The Configuration of GRB2 in Protein Interaction and Signal Transduction
Abstract
1. Introduction
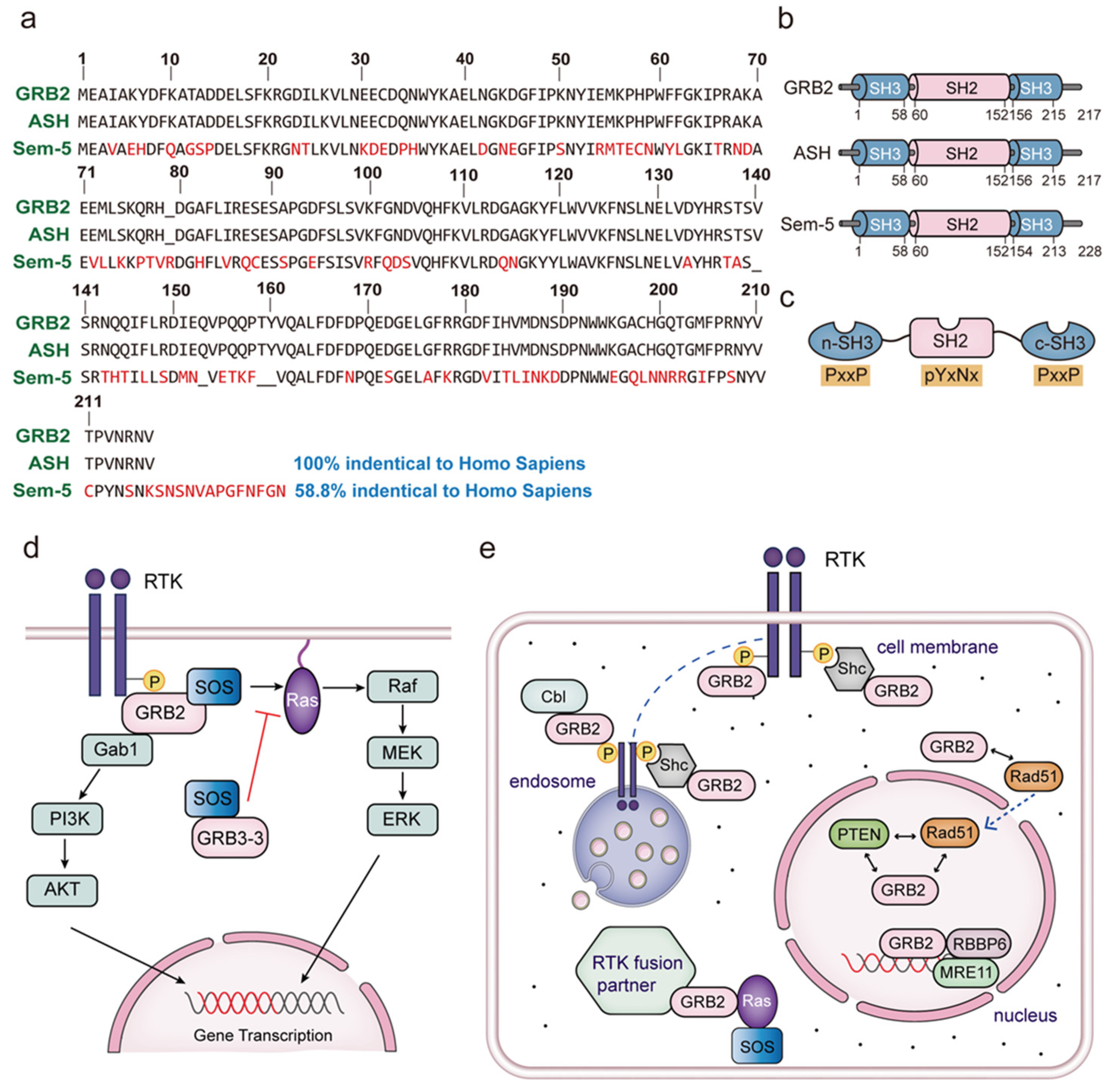
2. The Subcellular Location of GRB2
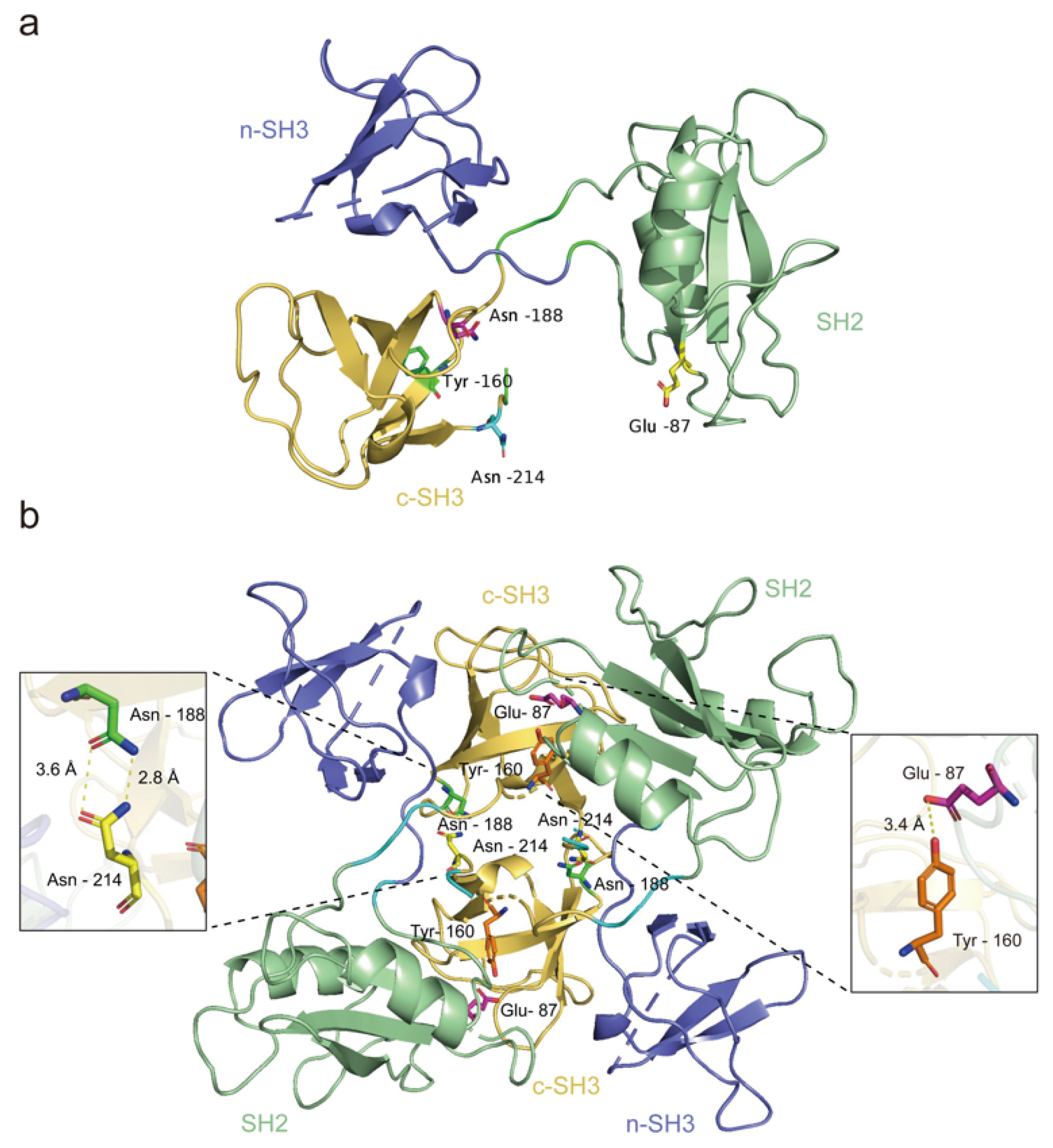
3. The Equilibrium between GRB2 Dimerization and Monomerization
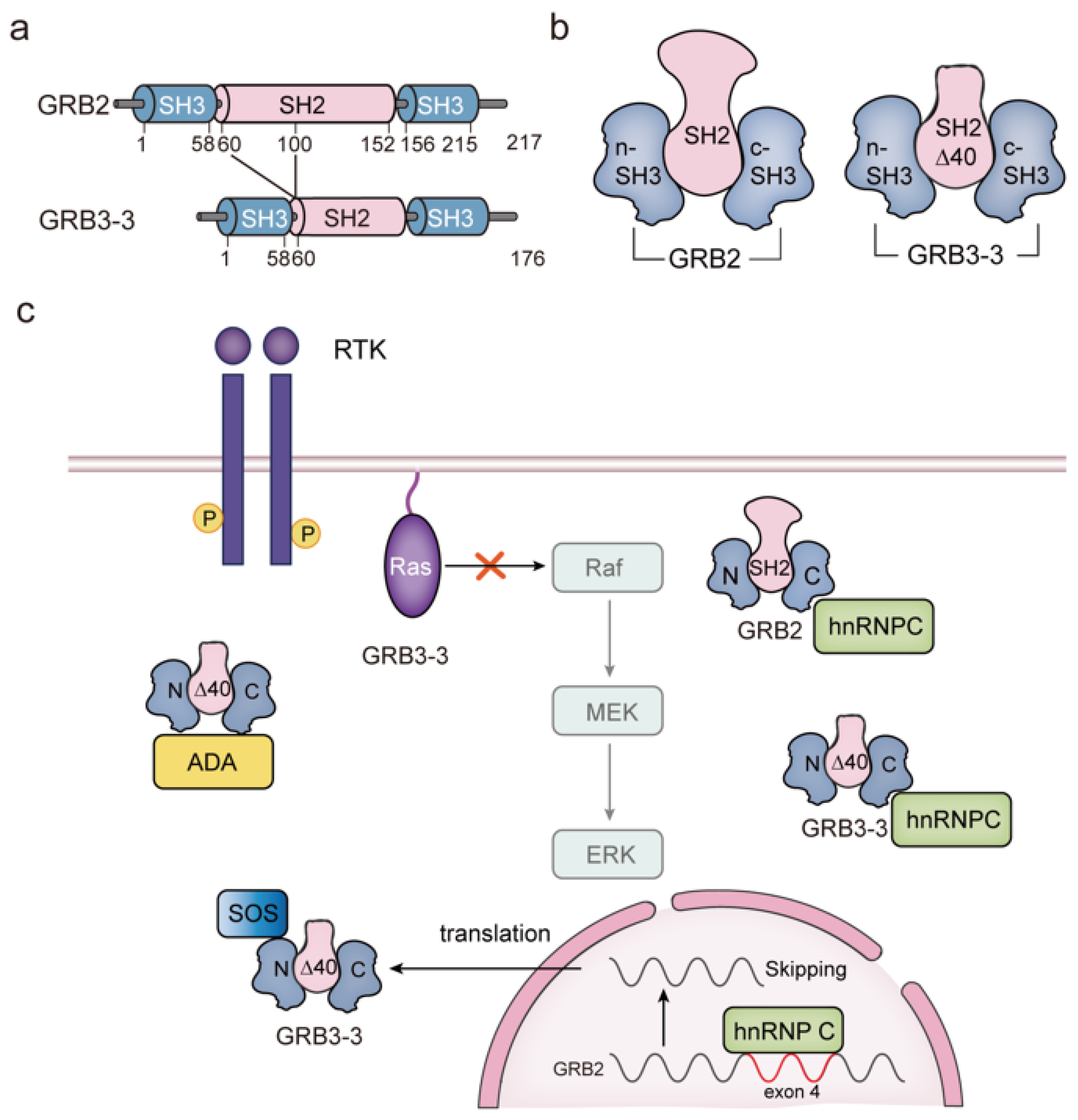
4. GRB3-3 Is a Splice Variant of GRB2
5. Overview of the Proteins Interacting with the GRB2 SH2 Domain
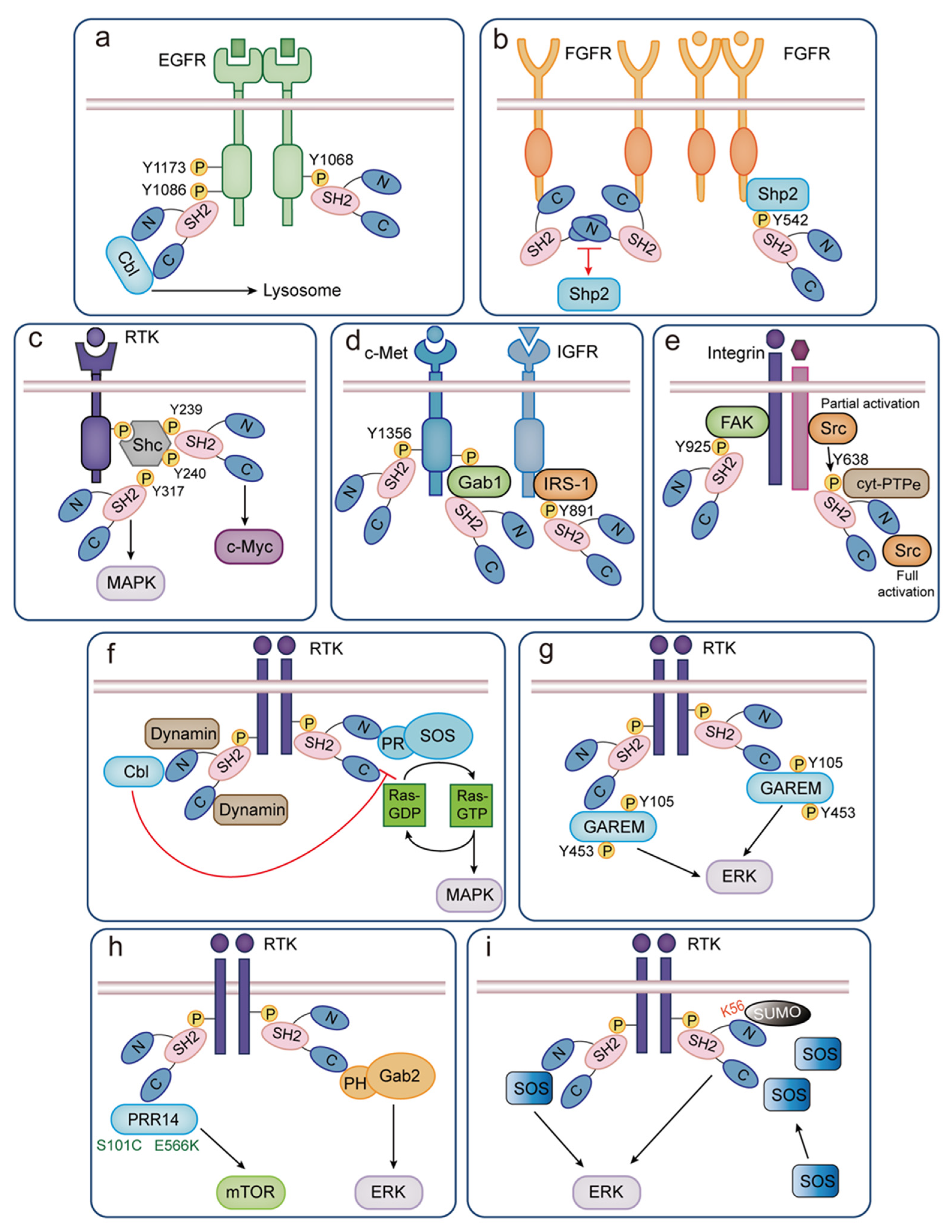
5.1. Role of GRB2 in EGF-Stimulated EGFR Signal Transduction
5.2. GRB2 Controls FGF-FGFR Signaling
5.3. The Function of GRB2-Shc Complexes
5.4. Role of GRB2 in Other Receptor Tyrosine Kinase Pathways
5.5. Role of GRB2 in the Non-Receptor Tyrosine Kinase Pathway
6. Overview the Proteins Interacting with the GRB2 SH3 Domain
6.1. Association between the GRB2-SOS Complex and Ras Signaling
6.2. GRB2 Binds to Cbl upon the Activation of Fyn
6.3. The Function of the GRB2-Dynamin Complex
6.4. GRB2 and GAREM
6.5. Gab2 Plays a Crucial Role in the Ras/ERK Pathway
6.6. GRB2 Facilitates the Activity of PRR14
7. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robinson, D.R.; Wu, Y.-M.; Lin, S.-F. The protein tyrosine kinase family of the human genome. Oncogene 2000, 19, 5548–5557. [Google Scholar] [CrossRef]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef]
- Regad, T. Targeting RTK signaling pathways in cancer. Cancers 2015, 7, 1758–1784. [Google Scholar] [CrossRef]
- Clark, S.G.; Stern, M.J.; Horvitz, H.R. C. elegans cell-signalling gene sem-5 encodes a protein with SH2 and SH3 domains. Nature 1992, 356, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Downward, J. The GRB2/Sem-5 adaptor protein. FEBS Lett. 1994, 338, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Lowenstein, E.J.; Daly, R.J.; Batzer, A.G.; Li, W.; Margolis, B.; Lammers, R.; Ullrich, A.; Skolnik, E.Y.; Bar-Sagi, D.; Schlessinger, J. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell 1992, 70, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Matuoka, K.; Shibata, M.; Yamakawa, A.; Takenawa, T. Cloning of ASH, a ubiquitous protein composed of one Src homology region (SH) 2 and two SH3 domains, from human and rat cDNA libraries. Proc. Natl. Acad. Sci. USA 1992, 89, 9015–9019. [Google Scholar] [CrossRef] [PubMed]
- Chardin, P.; Cussac, D.; Maignan, S.; Ducruix, A. The Grb2 adaptor. FEBS Lett. 1995, 369, 47–51. [Google Scholar] [CrossRef]
- Roy, M.J.; Surudoi, M.G.; Kropp, A.; Hou, J.; Dai, W.; Hardy, J.M.; Liang, L.Y.; Cotton, T.R.; Lechtenberg, B.C.; Dite, T.A.; et al. Structural mapping of PEAK pseudokinase interactions identifies 14-3-3 as a molecular switch for PEAK3 signaling. Nat. Commun. 2023, 14, 3542. [Google Scholar] [CrossRef] [PubMed]
- Schlessinger, J.; Lemmon, M.A. SH2 and PTB domains in tyrosine kinase signaling. Science’s STKE 2003, 2003, Re12. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, S.; Joy, S.T.; Arora, P.S.; BarSagi, D. An orthosteric inhibitor of the RAS-SOS interaction. Enzymes 2013, 34 Pt B, 25–39. [Google Scholar]
- Boguski, M.S.; McCormick, F. Proteins regulating Ras and its relatives. Nature 1993, 366, 643–654. [Google Scholar] [CrossRef]
- Mitin, N.; Rossman, K.L.; Der, C.J. Signaling interplay in Ras superfamily function. Curr. Biol. 2005, 15, R563–R574. [Google Scholar] [CrossRef]
- Fang, J.Y.; Richardson, B.C. The MAPK signalling pathways and colorectal cancer. Lancet Oncol. 2005, 6, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Ersahin, T.; Tuncbag, N.; Cetin-Atalay, R. The PI3K/AKT/mTOR interactive pathway. Mol. Biosyst. 2015, 11, 1946–1954. [Google Scholar] [CrossRef]
- Stainthorp, A.K.; Lin, C.C.; Wang, D.; Medhi, R.; Ahmed, Z.; Suen, K.M.; Miska, E.A.; Whitehouse, A.; Ladbury, J.E. Regulation of microRNA expression by the adaptor protein GRB2. Sci. Rep. 2023, 13, 9784. [Google Scholar] [CrossRef] [PubMed]
- Mitra, P.; Kalailingam, P.; Tan, H.B.; Thanabalu, T. Overexpression of GRB2 Enhances Epithelial to Mesenchymal Transition of A549 Cells by Upregulating SNAIL Expression. Cells 2018, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Sorkin, A. Coordinated traffic of Grb2 and Ras during epidermal growth factor receptor endocytosis visualized in living cells. Mol. Biol. Cell 2002, 13, 1522–1535. [Google Scholar] [CrossRef] [PubMed]
- Saxton, T.M.; Cheng, A.M.; Ong, S.H.; Lu, Y.; Sakai, R.; Cross, J.C.; Pawson, T. Gene dosage-dependent functions for phosphotyrosine-Grb2 signaling during mammalian tissue morphogenesis. Curr. Biol. 2001, 11, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Chardin, P.; Camonis, J.H.; Gale, N.W.; van Aelst, L.; Schlessinger, J.; Wigler, M.H.; Bar-Sagi, D. Human Sos1: A guanine nucleotide exchange factor for Ras that binds to GRB2. Science 1993, 260, 1338–1343. [Google Scholar] [CrossRef]
- Ramos-Morales, F.; Domínguez, A.; Rios, R.M.; Barroso, S.I.; Infante, C.; Schweighoffer, F.; Tocqué, B.; Pintor-Toro, J.A.; Tortolero, M. Adenosine deaminase is a specific partner for the Grb2 isoform Grb3-3. Biochem. Biophys. Res. Commun. 1997, 237, 735–740. [Google Scholar] [CrossRef]
- Yang, B.; Jung, D.; Motto, D.; Meyer, J.; Koretzky, G.; Campbell, K.P. SH3 domain-mediated interaction of dystroglycan and Grb2. J. Biol. Chem. 1995, 270, 11711–11714. [Google Scholar] [CrossRef]
- Zang, X.P.; Siwak, D.R.; Nguyen, T.X.; Tari, A.M.; Pento, J.T. KGF-induced motility of breast cancer cells is dependent on Grb2 and Erk1,2. Clin. Exp. Metastasis 2004, 21, 437–443. [Google Scholar] [CrossRef]
- Ijaz, M.; Wang, F.; Shahbaz, M.; Jiang, W.; Fathy, A.H.; Nesa, E.U. The Role of Grb2 in Cancer and Peptides as Grb2 Antagonists. Protein. Pept. Lett. 2018, 24, 1084–1095. [Google Scholar] [CrossRef] [PubMed]
- Feller, S.M.; Lewitzky, M. Potential disease targets for drugs that disrupt protein—Protein interactions of Grb2 and Crk family adaptors. Curr. Pharm. Des. 2006, 12, 529–548. [Google Scholar] [CrossRef] [PubMed]
- Pawson, T.; Gish, G.D.; Nash, P. SH2 domains, interaction modules and cellular wiring. Trends Cell Biol. 2001, 11, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.K.; Zhang, J.; Gu, H. Grb2, a simple adapter with complex roles in lymphocyte development, function, and signaling. Immunol. Rev. 2009, 232, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Verbeek, B.S.; Adriaansen-Slot, S.S.; Rijksen, G.; Vroom, T.M. Grb2 overexpression in nuclei and cytoplasm of human breast cells: A histochemical and biochemical study of normal and neoplastic mammary tissue specimens. J. Pathol. 1997, 183, 195–203. [Google Scholar] [CrossRef]
- Sorkin, A. Internalization of the epidermal growth factor receptor: Role in signalling. Biochem. Soc. Trans. 2001, 29 Pt 4, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chen, J.; He, L.; Stiles, B.L. PTEN: Tumor Suppressor and Metabolic Regulator. Front. Endocrinol. 2018, 9, 338. [Google Scholar] [CrossRef]
- Shen, W.H.; Balajee, A.S.; Wang, J.; Wu, H.; Eng, C.; Pandolfi, P.P.; Yin, Y. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell 2007, 128, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef]
- Demeyer, A.; Benhelli-Mokrani, H.; Chénais, B.; Weigel, P.; Fleury, F. Inhibiting homologous recombination by targeting RAD51 protein. Biochim. Biophys. Acta. Rev. Cancer. 2021, 1876, 188597. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Xu, S.; Xu, Y.; Gao, Q.; Zhang, C.; Liu, L.; Yang, H.; Jiang, X.; Che, Y. Grb2 binds to PTEN and regulates its nuclear translocation to maintain the genomic stability in DNA damage response. Cell Death Dis. 2019, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.; Tainer, J.A. The MRE11-RAD50-NBS1 Complex Conducts the Orchestration of Damage Signaling and Outcomes to Stress in DNA Replication and Repair. Annu. Rev. Biochem. 2018, 87, 263–294. [Google Scholar] [CrossRef]
- Ye, Z.; Xu, S.; Shi, Y.; Bacolla, A.; Syed, A.; Moiani, D.; Tsai, C.L.; Shen, Q.; Peng, G.; Leonard, P.G.; et al. GRB2 enforces homology-directed repair initiation by MRE11. Sci. Adv. 2021, 7, eabe9254. [Google Scholar] [CrossRef]
- Tomas, A.; Futter, C.E.; Eden, E.R. EGF receptor trafficking: Consequences for signaling and cancer. Trends Cell Biol. 2014, 24, 26–34. [Google Scholar] [CrossRef]
- Miaczynska, M.; Pelkmans, L.; Zerial, M. Not just a sink: Endosomes in control of signal transduction. Curr. Opin. Cell Biol. 2004, 16, 400–406. [Google Scholar] [CrossRef]
- Waterman, H.; Yarden, Y. Molecular mechanisms underlying endocytosis and sorting of ErbB receptor tyrosine kinases. FEBS Lett. 2001, 490, 142–152. [Google Scholar] [CrossRef]
- Wang, Y.; Pennock, S.D.; Chen, X.; Kazlauskas, A.; Wang, Z. Platelet-derived growth factor receptor-mediated signal transduction from endosomes. J. Biol. Chem. 2004, 279, 8038–8046. [Google Scholar] [CrossRef]
- Sorkin, A.; McClure, M.; Huang, F.; Carter, R. Interaction of EGF receptor and grb2 in living cells visualized by fluorescence resonance energy transfer (FRET) microscopy. Curr. Biol. 2000, 10, 1395–1398. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, K.S. Signaling via Shc family adapter proteins. Oncogene 2001, 20, 6322–6330. [Google Scholar] [CrossRef] [PubMed]
- Fortian, A.; Sorkin, A. Live-cell fluorescence imaging reveals high stoichiometry of Grb2 binding to the EGF receptor sustained during endocytosis. J. Cell Sci. 2014, 127 Pt 2, 432–444. [Google Scholar] [CrossRef]
- Raychaudhuri, M.; Roy, K.; Das, S.; Mukhopadhyay, D. The N-terminal SH3 domain of Grb2 is required for endosomal localization of AbetaPP. J. Alzheimers Dis. 2012, 32, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Alberti, S.; Gladfelter, A.; Mittag, T. Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell 2019, 176, 419–434. [Google Scholar] [CrossRef]
- Du, Z.; Lovly, C.M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer 2018, 17, 58. [Google Scholar] [CrossRef]
- Tulpule, A.; Guan, J.; Neel, D.S.; Allegakoen, H.R.; Lin, Y.P.; Brown, D.; Chou, Y.T.; Heslin, A.; Chatterjee, N.; Perati, S.; et al. Kinase-mediated RAS signaling via membraneless cytoplasmic protein granules. Cell 2021, 184, 2649–2664.e18. [Google Scholar] [CrossRef]
- Maignan, S.; Guilloteau, J.P.; Fromage, N.; Arnoux, B.; Becquart, J.; Ducruix, A. Crystal structure of the mammalian Grb2 adaptor. Science 1995, 268, 291–293. [Google Scholar] [CrossRef]
- Liao, T.J.; Jang, H.; Fushman, D.; Nussinov, R. SOS1 interacts with Grb2 through regions that induce closed nSH3 conformations. J. Chem. Phys. 2020, 153, 045106. [Google Scholar] [CrossRef]
- McDonald, C.B.; Seldeen, K.L.; Deegan, B.J.; Lewis, M.S.; Farooq, A. Grb2 adaptor undergoes conformational change upon dimerization. Arch. Biochem. Biophys. 2008, 475, 25–35. [Google Scholar] [CrossRef]
- Ahmed, Z.; Timsah, Z.; Suen, K.M.; Cook, N.P.; Lee, G.R.T.; Lin, C.C.; Gagea, M.; Marti, A.A.; Ladbury, J.E. Grb2 monomer-dimer equilibrium determines normal versus oncogenic function. Nat. Commun. 2015, 6, 7354. [Google Scholar] [CrossRef]
- Riera, L.; Lasorsa, E.; Ambrogio, C.; Surrenti, N.; Voena, C.; Chiarle, R. Involvement of Grb2 adaptor protein in nucleophosmin-anaplastic lymphoma kinase (NPM-ALK)-mediated signaling and anaplastic large cell lymphoma growth. J. Biol. Chem. 2010, 285, 26441–26450. [Google Scholar] [CrossRef]
- Fath, I.; Schweighoffer, F.; Rey, I.; Multon, M.C.; Boiziau, J.; Duchesne, M.; Tocqué, B. Cloning of a Grb2 isoform with apoptotic properties. Science 1994, 264, 971–974. [Google Scholar] [CrossRef]
- Seiler, C.; Stainthorp, A.K.; Ketchen, S.; Jones, C.M.; Marks, K.; Quirke, P.; Ladbury, J.E. The Grb2 splice variant, Grb3-3, is a negative regulator of RAS activation. Commun. Biol. 2022, 5, 1029. [Google Scholar] [CrossRef]
- Parker, F.; Maurier, F.; Delumeau, I.; Duchesne, M.; Faucher, D.; Debussche, L.; Dugue, A.; Schweighoffer, F.; Tocque, B. A Ras-GTPase-activating protein SH3-domain-binding protein. Mol. Cell. Biol. 1996, 16, 2561–2569. [Google Scholar] [CrossRef]
- Bustelo, X.R.; Suen, K.L.; Michael, W.M.; Dreyfuss, G.; Barbacid, M. Association of the vav proto-oncogene product with poly(rC)-specific RNA-binding proteins. Mol. Cell. Biol. 1995, 15, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, N.B.; Kishore, R.; Ismail, Z.; Zhang, Q.; Taylor, T.; Newman, S.A. cDNA cloning and spatiotemporal expression during avian embryogenesis of hnRNP A1, a regulatory factor in alternative splicing. Gene Expr. Patterns 2003, 3, 285–295. [Google Scholar] [CrossRef]
- Visa, N.; Alzhanova-Ericsson, A.T.; Sun, X.; Kiseleva, E.; Björkroth, B.; Wurtz, T.; Daneholt, B. A pre-mRNA-binding protein accompanies the RNA from the gene through the nuclear pores and into polysomes. Cell 1996, 84, 253–264. [Google Scholar] [CrossRef]
- Romero, F.; Ramos-Morales, F.; Dominguez, A.; Rios, R.M.; Schweighoffer, F.; Tocque, B.; Pintor-Toro, J.A.; Fischer, S.; Tortolero, M. Grb2 and its apoptotic isoform Grb3-3 associate with heterogeneous nuclear ribonucleoprotein C, and these interactions are modulated by poly(U) RNA. J. Biol. Chem. 1998, 273, 7776–7781. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Emdad, L.; Das, S.K.; Fisher, P.B. EGFR: An essential receptor tyrosine kinase-regulator of cancer stem cells. Adv. Cancer Res. 2020, 147, 161–188. [Google Scholar] [PubMed]
- Kozer, N.; Barua, D.; Henderson, C.; Nice, E.C.; Burgess, A.W.; Hlavacek, W.S.; Clayton, A.H. Recruitment of the adaptor protein Grb2 to EGFR tetramers. Biochemistry 2014, 53, 2594–2604. [Google Scholar] [CrossRef]
- Ogiso, H.; Ishitani, R.; Nureki, O.; Fukai, S.; Yamanaka, M.; Kim, J.H.; Saito, K.; Sakamoto, A.; Inoue, M.; Shirouzu, M.; et al. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell 2002, 110, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Kashishian, A.; Cooper, J.A. Phosphorylation sites at the C-terminus of the platelet-derived growth factor receptor bind phospholipase C gamma 1. Mol. Biol. Cell 1993, 4, 49–57. [Google Scholar] [CrossRef]
- Batzer, A.G.; Rotin, D.; Ureña, J.M.; Skolnik, E.Y.; Schlessinger, J. Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol. Cell. Biol. 1994, 14, 5192–5201. [Google Scholar]
- Arvidsson, A.K.; Rupp, E.; Nånberg, E.; Downward, J.; Rönnstrand, L.; Wennström, S.; Schlessinger, J.; Heldin, C.H.; Claesson-Welsh, L. Tyr-716 in the platelet-derived growth factor beta-receptor kinase insert is involved in GRB2 binding and Ras activation. Mol. Cell. Biol. 1994, 14, 6715–6726. [Google Scholar] [CrossRef] [PubMed]
- Nino, C.A.; Wollscheid, N.; Giangreco, G.; Maspero, E.; Polo, S. USP25 Regulates EGFR Fate by Modulating EGF-Induced Ubiquitylation Dynamics. Biomolecules 2020, 10, 1548. [Google Scholar] [CrossRef]
- Fukazawa, T.; Miyake, S.; Band, V.; Band, H. Tyrosine phosphorylation of Cbl upon epidermal growth factor (EGF) stimulation and its association with EGF receptor and downstream signaling proteins. J. Biol. Chem. 1996, 271, 14554–14559. [Google Scholar] [CrossRef]
- Yamazaki, T.; Zaal, K.; Hailey, D.; Presley, J.; Lippincott-Schwartz, J.; Samelson, L.E. Role of Grb2 in EGF-stimulated EGFR internalization. J. Cell Sci. 2002, 115 Pt 9, 1791–1802. [Google Scholar] [CrossRef] [PubMed]
- Furdui, C.M.; Lew, E.D.; Schlessinger, J.; Anderson, K.S. Autophosphorylation of FGFR1 kinase is mediated by a sequential and precisely ordered reaction. Mol. Cell 2006, 21, 711–717. [Google Scholar] [CrossRef]
- Takeda, M.; Arao, T.; Yokote, H.; Komatsu, T.; Yanagihara, K.; Sasaki, H.; Yamada, Y.; Tamura, T.; Fukuoka, K.; Kimura, H.; et al. AZD2171 shows potent antitumor activity against gastric cancer over-expressing fibroblast growth factor receptor 2/keratinocyte growth factor receptor. Clin. Cancer Res. 2007, 13, 3051–3057. [Google Scholar] [CrossRef][Green Version]
- Bryant, M.R.; Marta, C.B.; Kim, F.S.; Bansal, R. Phosphorylation and lipid raft association of fibroblast growth factor receptor-2 in oligodendrocytes. Glia 2009, 57, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Kunii, K.; Davis, L.; Gorenstein, J.; Hatch, H.; Yashiro, M.; Di Bacco, A.; Elbi, C.; Lutterbach, B. FGFR2-amplified gastric cancer cell lines require FGFR2 and Erbb3 signaling for growth and survival. Cancer Res. 2008, 68, 2340–2348. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Melo, F.A.; Ghosh, R.; Suen, K.M.; Stagg, L.J.; Kirkpatrick, J.; Arold, S.T.; Ahmed, Z.; Ladbury, J.E. Inhibition of Basal FGF Receptor Signaling by Dimeric Grb2. Cell 2012, 149, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, K.S.; Rosario, M.; Birchmeier, C.; Birchmeier, W. The tyrosine phosphatase Shp2 in development and cancer. Adv. Cancer Res. 2010, 106, 53–89. [Google Scholar] [PubMed]
- Tonks, N.K. PTP1B: From the sidelines to the front lines! FEBS Lett. 2003, 546, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.S.; Pawson, T. Phosphotyrosine phosphatases with SH2 domains: Regulators of signal transduction. Trends Genet. 1994, 10, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; George, R.; Lin, C.C.; Suen, K.M.; Levitt, J.A.; Suhling, K.; Ladbury, J.E. Direct binding of Grb2 SH3 domain to FGFR2 regulates SHP2 function. Cell Signal. 2010, 22, 23–33. [Google Scholar] [CrossRef]
- Ahmed, Z.; Lin, C.C.; Suen, K.M.; Melo, F.A.; Levitt, J.A.; Suhling, K.; Ladbury, J.E. Grb2 controls phosphorylation of FGFR2 by inhibiting receptor kinase and Shp2 phosphatase activity. J. Cell Biol. 2013, 200, 493–504. [Google Scholar] [CrossRef]
- Araki, T.; Nawa, H.; Neel, B.G. Tyrosyl Phosphorylation of Shp2 Is Required for Normal ERK Activation in Response to Some, but Not All, Growth Factors. J. Biol. Chem. 2003, 278, 41677–41684. [Google Scholar] [CrossRef]
- Margolis, B.; Skolnik, E.Y. Activation of Ras by receptor tyrosine kinases. J. Am. Soc. Nephrol. 1994, 5, 1288–1299. [Google Scholar] [CrossRef]
- Cattaneo, E.; Pelicci, P.G. Emerging roles for SH2/PTB-containing Shc adaptor proteins in the developing mammalian brain. Trends Neurosci. 1998, 21, 476–481. [Google Scholar] [CrossRef]
- Oku, S.; van der Meulen, T.; Copp, J.; Glenn, G.; van der Geer, P. Engineering NGF receptors to bind Grb2 directly uncovers differences in signaling ability between Grb2- and ShcA-binding sites. FEBS Lett. 2012, 586, 3658–3664. [Google Scholar] [CrossRef]
- Salcini, A.E.; McGlade, J.; Pelicci, G.; Nicoletti, I.; Pawson, T.; Pelicci, P.G. Formation of Shc-Grb2 complexes is necessary to induce neoplastic transformation by overexpression of Shc proteins. Oncogene 1994, 9, 2827–2836. [Google Scholar] [PubMed]
- Gotoh, N.; Tojo, A.; Shibuya, M. A novel pathway from phosphorylation of tyrosine residues 239/240 of Shc, contributing to suppress apoptosis by IL-3. EMBO J. 1996, 15, 6197–6204. [Google Scholar] [CrossRef]
- Duffy, M.J.; O’Grady, S.; Tang, M.; Crown, J. MYC as a target for cancer treatment. Cancer Treat. Rev. 2021, 94, 102154. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, N.; Toyoda, M.; Shibuya, M. Tyrosine phosphorylation sites at amino acids 239 and 240 of Shc are involved in epidermal growth factor-induced mitogenic signaling that is distinct from Ras/mitogen-activated protein kinase activation. Mol. Cell. Biol. 1997, 17, 1824–1831. [Google Scholar] [CrossRef] [PubMed]
- Harmer, S.L.; DeFranco, A.L. Shc contains two Grb2 binding sites needed for efficient formation of complexes with SOS in B lymphocytes. Mol. Cell. Biol. 1997, 17, 4087–4095. [Google Scholar] [CrossRef][Green Version]
- Birchmeier, C.; Birchmeier, W.; Gherardi, E.; Woude, G.F.V. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 2003, 4, 915–925. [Google Scholar] [CrossRef]
- Lai, J.F.; Kao, S.C.; Jiang, S.T.; Tang, M.J.; Chan, P.C.; Chen, H.C. Involvement of focal adhesion kinase in hepatocyte growth factor-induced scatter of Madin-Darby canine kidney cells. J. Biol. Chem. 2000, 275, 7474–7480. [Google Scholar] [CrossRef]
- Giubellino, A.; Burke, T.R., Jr.; Bottaro, D.P. Grb2 signaling in cell motility and cancer. Expert Opin. Ther. Targets 2008, 12, 1021–1033. [Google Scholar] [CrossRef]
- Hakuno, F.; Takahashi, S.I. IGF1 receptor signaling pathways. J. Mol. Endocrinol. 2018, 61, T69–T86. [Google Scholar] [CrossRef] [PubMed]
- Neet, K.; Hunter, T. Vertebrate non-receptor protein-tyrosine kinase families. Genes Cells 1996, 1, 147–169. [Google Scholar] [CrossRef] [PubMed]
- Cox, B.D.; Natarajan, M.; Stettner, M.R.; Gladson, C.L. New concepts regarding focal adhesion kinase promotion of cell migration and proliferation. J. Cell Biochem. 2006, 99, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Schlaepfer, D.D.; Hanks, S.K.; Hunter, T.; van der Geer, P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature 1994, 372, 786–791. [Google Scholar] [CrossRef]
- Avizienyte, E.; Wyke, A.W.; Jones, R.J.; McLean, G.W.; Westhoff, M.A.; Brunton, V.G.; Frame, M.C. Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signalling. Nat. Cell Biol. 2002, 4, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Granot-Attas, S.; Luxenburg, C.; Finkelshtein, E.; Elson, A. Protein tyrosine phosphatase epsilon regulates integrin-mediated podosome stability in osteoclasts by activating Src. Mol. Biol. Cell 2009, 20, 4324–4334. [Google Scholar] [CrossRef] [PubMed]
- Levy-Apter, E.; Finkelshtein, E.; Vemulapalli, V.; Li, S.S.; Bedford, M.T.; Elson, A. Adaptor protein GRB2 promotes Src tyrosine kinase activation and podosomal organization by protein-tyrosine phosphatase ϵ in osteoclasts. J. Biol. Chem. 2014, 289, 36048–36058. [Google Scholar] [CrossRef]
- Gale, N.W.; Kaplan, S.; Lowenstein, E.J.; Schlessinger, J.; Bar-Sagi, D. Grb2 mediates the EGF-dependent activation of guanine nucleotide exchange on Ras. Nature 1993, 363, 88–92. [Google Scholar] [CrossRef]
- Baltanas, F.C.; Garcia-Navas, R.; Santos, E. SOS2 Comes to the Fore: Differential Functionalities in Physiology and Pathology. Int. J. Mol. Sci. 2021, 22, 6613. [Google Scholar] [CrossRef]
- Baltanas, F.C.; Mucientes-Valdivieso, C.; Lorenzo-Martin, L.F.; Fernandez-Parejo, N.; Garcia-Navas, R.; Segrelles, C.; Calzada, N.; Fuentes-Mateos, R.; Paramio, J.M.; Bustelo, X.R.; et al. Functional Specificity of the Members of the Sos Family of Ras-GEF Activators: Novel Role of Sos2 in Control of Epidermal Stem Cell Homeostasis. Cancers 2021, 13, 2152. [Google Scholar] [CrossRef]
- Esteban, L.M.; Fernández-Medarde, A.; López, E.; Yienger, K.; Guerrero, C.; Ward, J.M.; Tessarollo, L.; Santos, E. Ras-guanine nucleotide exchange factor sos2 is dispensable for mouse growth and development. Mol. Cell. Biol. 2000, 20, 6410–6413. [Google Scholar] [CrossRef] [PubMed]
- Sondermann, H.; Nagar, B.; Bar-Sagi, D.; Kuriyan, J. Computational docking and solution x-ray scattering predict a membrane-interacting role for the histone domain of the Ras activator son of sevenless. Proc. Natl. Acad. Sci. USA 2005, 102, 16632–16637. [Google Scholar] [CrossRef] [PubMed]
- Bolgov, A.; Korban, S.; Luzik, D.; Zhemkov, V.; Kim, M.; Rogacheva, O.; Bezprozvanny, I. Crystal structure of the SH3 domain of growth factor receptor-bound protein 2. Acta Crystallogr. F Struct Biol. Commun. 2020, 76 Pt 6, 263–270. [Google Scholar] [CrossRef]
- Wittekind, M.; Mapelli, C.; Lee, V.; Goldfarb, V.; Friedrichs, M.S.; Meyers, C.A.; Mueller, L. Solution structure of the Grb2 N-terminal SH3 domain complexed with a ten-residue peptide derived from SOS: Direct refinement against NOEs, J-couplings and 1H and 13C chemical shifts. J. Mol. Biol. 1997, 267, 933–952. [Google Scholar] [CrossRef] [PubMed]
- Vidal, M.; Goudreau, N.; Cornille, F.; Cussac, D.; Gincel, E.; Garbay, C. Molecular and cellular analysis of Grb2 SH3 domain mutants: Interaction with Sos and dynamin. J. Mol. Biol. 1999, 290, 717–730. [Google Scholar] [CrossRef]
- Saksela, K.; Permi, P. SH3 domain ligand binding: What’s the consensus and where’s the specificity? FEBS Lett. 2012, 586, 2609–2614. [Google Scholar] [CrossRef]
- Liao, T.J.; Jang, H.; Nussinov, R.; Fushman, D. High-Affinity Interactions of the nSH3/cSH3 Domains of Grb2 with the C-Terminal Proline-Rich Domain of SOS1. J. Am. Chem. Soc. 2020, 142, 3401–3411. [Google Scholar] [CrossRef]
- Chook, Y.M.; Gish, G.D.; Kay, C.M.; Pai, E.F.; Pawson, T. The Grb2-mSos1 complex binds phosphopeptides with higher affinity than Grb2. J. Biol. Chem. 1996, 271, 30472–30478. [Google Scholar] [CrossRef]
- Dikic, I.; Giordano, S. Negative receptor signalling. Curr. Opin. Cell Biol. 2003, 15, 128–135. [Google Scholar] [CrossRef]
- Waterman, H.; Katz, M.; Rubin, C.; Shtiegman, K.; Lavi, S.; Elson, A.; Jovin, T.; Yarden, Y. A mutant EGF-receptor defective in ubiquitylation and endocytosis unveils a role for Grb2 in negative signaling. EMBO J. 2002, 21, 303–313. [Google Scholar] [CrossRef]
- da Silva, A.J.; Yamamoto, M.; Zalvan, C.H.; Rudd, C.E. Engagement of the TcR/CD3 complex stimulates p59fyn(T) activity: Detection of associated proteins at 72 and 120-130 kD. Mol. Immunol. 1992, 29, 1417–1425. [Google Scholar] [CrossRef]
- Tsygankov, A.Y.; Spana, C.; Rowley, R.B.; Penhallow, R.C.; Burkhardt, A.L.; Bolen, J.B. Activation-dependent tyrosine phosphorylation of Fyn-associated proteins in T lymphocytes. J. Biol. Chem. 1994, 269, 7792–7800. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, T.; Reedquist, K.A.; Trub, T.; Soltoff, S.; Panchamoorthy, G.; Druker, B.; Cantley, L.; Shoelson, S.E.; Band, H. The SH3 domain-binding T cell tyrosyl phosphoprotein p120. Demonstration of its identity with the c-cbl protooncogene product and in vivo complexes with Fyn, Grb2, and phosphatidylinositol 3-kinase. J. Biol. Chem. 1995, 270, 19141–19150. [Google Scholar] [CrossRef] [PubMed]
- Tsygankov, A.Y.; Mahajan, S.; Fincke, J.E.; Bolen, J.B. Specific association of tyrosine-phosphorylated c-Cbl with Fyn tyrosine kinase in T cells. J. Biol. Chem. 1996, 271, 27130–27137. [Google Scholar] [CrossRef] [PubMed]
- McClure, S.J.; Robinson, P.J. Dynamin, endocytosis and intracellular signalling (review). Mol. Membr. Biol. 1996, 13, 189–215. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.M.; De Camilli, P. Dynamin, a membrane-remodelling GTPase. Nat. Rev. Mol. Cell Biol. 2012, 13, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Seedorf, K.; Kostka, G.; Lammers, R.; Bashkin, P.; Daly, R.; Burgess, W.H.; van der Bliek, A.M.; Schlessinger, J.; Ullrich, A. Dynamin binds to SH3 domains of phospholipase C gamma and GRB-2. J. Biol. Chem. 1994, 269, 16009–16014. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.Y.; Jeong, M.J.; Yoo, J.; Lee, K.I.; Kwon, B.M.; Lim, D.S.; Lee, C.E.; Park, Y.M.; Han, M.Y. Grb2 dominantly associates with dynamin II in human hepatocellular carcinoma HepG2 cells. J. Cell Biochem. 2001, 84, 150–155. [Google Scholar] [CrossRef]
- Blagoev, B.; Ong, S.E.; Kratchmarova, I.; Mann, M. Temporal analysis of phosphotyrosine-dependent signaling networks by quantitative proteomics. Nat. Biotechnol. 2004, 22, 1139–1145. [Google Scholar] [CrossRef]
- Tashiro, K.; Tsunematsu, T.; Okubo, H.; Ohta, T.; Sano, E.; Yamauchi, E.; Taniguchi, H.; Konishi, H. GAREM, a novel adaptor protein for growth factor receptor-bound protein 2, contributes to cellular transformation through the activation of extracellular signal-regulated kinase signaling. J. Biol. Chem. 2009, 284, 20206–20214. [Google Scholar] [CrossRef]
- Taniguchi, T.; Tanaka, S.; Ishii, A.; Watanabe, M.; Fujitani, N.; Sugeo, A.; Gotoh, S.; Ohta, T.; Hiyoshi, M.; Matsuzaki, H.; et al. A brain-specific Grb2-associated regulator of extracellular signal-regulated kinase (Erk)/mitogen-activated protein kinase (MAPK) (GAREM) subtype, GAREM2, contributes to neurite outgrowth of neuroblastoma cells by regulating Erk signaling. J. Biol. Chem. 2013, 288, 29934–29942. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Neel, B.G. The “Gab” in signal transduction. Trends Cell Biol. 2003, 13, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jeong, E.G.; Nam, S.W.; Lee, J.Y.; Yoo, N.J.; Lee, S.H. Increased expression of Gab2, a scaffolding adaptor of the tyrosine kinase signalling, in gastric carcinomas. Pathology 2007, 39, 326–329. [Google Scholar] [PubMed]
- Xu, X.L.; Wang, X.; Chen, Z.L.; Jin, M.; Yang, W.; Zhao, G.F.; Li, J.W. Overexpression of Grb2-associated binder 2 in human lung cancer. Int. J. Biol. Sci. 2011, 7, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Wohrle, F.U.; Daly, R.J.; Brummer, T. Function, regulation and pathological roles of the Gab/DOS docking proteins. Cell Commun. Signal 2009, 7, 22. [Google Scholar] [CrossRef]
- Lewitzky, M.; Kardinal, C.; Gehring, N.H.; Schmidt, E.K.; Konkol, B.; Eulitz, M.; Birchmeier, W.; Schaeper, U.; Feller, S.M. The C-terminal SH3 domain of the adapter protein Grb2 binds with high affinity to sequences in Gab1 and SLP-76 which lack the SH3-typical P-x-x-P core motif. Oncogene 2001, 20, 1052–1062. [Google Scholar] [CrossRef]
- Harkiolaki, M.; Tsirka, T.; Lewitzky, M.; Simister, P.C.; Joshi, D.; Bird, L.E.; Jones, E.Y.; O’Reilly, N.; Feller, S.M. Distinct binding modes of two epitopes in Gab2 that interact with the SH3C domain of Grb2. Structure 2009, 17, 809–822. [Google Scholar] [CrossRef]
- Malagrino, F.; Coluccia, A.; Bufano, M.; Regina, G.; Puxeddu, M.; Toto, A.; Visconti, L.; Paone, A.; Magnifico, M.C.; Troilo, F.; et al. Targeting the Interaction between the SH3 Domain of Grb2 and Gab2. Cells 2020, 9, 2435. [Google Scholar] [CrossRef]
- Job, B.; Bernheim, A.; Beau-Faller, M.; Camilleri-Broet, S.; Girard, P.; Hofman, P.; Mazieres, J.; Toujani, S.; Lacroix, L.; Laffaire, J.; et al. Genomic aberrations in lung adenocarcinoma in never smokers. PLoS ONE 2010, 5, e15145. [Google Scholar] [CrossRef]
- Poleshko, A.; Mansfield, K.M.; Burlingame, C.C.; Andrake, M.D.; Shah, N.R.; Katz, R.A. The human protein PRR14 tethers heterochromatin to the nuclear lamina during interphase and mitotic exit. Cell Rep. 2013, 5, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lewinska, M.; Fan, X.; Zhu, J.; Yuan, Z.M. PRR14 is a novel activator of the PI3K pathway promoting lung carcinogenesis. Oncogene 2016, 35, 5527–5538. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.B.; Seldeen, K.L.; Deegan, B.J.; Farooq, A. SH3 domains of Grb2 adaptor bind to PXpsiPXR motifs within the Sos1 nucleotide exchange factor in a discriminate manner. Biochemistry 2009, 48, 4074–4085. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.B.; Seldeen, K.L.; Deegan, B.J.; Bhat, V.; Farooq, A. Binding of the cSH3 domain of Grb2 adaptor to two distinct RXXK motifs within Gab1 docker employs differential mechanisms. J. Mol. Recognit. 2011, 24, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Gill, G. SUMO and ubiquitin in the nucleus: Different functions, similar mechanisms? Genes Dev. 2004, 18, 2046–2059. [Google Scholar] [CrossRef]
- Hayashi, T.; Seki, M.; Maeda, D.; Wang, W.; Kawabe, Y.; Seki, T.; Saitoh, H.; Fukagawa, T.; Yagi, H.; Enomoto, T. Ubc9 is essential for viability of higher eukaryotic cells. Exp. Cell Res. 2002, 280, 212–221. [Google Scholar] [CrossRef]
- Qu, Y.; Chen, Q.; Lai, X.; Zhu, C.; Chen, C.; Zhao, X.; Deng, R.; Xu, M.; Yuan, H.; Wang, Y.; et al. SUMOylation of Grb2 enhances the ERK activity by increasing its binding with Sos1. Mol. Cancer 2014, 13, 95. [Google Scholar] [CrossRef]
| Domain | Inactive Mutant | Refs. |
|---|---|---|
| n-SH3 | W36K | Stainthorp et al. [16] |
| P49L | Mitra et al. [17] | |
| SH2 | R86K | Mitra et al. [17] |
| R86A | Jiang et al. [18] | |
| E89K | Saxton et al. [19] | |
| S90N | Chardin et al. [20] | |
| W139K | Stainthorp et al. [16] | |
| c-SH3 | G162R | Ramos-Morales et al. [21] |
| W193K | Stainthorp et al. [16] | |
| G203R | Yang et al. [22] | |
| P209L | Mitra et al. [17] |
| Domain | Protein Interaction | Domain or Site | Refs. |
|---|---|---|---|
| n-SH3 | SOS | PR domain | Liao et al. [107] |
| Cbl | - | Waterman et al. [110] | |
| Fukazawa et al. [113] | |||
| Dynamin | - | Yoon et al. [118] | |
| GAREM | Y105, Y453 | Blagoev et al. [119] | |
| ADA | - | Ramos-Morales et al. [21] | |
| cyt-PTPe | Y638 | Granot-Attas et al. [96] Levy-Apter et al. [97] | |
| SH2 | EGFR | Y1173, Y1086, Y1068 | Lowenstein et al. [6] Kashishian et al. [63] Batzer et al. [64] |
| PDGF | Y716 | Arvidsson et al. [65] | |
| FGFR | - | Lin et al. [73] | |
| Shp2 | Y542 | Araki et al. [79] | |
| Shc | Y239, Y240, Y317 | Salcini et al. [83] Gotoh et al. [84] | |
| FAK | Y925 | Schlaepfer et al. [94] | |
| cyt-PTPe | Y638 | Granot-Attas et al. [96] Levy-Apter et al. [97] | |
| c-Met | Y1356 | Giubellino et al. [90] | |
| IRS-1 | Y891 | Hakuno et al. [91] | |
| c-SH3 | FGFR | - | Ahmed et al. [77] |
| Cbl | - | Tsygankov et al. [114] | |
| Dynamin | - | Yoon et al. [118] | |
| Gab2 | PH domain | Harkiolaki et al. [127] | |
| GAREM | Y105, Y453 | Blagoev et al. [119] | |
| PRR14 | - | Yang et al. [131] | |
| ADA | - | Ramos-Morales et al. [21] | |
| hnRNP C | - | Romero et al. [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Liu, G.; Meng, Y.; Chen, H.; Ye, Z.; Jing, J. The Configuration of GRB2 in Protein Interaction and Signal Transduction. Biomolecules 2024, 14, 259. https://doi.org/10.3390/biom14030259
Wang D, Liu G, Meng Y, Chen H, Ye Z, Jing J. The Configuration of GRB2 in Protein Interaction and Signal Transduction. Biomolecules. 2024; 14(3):259. https://doi.org/10.3390/biom14030259
Chicago/Turabian StyleWang, Dingyi, Guoxia Liu, Yuxin Meng, Hongjie Chen, Zu Ye, and Ji Jing. 2024. "The Configuration of GRB2 in Protein Interaction and Signal Transduction" Biomolecules 14, no. 3: 259. https://doi.org/10.3390/biom14030259
APA StyleWang, D., Liu, G., Meng, Y., Chen, H., Ye, Z., & Jing, J. (2024). The Configuration of GRB2 in Protein Interaction and Signal Transduction. Biomolecules, 14(3), 259. https://doi.org/10.3390/biom14030259





