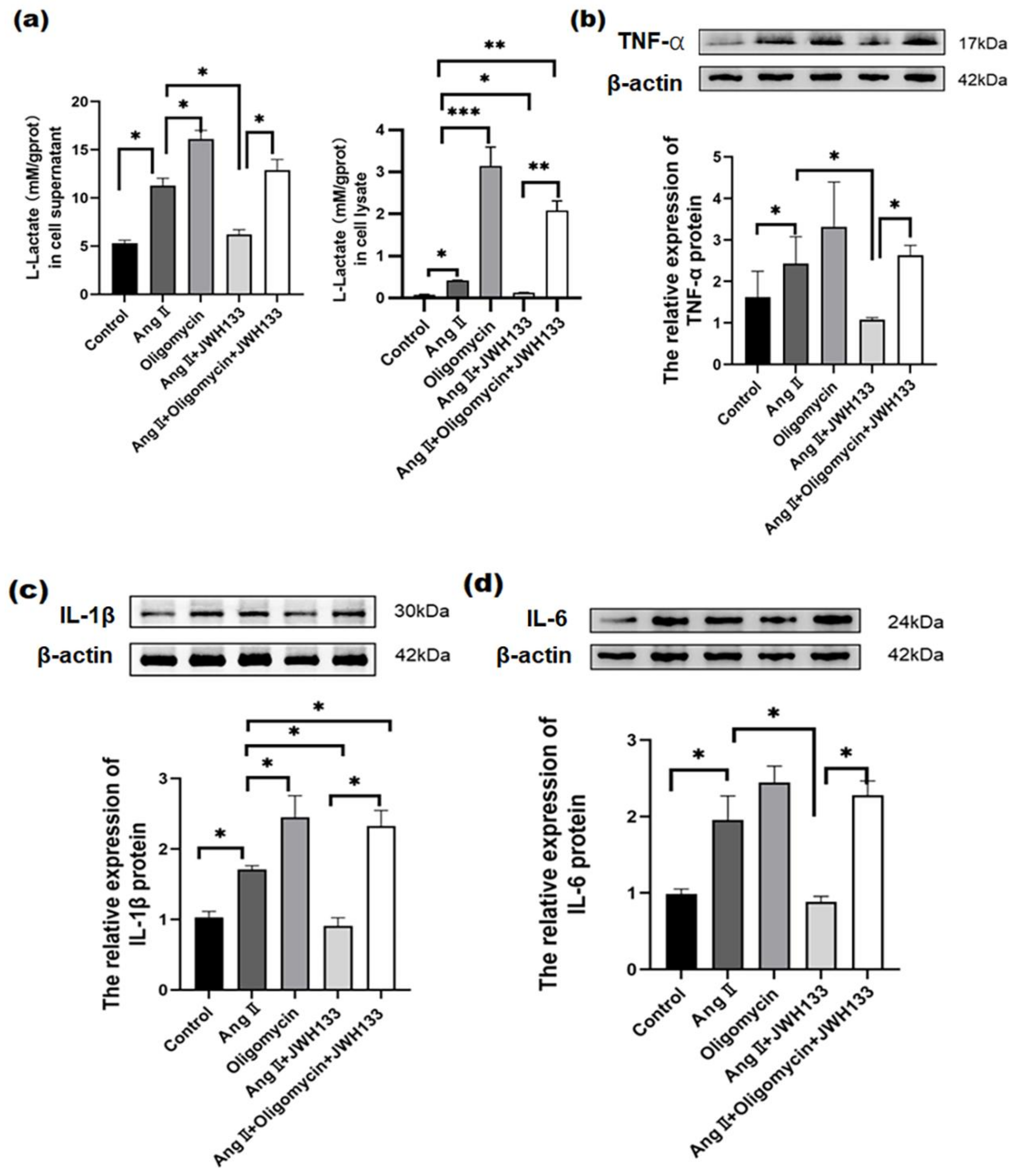
3.5. CB2 Receptor Agonist JWH133 Reversed Proinflammatory Cytokine Increase through Inhibiting Aerobic Glycolysis Enhancement Induced by AngII
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gebremedhin, D.; Lange, A.R.; Campbell, W.B.; Hillard, C.J.; Harder, D.R. Cannabinoid CB1 receptor of cat cerebral arterial muscle functions to inhibit L-type Ca2+ channel current. Am. J. Physiol. 1999, 276, H2085–H2093. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, B.; Mirshahi, F.; Sanyal, A.J.; Khanolkar, A.D.; Makriyannis, A.; Kunos, G. Functional CB1 cannabinoid receptors in human vascular endothelial cells. Biochem. J. 2000, 346 Pt 3, 835–840. [Google Scholar] [CrossRef]
- Bonz, A.; Laser, M.; Kullmer, S.; Kniesch, S.; Babin-Ebell, J.; Popp, V.; Ertl, G.; Wagner, J.A. Cannabinoids acting on CB1 receptors decrease contractile performance in human atrial muscle. J. Cardiovasc. Pharmacol. 2003, 41, 657–664. [Google Scholar] [CrossRef]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Mecha, M.; Carrillo-Salinas, F.J.; Feliu, A.; Mestre, L.; Guaza, C. Microglia activation states and cannabinoid system: Therapeutic implications. Pharmacol. Ther. 2016, 166, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Lake, K.D.; Compton, D.R.; Varga, K.; Martin, B.R.; Kunos, G. Cannabinoid-induced hypotension and bradycardia in rats mediated by CB1-like cannabinoid receptors. J. Pharmacol. Exp. Ther. 1997, 281, 1030–1037. [Google Scholar]
- Hillard, C.J. Endocannabinoids and vascular function. J. Pharmacol. Exp. Ther. 2000, 294, 27–32. [Google Scholar]
- Kunos, G.; Batkai, S.; Offertaler, L.; Mo, F.; Liu, J.; Karcher, J.; Harvey-White, J. The quest for a vascular endothelial cannabinoid receptor. Chem. Phys. Lipids 2002, 121, 45–56. [Google Scholar] [CrossRef]
- Randall, M.D.; Harris, D.; Kendall, D.A.; Ralevic, V. Cardiovascular effects of cannabinoids. Pharmacol. Ther. 2002, 95, 191–202. [Google Scholar] [CrossRef]
- Pacher, P.; Bátkai, S.; Kunos, G. The Endocannabinoid System as an Emerging Target of Pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A. Association between cannabis use and blood pressure levels according to comorbidities and socioeconomic status. Sci. Rep. 2023, 13, 2069. [Google Scholar] [CrossRef] [PubMed]
- Bátkai, S.; Pacher, P.; Osei-Hyiaman, D.; Radaeva, S.; Liu, J.; Harvey-White, J.; Offertáler, L.; Mackie, K.; Rudd, M.A.; Bukoski, R.D.; et al. Endocannabinoids Acting at Cannabinoid-1 Receptors Regulate Cardiovascular Function in Hypertension. Circulation 2004, 110, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.-K.; Guo, H.-C.; Liu, H.-Y.; Zhang, Z.-L.; Hu, M.-Y.; Zhang, Y.; Li, Q. Cannabinoid type 2 receptor agonist JWH133 decreases blood pressure of spontaneously hypertensive rats through relieving inflammation in the rostral ventrolateral medulla of the brain. J. Hypertens. 2020, 38, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Victor, R.G.; Shafiq, M.M. Sympathetic neural mechanisms in human hypertension. Curr. Hypertens. Rep. 2008, 10, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, E.A.; Sved, A.F.; Speth, R.C. Angiotensin modulation of rostral ventrolateral medulla (RVLM) in cardiovascular regulation. Mol. Cell. Endocrinol. 2009, 302, 167–175. [Google Scholar] [CrossRef]
- Takeda, K.; Nakata, T.; Takesako, T.; Itoh, H.; Hirata, M.; Kawasaki, S.; Hayashi, J.; Oguro, M.; Sasaki, S.; Nakagawa, M. Sympathetic inhibition and attenuation of spontaneous hypertension by PVN lesions in rats. Brain Res. 1991, 543, 296–300. [Google Scholar] [CrossRef]
- Shi, P.; Diez-Freire, C.; Jun, J.Y.; Qi, Y.; Katovich, M.J.; Li, Q.; Sriramula, S.; Francis, J.; Sumners, C.; Raizada, M.K. Brain microglial cytokines in neurogenic hypertension. Hypertension 2010, 56, 297–303. [Google Scholar] [CrossRef]
- Shen, X.Z.; Li, Y.; Li, L.; Shah, K.H.; Bernstein, K.E.; Lyden, P.; Shi, P. Microglia participate in neurogenic regulation of hypertension. Hypertension 2015, 66, 309–316. [Google Scholar] [CrossRef]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef]
- Tan, X.; Jiao, P.L.; Wang, Y.K.; Wu, Z.T.; Zeng, X.R.; Li, M.L.; Wang, W.Z. The phosphoinositide-3 kinase signaling is involved in neuroinflammation in hypertensive rats. CNS Neurosci. Ther. 2017, 23, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.L.; Chan, S.H.; Chan, J.Y. Neuroinflammation and oxidative stress in rostral ventrolateral medulla contribute to neurogenic hypertension induced by systemic inflammation. J. Neuroinflamm. 2012, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Fung, K.P.; Kwok, T.T.; Lee, C.Y.; Suen, Y.K.; Kong, S.K. Mitochondria-targeting drug oligomycin blocked P-glycoprotein activity and triggered apoptosis in doxorubicin-resistant HepG2 cells. Chemotherapy 2004, 50, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Li, D.P.; Pan, H.L. Glutamatergic Regulation of Hypothalamic Presympathetic Neurons in Hypertension. Curr. Hypertens. Rep. 2017, 19, 78. [Google Scholar] [CrossRef] [PubMed]
- Cuadra, A.E.; Shan, Z.; Sumners, C.; Raizada, M.K. A current view of brain renin–angiotensin system: Is the (pro)renin receptor the missing link? Pharmacol. Ther. 2009, 125, 27–38. [Google Scholar] [CrossRef]
- Abegaz, B.; Davern, P.J.; Jackson, K.L.; Nguyen-Huu, T.P.; Bassi, J.K.; Connelly, A.; Choong, Y.T.; Allen, A.M.; Head, G.A. Cardiovascular role of angiotensin type1A receptors in the nucleus of the solitary tract of mice. Cardiovasc. Res. 2013, 100, 181–191. [Google Scholar] [CrossRef]
- Fujita, Y.; Yamashita, T. Neuroprotective function of microglia in the developing brain. Neuronal Signal 2021, 5, NS20200024. [Google Scholar] [CrossRef]
- Kaur, C.; Rathnasamy, G.; Ling, E.A. Biology of Microglia in the Developing Brain. J. Neuropathol. Exp. Neurol. 2017, 76, 736–753. [Google Scholar] [CrossRef]
- Klawonn, A.M.; Fritz, M.; Castany, S.; Pignatelli, M.; Canal, C.; Simila, F.; Tejeda, H.A.; Levinsson, J.; Jaarola, M.; Jakobsson, J.; et al. Microglial activation elicits a negative affective state through prostaglandin-mediated modulation of striatal neurons. Immunity 2021, 54, 225–234.6. [Google Scholar] [CrossRef]
- Krasemann, S.; Madore, C.; Cialic, R.; Baufeld, C.; Calcagno, N.; El Fatimy, R.; Beckers, L.; O’Loughlin, E.; Xu, Y.; Fanek, Z.; et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 2017, 47, 566–581.e9. [Google Scholar] [CrossRef]
- Werneburg, S.; Jung, J.; Kunjamma, R.B.; Ha, S.K.; Luciano, N.J.; Willis, C.M.; Gao, G.; Biscola, N.P.; Havton, L.A.; Crocker, S.J.; et al. Targeted Complement Inhibition at Synapses Prevents Microglial Synaptic Engulfment and Synapse Loss in Demyelinating Disease. Immunity 2020, 52, 167–182.e7. [Google Scholar] [CrossRef]
- Calvillo, L.; Gironacci, M.M.; Crotti, L.; Meroni, P.L.; Parati, G. Neuroimmune crosstalk in the pathophysiology of hypertension. Nat. Rev. Cardiol. 2019, 16, 476–490. [Google Scholar] [CrossRef] [PubMed]
- Talarico, G.; Trebbastoni, A.; Bruno, G.; de Lena, C. Modulation of the Cannabinoid System: A New Perspective for the Treatment of the Alzheimer’s Disease. Curr. Neuropharmacol. 2019, 17, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. Regulation of Immune Cell Functions by Metabolic Reprogramming. J. Immunol. Res. 2018, 2018, 8605471. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, R.C.; Angiari, S. Microglia immunometabolism: From metabolic disorders to single cell metabolism. Semin. Cell Dev. Biol. 2019, 94, 129–137. [Google Scholar] [CrossRef]
- Stefan, S.; Daniel, B.; Philip, M.; Heiko, S.; Christoph, K. Mathematical models for explaining the Warburg effect: A review focussed on ATP and biomass production. Biochem. Soc. Trans. 2015, 43, 1187–1194. [Google Scholar]
- Bart, E.; Eyal, A.; Huang, S.C.; Smith, A.M.; Chang, C.H.; Lam, W.Y.; Veronika, R.; Freitas, T.C.; Julianna, B.; van der Windt, G.J.; et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKε supports the anabolic demands of dendritic cell activation. Nat. Immunol. 2014, 15, 323–332. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, R.; Zhang, Y.; Shi, Y.; Wang, X.; Wang, X.; Ma, G.; Li, Q. Activation of Cannabinoid Type 2 Receptor in Microglia Reduces Neuroinflammation through Inhibiting Aerobic Glycolysis to Relieve Hypertension. Biomolecules 2024, 14, 333. https://doi.org/10.3390/biom14030333
Shan R, Zhang Y, Shi Y, Wang X, Wang X, Ma G, Li Q. Activation of Cannabinoid Type 2 Receptor in Microglia Reduces Neuroinflammation through Inhibiting Aerobic Glycolysis to Relieve Hypertension. Biomolecules. 2024; 14(3):333. https://doi.org/10.3390/biom14030333
Chicago/Turabian StyleShan, Ruohan, Yuxiang Zhang, Yiping Shi, Xiaowen Wang, Xueke Wang, Guanying Ma, and Qian Li. 2024. "Activation of Cannabinoid Type 2 Receptor in Microglia Reduces Neuroinflammation through Inhibiting Aerobic Glycolysis to Relieve Hypertension" Biomolecules 14, no. 3: 333. https://doi.org/10.3390/biom14030333
APA StyleShan, R., Zhang, Y., Shi, Y., Wang, X., Wang, X., Ma, G., & Li, Q. (2024). Activation of Cannabinoid Type 2 Receptor in Microglia Reduces Neuroinflammation through Inhibiting Aerobic Glycolysis to Relieve Hypertension. Biomolecules, 14(3), 333. https://doi.org/10.3390/biom14030333





