Sex as a Critical Variable in Basic and Pre-Clinical Studies of Fibrodysplasia Ossificans Progressiva
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Mouse Models and Genotyping
2.2. Tamoxifen Administration
2.3. Muscle Injury
2.4. Ligand and Antibody Injections
2.5. µCT and HO Quantification
2.6. Cell Isolation
2.7. Flow Cytometry Analysis
2.8. Fluorescence-Activated Cell Sorting
2.9. Reciprocal Transplantation
2.10. Castration Surgery
2.11. Ovariectomy
2.12. Luciferase Proliferation Assay
2.13. Statistics
3. Results
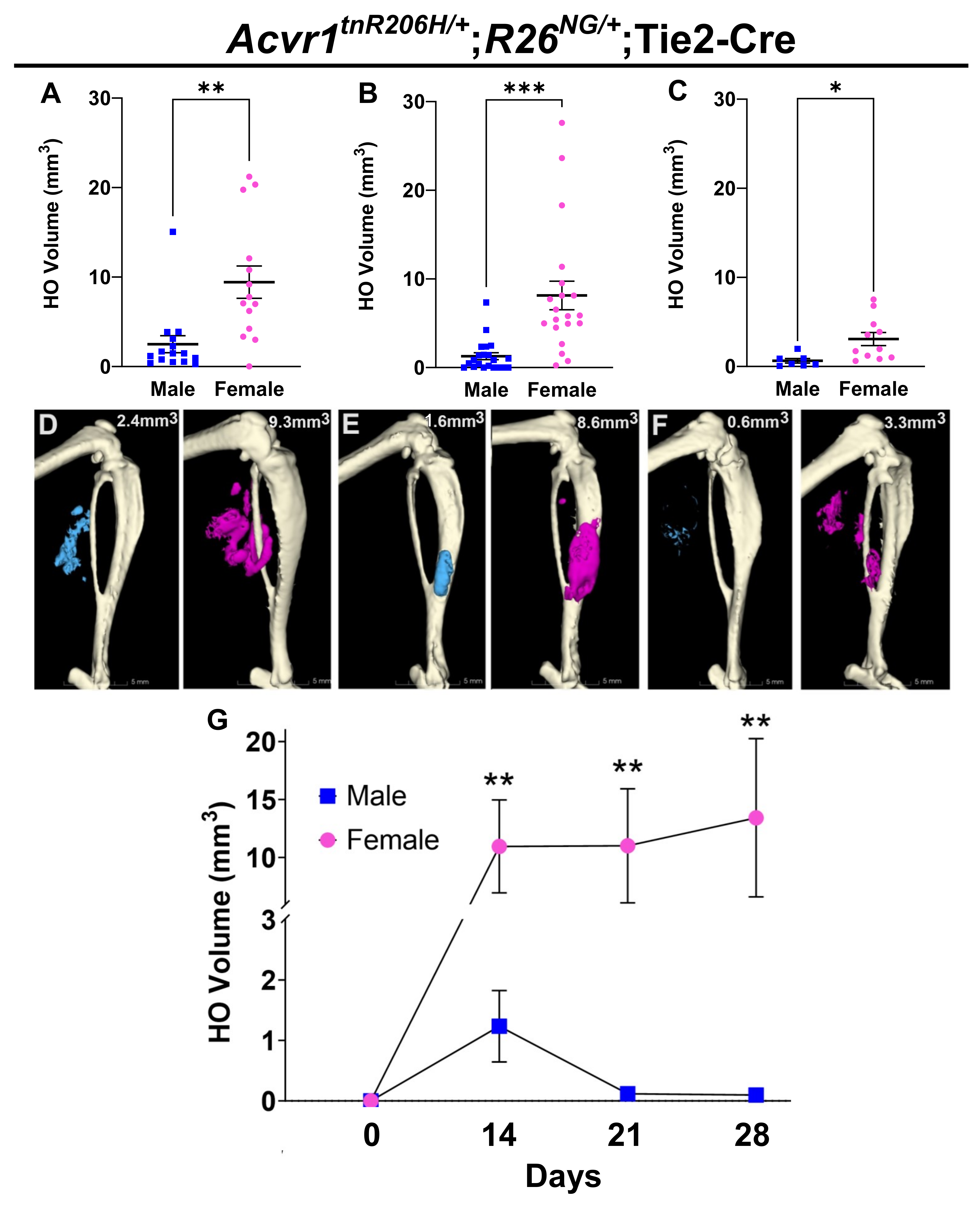
3.1. Female FOP Mice Develop More Heterotopic Bone and Exhibit a More Variable Response to Muscle Injury Than Males
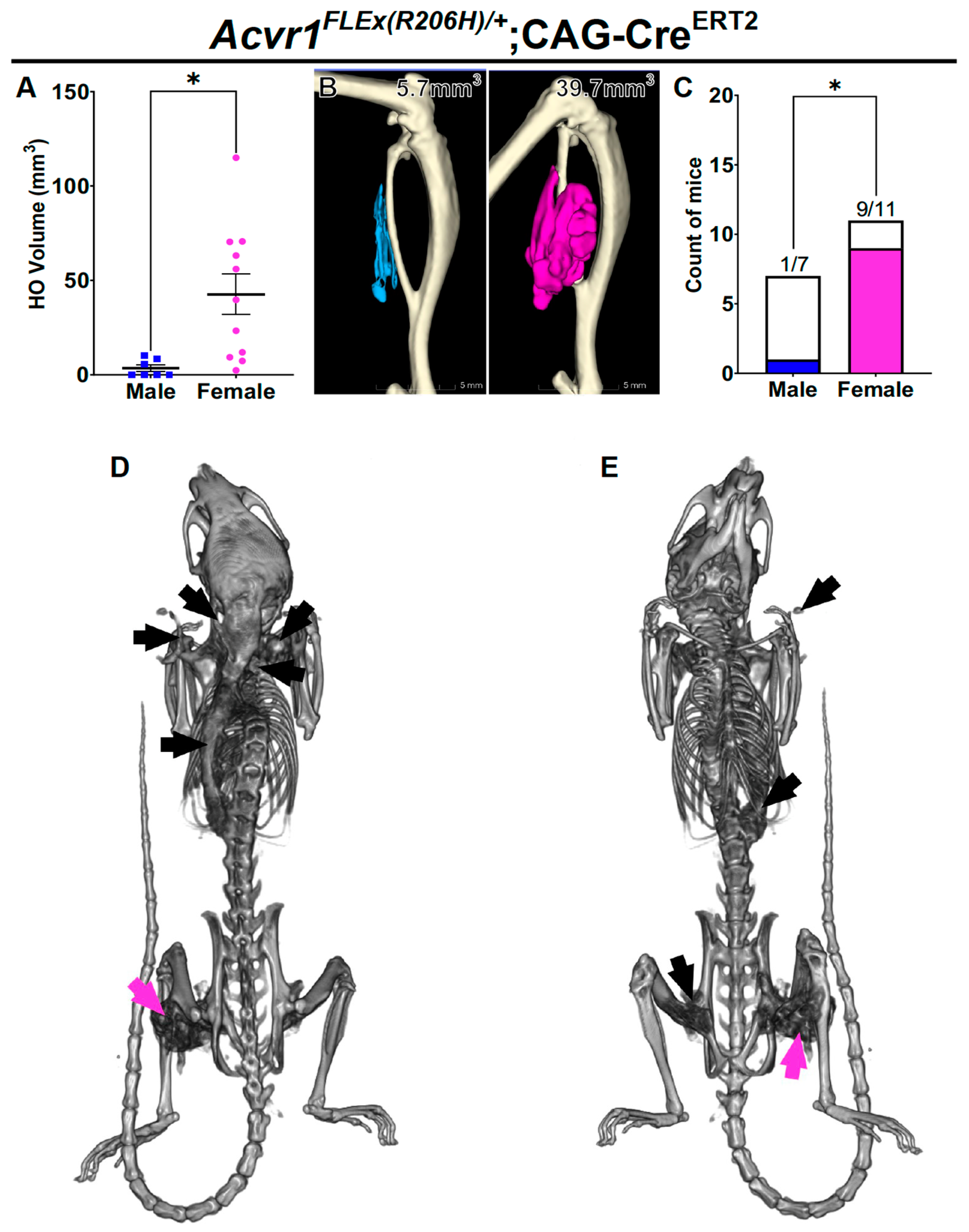
3.2. Female Bias in Both Injury-Induced and Spontaneous HO in an Independently Derived FOP Mouse Model
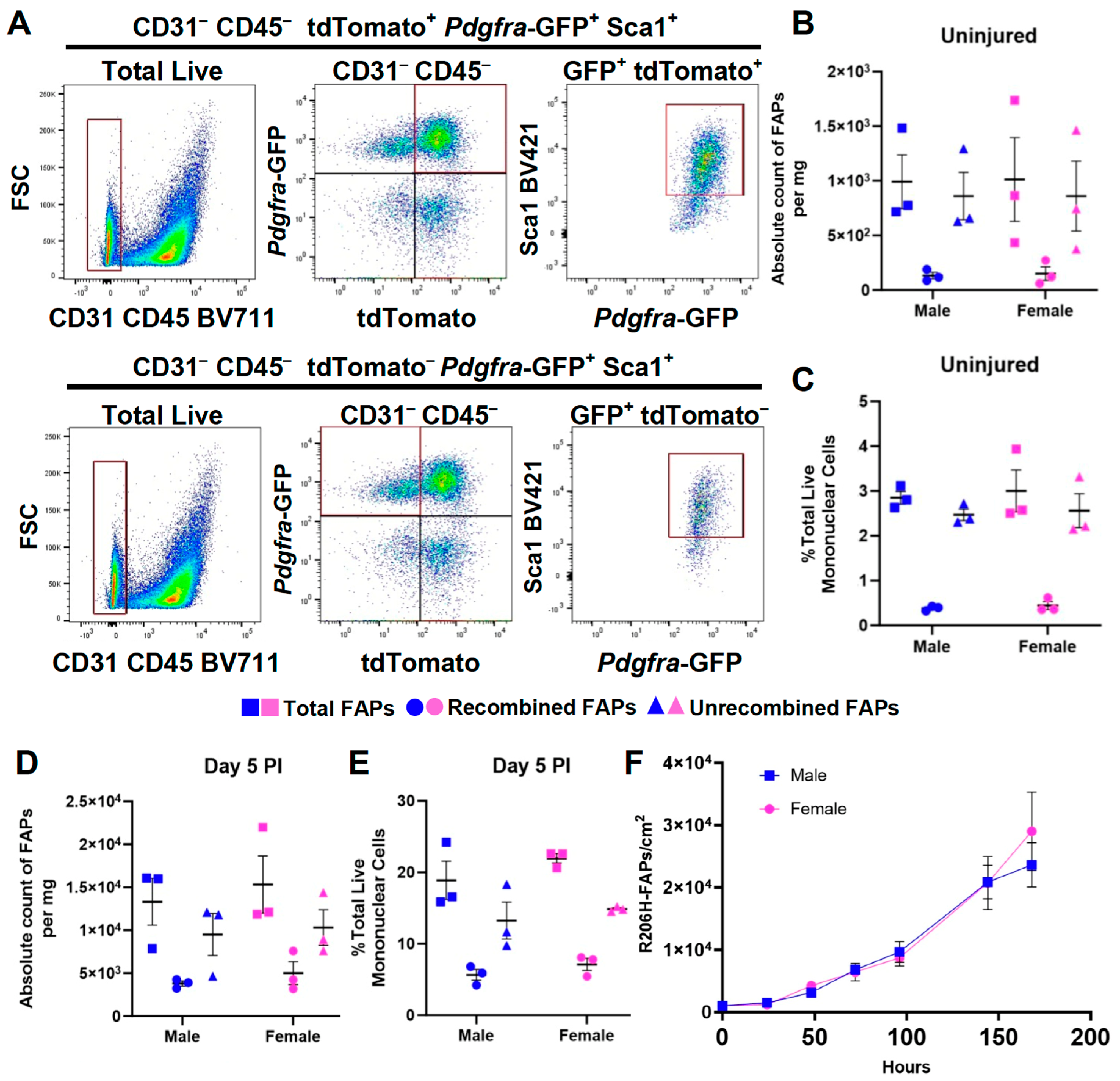
3.3. The Female Sex Bias in HO Is Not Due to a Difference in the Abundance of FAPs or to a Greater Efficiency of Cre-Dependent Recombination of the Acvr1tnR206H Allele
3.4. The Female Bias in FAP-Directed HO Is Driven by Cell-Autonomous Factors
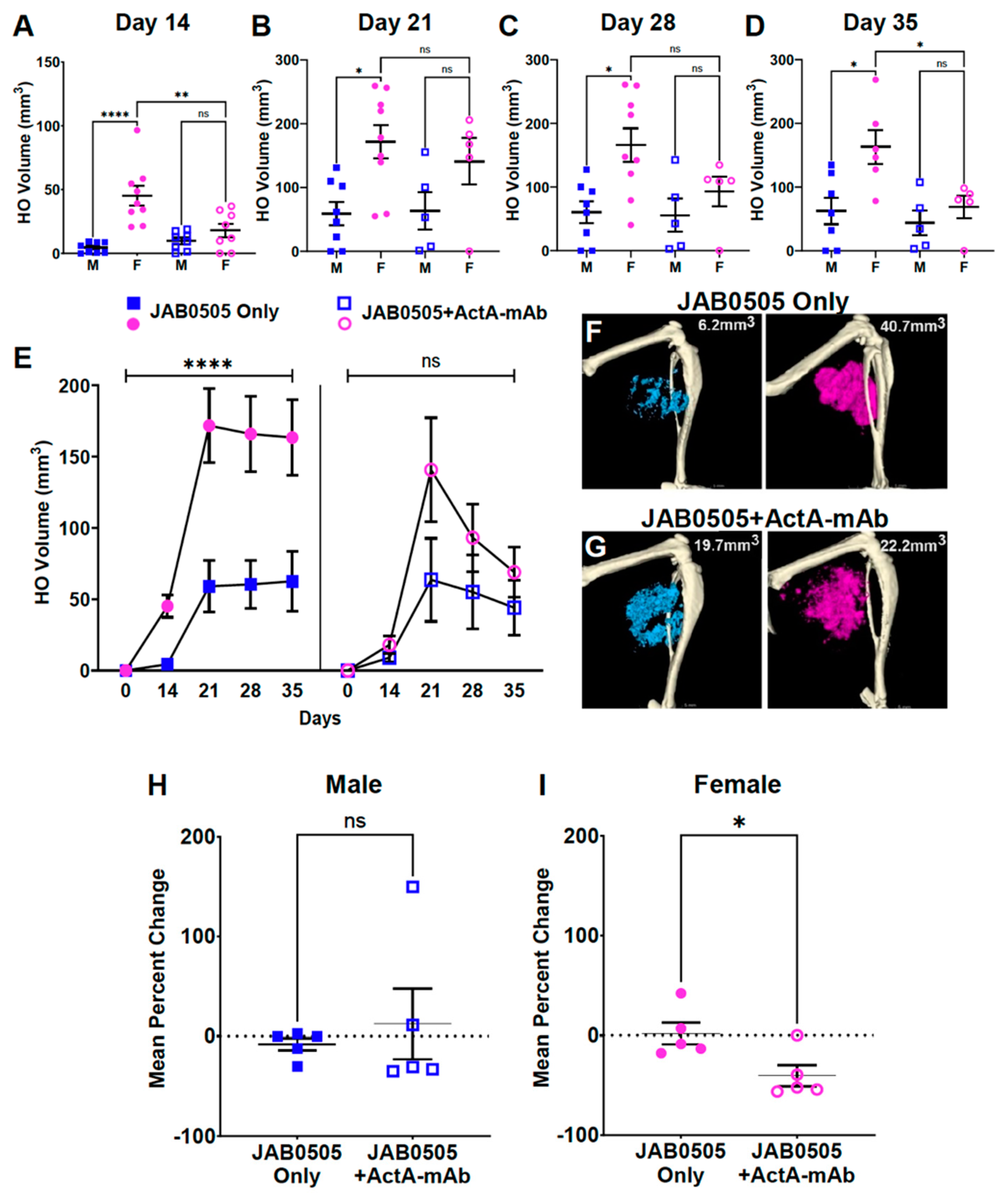
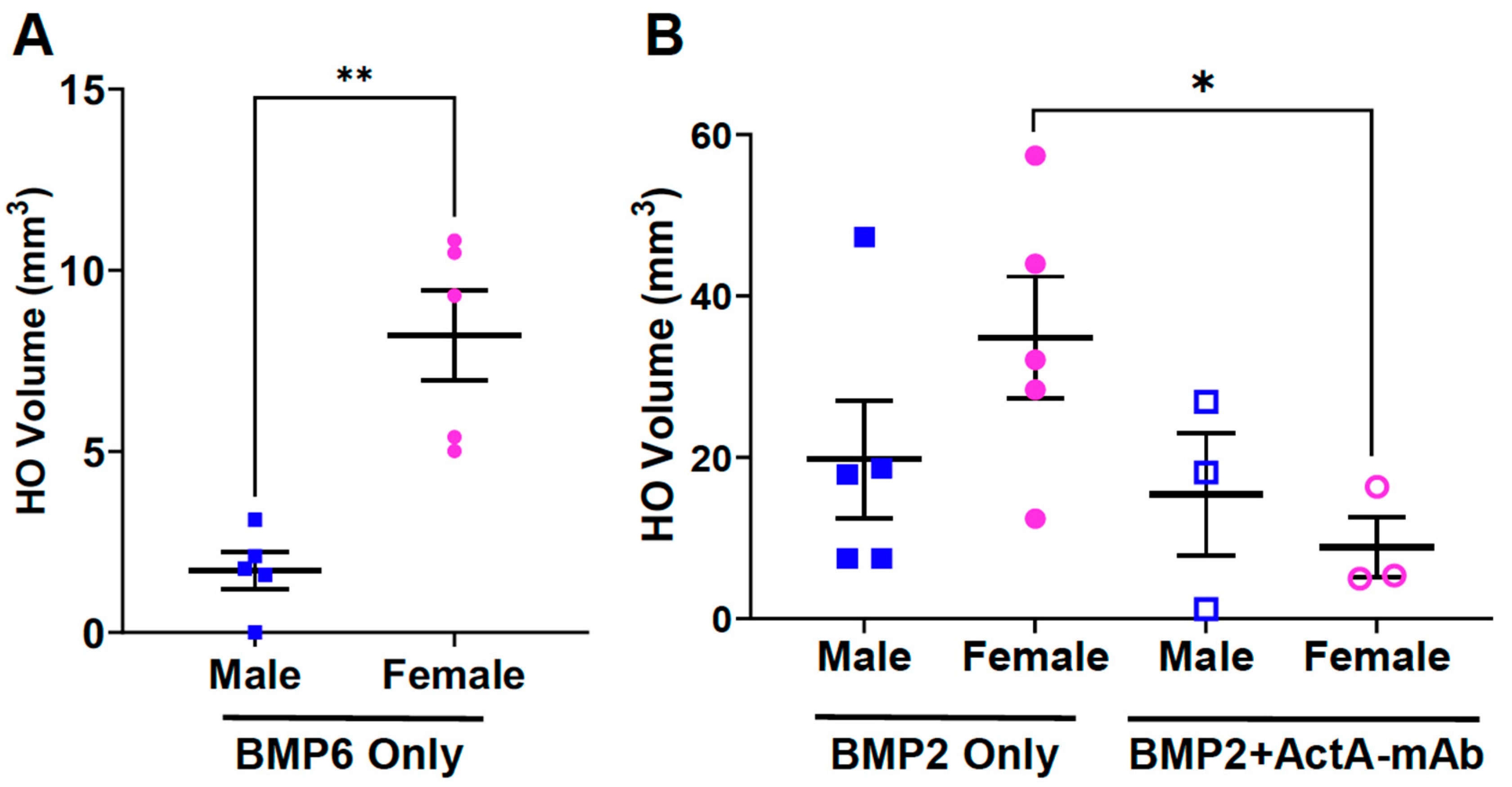
3.5. Activin A Inhibition Attenuates HO Formation and Reduces the Stability of Nascent HO in Female FOP Mice
3.6. Biological Sex Impacts on BMP-Induced HO and the Effect of Activin A Inhibition
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.K. Sex as an Important Biological Variable in Biomedical Research. BMB Rep. 2018, 51, 167–173. [Google Scholar] [CrossRef]
- Soldin, O.P.; Mattison, D.R. Sex Differences in Pharmacokinetics and Pharmacodynamics. Clin. Pharmacokinet. 2009, 48, 143–157. [Google Scholar] [CrossRef]
- Morris, M.E.; Lee, H.-J.; Predko, L.M. Gender Differences in the Membrane Transport of Endogenous and Exogenous Compounds. Pharmacol. Rev. 2003, 55, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Soldin, O.P.; Chung, S.H.; Mattison, D.R. Sex Differences in Drug Disposition. J. Biomed. Biotechnol. 2011, 2011, 187103. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health NOT-OD-15-102: Consideration of Sex as a Biological Variable in NIH-Funded Research. Available online: https://grants.nih.gov/grants/guide/notice-files/not-od-15-102.html (accessed on 18 September 2023).
- Bongetta, D.; Bua, M.; Bruno, R.; Colombo, E.V.; de Laurentis, C.; Versace, A.; Locatelli, M.; Assietti, R. Is Gender a Factor Affecting Long-Term Heterotopic Ossification Incidence After Single-Level Cervical Disc Arthroplasty? World Neurosurg. 2022, 165, 6–12. [Google Scholar] [CrossRef]
- Leung, C.; Casey, A.T.; Goffin, J.; Kehr, P.; Liebig, K.; Lind, B.; Logroscino, C.; Pointillart, V. Clinical Significance of Heterotopic Ossification in Cervical Disc Replacement: A Prospective Multicenter Clinical Trial. Neurosurgery 2005, 57, 759–763. [Google Scholar] [CrossRef]
- Yi, S.; Shin, D.A.; Kim, K.N.; Choi, G.; Shin, H.C.; Kim, K.S.; Yoon, D.H. The Predisposing Factors for the Heterotopic Ossification after Cervical Artificial Disc Replacement. Spine J. 2013, 13, 1048–1054. [Google Scholar] [CrossRef]
- Kjægaard-Andersen, P.; Steinke, M.S.; Hougaard, K.; Søjbjerg, J.O.; Jensen, J. Heterotopic Bone Formation Following Hip Arthroplasty: A Retrospective Study of 65 Bilateral Cases. Acta Orthop. Scand. 2009, 62, 223–225. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, F.; Chen, W.; Zhang, Q.; Liu, S.; Zhang, Y. Incidence and Risk Factors for Heterotopic Ossification after Total Hip Arthroplasty: A Meta-Analysis. Arch. Orthop. Trauma Surg. 2015, 135, 1307–1314. [Google Scholar] [CrossRef]
- Steinberg, G.G.; Hubbard, C. Heterotopic Ossification after Femoral Intramedullary Rodding. J. Orthop. Trauma 1993, 7, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Bargellesi, S.; Cavasin, L.; Scarponi, F.; Tanti, A.D.; Bonaiuti, D.; Bartolo, M.; Boldrini, P.; Estraneo, A.; Heterotopic Ossification Cross Sectional Survey group (HOCSS). Occurrence and Predictive Factors of Heterotopic Ossification in Severe Acquired Brain Injured Patients during Rehabilitation Stay: Cross-Sectional Survey. Clin. Rehabil. 2017, 32, 255–262. [Google Scholar] [CrossRef]
- Hu, X.; Sun, Z.; Li, F.; Jiang, C.; Yan, W.; Sun, Y. Burn-Induced Heterotopic Ossification from Incidence to Therapy: Key Signaling Pathways Underlying Ectopic Bone Formation. Cell. Mol. Biol. Lett. 2021, 26, 34. [Google Scholar] [CrossRef]
- Papanagiotou, M.; Dailiana, Z.H.; Karachalios, T.; Varitimidis, S.; Hantes, M.; Dimakopoulos, G.; Vlychou, M.; Malizos, K.N. Heterotopic Ossification after the Use of Recombinant Human Bone Morphogenetic Protein-7. World J. Orthop. 2017, 8, 36–41. [Google Scholar] [CrossRef]
- Salemi, P.; Olson, J.M.S.; Dickson, L.E.; Germain-Lee, E.L. Ossifications in Albright Hereditary Osteodystrophy: Role of Genotype, Inheritance, Sex, Age, Hormonal Status, and BMI. J. Clin. Endocrinol. Metab. 2017, 103, 158–168. [Google Scholar] [CrossRef]
- Huso, D.L.; Edie, S.; Levine, M.A.; Schwindinger, W.; Wang, Y.; Jüppner, H.; Germain-Lee, E.L. Heterotopic Ossifications in a Mouse Model of Albright Hereditary Osteodystrophy. PLoS ONE 2011, 6, e21755. [Google Scholar] [CrossRef]
- Ranganathan, K.; Peterson, J.; Agarwal, S.; Oluwatobi, E.; Loder, S.; Forsberg, J.A.; Davis, T.A.; Buchman, S.R.; Wang, S.C.; Levi, B. Role of Gender in Burn-Induced Heterotopic Ossification and Mesenchymal Cell Osteogenic Differentiation. Plast. Reconstr. Surg. 2015, 135, 1631–1641. [Google Scholar] [CrossRef]
- Corsi, K.A.; Pollett, J.B.; Phillippi, J.A.; Usas, A.; Li, G.; Huard, J. Osteogenic Potential of Postnatal Skeletal Muscle–Derived Stem Cells Is Influenced by Donor Sex. J. Bone Miner. Res. 2007, 22, 1592–1602. [Google Scholar] [CrossRef]
- Wang, L.; Carroll, D.O.; Liu, X.; Roth, T.; Kim, H.; Halloran, B.; Nissenson, R.A. Effects of Blockade of Endogenous Gi Signaling in Tie2-expressing Cells on Bone Formation in a Mouse Model of Heterotopic Ossification. J. Orthop. Res. 2015, 33, 1212–1217. [Google Scholar] [CrossRef]
- Shore, E.M.; Xu, M.; Feldman, G.J.; Fenstermacher, D.A.; Cho, T.-J.; Choi, I.H.; Connor, J.M.; Delai, P.; Glaser, D.L.; LeMerrer, M.; et al. A Recurrent Mutation in the BMP Type I Receptor ACVR1 Causes Inherited and Sporadic Fibrodysplasia Ossificans Progressiva. Nat. Genet. 2006, 38, 525–527. [Google Scholar] [CrossRef]
- Kaplan, F.S.; Xu, M.; Seemann, P.; Connor, J.M.; Glaser, D.L.; Carroll, L.; Delai, P.; Fastnacht-Urban, E.; Forman, S.J.; Gillessen-Kaesbach, G.; et al. Classic and Atypical Fibrodysplasia Ossificans Progressiva (FOP) Phenotypes Are Caused by Mutations in the Bone Morphogenetic Protein (BMP) Type I Receptor ACVR1. Hum. Mutat. 2009, 30, 379–390. [Google Scholar] [CrossRef]
- Hatsell, S.J.; Idone, V.; Wolken, D.M.A.; Huang, L.; Kim, H.J.; Wang, L.; Wen, X.; Nannuru, K.C.; Jimenez, J.; Xie, L.; et al. ACVR1R206H Receptor Mutation Causes Fibrodysplasia Ossificans Progressiva by Imparting Responsiveness to Activin A. Sci. Transl. Med. 2015, 7, 303ra137. [Google Scholar] [CrossRef]
- Hino, K.; Ikeya, M.; Horigome, K.; Matsumoto, Y.; Ebise, H.; Nishio, M.; Sekiguchi, K.; Shibata, M.; Nagata, S.; Matsuda, S.; et al. Neofunction of ACVR1 in Fibrodysplasia Ossificans Progressiva. Proc. Natl. Acad. Sci. USA 2015, 112, 15438–15443. [Google Scholar] [CrossRef]
- Mantick, N.; Bachman, E.; Baujat, G.; Brown, M.; Collins, O.; Cunto, C.D.; Delai, P.; Eekhoff, M.; Felde, R.Z.; Grogan, D.R.; et al. The FOP Connection Registry: Design of an International Patient-Sponsored Registry for Fibrodysplasia Ossificans Progressiva. Bone 2018, 109, 285–290. [Google Scholar] [CrossRef]
- Peng, K.; Cheung, K.; Lee, A.; Sieberg, C.; Borsook, D.; Upadhyay, J. Longitudinal Evaluation of Pain, Flare-Up, and Emotional Health in Fibrodysplasia Ossificans Progressiva: Analyses of the International FOP Registry. JBMR Plus 2019, 3, e10181. [Google Scholar] [CrossRef]
- Pignolo, R.J.; Hsiao, E.C.; Baujat, G.; Lapidus, D.; Sherman, A.; Kaplan, F.S. Prevalence of Fibrodysplasia Ossificans Progressiva (FOP) in the United States: Estimate from Three Treatment Centers and a Patient Organization. Orphanet J. Rare Dis. 2021, 16, 350. [Google Scholar] [CrossRef]
- Joe, A.W.B.; Yi, L.; Natarajan, A.; Grand, F.L.; So, L.; Wang, J.; Rudnicki, M.A.; Rossi, F.M.V. Muscle Injury Activates Resident Fibro/Adipogenic Progenitors That Facilitate Myogenesis. Nat. Cell Biol. 2010, 12, 153–163. [Google Scholar] [CrossRef]
- Uezumi, A.; Fukada, S.; Yamamoto, N.; Takeda, S.; Tsuchida, K. Mesenchymal Progenitors Distinct from Satellite Cells Contribute to Ectopic Fat Cell Formation in Skeletal Muscle. Nat. Cell Biol. 2010, 12, 143–152. [Google Scholar] [CrossRef]
- Eisner, C.; Cummings, M.; Johnston, G.; Tung, L.W.; Groppa, E.; Chang, C.; Rossi, F.M. Murine Tissue-Resident PDGFRα+ Fibro-Adipogenic Progenitors Spontaneously Acquire Osteogenic Phenotype in an Altered Inflammatory Environment. J. Bone Miner. Res. 2020, 35, 1525–1534. [Google Scholar] [CrossRef]
- Lees-Shepard, J.B.; Yamamoto, M.; Biswas, A.A.; Stoessel, S.J.; Nicholas, S.-A.E.; Cogswell, C.A.; Devarakonda, P.M.; Schneider, M.J.; Cummins, S.M.; Legendre, N.P.; et al. Activin-Dependent Signaling in Fibro/Adipogenic Progenitors Causes Fibrodysplasia Ossificans Progressiva. Nat. Commun. 2018, 9, 471. [Google Scholar] [CrossRef]
- Lees-Shepard, J.B.; Nicholas, S.-A.E.; Stoessel, S.J.; Devarakonda, P.M.; Schneider, M.J.; Yamamoto, M.; Goldhamer, D.J. Palovarotene Reduces Heterotopic Ossification in Juvenile FOP Mice but Exhibits Pronounced Skeletal Toxicity. eLife 2018, 7, 305. [Google Scholar] [CrossRef]
- Wosczyna, M.N.; Biswas, A.A.; Cogswell, C.A.; Goldhamer, D.J. Multipotent Progenitors Resident in the Skeletal Muscle Interstitium Exhibit Robust BMP-Dependent Osteogenic Activity and Mediate Heterotopic Ossification. J. Bone Miner. Res. 2012, 27, 1004–1017. [Google Scholar] [CrossRef]
- Yamamoto, M.; Stoessel, S.J.; Yamamoto, S.; Goldhamer, D.J. Overexpression of Wild-Type ACVR1 in Fibrodysplasia Ossificans Progressiva Mice Rescues Perinatal Lethality and Inhibits Heterotopic Ossification. J. Bone Miner. Res. 2022, 37, 2077–2093. [Google Scholar] [CrossRef]
- Kisanuki, Y.Y.; Hammer, R.E.; Miyazaki, J.; Williams, S.C.; Richardson, J.A.; Yanagisawa, M. Tie2-Cre Transgenic Mice: A New Model for Endothelial Cell-Lineage Analysis in Vivo. Dev. Biol. 2001, 230, 230–242. [Google Scholar] [CrossRef]
- Hamilton, T.G.; Klinghoffer, R.A.; Corrin, P.D.; Soriano, P. Evolutionary Divergence of Platelet-Derived Growth Factor Alpha Receptor Signaling Mechanisms. Mol. Cell. Biol. 2003, 23, 4013–4025. [Google Scholar] [CrossRef]
- Yamamoto, M.; Shook, N.A.; Kanisicak, O.; Yamamoto, S.; Wosczyna, M.N.; Camp, J.R.; Goldhamer, D.J. A Multifunctional Reporter Mouse Line for Cre- and FLP-Dependent Lineage Analysis. Genesis 2009, 47, 107–114. [Google Scholar] [CrossRef]
- Safran, M.; Kim, W.Y.; Kung, A.L.; Horner, J.W.; DePinho, R.A.; Kaelin, W.G. Mouse Reporter Strain for Noninvasive Bioluminescent Imaging of Cells That Have Undergone Cre-Mediated Recombination. Mol. Imaging 2003, 2, 15353500200303154. [Google Scholar] [CrossRef]
- Hayashi, S.; McMahon, A.P. Efficient Recombination in Diverse Tissues by a Tamoxifen-Inducible Form of Cre: A Tool for Temporally Regulated Gene Activation/Inactivation in the Mouse. Dev. Biol. 2002, 244, 305–318. [Google Scholar] [CrossRef]
- Lees-Shepard, J.B.; Stoessel, S.J.; Chandler, J.T.; Bouchard, K.; Bento, P.; Apuzzo, L.N.; Devarakonda, P.M.; Hunter, J.W.; Goldhamer, D.J. An Anti-ACVR1 Antibody Exacerbates Heterotopic Ossification by Fibro-Adipogenic Progenitors in Fibrodysplasia Ossificans Progressiva Mice. J. Clin. Investig. 2022, 132, e153795. [Google Scholar] [CrossRef]
- Biswas, A.A.; Goldhamer, D.J. FACS Fractionation and Differentiation of Skeletal-Muscle Resident Multipotent Tie2+ Progenitors. Methods Mol. Biol. 2016, 1460, 255–267. [Google Scholar] [CrossRef]
- Nagy, A.; Gertsenstein, M.; Vintersten, K.; Behringer, R. Castration. Cold Spring Harb. Protoc. 2006, 2006. [Google Scholar] [CrossRef]
- Souza, V.R.; Mendes, E.; Casaro, M.; Antiorio, A.T.F.B.; Oliveira, F.A.; Ferreira, C.M. Pre-Clinical Models, Techniques and Protocols. Methods Mol. Biol. 2018, 1916, 303–309. [Google Scholar] [CrossRef]
- Lounev, V.Y.; Ramachandran, R.; Wosczyna, M.N.; Yamamoto, M.; Maidment, A.D.A.; Shore, E.M.; Glaser, D.L.; Goldhamer, D.J.; Kaplan, F.S. Identification of Progenitor Cells That Contribute to Heterotopic Skeletogenesis. J. Bone Jt. Surg. 2009, 91, 652–663. [Google Scholar] [CrossRef]
- Dey, D.; Bagarova, J.; Hatsell, S.J.; Armstrong, K.A.; Huang, L.; Ermann, J.; Vonner, A.J.; Shen, Y.; Mohedas, A.H.; Lee, A.; et al. Two Tissue-Resident Progenitor Lineages Drive Distinct Phenotypes of Heterotopic Ossification. Sci. Transl. Med. 2016, 8, 366ra163. [Google Scholar] [CrossRef]
- Rowe, R.W.; Goldspink, G. Muscle Fibre Growth in Five Different Muscles in Both Sexes of Mice. J. Anat. 1969, 104, 519–530. [Google Scholar]
- Griffin, G.E.; Goldspink, G. The Increase in Skeletal Muscle Mass in Male and Female Mice. Anat. Rec. 1973, 177, 465–469. [Google Scholar] [CrossRef]
- Pignolo, R.J.; Bedford-Gay, C.; Liljesthröm, M.; Durbin-Johnson, B.P.; Shore, E.M.; Rocke, D.M.; Kaplan, F.S. The Natural History of Flare-Ups in Fibrodysplasia Ossificans Progressiva (FOP): A Comprehensive Global Assessment. J. Bone Miner. Res. 2016, 31, 650–656. [Google Scholar] [CrossRef]
- Upadhyay, J.; Xie, L.; Huang, L.; Das, N.; Stewart, R.C.; Lyon, M.C.; Palmer, K.; Rajamani, S.; Graul, C.; Lobo, M.; et al. The Expansion of Heterotopic Bone in Fibrodysplasia Ossificans Progressiva Is Activin A-Dependent. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2017, 38, 525. [Google Scholar] [CrossRef]
- Aykul, S.; Huang, L.; Wang, L.; Das, N.M.; Reisman, S.; Ray, Y.; Zhang, Q.; Rothman, N.; Nannuru, K.C.; Kamat, V.; et al. Anti-ACVR1 Antibodies Exacerbate Heterotopic Ossification in Fibrodysplasia Ossificans Progressiva (FOP) by Activating FOP-Mutant ACVR1. J. Clin. Investig. 2022, 132, e153792. [Google Scholar] [CrossRef]
- Mundy, C.; Yao, L.; Sinha, S.; Chung, J.; Rux, D.; Catheline, S.E.; Koyama, E.; Qin, L.; Pacifici, M. Activin A Promotes the Development of Acquired Heterotopic Ossification and Is an Effective Target for Disease Attenuation in Mice. Sci. Signal. 2021, 14, eabd0536. [Google Scholar] [CrossRef]
- Mundy, C.; Yao, L.; Shaughnessy, K.A.; Saunders, C.; Shore, E.M.; Koyama, E.; Pacifici, M. Palovarotene Action Against Heterotopic Ossification Includes a Reduction of Local Participating Activin A-Expressing Cell Populations. JBMR Plus 2023, 7, e10821. [Google Scholar] [CrossRef]
- Lin, S.; Svoboda, K.K.H.; Feng, J.Q.; Jiang, X. The Biological Function of Type I Receptors of Bone Morphogenetic Protein in Bone. Bone Res. 2016, 4, 16005. [Google Scholar] [CrossRef]
- Nickel, J.; Mueller, T.D. Specification of BMP Signaling. Cells 2019, 8, 1579. [Google Scholar] [CrossRef]
- Katagiri, T.; Watabe, T. Bone Morphogenetic Proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a021899. [Google Scholar] [CrossRef]
- Kitterman, J.A.; Strober, J.B.; Kan, L.; Rocke, D.M.; Cali, A.; Peeper, J.; Snow, J.; Delai, P.L.R.; Morhart, R.; Pignolo, R.J.; et al. Neurological Symptoms in Individuals with Fibrodysplasia Ossificans Progressiva. J. Neurol. 2012, 259, 2636–2643. [Google Scholar] [CrossRef]
- Pignolo, R.J.; Cheung, K.; Kile, S.; Fitzpatrick, M.A.; Cunto, C.D.; Mukaddam, M.A.; Hsiao, E.C.; Baujat, G.; Delai, P.; Eekhoff, E.M.W.; et al. Self-Reported Baseline Phenotypes from the International Fibrodysplasia Ossificans Progressiva (FOP) Association Global Registry. Bone 2020, 134, 115274. [Google Scholar] [CrossRef]
- Pignolo, R.J.; Baujat, G.; Brown, M.A.; Cunto, C.D.; DiRocco, M.; Hsiao, E.C.; Keen, R.; Mukaddam, M.A.; Sang, K.-H.L.Q.; Wilson, A.; et al. Natural History of Fibrodysplasia Ossificans Progressiva: Cross-Sectional Analysis of Annotated Baseline Phenotypes. Orphanet J. Rare Dis. 2019, 14, 98. [Google Scholar] [CrossRef]
- Chakkalakal, S.A.; Uchibe, K.; Convente, M.R.; Zhang, D.; Economides, A.N.; Kaplan, F.S.; Pacifici, M.; Iwamoto, M.; Shore, E.M. Palovarotene Inhibits Heterotopic Ossification and Maintains Limb Mobility and Growth in Mice With the Human ACVR1(R206H) Fibrodysplasia Ossificans Progressiva (FOP) Mutation. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2016, 31, 1666–1675. [Google Scholar] [CrossRef]
- Goldhamer, D.J.; Lees-Shepard, J.B. Response to Comment on “Palovarotene Reduces Heterotopic Ossification in Juvenile FOP Mice but Exhibits Pronounced Skeletal Toxicity”. eLife 2019, 8, e43928. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, T.; Tsukamoto, S.; Kuratani, M.; Tsuji, S.; Nakamura, K.; Ohte, S.; Kawaguchi, Y.; Takaishi, K. A Blocking Monoclonal Antibody Reveals Dimerization of Intracellular Domains of ALK2 Associated with Genetic Disorders. Nat. Commun. 2023, 14, 2960. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-S.; Lin, C.; Ma, H.; Xie, J.; Kaplan, F.S.; Gao, G.; Shim, J.-H. AAV-Mediated Targeting of the Activin A-ACVR1R206H Signaling in Fibrodysplasia Ossificans Progressiva. Biomolecules 2023, 13, 1364. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-S.; Kim, J.-M.; Xie, J.; Chaugule, S.; Lin, C.; Ma, H.; Hsiao, E.; Hong, J.; Chun, H.; Shore, E.M.; et al. Suppression of Heterotopic Ossification in Fibrodysplasia Ossificans Progressiva Using AAV Gene Delivery. Nat. Commun. 2022, 13, 6175. [Google Scholar] [CrossRef]
- Malecova, B.; Gatto, S.; Etxaniz, U.; Passafaro, M.; Cortez, A.; Nicoletti, C.; Giordani, L.; Torcinaro, A.; Bardi, M.D.; Bicciato, S.; et al. Dynamics of Cellular States of Fibro-Adipogenic Progenitors during Myogenesis and Muscular Dystrophy. Nat. Commun. 2018, 9, 3670. [Google Scholar] [CrossRef] [PubMed]
- Lees-Shepard, J.B.; Goldhamer, D.J. Stem Cells and Heterotopic Ossification: Lessons from Animal Models. Bone 2018, 109, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Qu-Petersen, Z.; Deasy, B.; Jankowski, R.; Ikezawa, M.; Cummins, J.; Pruchnic, R.; Mytinger, J.; Cao, B.; Gates, C.; Wernig, A.; et al. Identification of a Novel Population of Muscle Stem Cells in Mice. J. Cell Biol. 2002, 157, 851–864. [Google Scholar] [CrossRef]
- Stanley, A.; Tichy, E.D.; Kocan, J.; Roberts, D.W.; Shore, E.M.; Mourkioti, F. Dynamics of Skeletal Muscle-Resident Stem Cells during Myogenesis in Fibrodysplasia Ossificans Progressiva. Npj Regen. Med. 2022, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Lemos, D.R.; Babaeijandaghi, F.; Low, M.; Chang, C.-K.; Lee, S.T.; Fiore, D.; Zhang, R.-H.; Natarajan, A.; Nedospasov, S.A.; Rossi, F.M.V. Nilotinib Reduces Muscle Fibrosis in Chronic Muscle Injury by Promoting TNF-Mediated Apoptosis of Fibro/Adipogenic Progenitors. Nat. Med. 2015, 21, 786–794. [Google Scholar] [CrossRef]
- Compston, J.E. Sex Steroids and Bone. Physiol. Rev. 2001, 81, 419–447. [Google Scholar] [CrossRef]
- Deasy, B.M.; Lu, A.; Tebbets, J.C.; Feduska, J.M.; Schugar, R.C.; Pollett, J.B.; Sun, B.; Urish, K.L.; Gharaibeh, B.M.; Cao, B.; et al. A Role for Cell Sex in Stem Cell-Mediated Skeletal Muscle Regeneration: Female Cells Have Higher Muscle Regeneration Efficiency. J. Cell Biol. 2007, 177, 73–86. [Google Scholar] [CrossRef]
- Kaipia, A.; Toppari, J.; Huhtaniemi, I.; Paranko, J. Sex Difference in the Action of Activin-A on Cell Proliferation of Differentiating Rat Gonad. Endocrinology 1994, 134, 2165–2170. [Google Scholar] [CrossRef]
- Zhong, X.; Narasimhan, A.; Silverman, L.M.; Young, A.R.; Shahda, S.; Liu, S.; Wan, J.; Liu, Y.; Koniaris, L.G.; Zimmers, T.A. Sex Specificity of Pancreatic Cancer Cachexia Phenotypes, Mechanisms, and Treatment in Mice and Humans: Role of Activin. J. Cachexia Sarcopenia Muscle 2022, 13, 2146–2161. [Google Scholar] [CrossRef]
- Aykul, S.; Corpina, R.A.; Goebel, E.J.; Cunanan, C.J.; Dimitriou, A.; Kim, H.; Zhang, Q.; Rafique, A.; Leidich, R.; Wang, X.; et al. Activin A Forms a Non-Signaling Complex with ACVR1 and Type II Activin/BMP Receptors via Its Finger 2 Tip Loop. elife 2020, 9, e54582. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, F.; Xie, L.; Crane, J.; Zhen, G.; Mishina, Y.; Deng, R.; Gao, B.; Chen, H.; Liu, S.; et al. Inhibition of Overactive TGF-β Attenuates Progression of Heterotopic Ossification in Mice. Nat. Commun. 2018, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Bloise, E.; Ciarmela, P.; Cruz, C.D.; Luisi, S.; Petraglia, F.; Reis, F.M. Activin A in Mammalian Physiology. Physiol. Rev. 2019, 99, 739–780. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burdick, L.N.; DelVichio, A.H.; Hanson, L.R.; Griffith, B.B.; Bouchard, K.R.; Hunter, J.W.; Goldhamer, D.J. Sex as a Critical Variable in Basic and Pre-Clinical Studies of Fibrodysplasia Ossificans Progressiva. Biomolecules 2024, 14, 177. https://doi.org/10.3390/biom14020177
Burdick LN, DelVichio AH, Hanson LR, Griffith BB, Bouchard KR, Hunter JW, Goldhamer DJ. Sex as a Critical Variable in Basic and Pre-Clinical Studies of Fibrodysplasia Ossificans Progressiva. Biomolecules. 2024; 14(2):177. https://doi.org/10.3390/biom14020177
Chicago/Turabian StyleBurdick, Lorraine N., Amanda H. DelVichio, L. Russell Hanson, Brenden B. Griffith, Keith R. Bouchard, Jeffrey W. Hunter, and David J. Goldhamer. 2024. "Sex as a Critical Variable in Basic and Pre-Clinical Studies of Fibrodysplasia Ossificans Progressiva" Biomolecules 14, no. 2: 177. https://doi.org/10.3390/biom14020177
APA StyleBurdick, L. N., DelVichio, A. H., Hanson, L. R., Griffith, B. B., Bouchard, K. R., Hunter, J. W., & Goldhamer, D. J. (2024). Sex as a Critical Variable in Basic and Pre-Clinical Studies of Fibrodysplasia Ossificans Progressiva. Biomolecules, 14(2), 177. https://doi.org/10.3390/biom14020177





