Functional Implications of the Prosomeric Brain Model
Abstract
1. Introduction
2. Brain Models
2.1. The Columnar Model
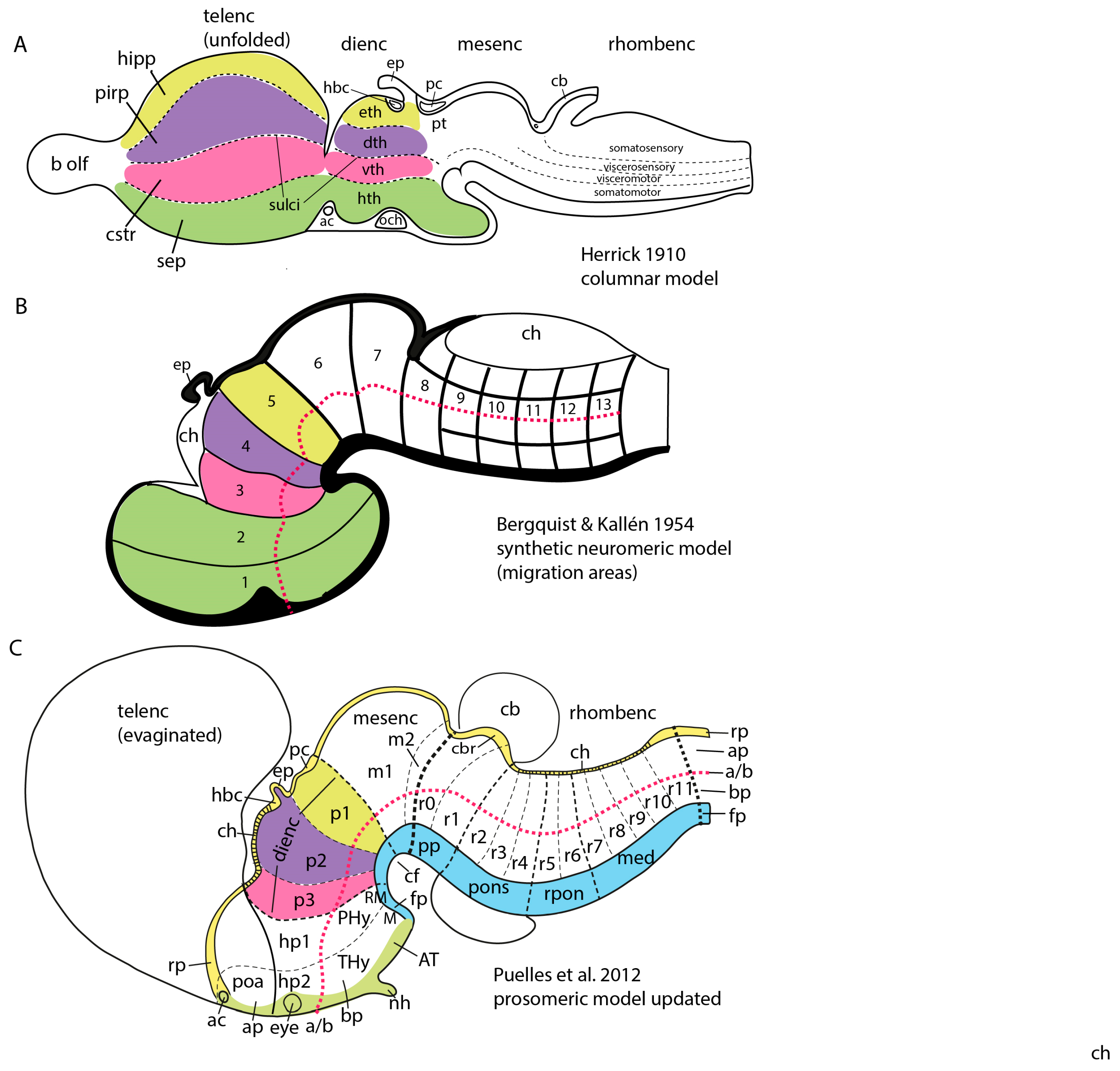
2.2. The Main Developmental Models Available
2.3. The Early Neuromeric Model
2.4. His’s Model
2.5. The Synthetic School of Neuromery
2.6. The Birth of the Molecular Prosomeric Model
3. Functions
3.1. General Functional Implications of the Prosomeric Brain Model
3.2. The Case of Vision
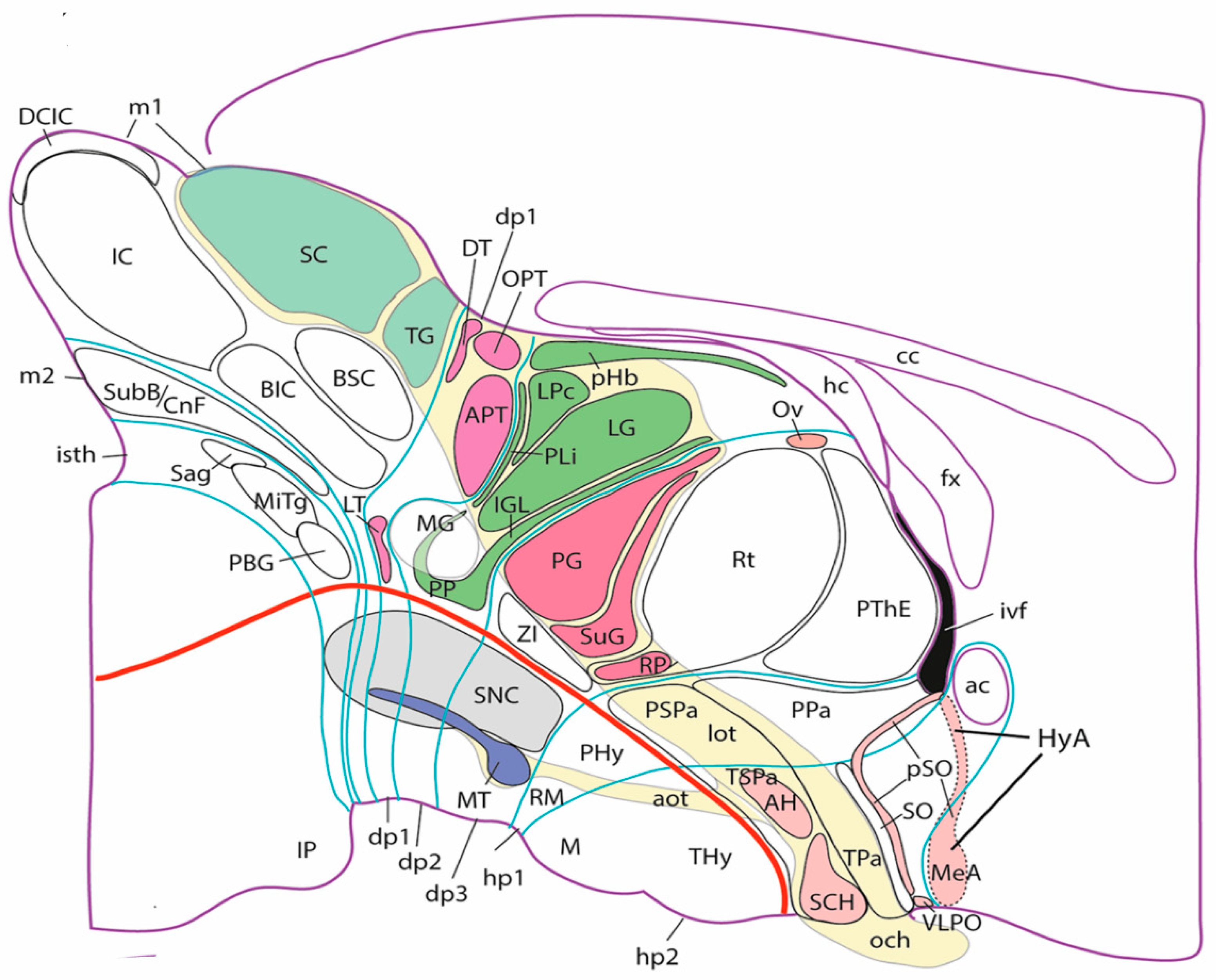
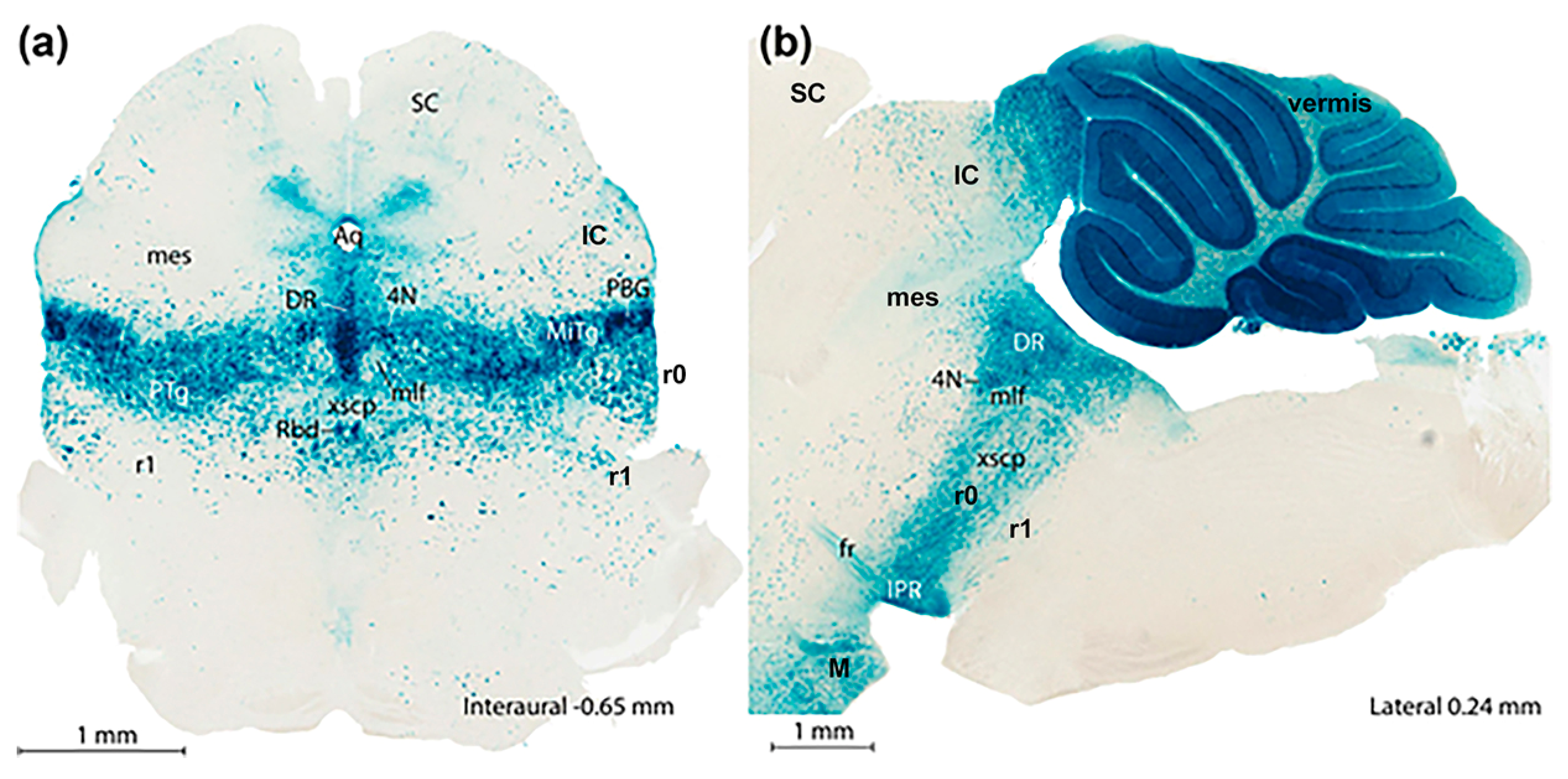
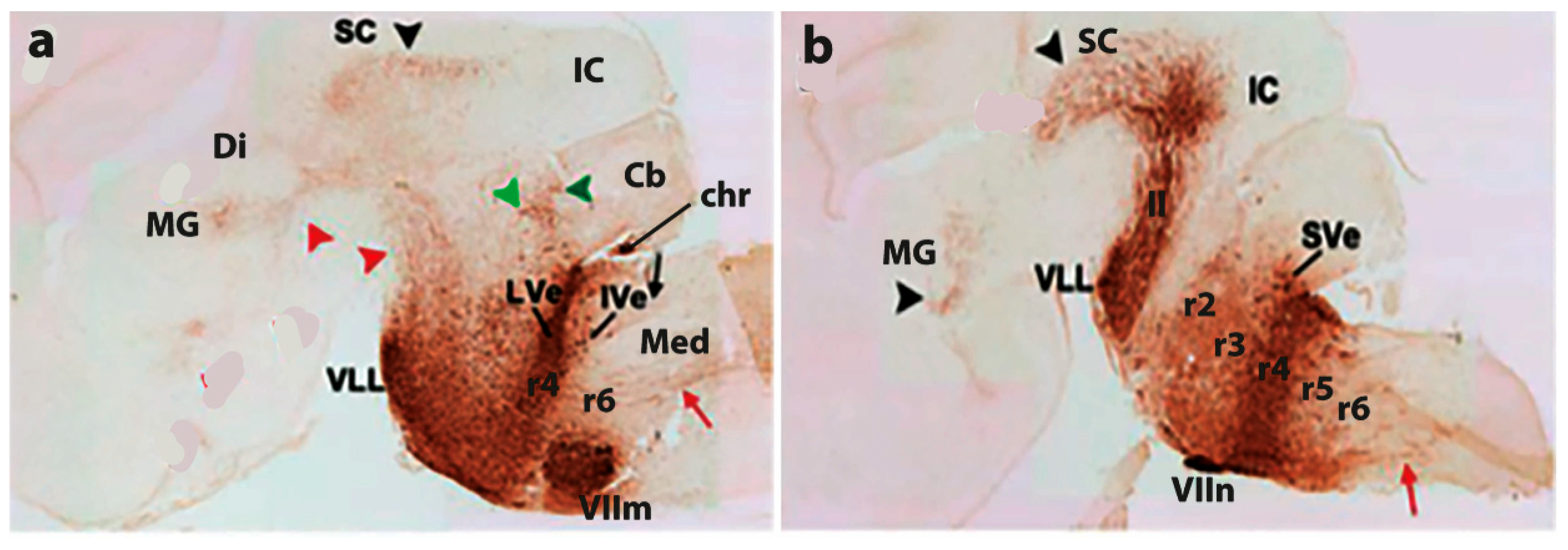
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 4N | trochlear motor nucleus |
| ac | anterior commissure |
| AH | anterior hypothalamic nucleus |
| aot | accessory optic tract |
| APt | anterior pituitary (adenohypophysis) |
| APT | anterior pretectal nucleus |
| BIC | interstitial nucleus of the inferior collicular brachium conjunctivum |
| BSC | interstitial nucleus of the superior collicular or tectal brachium |
| Cb | cerebellum |
| cc | corpus callosum |
| chr | chorioidal roofplate |
| DCIC | dorsal cap of inferior colliculus |
| Di | diencephalon |
| dp1–dp3 | diencephalic prosomeres 1–3 |
| DPal | dorsal pallium |
| DR | dorsal raphe nucleus |
| DT | dorsal terminal nucleus of the accessory optic pathway |
| fr | fasciculus retroflexus (habenulo-interpeduncular tract) |
| hc | hippocampal commissure |
| hp1 | hypothalamo-telencephalic prosomere 1 |
| hp2 | hypothalamo-telencephalic prosomere 2 |
| HyA | hypothalamo-amygdalar corridor |
| IC | inferior colliculus |
| icc | intercollicular commissure |
| IGL | intergeniculate leaflet |
| IP | interpeduncular nucleus |
| IPN | interpeduncular nucleus |
| is | isthmic rhombomere (r0) |
| isth | isthmus |
| IVe | inferior vestibular nucleus |
| ivf | interventricular foramen |
| LG | lateral geniculate nucleus |
| ll | lateral lemniscus tract |
| lot | lateral optic tract |
| LPal | lateral pallium |
| LPc | lateral posterior nucleus, caudal part (pulvinar homolog in mouse) |
| LT | lateral terminal nucleus |
| LVe | lateral vestibular nuleus |
| M | mamillary body |
| m1–m2 | midbrain prosomeres 1–2 |
| mes | mesencephalon (midbrain) |
| MG | medual geniculate nucleus |
| MiTg | microcellular tegmental nucleus |
| mlf | medial longitudinal tract |
| mp1–mp2 | midbraon prosomeres 1–2 |
| MPal | medial pallium |
| MT | medial terminal nucleus of the accessory optic pathway |
| OB | olfactory bulb |
| och | optic chiasma |
| on | optic nerve |
| OPT | olivary pretectal nucleus |
| Ov | ovoid retinorecipient nucleus |
| p1–p3 | diencephalic prosomeres 1–3 |
| PalSe | pallial septum (now interpreted as ‘subpallial dorsal septum’) |
| PBG | parabigeminal nucleus |
| pc | posterior commissure |
| PG | pregeniculate nucleus |
| pHb | parahabenular retinorecipient nucleus |
| Pi | pineal gland |
| PLi | posterior lineal thalamic nucleus |
| POA | preoptic area |
| PP | posterior peduncular nucleus |
| PPa | peduncular paraventricular area |
| PPt | posterior pituitary (neurohypophysis) |
| PSO | parasupraoptic retinorecipient nucleus |
| PSPa | peduncular subparaventricular hypothalamic area |
| PThE | prethalamic eminence |
| r0–r11 | rhombomeres 0 (isthmus) to 11 |
| Rbd | rhabdoid nucleus |
| RM | retromamillary área |
| RP | retropeduncular nucleus |
| Rt | reticular nucleus |
| Sag | sagular nucleus |
| SC | superior colliculus |
| SCH | suprachiasmatic nucleus |
| SNC | substantia nigra compacta |
| SO | supraoptic nucleus |
| SPallSe | subpallial septum |
| SubB/Cnf | subbrachial nucleus/cuneiform nucleus or area |
| SuG | subgeniculate nucleus |
| SVe | superior vestibular nucleus |
| tc | tectal commissure |
| TG | tectal gray retinorecipient formation |
| THy | terminal hypothalamus |
| TPa | terminal paraventricular area |
| TSPa | terminal subparaventricular hypothalamic area |
| VIIm | facial motor nucleus (7th cranial nerve) |
| VIIn | facial nerve root |
| VLL | ventral nucleus of the lateral lemniscus |
| VLPO | ventrolateral preoptic nucleus |
| VPal | ventral pallium |
| xscp | decussation of superior cerebellar peduncle |
| Zi | zona incerta |
References
- Watson, C.; Mitchelle, A.; Puelles, L. A new mammalian brain ontology based on developmental gene expression. In Evolution of Nervous Systems, 2nd ed.; Kaas, J., Ed.; Elsevier: Oxford, UK, 2017; Volume 2, pp. 53–75. [Google Scholar]
- Tomás-Roca, L.; Corral-San-Miguel, R.; Aroca, P.; Puelles, L.; Marín, F. Crypto-rhombomeres of the mouse medulla oblongata, defined by molecular and morphological features. Anat. Embryol. 2014, 221, 815–838. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.; Shimogori, T.; Puelles, L. Mouse Fgf8-Cre-LacZ lineage analysis defines the territory of the postnatal mammalian isthmus. J. Comp. Neurol. 2017, 525, 2782–2799. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.; Kirkcaldie, M.; Puelles, L. Developmental gene expression redefines the mammalian brain stem. In Evolution of Nervous Systems, 2nd ed.; Elsevier: Oxford, UK, 2017; Volume 2, pp. 467–475. [Google Scholar]
- Puelles, L. Recollections on the Origins and Development of the Prosomeric Model. Front. Neuroanat. 2021, 15, 787913. [Google Scholar] [CrossRef] [PubMed]
- Clarke, E.; O’Malley, C.D. The Human Brain and Spinal Cord. In A Historical Study Illustrated by Writings from Antiquity to the Twentieth Century, 2nd ed.; Revised and Enlarged (1st Edit in 1968); Norman Publishing: San Francisco, CA, USA, 1995. [Google Scholar]
- Meyer, A. Historical Aspects of Cerebral Anatomy; Oxford University Press: London, UK, 1971. [Google Scholar]
- Gaskell, W.H. On the Relation between the Structure, Function, Distribution and Origin of the Cranial Nerves; together with a Theory of the Origin of the Nervous System of Vertebrata. J. Physiol. 1889, 10, 153–212. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.B. An attempt to define the primitive functional divisions of the central nervous system. J. Comp. Neurol. 1902, 12, 87–106. [Google Scholar] [CrossRef]
- Herrick, C.J. The doctrine of nerve components and some of its applications. J. Comp. Neurol. 1903, 13, 301–312. [Google Scholar] [CrossRef]
- Herrick, C.J. The morphology of the forebrain in amphibia and reptilia. J. Comp. Neurol. Psychol. 1910, 20, 413–547. [Google Scholar] [CrossRef]
- Nieuwenhuys, R. Principles of Current Vertebrate Neuromorphology. Brain Behav. Evol. 2017, 90, 117–130. [Google Scholar] [CrossRef]
- Kuhlenbeck, H. Vorlesungen über das Zentralnervensystem der Wirbeltiere. In Eine Einführung in die Gehirnanatomie auf Vergleichender Grundlage; Gustav Fischer Verlag: Jena, Germany, 1927. [Google Scholar]
- Kuhlenbeck, H. The Central Nervous System of Vertebrates; Part II: Overall Morphological Pattern; S. Karger: Basel, Switzerland, 1973; Volume 3. [Google Scholar]
- Bergquist, H.; Källěn, B. Notes on the early histogenesis and morphogenesis of the central nervous system in vertebrates. J. Comp. Neurol. 1954, 100, 627–659. [Google Scholar] [CrossRef]
- Puelles, L. Plan of the developing vertebrate nervous system relating embryology to the adult nervous system (prosomere model, overview of brain organization). In Comprehensive Developmental Neuroscience: Patterning and Cell Type Specifi-cation in the Developing CNS and PNS; Rubenstein, J.L.R., Rakic, P., Eds.; Academic Press: Amsterdam, The Netherlands, 2013; pp. 187–209. [Google Scholar]
- Puelles, L.; Harrison, M.; Paxinos, G.; Watson, C. A developmental ontology for the mammalian brain based on the prosomeric model. Trends Neurosci. 2013, 36, 570–578. [Google Scholar] [CrossRef]
- Puelles, L.; Rubenstein, J.L.R. A new scenario of hypothalamic organization: Rationale of new hypotheses introduced in the updated prosomeric model. Front. Neuroanat. 2015, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuys, R.; Puelles, L. Towards a New Neuromorphology; Springer Science and Business Media LLC: Dordrecht, The Netherlands, 2016; ISBN 9783319256924. [Google Scholar]
- Puelles, L. Developmental studies of avian brain organization. Int. J. Dev. Biol. 2018, 62, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Herrick, C.J. Neurological Foundations of Animal Behavior; Biodiversity Heritage Library: Washington, DC, USA, 1924. [Google Scholar]
- Herrick, C.J. Brains of Rats and Men; University of Chicago Press: Chicago, IL, USA, 1926. [Google Scholar]
- Herrick, C.J. An Introduction to Neurology, 5th ed.; Saunders: Philadelphia, PA, USA, 1931. [Google Scholar]
- Herrick, C.J. The Thinking Machine, 2nd ed.; University of Chicago Press: Chicago, IL, USA, 1932. [Google Scholar]
- Herrick, C.J. The Brain of the Tiger Salamander, Ambystoma tigrinum; University of Chicago Press: Chicago, IL, USA, 1948. [Google Scholar]
- Herrick, C.J. George Elliott Coghill, Naturalist and Philosopher; University of Chicago Press: Chicago, IL, USA, 1949. [Google Scholar]
- Herrick, C.J. The Evolution of Human Nature; University of Texas Press: Austin, TX, USA, 1956. [Google Scholar]
- Johnston, J.B. The Nervous System of Vertebrates; John Murray: London, UK, 1908. [Google Scholar]
- Swanson, L.W. Brain Architecture. Understanding the Basic Plan, 2nd ed.; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Puelles, L. Prosomeric classification of retinorecipient centers: A new causal scenario. Anat. Embryol. 2022, 227, 1171–1193. [Google Scholar] [CrossRef] [PubMed]
- Puelles, L.; Martinez-de-la-Torre, M.; Martinez, S.; Watson, C.; Paxinos, G. The Chick Brain in Stereotaxic Coordinates and Alternate Stains, 2nd ed.; Academic Press: New York, NY, USA, 2019. [Google Scholar]
- His, W. Vorschläge zur Eintheilung des Gehirns. Arch. Anat. EntwicklGesch 1893, 1893, 173–179. [Google Scholar]
- His, W. Die Anatomische Nomenclatur, Nomina Anatomica. Neurol. Suppl. Bd. Arch. Anat. Entwickl-Gesch 1895, 1895, 155–177. [Google Scholar]
- His, W. Die Entwicklung des Menschliches Gehirns während der Ersten Monaten; Hirzel: Leipzig, Germany, 1904. [Google Scholar]
- Orr, H. Contribution to the embryology of the lizard, with special reference to the central nervous system and some organs of the head, together with observations on the origin of vertebrates. J. Morphol. 1887, 1, 311–372. [Google Scholar] [CrossRef]
- Von Kupffer, K. Die Morphogenie des Zentralnervensystems. In Handbuch der Vergleichenden und Experimentellen Entwicklungslehere der Wirbeltiere; Bd. 2, Teil 3; Hertwig, O., Ed.; Gustav Fischer: Jena, Germany, 1906; pp. 1–272. [Google Scholar]
- Ziehen, T. Die Morphogenie des Zentralnervensystems der Säugetiere. In Handbuch der Vergleichenden und Experi-mentellen Entwicklungslehere der Wirbeltiere; Bd. 2, Teil 3; Hertwig, O., Ed.; Gustav Fischer: Jena, Germany, 1906; pp. 273–394. [Google Scholar]
- Cambronero, F.; Puelles, L. Rostrocaudal nuclear relationships in the avian medulla oblongata: A fate map with quail chick chimeras. J. Comp. Neurol. 2000, 427, 522–545. [Google Scholar] [CrossRef]
- Marín, F.; Aroca, P.; Puelles, L. Hox gene colinear expression in the avian medulla oblongata is correlated with pseu-dorhombomeric domains. Dev. Biol. 2008, 323, 230–247. [Google Scholar] [CrossRef]
- Puelles, L.; Martínez-Marin, R.; Melgarejo-Otalora, P.; Ayad, A.; Valavanis, A.; Ferran, J.L. Patterned vascularization of embryonic mouse forebrain, and neuromeric topology of major human subarachnoidal arterial branches: A prosomeric mapping. Front. Neuroanat. 2019, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Amat, J.A.; Martínez-De-La-Torre, M.; Trujillo, C.M.; Fernández, B.; Puelles, L. Neurogenetic heterochrony in chick, lizard, and rat mapped with wholemount acetylcholinesterase and the prosomeric model. Brain, Behav. Evol. 2022, 97, 48–82. [Google Scholar] [CrossRef] [PubMed]
- Di Bonito, M.; Studer, M.; Puelles, L. Nuclear derivatives and axonal projections originating from rhombomere 4 in the mouse hindbrain. Anat. Embryol. 2017, 222, 3509–3542. [Google Scholar] [CrossRef]
- Sherrington, C. The Integrative Action of the Nervous System, 1st ed.; Cambridge University Press: Cambridge, UK, 1906. [Google Scholar]
- Ruiz-Cabrera, S.; Pérez-Santos, I.; Zaldivar-Diez, J.; García-Cabezas, M. Expansion modes of primate nervous system structures in the light of the prosomeric model. Front. Mammal Sci. 2023, 2, 1241573. [Google Scholar] [CrossRef]
- Palmgren, A. Embryological and morphological studies on the mid-brain and cerebellum of vertebrates. Acta Zool. 1921, 2, 1–94. [Google Scholar] [CrossRef]
- Rendahl, H. Embryologische und morphologische Studien über das Zwischenhirn beim Huhn. Acta Zool. 1924, 5, 241–344. [Google Scholar] [CrossRef]
- Vaage, S. The segmentation of the primitive neural tube in chick embryos (Gallus domesticus): A morphological, histochemical and autoradiographical investigation. Ergebn. Anat. EntwGesch. 1969, 41, 3–87. [Google Scholar]
- Vaage, S. The histogenesis of the isthmic nuclei in chick embryos (Gallus domesticus). Anat. Embryol. 1973, 142, 283–314. [Google Scholar] [CrossRef]
- Keyser, A. The development of the diencephalon of the Chinese hamster: An investigation of the validity of the criteria of subdivision of the brain. Acta Anat. 1972, 83 (Suppl. S59), 1–178. [Google Scholar]
- Gribnau, A.A.M.; Geijsberts, L.G.M. Morphogenesis of the Brain in Staged Rhesus Monkey Embryos; Springer Science and Business Media LLC: Dordrecht, The Netherlands, 1985; pp. 1–69. ISBN 3540244743. [Google Scholar]
- Puelles, L.; Amat, J.A.; Martínez de la Torre, M. Segment-related, mosaic neurogenetic pattern in the forebrain and mesen-cephalon of early chick embryos. I. Topography of AChE-positive neuroblasts up to stage HH18. J. Comp. Neurol. 1987, 266, 147–268. [Google Scholar] [CrossRef] [PubMed]
- Puelles, L.; Rubenstein, J.L. Expression patterns of homeobox and other putative regulatory genes in the embryonic mouse forebrain suggest a neuromeric organization. Trends Neurosci. 1993, 16, 472–479. [Google Scholar] [CrossRef]
- Puelles, L.; Rubenstein, J.L. Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci. 2003, 26, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; van Velthoven, C.T.J.; Kunst, M.; Zhang, M.; McMillen, D.; Lee, C.; Jung, W.; Goldy, J.; Abdelhak, A.; Aitken, M.; et al. A high-resolution transcriptomic and spatial atlas of cell types in the whole mouse brain. Nature 2023, 624, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Cisek, P.; Kalaska, J.F. Neural mechanisms for interacting with a world full of action choices. Annu. Rev. Neurosci. 2010, 33, 269–298. [Google Scholar] [CrossRef] [PubMed]
- Cisek, P. Resynthesizing behavior through phylogenetic refinement. Attention, Perception, Psychophys. 2019, 81, 2265–2287. [Google Scholar] [CrossRef] [PubMed]
- Alexander, P.A. The Making of a Fly: The Genetics of Animal Design; Blackwell Scientific Publications: Oxford, UK, 1992. [Google Scholar]
- Kaufman, S.A. The Origins of Order: Self-Organization and Selection in Evolution; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- Davidson, E.H. Genomic Regulatory Systems. In Development and Evolution; Academic Press: San Diego, CA, USA, 2001. [Google Scholar]
- Davidson, E.H. The Regulatory Genome. In Gene Regulatory Networks in Development and Evolution; Academic Press: San Diego, CA, USA, 2006. [Google Scholar]
- Albuixech-Crespo, B.; Blanch, L.L.; Burguera, D.; Maeso, I.; Sánchez-Arrones, L.; Moreno-Bravo, J.A.; Somorjai, I.; Pascual-Anaya, J.; Puelles, E.; Bovolenta, P.; et al. Molecular regionalization of the developing amphioxus neural tube challenges major partitions of the vertebrate brain. PLoS Biol. 2017, 15, e2001573. [Google Scholar] [CrossRef] [PubMed]
- Bulfone, A.; Puelles, L.; Porteus, M.; Frohman, M.; Martin, G.; Rubenstein, J. Spatially restricted expression of Dlx-1, Dlx-2 (Tes-1), Gbx-2, and Wnt-3 in the embryonic day 12.5 mouse forebrain defines potential transverse and longitudinal segmental boundaries. J. Neurosci. 1993, 13, 3155–3172. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, J.L.R.; Martinez, S.; Shimamura, K.; Puelles, L. The Embryonic Vertebrate Forebrain: The Prosomeric Model. Science 1994, 266, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Diaz, C.; Puelles, L. Developmental genes and malformations in the hypothalamus. Front. Neuroanat. 2020, 14, 1–28. [Google Scholar] [CrossRef]
- Puelles, L.; Martinez-de-la-Torre, M.; Bardet, S.; Rubenstein, J.L.R. Hypothalamus. In The Mouse Nervous System; Watson, C., Paxinos, G., Puelles, L., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2012; pp. 221–312. [Google Scholar]
- Puelles, L. Role of secondary organizers in the evolution of forebrain development in vertebrates. In Handbook of Evolutionary Neuroscience; Shepherd, S.V., Ed.; Blackwell-Wiley: Chichester, UK, 2017; pp. 350–387. [Google Scholar]
- Thompson, C.L.; Ng, L.; Menon, V.; Martinez, S.; Lee, C.-K.; Glattfelder, K.; Sunkin, S.M.; Henry, A.; Lau, C.; Dang, C.; et al. A high resolution spatiotemporal atlas of gene expression of the C57Bl/6J developing mouse brain. Neuron 2014, 83, 309–323. [Google Scholar] [CrossRef]
- Martínez, S.; Puelles, E.; Puelles, L.; Echevarria, D. Molecular regionalization of developing neural tube. In The Mouse Nervous System; Chapter 1; Watson, C., Paxinos, G., Puelles, L., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2012; pp. 2–18. [Google Scholar]
- Kronman, F.A.; Liwang, J.K.; Betty, R.; Vanselow, D.J.; Wu, Y.-T.; Tustison, N.J.; Bhandiwad, A.; Manjila, S.B.; Minteer, J.A.; Shin, D.; et al. Developmental mouse brain common coordinate framework. BioRchiV 2024. submitted. [Google Scholar] [CrossRef]
- Ferrán, J.L.; Dutra de Oliveira, E.; Sánchez-Arrones, L.; Sandoval, J.E.; Martínez-de-la-Torre, M.; Puelles, L. Genoarchitectonic profile of developing nuclear groups in the chicken pretectum. J. Comp. Neurol. 2009, 517, 405–451. [Google Scholar] [CrossRef]
- Puelles, L.; Diaz, C.; Stühmer, T.; Ferran, J.L.; Martínez-de la Torre, M.; Rubenstein, J.L.R. LacZ-reporter mapping of Dlx5/6 expression and genoarchitectural analysis of the postnatal mouse prethalamus. J. Comp. Neurol. 2021, 529, 367–420. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates, 4th ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Redies, C.; Puelles, L. Modularity in CNS development. Bioessays 2001, 23, 1100–1111. [Google Scholar] [CrossRef] [PubMed]
- Díaz, C.; Glover, J.C.; Puelles, L.; Bjaalie, J.G. The relationship between hodological and cytoarchitectonic organization in the vestibular complex of the 11-day chicken embryo. J. Comp. Neurol. 2003, 457, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Wiener, S.I.; Taube, J.S. (Eds.) Head Direction Cells and the Neural Mechanisms of Spatial Orientation; A Bradford Book; MIT Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Mizumori, S.J.Y. (Ed.) Hippocampal Place Fields. In Relevance to Learning and Memory; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Puelles, L.; Medina, L. Development of neurons expressing tyrosine hydroxylase and dopamine in the chicken brain: A comparative segmental analysis. In Phylogeny and Development of Catecholamine Systems in the CNS of Vertebrates; Reiner, A., Smeets, W.J.A.J., Eds.; Cambridge University Press: Cambridge, UK, 1994; pp. 381–406. [Google Scholar]
- Alonso, A.; Merchán, P.; Sandoval, J.E.; Sánchez-Arrones, L.; Garcia-Cazorla, A.; Artuch, R.; Ferrán, J.L.; Martínez-De-La-Torre, M.; Puelles, L. Development of the serotonergic cells in murine raphe nuclei and their relations with rhombomeric domains. Anat. Embryol. 2012, 218, 1229–1277. [Google Scholar] [CrossRef]
- Lorente-Cánovas, B.; Marín, F.; Corral-San-Miguel, R.; Hidalgo-Sánchez, M.; Ferrán, J.L.; Puelles, L.; Aroca, P. Multiple origins, migratory paths and molecular profiles of cells populating the avian interpeduncular nucleus. Dev. Biol. 2012, 361, 12–26. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puelles, L. Functional Implications of the Prosomeric Brain Model. Biomolecules 2024, 14, 331. https://doi.org/10.3390/biom14030331
Puelles L. Functional Implications of the Prosomeric Brain Model. Biomolecules. 2024; 14(3):331. https://doi.org/10.3390/biom14030331
Chicago/Turabian StylePuelles, Luis. 2024. "Functional Implications of the Prosomeric Brain Model" Biomolecules 14, no. 3: 331. https://doi.org/10.3390/biom14030331
APA StylePuelles, L. (2024). Functional Implications of the Prosomeric Brain Model. Biomolecules, 14(3), 331. https://doi.org/10.3390/biom14030331





