Tricarboxylic Acid Cycle Intermediates and Individual Ageing
Abstract
1. Introduction
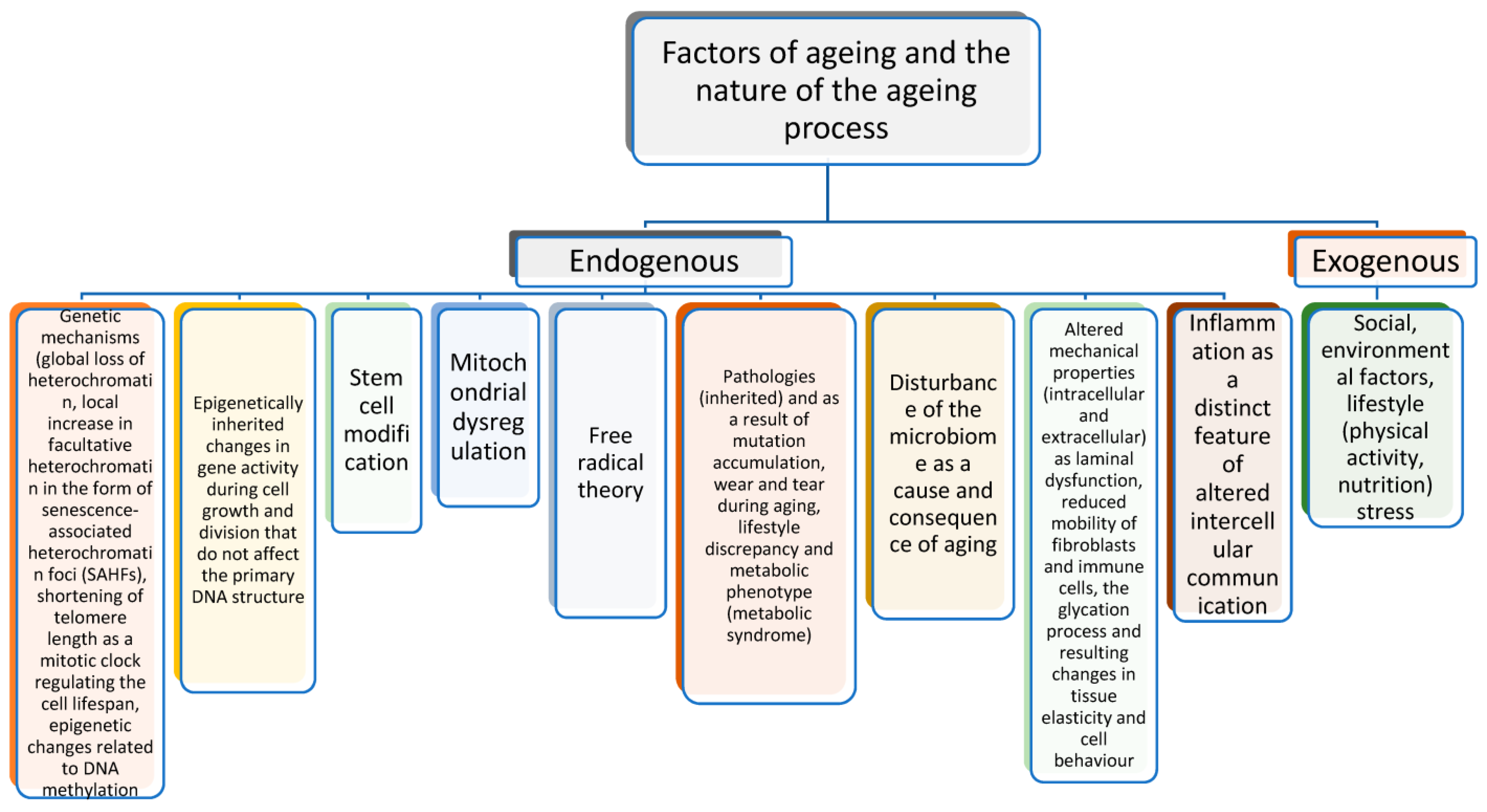
2. Biological Mechanisms of the Ageing Process
3. Biomarkers of Ageing
4. Accelerated Ageing and Age-Related Pathology
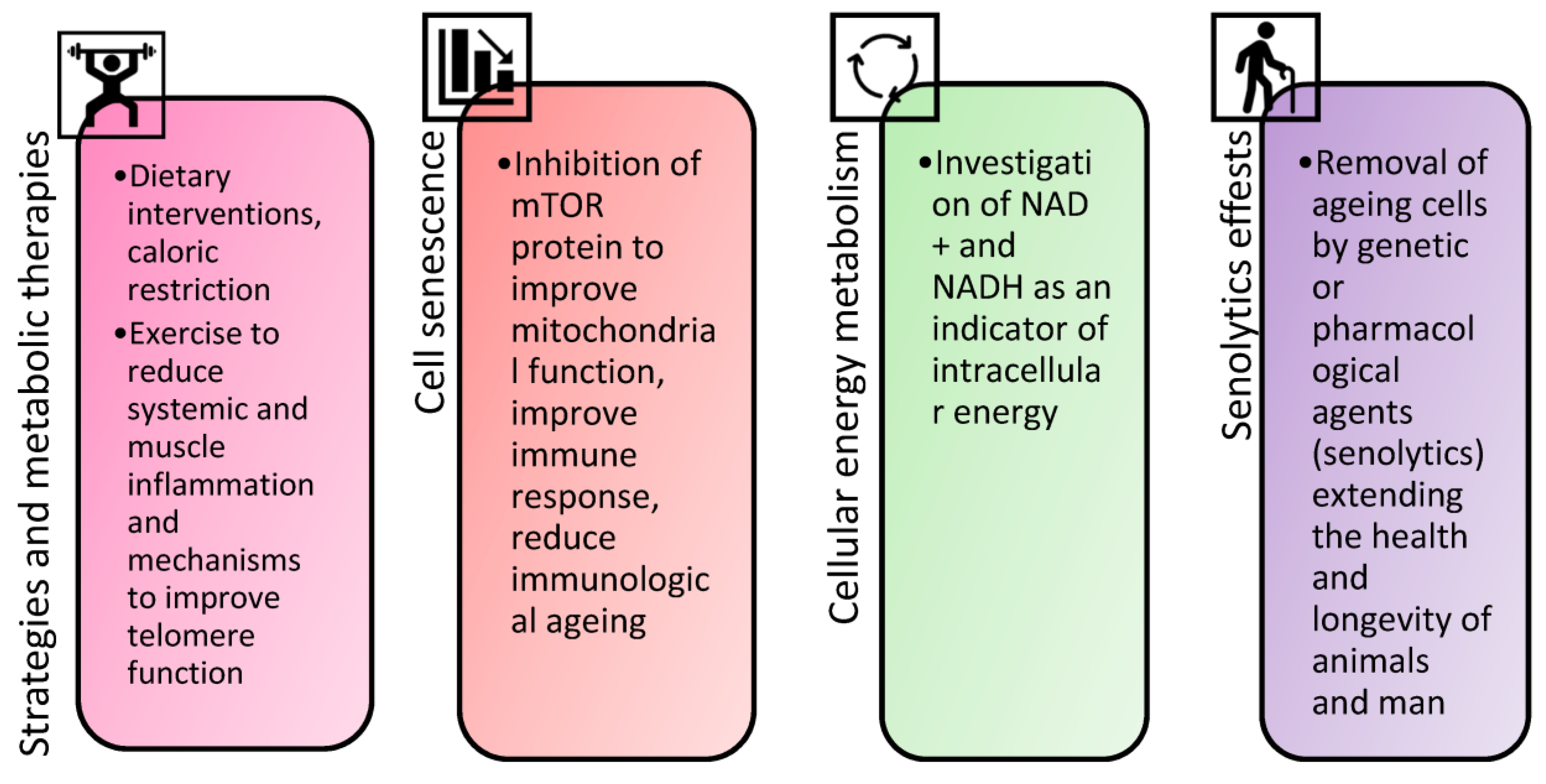
5. Krebs Cycle as the Key Component of Cellular Energy and Ageing
6. mTOR, Ageing, and Metabolism
7. α-Ketoglutarate, Energy Metabolism, and Ageing
8. Succinic Acid and Age-Related Pathologies
9. Different Levels of Initial Physiological Reactivity of the Organism Potentially Determine the Mechanisms of Ageing
10. Individual Ageing, Energy Metabolism, Hormonal Status, and Receptor Control
11. Biomarkers of Ageing and the Krebs Cycle
12. Neurohumoral Regulation, Metabolic Disorders, Substrates of the Krebs Cycle
13. Conclusions
Funding
Conflicts of Interest
References
- Lipsky, M.S.; King, M. Biological theories of aging. Dis. Mon. 2015, 61, 460–466. [Google Scholar] [CrossRef]
- Atchley, R.C. A continuity theory of normal aging. Gerontologist 1989, 29, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, J.M.; Lemaître, J.F. An integrative view of senescence in nature. Funct. Ecol. 2020, 34, 4–16. [Google Scholar] [CrossRef]
- Purchase, C.F.; Rooke, A.C.; Gaudry, M.J.; Treberg, J.R.; Mittell, E.A.; Morrissey, M.B.; Rennie, M.D. A synthesis of senescence predictions for indeterminate growth, and support from multiple tests in wild lake trout. Proc. Biol. Sci. 2022, 289, 20212146. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Møller, A.P. An explanation for negligible senescence in animals. Ecol. Evol. 2022, 12, e8970. [Google Scholar] [CrossRef] [PubMed]
- Tully, T.; Le Galliard, J.F.; Baron, J.P. Micro-geographic shift between negligible and actuarial senescence in a wild snake. J. Anim. Ecol. 2020, 89, 2704–2716. [Google Scholar] [CrossRef]
- Rejeski, W.J.; Fanning, J. Models and theories of health behavior and clinical interventions in aging: A contemporary, integrative approach. Clin. Interv. Aging 2019, 14, 1007–1019. [Google Scholar] [CrossRef]
- Pang, L.; Jiang, X.; Lian, X.; Chen, J.; Song, E.F.; Jin, L.G.; Xia, Z.Y.; Ma, H.C.; Cai, Y. Caloric restriction-mimetics for the reduction of heart failure risk in aging heart: With consideration of gender-related differences. Mil. Med. Res. 2022, 9, 33. [Google Scholar] [CrossRef]
- Martin, B.; Mattson, M.P.; Maudsley, S. Caloric restriction and intermittent fasting: Two potential diets for successful brain aging. Ageing Res. Rev. 2006, 5, 332–353. [Google Scholar] [CrossRef]
- McKendry, J.; Stokes, T.; Mcleod, J.C.; Phillips, S.M. Resistance Exercise, Aging, Disuse, and Muscle Protein Metabolism. Compr. Physiol. 2021, 11, 2249–2278. [Google Scholar] [CrossRef]
- Devranis, P.; Vassilopoulou, Ε.; Tsironis, V.; Sotiriadis, P.M.; Chourdakis, M.; Aivaliotis, M.; Tsolaki, M. Mediterranean Diet, Ketogenic Diet or MIND Diet for Aging Populations with Cognitive Decline: A Systematic Review. Life 2023, 13, 173. [Google Scholar] [CrossRef]
- Cummings, N.E.; Lamming, D.W. Regulation of metabolic health and aging by nutrient-sensitive signaling pathways. Mol. Cell. Endocrinol. 2017, 455, 13–22. [Google Scholar] [CrossRef]
- Ho, E.; Drake, V.J.; Michels, A.J.; Nkrumah-Elie, Y.M.; Brown, L.L.; Scott, J.M.; Newman, J.W.; Shukitt-Hale, B.; Soumyanath, A.; Chilton, F.H.; et al. Perspective: Council for Responsible Nutrition Science in Session. Optimizing Health with Nutrition-Opportunities, Gaps, and the Future. Adv. Nutr. 2023, 14, 948–958. [Google Scholar] [CrossRef]
- Bürkle, A.; Moreno-Villanueva, M.; Bernhard, J.; Blasco, M.; Zondag, G.; Hoeijmakers, J.H.; Toussaint, O.; Grubeck-Loebenstein, B.; Mocchegiani, E.; Collino, S.; et al. MARK-AGE biomarkers of ageing. Mech. Ageing Dev. 2015, 151, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, H.R.; Augustyniak, E.M.; Bennett, S.J.; Debacq-Chainiaux, F.; Dunston, C.R.; Kristensen, P.; Melchjorsen, C.J.; Navarrete, S.A.; Simm, A.; Toussaint, O. Novel ageing-biomarker discovery using data-intensive technologies. Mech. Ageing Dev. 2015, 151, 114–121. [Google Scholar] [CrossRef]
- Lukianova, L.D. Current issues of adaptation to hypoxia. Signal mechanisms and their role in system regulation. Patol. Fiziol. Eksp. Ter. 2011, 1, 3–19. (In Russian) [Google Scholar]
- Luk’ianova, L.D. Molecular mechanisms of tissue hypoxia and organism adaptation. Fiziol. Zh. 2003, 49, 17–35. (In Russian) [Google Scholar]
- Dzhalilova, D.S.; Silina, M.V.; Kosyreva, A.M.; Tsvetkov, I.S.; Makarova, O.V. Morphological and Molecular Biological Features of the Systemic Inflammatory Response in Old Wistar Rats with High and Low Resistance to Hypoxia. Bull. Exp. Biol. Med. 2023, 175, 704–710. [Google Scholar] [CrossRef]
- Kurhaluk, N.; Lukash, O.; Tkaczenko, H. Do the Effects of Krebs Cycle Intermediates on Oxygen-Dependent Processes in Hypoxia Mediated by the Nitric Oxide System Have Reciprocal or Competitive Relationships? Cell Physiol. Biochem. 2023, 57, 426–451. [Google Scholar] [CrossRef]
- Kurhaluk, N. Supplementation with l-arginine and nitrates vs. age and individual physiological reactivity. Nutr. Rev. 2023, nuad131. [Google Scholar] [CrossRef]
- da Costa, J.P.; Vitorino, R.; Silva, G.M.; Vogel, C.; Duarte, A.C.; Rocha-Santos, T. A synopsis on aging-Theories, mechanisms and future prospects. Ageing Res. Rev. 2016, 29, 90–112. [Google Scholar] [CrossRef]
- Mohamad Kamal, N.S.; Safuan, S.; Shamsuddin, S.; Foroozandeh, P. Aging of the cells: Insight into cellular senescence and detection Methods. Eur. J. Cell Biol. 2020, 99, 151108. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.S.D.S.A.; Maciel, Á.C.C.; Rolland, Y.; Vellas, B.; de Souto Barreto, P. Frailty biomarkers under the perspective of geroscience: A narrative review. Ageing Res. Rev. 2022, 81, 101737. [Google Scholar] [CrossRef]
- Boyd-Kirkup, J.D.; Green, C.D.; Wu, G.; Wang, D.; Han, J.D. Epigenomics and the regulation of aging. Epigenomics 2013, 5, 205–227. [Google Scholar] [CrossRef] [PubMed]
- Mc Auley, M.T. DNA methylation in genes associated with the evolution of ageing and disease: A critical review. Ageing Res. Rev. 2021, 72, 101488. [Google Scholar] [CrossRef]
- Simar, D.; Malatesta, D.; Koechlin, C.; Cristol, J.P.; Vendrell, J.P.; Caillaud, C. Effect of age on Hsp72 expression in leukocytes of healthy active people. Exp. Gerontol. 2004, 39, 1467–1474. [Google Scholar] [CrossRef]
- Maklakov, A.A.; Chapman, T. Evolution of ageing as a tangle of trade-offs: Energy versus function. Proc. Biol. Sci. 2019, 286, 20191604. [Google Scholar] [CrossRef]
- Pamplona, R.; Jové, M.; Gómez, J.; Barja, G. Whole organism aging: Parabiosis, inflammaging, epigenetics, and peripheral and central aging clocks. The ARS of aging. Exp. Gerontol. 2023, 174, 112137. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef]
- Marzetti, E.; Calvani, R.; Cesari, M.; Buford, T.W.; Lorenzi, M.; Behnke, B.J.; Leeuwenburgh, C. Mitochondrial dysfunction and sarcopenia of aging: From signaling pathways to clinical trials. Int. J. Biochem. Cell Biol. 2013, 45, 2288–2301. [Google Scholar] [CrossRef]
- Maruyama, Y. Aging and arterial-cardiac interactions in the elderly. Int. J. Cardiol. 2012, 155, 14–19. [Google Scholar] [CrossRef]
- Chang, J.Y.; Hong, H.J.; Kang, S.G.; Kim, J.T.; Zhang, B.Y.; Shong, M. The Role of Growth Differentiation Factor 15 in Energy Metabolism. Diabetes Metab. J. 2020, 44, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Kritsilis, M.; V Rizou, S.; Koutsoudaki, P.N.; Evangelou, K.; Gorgoulis, V.G.; Papadopoulos, D. Ageing, Cellular Senescence and Neurodegenerative Disease. Int. J. Mol. Sci. 2018, 19, 2937. [Google Scholar] [CrossRef]
- Wissler Gerdes, E.O.; Zhu, Y.; Weigand, B.M.; Tripathi, U.; Burns, T.C.; Tchkonia, T.; Kirkland, J.L. Cellular senescence in aging and age-related diseases: Implications for neurodegenerative diseases. Int. Rev. Neurobiol. 2020, 155, 203–234. [Google Scholar] [CrossRef]
- Lee, G. Cellular Senescence: The Villain of Metabolic Disease?: Discovery of a distinct senescent cell population in obesity-induced metabolic dysfunction. Mol. Cells 2022, 45, 531–533. [Google Scholar] [CrossRef]
- Schaum, N.; Lehallier, B.; Hahn, O.; Pálovics, R.; Hosseinzadeh, S.; Lee, S.E.; Sit, R.; Lee, D.P.; Losada, P.M.; Zardeneta, M.E.; et al. Ageing hallmarks exhibit organ-specific temporal signatures. Nature 2020, 583, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Young, L.; Barsotti, R. Mitochondria in cell senescence: A Friend or Foe? Adv. Protein Chem. Struct. Biol. 2023, 136, 35–91. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients 2019, 11, 1633. [Google Scholar] [CrossRef]
- Aging Biomarker Consortium; Bao, H.; Cao, J.; Chen, M.; Chen, M.; Chen, W.; Chen, X.; Chen, Y.; Chen, Y.; Chen, Y.; et al. Biomarkers of aging. Sci. China Life Sci. 2023, 66, 893–1066. [Google Scholar] [CrossRef] [PubMed]
- Junnila, R.K.; List, E.O.; Berryman, D.E.; Murrey, J.W.; Kopchick, J.J. The GH/IGF-1 axis in ageing and longevity. Nat. Rev. Endocrinol. 2013, 9, 366–376. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.V. Recent Progress in Regulation of Aging by Insulin/IGF-1 Signaling in Caenorhabditis elegans. Mol. Cells 2022, 45, 763–770. [Google Scholar] [CrossRef]
- Xu, C.; Wang, L.; Fozouni, P.; Evjen, G.; Chandra, V.; Jiang, J.; Lu, C.; Nicastri, M.; Bretz, C.; Winkler, J.D.; et al. SIRT1 is downregulated by autophagy in senescence and ageing. Nat. Cell Biol. 2020, 22, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Novak, R.M.; Armon, C.; Battalora, L.; Buchacz, K.; Li, J.; Ward, D.; Carlson, K.; Palella, F.J., Jr.; HIV Outpatient Study (HOPS) Investigators. Aging, trends in CD4+/CD8+ cell ratio, and clinical outcomes with persistent HIV suppression in a dynamic cohort of ambulatory HIV patients. AIDS 2022, 36, 815–827. [Google Scholar] [CrossRef]
- Lu, A.T.; Quach, A.; Wilson, J.G.; Reiner, A.P.; Aviv, A.; Raj, K.; Hou, L.; Baccarelli, A.A.; Li, Y.; Stewart, J.D.; et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging 2019, 11, 303–327. [Google Scholar] [CrossRef]
- Shultz, L.D.; Lyons, B.L.; Burzenski, L.M.; Gott, B.; Chen, X.; Chaleff, S.; Kotb, M.; Gillies, S.D.; King, M.; Mangada, J.; et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 2005, 174, 6477–6489. [Google Scholar] [CrossRef]
- Tyrrell, D.J.; Goldstein, D.R. Ageing and atherosclerosis: Vascular intrinsic and extrinsic factors and potential role of IL-6. Nat. Rev. Cardiol. 2021, 18, 58–68. [Google Scholar] [CrossRef]
- Tran Van Hoi, E.; De Glas, N.A.; Portielje, J.E.A.; Van Heemst, D.; Van Den Bos, F.; Jochems, S.P.; Mooijaart, S.P. Biomarkers of the ageing immune system and their association with frailty—A systematic review. Exp. Gerontol. 2023, 176, 112163. [Google Scholar] [CrossRef]
- Nyman, E.; Liv, P.; Wester, P.; Näslund, U.; Grönlund, C. Carotid wall echogenicity at baseline associates with accelerated vascular aging in a middle-aged population. Int. J. Cardiovasc. Imaging 2023, 39, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Wohlin, M.; Sundström, J.; Andrén, B.; Larsson, A.; Lind, L. An echolucent carotid artery intima-media complex is a new and independent predictor of mortality in an elderly male cohort. Atherosclerosis 2009, 205, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Livshits, G.; Kalinkovich, A. A cross-talk between sestrins, chronic inflammation and cellular senescence governs the development of age-associated sarcopenia and obesity. Ageing Res. Rev. 2023, 86, 101852. [Google Scholar] [CrossRef]
- Snyder, B.; Shell, B.; Cunningham, J.T.; Cunningham, R.L. Chronic intermittent hypoxia induces oxidative stress and inflammation in brain regions associated with early-stage neurodegeneration. Physiol. Rep. 2017, 5, e13258. [Google Scholar] [CrossRef]
- Cardoso, A.L.; Fernandes, A.; Aguilar-Pimentel, J.A.; de Angelis, M.H.; Guedes, J.R.; Brito, M.A.; Ortolano, S.; Pani, G.; Athanasopoulou, S.; Gonos, E.S.; et al. Towards frailty biomarkers: Candidates from genes and pathways regulated in aging and age-related diseases. Ageing Res. Rev. 2018, 47, 214–277. [Google Scholar] [CrossRef]
- Johnson, S.C.; Rabinovitch, P.S.; Kaeberlein, M. mTOR is a key modulator of ageing and age-related disease. Nature 2013, 493, 338–345. [Google Scholar] [CrossRef]
- Bjedov, I.; Rallis, C. The Target of Rapamycin Signalling Pathway in Ageing and Lifespan Regulation. Genes 2020, 11, 1043. [Google Scholar] [CrossRef]
- Johnson, S.C. Nutrient Sensing, Signaling and Ageing: The Role of IGF-1 and mTOR in Ageing and Age-Related Disease. Subcell. Biochem. 2018, 90, 49–97. [Google Scholar] [CrossRef]
- Kitada, M.; Xu, J.; Ogura, Y.; Monno, I.; Koya, D. Mechanism of Activation of Mechanistic Target of Rapamycin Complex 1 by Methionine. Front. Cell Dev. Biol. 2020, 8, 715. [Google Scholar] [CrossRef] [PubMed]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in healthy aging and disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef] [PubMed]
- Gans, I.; Hartig, E.I.; Zhu, S.; Tilden, A.R.; Hutchins, L.N.; Maki, N.J.; Graber, J.H.; Coffman, J.A. Klf9 is a key feedforward regulator of the transcriptomic response to glucocorticoid receptor activity. Sci. Rep. 2020, 10, 11415. [Google Scholar] [CrossRef]
- Shirakabe, A.; Ikeda, Y.; Sciarretta, S.; Zablocki, D.K.; Sadoshima, J. Aging and Autophagy in the Heart. Circ. Res. 2016, 118, 1563–1576. [Google Scholar] [CrossRef] [PubMed]
- Leon, L.J.; Gustafsson, Å.B. Staying young at heart: Autophagy and adaptation to cardiac aging. J. Mol. Cell. Cardiol. 2016, 95, 78–85. [Google Scholar] [CrossRef]
- Goldsmith, T.C. Evolution of Aging Theories: Why Modern Programmed Aging Concepts Are Transforming Medical Research. Biochemistry 2016, 81, 1406–1412. [Google Scholar] [CrossRef] [PubMed]
- Morsli, S.; Bellantuono, I. The use of geroprotectors to prevent multimorbidity: Opportunities and challenges. Mech. Ageing Dev. 2021, 193, 111391. [Google Scholar] [CrossRef] [PubMed]
- Trendelenburg, A.U.; Scheuren, A.C.; Potter, P.; Müller, R.; Bellantuono, I. Geroprotectors: A role in the treatment of frailty. Mech. Ageing Dev. 2019, 180, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Borkum, J.M. The Tricarboxylic Acid Cycle as a Central Regulator of the Rate of Aging: Implications for Metabolic Interventions. Adv. Biol. 2023, 7, e2300095. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.K.; Finley, L.W.S. Regulation and function of the mammalian tricarboxylic acid cycle. J. Biol. Chem. 2023, 299, 102838. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef]
- Bhullar, S.K.; Dhalla, N.S. Status of Mitochondrial Oxidative Phosphorylation during the Development of Heart Failure. Antioxidants 2023, 12, 1941. [Google Scholar] [CrossRef]
- Iverson, T.M.; Singh, P.K.; Cecchini, G. An evolving view of complex II-noncanonical complexes, megacomplexes, respiration, signaling, and beyond. J. Biol. Chem. 2023, 299, 104761. [Google Scholar] [CrossRef] [PubMed]
- Fedotcheva, N.I.; Sokolov, A.P.; Kondrashova, M.N. Nonezymatic formation of succinate in mitochondria under oxidative stress. Free Radic. Biol. Med. 2006, 41, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Mookerjee, S.A.; Gerencser, A.A.; Watson, M.A.; Brand, M.D. Controlled power: How biology manages succinate-driven energy release. Biochem. Soc. Trans. 2021, 49, 2929–2939. [Google Scholar] [CrossRef]
- Sheu, K.F.; Blass, J.P. The alpha-ketoglutarate dehydrogenase complex. Ann. N. Y. Acad. Sci. 1999, 893, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Omini, J.; Wojciechowska, I.; Skirycz, A.; Moriyama, H.; Obata, T. Association of the malate dehydrogenase-citrate synthase metabolon is modulated by intermediates of the Krebs tricarboxylic acid cycle. Sci. Rep. 2021, 11, 18770. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.; Ki, S.H.; Shin, S.M. Sestrin2-AMPK activation protects mitochondrial function against glucose deprivation-induced cytotoxicity. Cell. Signal. 2015, 27, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Parmigiani, A.; Divakaruni, A.S.; Archer, K.; Murphy, A.N.; Budanov, A.V. Sestrin2 is induced by glucose starvation via the unfolded protein response and protects cells from non-canonical necroptotic cell death. Sci. Rep. 2016, 6, 22538. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, L.B.; Luengo, A.; Danai, L.V.; Bush, L.N.; Diehl, F.F.; Hosios, A.M.; Lau, A.N.; Elmiligy, S.; Malstrom, S.; Lewis, C.A.; et al. Aspartate is an endogenous metabolic limitation for tumour growth. Nat. Cell Biol. 2018, 20, 782–788. [Google Scholar] [CrossRef]
- Tan, H.W.S.; Sim, A.Y.L.; Long, Y.C. Glutamine metabolism regulates autophagy-dependent mTORC1 reactivation during amino acid starvation. Nat. Commun. 2017, 8, 338. [Google Scholar] [CrossRef] [PubMed]
- Brace, L.E.; Vose, S.C.; Stanya, K.; Gathungu, R.M.; Marur, V.R.; Longchamp, A.; Treviño-Villarreal, H.; Mejia, P.; Vargas, D.; Inouye, K.; et al. Increased oxidative phosphorylation in response to acute and chronic DNA damage. NPJ Aging Mech. Dis. 2016, 2, 16022. [Google Scholar] [CrossRef]
- Lu, C.; Jiang, Y.; Xu, W.; Bao, X. Sestrin2: Multifaceted functions, molecular basis, and its implications in liver diseases. Cell Death Dis. 2023, 14, 160. [Google Scholar] [CrossRef]
- Garaeva, A.A.; Kovaleva, I.E.; Chumakov, P.M.; Evstafieva, A.G. Mitochondrial dysfunction induces SESN2 gene expression through Activating Transcription Factor 4. Cell Cycle 2016, 15, 64–71. [Google Scholar] [CrossRef]
- Barja, G.; Pamplona, R. mTORC1 is also involved in longevity between species. Aging 2021, 13, 14544–14545. [Google Scholar] [CrossRef]
- Wu, Z.; Isik, M.; Moroz, N.; Steinbaugh, M.J.; Zhang, P.; Blackwell, T.K. Dietary Restriction Extends Lifespan through Metabolic Regulation of Innate Immunity. Cell Metab. 2019, 29, 1192–1205.e8. [Google Scholar] [CrossRef]
- Kume, S. Pathophysiological roles of nutrient-sensing mechanisms in diabetes and its complications. Diabetol. Int. 2019, 10, 245–249. [Google Scholar] [CrossRef]
- Park, J.W.; Jeong, J.; Bae, Y.S. Protein Kinase CK2 Is Upregulated by Calorie Restriction and Induces Autophagy. Mol. Cells 2022, 45, 112–121. [Google Scholar] [CrossRef]
- Uvdal, P.; Shashkova, S. The Effect of Calorie Restriction on Protein Quality Control in Yeast. Biomolecules 2023, 13, 841. [Google Scholar] [CrossRef]
- Carosi, J.M.; Fourrier, C.; Bensalem, J.; Sargeant, T.J. The mTOR-lysosome axis at the centre of ageing. FEBS Open Biol. 2022, 12, 739–757. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, M.; Miyazaki, I. Glutathione and Related Molecules in Parkinsonism. Int. J. Mol. Sci. 2021, 22, 8689. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, S.; Ping, Z.; Li, Y.; Jiang, T.; Zheng, X.; Zhang, Z.; Wang, G.; Liu, Z.; Sun, H.; et al. Age-Induced Accumulation of Succinate Promotes Cardiac Fibrogenesis. Circ. Res. 2023, 1–23. [Google Scholar] [CrossRef]
- Oh, C.J.; Kim, M.J.; Lee, J.M.; Kim, D.H.; Kim, I.Y.; Park, S.; Kim, Y.; Lee, K.B.; Lee, S.H.; Lim, C.W.; et al. Inhibition of pyruvate dehydrogenase kinase 4 ameliorates kidney ischemia-reperfusion injury by reducing succinate accumulation during ischemia and preserving mitochondrial function during reperfusion. Kidney Int. 2023, 104, 724–739. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Yang, H.; Dai, C.; Xu, F.; Peng, S.; Zhao, Y.; Zhao, C.; Zhao, L. Expression of hypoxia inducible factor-1α and its correlation with phosphoenolpyruvate carboxykinase after portal vein ligation in rats. Life Sci. 2017, 190, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Balazs, R. Control of glutamate metabolism. The effect of pyruvate. J. Neurochem. 1965, 12, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Balazs, R. Control of glutamate oxidation in brain and liver mitochondrial systems. Biochem. J. 1965, 95, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Yudkoff, M.; Nelson, D.; Daikhin, Y.; Erecińska, M. Tricarboxylic acid cycle in rat brain synaptosomes. Fluxes and interactions with aspartate aminotransferase and malate/aspartate shuttle. J. Biol. Chem. 1994, 269, 27414–27420. [Google Scholar] [CrossRef]
- Panov, A.; Orynbayeva, Z. Determination of mitochondrial metabolic phenotype through investigation of the intrinsic inhibition of succinate dehydrogenase. Anal. Biochem. 2018, 552, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.; Walsh, J.M.; Dennis, S.C.; Clark, J.B. Synaptic and non-synaptic mitochondria from rat brain: Isolation and characterization. J. Neurochem. 1977, 28, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Panov, A.; Scarpa, A. Independent modulation of the activity of alpha-ketoglutarate dehydrogenase complex by Ca2+ and Mg2+. Biochemistry 1996, 35, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Zeng, L.; Yao, K.; Kong, X.; Wu, G.; Yin, Y. The glutamine-alpha-ketoglutarate (AKG) metabolism and its nutritional implications. Amino Acids 2016, 48, 2067–2080. [Google Scholar] [CrossRef]
- Liu, S.; He, L.; Yao, K. The Antioxidative Function of Alpha-Ketoglutarate and Its Applications. BioMed Res. Int. 2018, 2018, 3408467. [Google Scholar] [CrossRef]
- He, L.; Xu, Z.; Yao, K.; Wu, G.; Yin, Y.; Nyachoti, C.M.; Kim, S.W. The Physiological Basis and Nutritional Function of Alpha-ketoglutarate. Curr. Protein Pept. Sci. 2015, 16, 576–581. [Google Scholar] [CrossRef]
- Guo, S.; Duan, R.; Wang, L.; Hou, Y.; Tan, L.; Cheng, Q.; Liao, M.; Ding, B. Dietary α-ketoglutarate supplementation improves hepatic and intestinal energy status and anti-oxidative capacity of Cherry Valley ducks. Anim. Sci. J. 2017, 88, 1753–1762. [Google Scholar] [CrossRef]
- Chin, R.M.; Fu, X.; Pai, M.Y.; Vergnes, L.; Hwang, H.; Deng, G.; Diep, S.; Lomenick, B.; Meli, V.S.; Monsalve, G.C.; et al. The metabolite α-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature 2014, 510, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Chitalia, V. α-Ketoglutarate-A New Currency of Longevity. Sci. Transl. Med. 2014, 6, 244ec117. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wang, T.; Wu, N.; Li, D.; Fan, X.; Xu, Z.; Mishra, S.K.; Yang, M. Alpha-ketoglutarate extends Drosophila lifespan by inhibiting mTOR and activating AMPK. Aging 2019, 11, 4183–4197. [Google Scholar] [CrossRef] [PubMed]
- Naeini, S.H.; Mavaddatiyan, L.; Kalkhoran, Z.R.; Taherkhani, S.; Talkhabi, M. Alpha-ketoglutarate as a potent regulator for lifespan and healthspan: Evidences and perspectives. Exp. Gerontol. 2023, 175, 112154. [Google Scholar] [CrossRef] [PubMed]
- Gyanwali, B.; Lim, Z.X.; Soh, J.; Lim, C.; Guan, S.P.; Goh, J.; Maier, A.B.; Kennedy, B.K. Alpha-Ketoglutarate dietary supplementation to improve health in humans. Trends Endocrinol. Metab. 2022, 33, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Bayliak, M.M.; Lushchak, V.I. Pleiotropic effects of alpha-ketoglutarate as a potential anti-ageing agent. Ageing Res. Rev. 2021, 66, 101237. [Google Scholar] [CrossRef] [PubMed]
- Asadi Shahmirzadi, A.; Edgar, D.; Liao, C.Y.; Hsu, Y.M.; Lucanic, M.; Asadi Shahmirzadi, A.; Wiley, C.D.; Gan, G.; Kim, D.E.; Kasler, H.G.; et al. Alpha-Ketoglutarate, an Endogenous Metabolite, Extends Lifespan and Compresses Morbidity in Aging Mice. Cell Metab. 2020, 32, 447–456.e6. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, T.W.; Anderson, R.M. Alpha-Ketoglutarate, the Metabolite that Regulates Aging in Mice. Cell Metab. 2020, 32, 323–325. [Google Scholar] [CrossRef]
- Zdzisińska, B.; Żurek, A.; Kandefer-Szerszeń, M. Alpha-Ketoglutarate as a Molecule with Pleiotropic Activity: Well-Known and Novel Possibilities of Therapeutic Use. Arch. Immunol. Ther. Exp. 2017, 65, 21–36. [Google Scholar] [CrossRef]
- Yang, F.; Zhou, Z.; Guo, M.; Zhou, Z. The study of skin hydration, anti-wrinkles function improvement of anti-aging cream with alpha-ketoglutarate. J. Cosmet. Dermatol. 2022, 21, 1736–1743. [Google Scholar] [CrossRef]
- Kaławaj, K.; Sławińska-Brych, A.; Mizerska-Kowalska, M.; Żurek, A.; Bojarska-Junak, A.; Kandefer-Szerszeń, M.; Zdzisińska, B. Alpha Ketoglutarate Exerts In Vitro Anti-Osteosarcoma Effects through Inhibition of Cell Proliferation, Induction of Apoptosis via the JNK and Caspase 9-Dependent Mechanism, and Suppression of TGF-β and VEGF Production and Metastatic Potential of Cells. Int. J. Mol. Sci. 2020, 21, 9406. [Google Scholar] [CrossRef] [PubMed]
- Grimolizzi, F.; Arranz, L. Multiple faces of succinate beyond metabolism in blood. Haematologica 2018, 103, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, J.; Buckingham, J.A.; Roebuck, S.; Brand, M.D. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 2002, 277, 44784–44790. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, C.L.; Perevoshchikova, I.V.; Hey-Mogensen, M.; Orr, A.L.; Brand, M.D. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol. 2013, 1, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Else, T. 15 years of paraganglioma: Pheochromocytoma, paraganglioma and genetic syndromes: A historical perspective. Endocr. Relat. Cancer 2015, 22, T147–T159. [Google Scholar] [CrossRef] [PubMed]
- Boulahbel, H.; Durán, R.V.; Gottlieb, E. Prolyl hydroxylases as regulators of cell metabolism. Biochem. Soc. Trans. 2009, 37 Pt 1, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Piruat, J.I.; Millán-Uclés, A. Genetically modeled mice with mutations in mitochondrial metabolic enzymes for the study of cancer. Front. Oncol. 2014, 4, 200. [Google Scholar] [CrossRef]
- Piantadosi, C.A.; Suliman, H.B. Transcriptional Regulation of SDHa flavoprotein by nuclear respiratory factor-1 prevents pseudo-hypoxia in aerobic cardiac cells. J. Biol. Chem. 2008, 283, 10967–10977. [Google Scholar] [CrossRef]
- Domalpally, A.; Whittier, S.A.; Pan, Q.; Dabelea, D.M.; Darwin, C.H.; Knowler, W.C.; Lee, C.G.; Luchsinger, J.A.; White, N.H.; Chew, E.Y. Diabetes Prevention Program Research (DPPOS) Group. Association of Metformin With the Development of Age-Related Macular Degeneration. JAMA Ophthalmol. 2023, 141, 140–147. [Google Scholar] [CrossRef]
- Nishima, N.; Tanaka, S. Suppressing succinate accumulation during ischemia protects the kidney from IRI. Kidney Int. 2023, 104, 646–649. [Google Scholar] [CrossRef]
- Bogolepova, A.N. Posleoperatsionnaya kognitivnaya disfunktsiya [Postoperative cognitive dysfunction]. Zh. Nevrol. Psikhiatr. Im. S S Korsakova. 2022, 122, 7–11. (In Russian) [Google Scholar] [CrossRef] [PubMed]
- Mateska, I.; Witt, A.; Hagag, E.; Sinha, A.; Yilmaz, C.; Thanou, E.; Sun, N.; Kolliniati, O.; Patschin, M.; Abdelmegeed, H.; et al. Succinate mediates inflammation-induced adrenocortical dysfunction. Elife 2023, 12, e83064. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, S.; Suresh, A.P.; Mangal, J.L.; Ng, N.D.; Sundem, A.; Behbahani, H.S.; Rubino, T.E., Jr.; Yaron, J.R.; Khodaei, T.; Green, M.; et al. Succinate in the tumor microenvironment affects tumor growth and modulates tumor associated macrophages. Biomaterials 2023, 301, 122292. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Y.; Zhang, X.; Xiong, X.; Zhou, F.; Li, X.; Fan, J.; Liang, X.; Li, G.; Peng, Y.; et al. Mitochondrial damage-induced abnormal glucose metabolism with ageing in the hippocampus of APP/PS1 mice. Metabolomics 2023, 19, 56. [Google Scholar] [CrossRef] [PubMed]
- Mota-Martorell, N.; Jové, M.; Berdún, R.; Pamplona, R. Plasma methionine metabolic profile is associated with longevity in mammals. Commun. Biol. 2021, 4, 725. [Google Scholar] [CrossRef]
- Knauf, F.; Mohebbi, N.; Teichert, C.; Herold, D.; Rogina, B.; Helfand, S.; Gollasch, M.; Luft, F.C.; Aronson, P.S. The life-extending gene Indy encodes an exchanger for Krebs-cycle intermediates. Biochem. J. 2006, 397, 25–29. [Google Scholar] [CrossRef]
- Serebrovskaya, T.V.; Xi, L. Individualized intermittent hypoxia training: Principles and practices. In Intermittent Hypoxia and Human Diseases; Xi, L., Serebrovskaya, T., Eds.; Springer: London, UK, 2012. [Google Scholar]
- Strauss, E.; Waliszewski, K.; Oszkinis, G.; Staniszewski, R. Polymorphisms of genes involved in the hypoxia signaling pathway and the development of abdominal aortic aneurysms or large-artery atherosclerosis. J. Vasc. Surg. 2015, 61, 1105–1113. [Google Scholar] [CrossRef]
- Hou, Y.P.; Wu, J.L.; Tan, C.; Chen, Y.; Guo, R.; Luo, Y.J. Sex-based differences in the prevalence of acute mountain sickness: A meta-analysis. Mil. Med. Res. 2019, 6, 38. [Google Scholar] [CrossRef]
- Chernobaeva, G.N.; Lukyanova, L.D. The role of individual resistance to the hypoxic factor in the search for antihypoxic agents and assessing the effectiveness of their action. In Pharmacological Correction of Hypoxic Conditions; Lukyanova, L.D., Ed.; ACS Publications: Moskow, Russia, 1989. [Google Scholar]
- Hordiĭ, S.K.; Shostakovska, I.V.; Doliba, M.M.; Babskyĭ, A.M.; Muzyka, F.V.; Kondrashova, M.M.; Stefankiv, I.S. Adrenergic and cholinergic regulation of respiratory efficiency of secretory cells. Fiziol. Zh. 1994, 40, 46–56. (In Ukrainian) [Google Scholar]
- Kurhaluk, N.; Lukash, O.; Nosar, V.; Portnychenko, A.; Portnichenko, V.; Wszedybyl-Winklewska, M.; Winklewski, P.J. Liver mitochondrial respiratory plasticity and oxygen uptake evoked by cobalt chloride in rats with low and high resistance to extreme hypobaric hypoxia. Can. J. Physiol. Pharmacol. 2019, 97, 392–399. [Google Scholar] [CrossRef]
- Doliba, M.M.; Kurhaliuk, N.M.; Muzyka, F.V.; Shostakovska, I.V.; Kondrashova, M.M. Effect of alpha-ketoglutarate and acetylcholine synergism on energy metabolism in mitochondria. Fiziol. Zh. 1993, 39, 65–70. (In Ukrainian) [Google Scholar]
- Shostakovs’ka, I.V.; Kurhaliuk, N.M.; Berhtraum, D.I.; Doliba, M.M. The effect of the parenteral administration of alpha-ketoglutarate on the resistance of rats to ionizing radiation and on their cholinergic systems. Fiziol. Zh. 1999, 45, 119–126. [Google Scholar]
- Kurhaluk, N. The Effectiveness of L-arginine in Clinical Conditions Associated with Hypoxia. Int. J. Mol. Sci. 2023, 24, 8205. [Google Scholar] [CrossRef]
- Kurhaliuk, N.M.; Kotsiuruba, A.V.; Sahach, V.F. The modification of nitric oxide production by exogenous substrates of Krebs cycle during acute hypoxia. Fiziolohichnyi Zhurnal 2005, 51, 20–28. [Google Scholar] [PubMed]
- Kurhaliuk, N.M.; Serebrovs’ka, T.V. Tricarboxylic acid cycle in energy metabolism and antioxidant cell defense in acute hypoxia. Fiziol. Zh. 2003, 49, 104–109. [Google Scholar]
- Salminen, A.; Kauppinen, A.; Hiltunen, M.; Kaarniranta, K. Krebs cycle intermediates regulate DNA and histone methylation: Epigenetic impact on the aging process. Ageing Res. Rev. 2014, 16, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Crake, R.L.I.; Burgess, E.R.; Royds, J.A.; Phillips, E.; Vissers, M.C.M.; Dachs, G.U. The Role of 2-Oxoglutarate Dependent Dioxygenases in Gliomas and Glioblastomas: A Review of Epigenetic Reprogramming and Hypoxic Response. Front. Oncol. 2021, 11, 619300. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Hiltunen, M.; Kauppinen, A. Krebs cycle dysfunction shapes epigenetic landscape of chromatin: Novel insights into mitochondrial regulation of aging process. Cell. Signal. 2014, 26, 1598–1603. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. 2-Oxoglutarate-dependent dioxygenases are sensors of energy metabolism, oxygen availability, and iron homeostasis: Potential role in the regulation of aging process. Cell. Mol. Life Sci. 2015, 72, 3897–3914. [Google Scholar] [CrossRef] [PubMed]
- Karuppagounder, S.S.; Kumar, A.; Shao, D.S.; Zille, M.; Bourassa, M.W.; Caulfield, J.T.; Alim, I.; Ratan, R.R. Metabolism and epigenetics in the nervous system: Creating cellular fitness and resistance to neuronal death in neurological conditions via modulation of oxygen-, iron-, and 2-oxoglutarate-dependent dioxygenases. Brain Res. 2015, 1628 Pt B, 273–287. [Google Scholar] [CrossRef]
- O’Neill, L.A. Biochemistry: Succinate strikes. Nature 2014, 515, 350–351. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, P.K.; Wise, S.Y.; Seed, T.M. Mobilized progenitor cells as a bridging therapy for radiation casualties: A brief review of tocopherol succinate-based approaches. Int. Immunopharmacol. 2011, 11, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Khazanov, V.A.; Kiseliova, A.A.; Vasiliev, K.Y.; Chernyschova, G.A. Cardioprotective effects of trimetazidine and a combination of succinic and malic acids in acute myocardial ischemia. Bull. Exp. Biol. Med. 2008, 146, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Effendi, N.; Mishiro, K.; Wakabayashi, H.; Gabryel-Skrodzka, M.; Shiba, K.; Taki, J.; Jastrząb, R.; Kinuya, S.; Ogawa, K. Synthesis and evaluation of radiogallium-labeled long-chain fatty acid derivatives as myocardial metabolic imaging agents. PLoS ONE 2021, 16, e0261226. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, N.; Morita, K.; Kuge, Y.; Tsukamoto, E. The role of fatty acids in cardiac imaging. J. Nucl. Med. 2000, 41, 1525–1534. [Google Scholar] [PubMed]
- Dyck, J.R.; Cheng, J.F.; Stanley, W.C.; Barr, R.; Chandler, M.P.; Brown, S.; Wallace, D.; Arrhenius, T.; Harmon, C.; Yang, G.; et al. Malonyl coenzyme a decarboxylase inhibition protects the ischemic heart by inhibiting fatty acid oxidation and stimulating glucose oxidation. Circ. Res. 2004, 94, e78–e84. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Lee, H.; Chanda, D.; Thoudam, T.; Kang, H.J.; Harris, R.A.; Lee, I.K. The Role of Pyruvate Metabolism in Mitochondrial Quality Control and Inflammation. Mol. Cells 2023, 46, 259–267. [Google Scholar] [CrossRef]
- Labarthe, F.; Khairallah, M.; Bouchard, B.; Stanley, W.C.; Des Rosiers, C. Fatty acid oxidation and its impact on response of spontaneously hypertensive rat hearts to an adrenergic stress: Benefits of a medium-chain fatty acid. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1425–H1436. [Google Scholar] [CrossRef]
- Goodwin, G.W.; Taegtmeyer, H. Improved energy homeostasis of the heart in the metabolic state of exercise. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H1490–H1501. [Google Scholar] [CrossRef]
- Eelen, G.; de Zeeuw, P.; Treps, L.; Harjes, U.; Wong, B.W.; Carmeliet, P. Endothelial Cell Metabolism. Physiol. Rev. 2018, 98, 3–58. [Google Scholar] [CrossRef]
- Okovityĭ, S.V.; Sukhanov, D.S.; Zaplutanov, V.A.; Smagina, A.N. Antihypoxants in current clinical practice. Klin. Med. 2012, 90, 63–68. [Google Scholar]
- Panconesi, R.; Widmer, J.; Carvalho, M.F.; Eden, J.; Dondossola, D.; Dutkowski, P.; Schlegel, A. Mitochondria and ischemia reperfusion injury. Curr. Opin. Organ. Transplant. 2022, 27, 434–445. [Google Scholar] [CrossRef]
- Dambrova, M.; Zuurbier, C.J.; Borutaite, V.; Liepinsh, E.; Makrecka-Kuka, M. Energy substrate metabolism and mitochondrial oxidative stress in cardiac ischemia/reperfusion injury. Free Radic. Biol. Med. 2021, 165, 24–37. [Google Scholar] [CrossRef]
- Mills, E.; O’Neill, L.A. Succinate: A metabolic signal in inflammation. Trends Cell Biol. 2014, 24, 313–320. [Google Scholar] [CrossRef]
- Jiang, S.; Yan, W. Succinate in the cancer-immune cycle. Cancer Lett. 2017, 390, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.S.; Bagwe Parab, S.; Kaur, G. Promising effects of emoxypine and its succinate derivative in the management of various diseases-with insights on recent patent applications. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100121. [Google Scholar] [CrossRef] [PubMed]
- Zuurbier, C.J.; Bertrand, L.; Beauloye, C.R.; Andreadou, I.; Ruiz-Meana, M.; Jespersen, N.R.; Kula-Alwar, D.; Prag, H.A.; Eric Botker, H.; Dambrova, M.; et al. Cardiac metabolism as a driver and therapeutic target of myocardial infarction. J. Cell. Mol. Med. 2020, 24, 5937–5954. [Google Scholar] [CrossRef] [PubMed]


| Model | Description | Possible Mechanisms | Authors |
|---|---|---|---|
| Lifespan of adult Caenorhabditis elegans, proposal of new strategies for the prevention and treatment of ageing and age-related diseases in both C. elegans and mammalian cells | Molecular mechanisms underlying ad libitum feeding processes, dietary restriction connected with the lifespan and age-related diseases in evolutionarily diverse organisms | AKG impact inhibited ATP synthase and reduced the ATP content, which decreased oxygen consumption and increased autophagy processes | [101] |
| Caenorhabditis elegans as a model system, C. elegans orthologue of the ATP5B model, effects of modulators of worm longevity | Critical role of AKG in prolonging the lifespan, mediator of autophagy by inhibition of the TOR mechanism | ATP synthase as a potential target, mechanism of inhibition of TOR by AKG, the relationship of ATP58 inhibition with TOR target of rapamycin (TOR), 5′ adenosine monophosphate-activated protein kinase (AMPK), and FoxO | [102] |
| Drosophila fruit fly model receiving diets with 5 μM AKG | Heat shock protein genes (Hsp22 and Hsp70), mRNA expression, cry, FoxO, HNF4, p300, Sirt1, AMPKα, HDAC4, PI3K, TORC, PGC, and SREBP genes | ATP/ADP ratio, increased autophagy processes, AMPK, activation of AMPK signalling, inhibition of the mTOR pathway | [103] |
| Analysis of AKG effects on roundworm Caenorhabditis elegans (C. elegans), Drosophila, mice, and humans as models in different ageing and longevity studies | The review discusses different AKG effects on the lifespan depending on the animal (C. elegans, Drosophila, mice) and human models | Regulation of stem cell behaviours, modulation of the level of inflammation processes, activation of the Nrf2/ARE signalling pathway, and ROS reduction, the AMPK signalling pathway and downregulation of mTOR | [104] |
| Potential therapeutic use in humans to treat age-related diseases, clinical studies of therapeutic interventions | Potential positive effects of AKG on muscle growth, wound healing, and promotion of faster recovery after surgery; dietary supplementation in humans | Antioxidant properties; cellular respiration processes, one of the key regulators of the citric acid cycle | [105] |
| Analysis and discussion of data from different sources obtained in different models: nematodes, fruit flies, yeast, and mice, and limitations of AKG use as a geroprotective agent | Potential anti-ageing effects and geroprotective action of AKG through mimicking of calorie restriction and properties of a hormesis agent | Modulated energy production mechanisms connected with Krebs cycle functioning, production of moderate levels of ROS according to the hormesis conception, impact on DNA obligate substrate and histone demethylases processes, direct antioxidant properties | [106] |
| Analysis of alpha-ketoglutarate calcium salt on the healthspan and lifespan in C57BL/6 mice | Series of longitudinal and clinical experiments on a longer and healthier life in the murine model, by a mechanism reducing frailty and enhancing longevity, and compression of morbidity | Decrease in the levels of systemic inflammatory cytokines, namely IL-10 | [107] |
| Calcium alpha-ketoglutarate salt dietary supplementation in the murine model, frailty index study, analysis of the efficacy of processes in boosting health and longevity models | Significant increase in the median and maximal lifespan in mice, a decrease in the proportion of life in which mice were frail, reduction in frailty scores of females and males after the impact of the AKG diet. Only AKG-fed females were protected against age-dependent increases in circulating inflammatory cytokines | AKG as an important agent of the ageing regulatory pathway, an amino acid metabolism player, and a partner in aminotransferase reactions, reactions involved in chromatin modification, immune and inflammatory pathways, growth regulation, and epigenetic regulation of gene expression | [108] |
| Therapeutic use of AKG in different metabolic pathological processes and for treatment of diseases | The involvement of AKG in multiple metabolic and cellular pathways; the metabolite as an important key factor in amino acid biosynthesis, epigenetic processes, cellular signalling, a transcription factor in cancer development and progression, protein deficiency oxidative stress conditions, an immunomodulatory agent, and a bone anabolic factor | Endogenous intermediary metabolite in the Krebs cycle, hydroxylation reactions on various types of substrates, hypoxia-inducible factor, oxidative stress | [109] |
| Clinical study testing in vitro methods; 28 days of treatment with the use of AKG-containing cream; epidermal keratinocyte proliferation assays | Assessment of skin wrinkles, texture, elasticity, and firmness in vitro using capillary electrophoresis time-of-flight mass spectrometry assays and rice-fermented liquid | AKG significantly reduced skin wrinkles and had anti-ageing effects on epidermal keratinocyte proliferation in the skin | [110] |
| Human osteosarcoma (OS) cell lines Saos-2 (HTB-85TM) and HOS (CRL-1543TM) model; alpha-ketoglutarate disodium salt dihydrate impact, in vitro study | Anti-osteosarcoma effects of AKG supplementation in an in vitro study analysis; JNK pathway, Bax/Bcl-2 ratio, caspase-9 and caspase-3; pro-metastatic TGF-β, and pro-angiogenic VEGF cytokines | Pro-apoptotic effect of AKG, with its anti-metastatic potential linked with inhibition of OS cell motility | [111] |
| Model | Description | Possible Mechanisms | Authors |
|---|---|---|---|
| SUCNR1−/− mouse model and an AAV9-based approach, older mice/human model, succinate receptor as SUCNR1/GPR91 in analysis of fibrosis processes in old animals, diastolic dysfunction process depending on age | Succinate promoted fibroblast activation and apoptosis resistance in both young and old mice via succinate receptor SUCNR1 and stimulation of PKM2 dimerisation | Dimeric PKM2 translocated to the nucleus and mitochondria, where it promoted fibroblast activation and apoptosis resistance via interaction with HIF-1α; the metformin impact as a mediated succinate-dependent mechanism agent may inhibit fibroblast activation and apoptosis resistance in a murine model | [88] |
| In vitro model of ischaemia–reperfusion kidney injury in mice; proximal tubule cell-specific Pdk4 knockout (Pdk4ptKO) murine model, pyruvate dehydrogenase kinase 4 deficiency analysis | Knockout or pharmacological inhibition of the PDK4 pathway ameliorated ischaemia–reperfusion kidney damage caused by a cell-permeable form of succinate, i.e., dimethyl succinate, and mitochondrial ROS generation processes | Inhibition of PDK4 prevents in vitro ischaemia–reperfusion kidney injury via the reduction in succinate accumulation and mitochondrial dysfunction | [89] |
| Analysis of postoperative cognitive dysfunction processes, gerontological patients after cardiac surgery, and a cognitive impairment model, people over 60 years of age, Cytoflavin containing succinic acid | Cytoflavin containing inosine, nicotinamide, riboflavin, and succinic acid was used in elderly postoperative patients in a multicenter, double-blind, placebo-controlled, and randomised study | Improvement of gerontological patients’ condition | [121] |
| Study of steroidogenic adrenocortical cells in LPS-induced systemic inflammation processes in a murine model, succinate–succinate dehydrogenase relationship | Increased succinate levels by disruption of oxidative phosphorylation and increased ATP synthesis connected with high ROS production | SDHB expression via upregulation of DNA methyltransferase 1 and methylation processes in the SDHB promoter | [122] |
| Review analysis of the physiological and pathophysiological condition connected with succinic acid metabolism and SDH functions | Succinate functions and hypoxia-inducible factor (HIF)-1α, development of pseudohypoxia and tumours via mutated SDH, succinate functions in metabolic or non-metabolic pathways, lysine succinylation process as proteins and immunomodulatory modification levels, blood formation or haematopoiesis | Activation of succinate receptor 1 (SUCNR1), G protein-coupled receptor 91 (GPR91), or hypoxia-inducible factor-1α, (HIF)-1α | [112] |
| In vivo ischaemia–reperfusion of heart in an open-chest mouse model, metabolomic analysis of ex vivo Langendorff heart experiments | Study of succinate-dependent mitochondrial superoxide production in myoblasts | Inhibition of ischaemic succinate accumulation and its oxidation as an effective way in ischaemia–reperfusion conditions | [123] |
| Immune-defective ageing murine model, clinically relevant BRAFV600E mutated YUMM1.1 melanoma tumour model, cancer immunotherapies | Tumour microenvironment study using polyethylene succinate microparticle biomaterial | Succinate-mediated immune and cancer cell responses in a tumour model and immunotherapies | [124] |
| Hippocampus of different aged APP/PS1 double transgenic AD mice, analysis of the β-amyloid level with the immunohistochemistry method | 3, 6, 9, and 12-month-old mice groups, learning and memory test analysis, mitochondrial damage, and autophagosome accumulation assays | Abnormal accumulation of succinic acid and citric acid associated with age-related damage to hippocampal mitochondria in the APP/PS1 murine model | [125] |
| Comparative approach aimed at determination of the plasma methionine metabolic profile using an LC-MS/MS platform from 11 mammalian species with a longevity ranging from 3.5 to 120 years | Species longevity-specific plasma profile of methionine metabolism dependencies | Longevity connected with reduced succinate and malate levels | [126] |
| Mature female Xenopus laevis frogs INDY-expressing oocyte model, longevity gene Indy | Succinate-stimulated [14C] citrate efflux | Longevity gene Indy functions as an exchanger of dicarboxylate and tricarboxylate Krebs cycle intermediates | [127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurhaluk, N. Tricarboxylic Acid Cycle Intermediates and Individual Ageing. Biomolecules 2024, 14, 260. https://doi.org/10.3390/biom14030260
Kurhaluk N. Tricarboxylic Acid Cycle Intermediates and Individual Ageing. Biomolecules. 2024; 14(3):260. https://doi.org/10.3390/biom14030260
Chicago/Turabian StyleKurhaluk, Natalia. 2024. "Tricarboxylic Acid Cycle Intermediates and Individual Ageing" Biomolecules 14, no. 3: 260. https://doi.org/10.3390/biom14030260
APA StyleKurhaluk, N. (2024). Tricarboxylic Acid Cycle Intermediates and Individual Ageing. Biomolecules, 14(3), 260. https://doi.org/10.3390/biom14030260






