Yap Is a Nutrient Sensor Sensitive to the Amino Acid L-Isoleucine and Regulates the Expression of Ctgf in Cardiomyocytes
Abstract
1. Introduction
2. Methods
2.1. In Silico Analyses
2.2. Animal Care
2.3. Pre-Clinical Models
2.4. Tissue Collection and Preparation
2.5. Immunohistochemistry
2.6. Cell Culture
2.7. Cell Stress Treatments
2.8. Immunoblotting
2.8.1. Cell Collection and Whole-Cell Lysate Preparation
2.8.2. Nuclear and Cytoplasmic Protein Extraction
2.9. Western Blotting
2.9.1. Isolation and Measurement of Free Amino Acids
2.9.2. Quantitative Polymerase Chain Reaction (qPCR)
2.10. Immunocytochemistry
2.11. Statistical Analysis:
3. Results
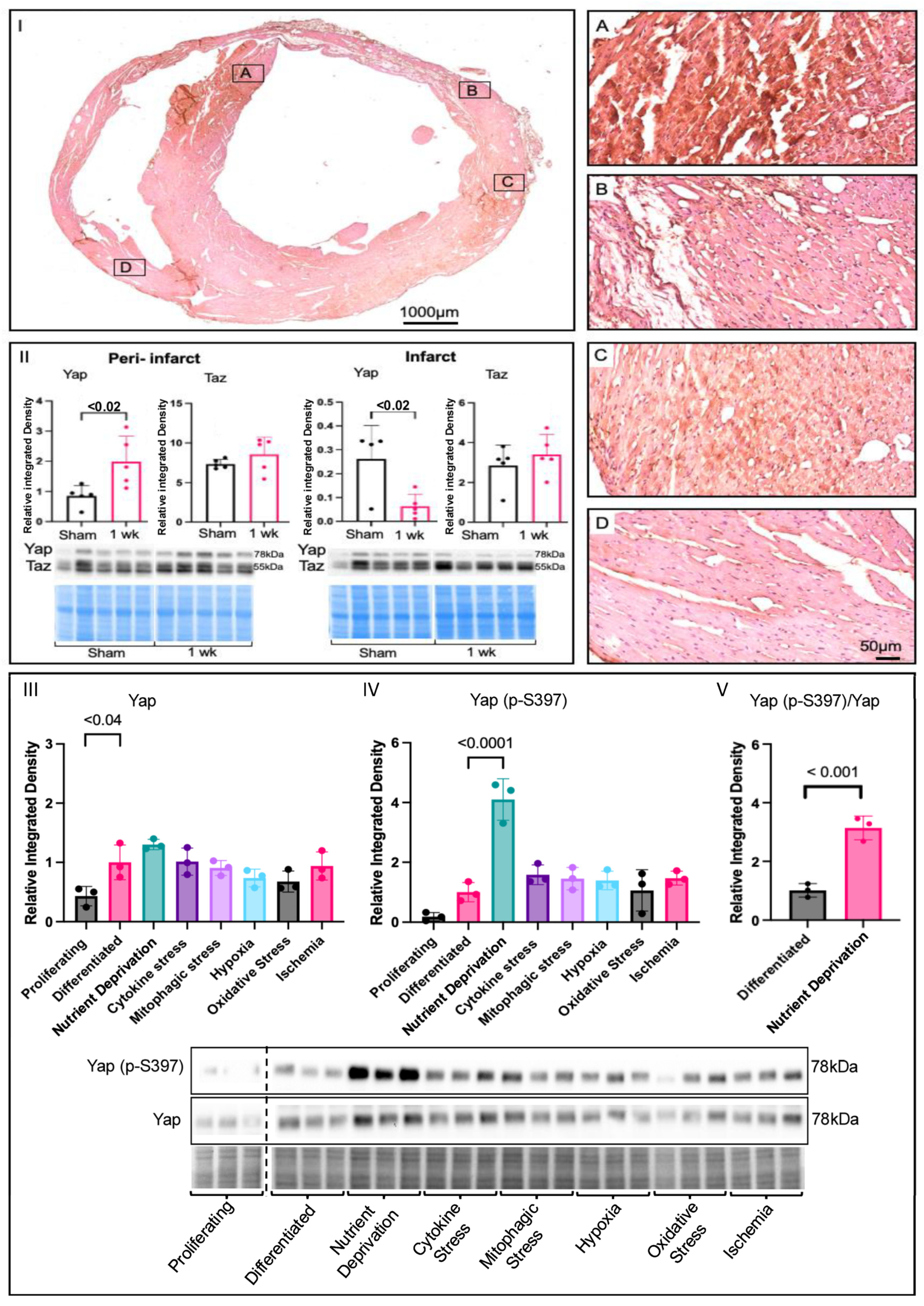
3.1. Yap Signalling in Myocardial Infarction and Associated Stressors
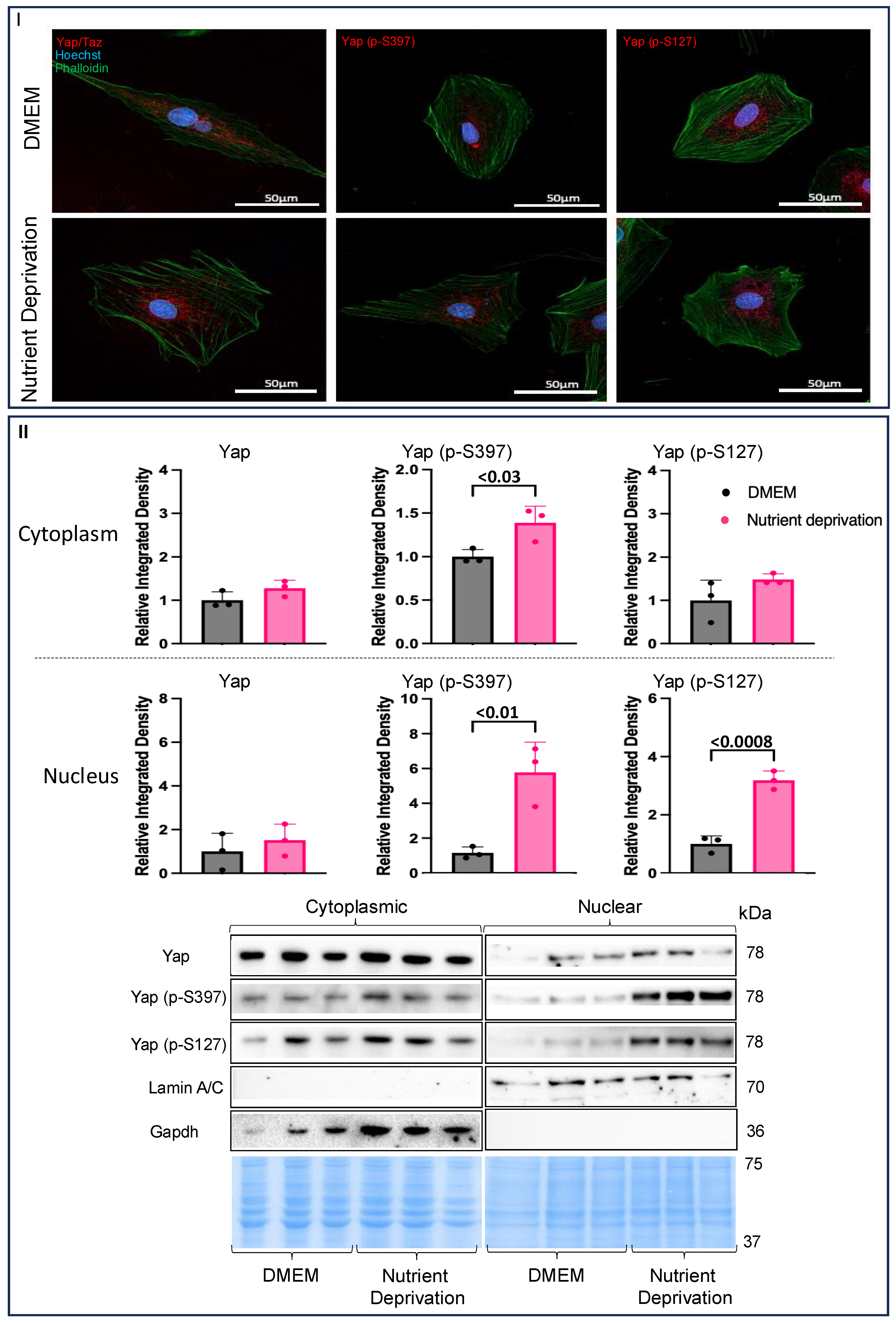
3.2. Nutrient Deprivation Affects Yap Post-Translation Modification and Compartmentation
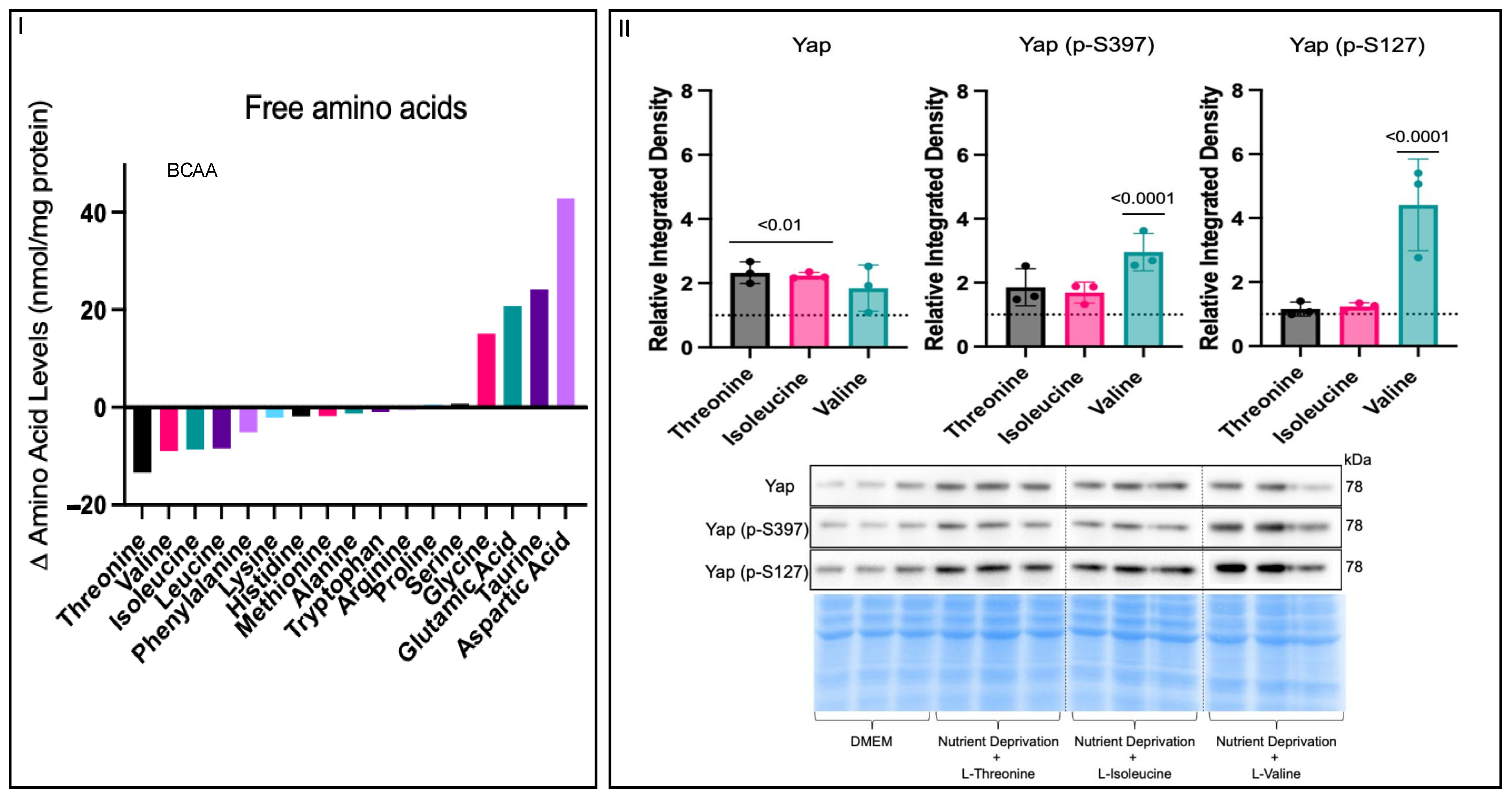
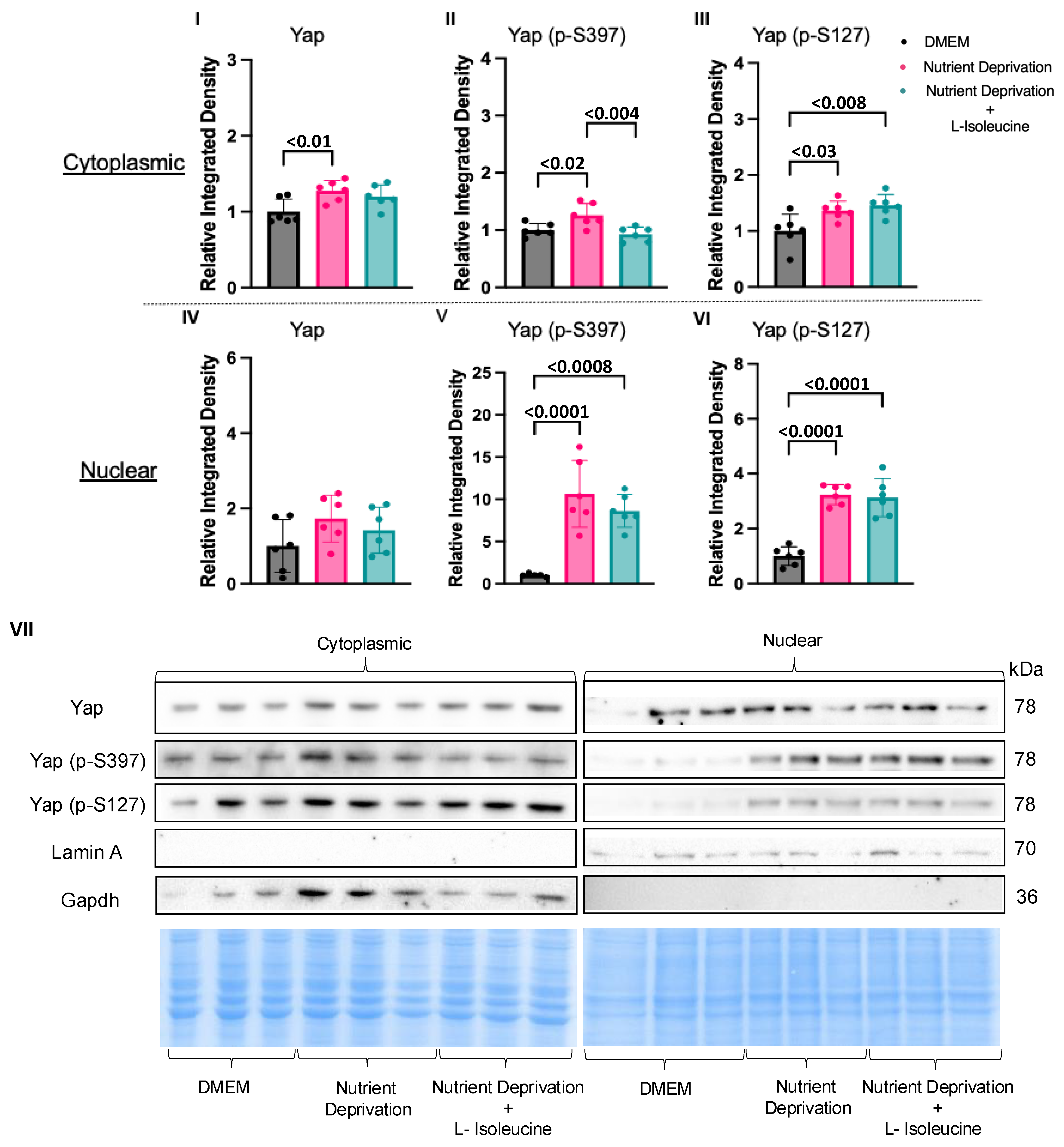
3.3. Amino Acid Nutrient Sensitivity Impacts Yap Signalling
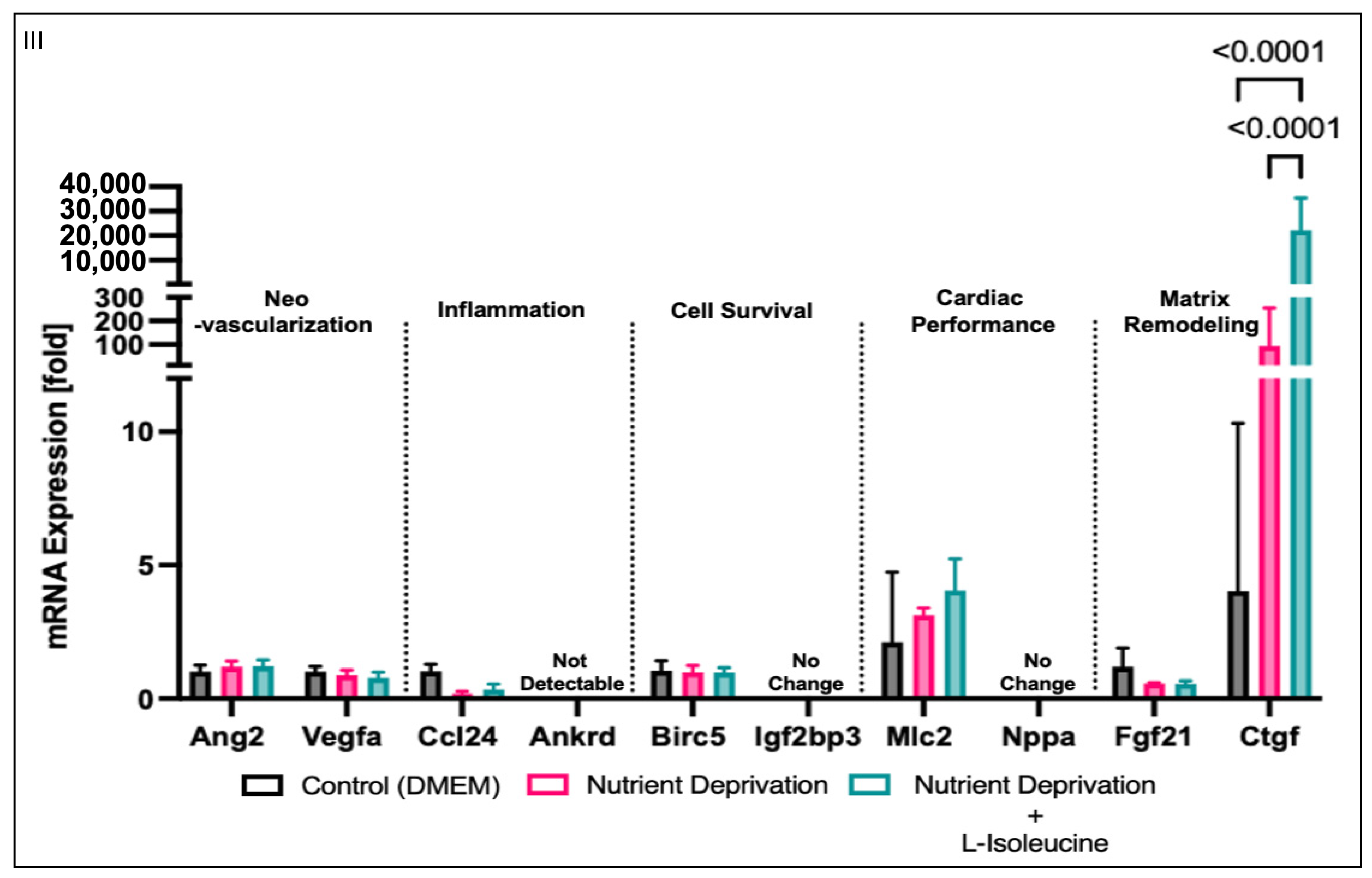
3.4. Yap-Associated Gene Targets Expression Relevant to Myocardial Infarction
4. Discussion
4.1. The Effects of Nutrient Deprivation on Yap Signalling
4.2. Compartmentalization of Yap in Rat Cardiomyotubes after Nutrient Deprivation
4.3. Connective Tissue Growth Factor, Yap Signalling, and the Heart
4.4. Clinical Relevance
5. Limitations and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth universal definition of myocardial infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef] [PubMed]
- Ojha, N.; Dhamoon, A.S.; Chapagain, R. Myocardial Infarction (Nursing); StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Burke, A.P.; Virmani, R. Pathophysiology of acute myocardial infarction. Med. Clin. N. Am. 2007, 91, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Suchard, M.A.; Krumholz, H.M.; Schuemie, M.J.; Shea, S.; Duke, J.; Pratt, N.; Reich, C.G.; Madigan, D.; You, S.C.; et al. Comparative First-Line Effectiveness and Safety of ACE (Angiotensin-Converting Enzyme) Inhibitors and Angiotensin Receptor Blockers: A Multinational Cohort Study. Hypertens 2021, 78, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Del Re, D.P.; Amgalan, D.; Linkermann, A.; Liu, Q.; Kitsis, R.N. Fundamental mechanisms of regulated cell death and implications for heart disease. Physiol. Rev. 2019, 99, 1765–1817. [Google Scholar] [CrossRef]
- Nakamura, M.; Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018, 15, 387–407. [Google Scholar] [CrossRef]
- Nauta, J.F.; Hummel, Y.M.; Tromp, J.; Ouwerkerk, W.; van der Meer, P.; Jin, X.; Lam, C.S.P.; Bax, J.J.; Metra, M.; Samani, N.J.; et al. Concentric vs. eccentric remodelling in heart failure with reduced ejection fraction: Clinical characteristics, pathophysiology and response to treatment. Eur. J. Heart Fail. 2020, 22, 1147–1155. [Google Scholar] [CrossRef]
- Kim, W.; Jho, E.H. The history and regulatory mechanism of the Hippo pathway. BMB Rep. 2018, 51, 106–118. [Google Scholar] [CrossRef]
- Pocaterra, A.; Romani, P.; Dupont, S. YAP/TAZ functions and their regulation at a glance. J. Cell Sci. 2020, 133, jcs230425. [Google Scholar] [CrossRef]
- Kim, M.K.; Jang, J.W.; Bae, S.C. DNA binding partners of YAP/TAZ. BMB Rep. 2018, 51, 126–133. [Google Scholar] [CrossRef]
- Wang, P.; Gong, Y.; Guo, T.; Li, M.; Fang, L.; Yin, S.; Kamran, M.; Liu, Y.; Xu, J.; Xu, L.; et al. Activation of Aurora A kinase increases YAP stability via blockage of autophagy. Cell Death Dis. 2019, 10, 432. [Google Scholar] [CrossRef]
- Moon, S.; Kim, W.; Kim, S.; Kim, Y.; Song, Y.; Bilousov, O.; Kim, J.; Lee, T.; Cha, B.; Kim, M.; et al. Phosphorylation by NLK inhibits YAP -14-3-3-interactions and induces its nuclear localization. EMBO Rep. 2017, 18, 61–71. [Google Scholar] [CrossRef]
- Deng, F.; Wu, Z.; Zou, F.; Wang, S.; Wang, X. The Hippo-YAP/TAZ Signaling Pathway in Intestinal Self-Renewal and Regeneration After Injury. Front. Cell Dev. Biol. 2022, 10, 894737. [Google Scholar] [CrossRef] [PubMed]
- Hora, S.; Wuestefeld, T. Liver Injury and Regeneration: Current Understanding, New Approaches, and Future Perspectives. Cells 2023, 12, 2129. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, S.; Heallen, T.; Martin, J.F. The Hippo pathway in the heart: Pivotal roles in development, disease, and regeneration. Nat. Rev. Cardiol. 2018, 15, 672–684. [Google Scholar] [CrossRef]
- Xiao, Y.; Leach, J.; Wang, J.; Martin, J.F. Hippo/Yap Signaling in Cardiac Development and Regeneration. Curr. Treat. Opt. Cardiovasc. Med. 2016, 18, 38. [Google Scholar] [CrossRef]
- Xin, M.; Kim, Y.; Sutherland, L.B.; Murakami, M.; Qi, X.; McAnally, J.; Porrello, E.R.; Mahmoud, A.I.; Tan, W.; Shelton, J.M.; et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 13839–13844. [Google Scholar] [CrossRef]
- Gong, R.; Jiang, Z.; Zagidullin, N.; Liu, T.; Cai, B. Regulation of cardiomyocyte fate plasticity: A key strategy for cardiac regeneration. Signal Transduct. Target. Ther. 2021, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Brazma, A.; Hingamp, P.; Quackenbush, J.; Sherlock, G.; Spellman, P.; Stoeckert, C.; Aach, J.; Ansorge, W.; Ball, C.A.; Causton, H.C.; et al. Minimum information about a microarray experiment (MIAME)—Toward standards for microarray data. Nat. Genet. 2001, 29, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Tulacz, D.; Mackiewicz, U.; Maczewski, M.; Maciejak, A.; Gora, M.; Burzynska, B. Transcriptional profiling of left ventricle and peripheral blood mononuclear cells in a rat model of postinfarction heart failure. BMC Med. Genom. 2013, 6, 49. [Google Scholar] [CrossRef]
- Isogai, S.; Nishimura, A.; Kotaka, A.; Murakami, N.; Hotta, N.; Ishida, H.; Takagi, H. High-Level Production of Isoleucine and Fusel Alcohol by Expression of the Feedback Inhibition-Insensitive Threonine Deaminase in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2022, 88, e0213021. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Von Gise, A.; Zhou, P.; Gu, F.; Ma, Q.; Jiang, J.; Yau, A.L.; Buck, J.N.; Gouin, K.A.; Van Gorp, P.R.R.; et al. Cardiac-specific YAP activation improves cardiac function and survival in an experimental murine MI model. Circ. Res. 2014, 115, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Heallen, T.; Morikawa, Y.; Leach, J.; Tao, G.; Willerson, J.T.; Johnson, R.L.; Martin, J.F. Hippo signaling impedes adult heart regeneration. Development 2013, 140, 4683–4690. [Google Scholar] [CrossRef] [PubMed]
- Correction (JACC: Basic to Translational Science (2020) 5(9) (931–945), (S2452302X20303417), (10.1016/j.jacbts.2020.07.009)). JACC Basic Transl. Sci. 2021, 6, 629. [CrossRef]
- Wang, M.; Lin, B.Y.; Sun, S.; Dai, C.; Long, F.; Butcher, J.T. Shear and hydrostatic stress regulate fetal heart valve remodeling through YAP-mediated mechanotransduction. eLife 2023, 12, e83209. [Google Scholar] [CrossRef]
- Wong, D.C.P.; Xiao, J.; Chew, T.W.; Pan, M.; Lee, C.J.M.; Ang, J.W.; Yow, I.; Thivakar, T.; Ackers-Johnson, M.; Lee, N.J.W.; et al. BNIP-2 Activation of Cellular Contractility Inactivates YAP for H9c2 Cardiomyoblast Differentiation. Adv. Sci. 2022, 9, e2202834. [Google Scholar] [CrossRef]
- Koo, J.H.; Guan, K.L. Interplay between YAP/TAZ and Metabolism. Cell Metab. 2018, 28, 196–206. [Google Scholar] [CrossRef]
- Dimou, A.; Tsimihodimos, V.; Bairaktari, E. The Critical Role of the Branched Chain Amino Acids (BCAAs) Catabolism-Regulating Enzymes, Branched-Chain Aminotransferase (BCAT) and Branched-Chain α-Keto Acid Dehydrogenase (BCKD), in Human Pathophysiology. Int. J. Mol. Sci. 2022, 23, 4022. [Google Scholar] [CrossRef]
- Wada, K.I.; Itoga, K.; Okano, T.; Yonemura, S.; Sasaki, H. Hippo pathway regulation by cell morphology and stress fibers. Development 2011, 138, 3907–3914. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Wang, J.; Sinnett-Smith, J.; Stevens, J.V.; Young, S.H.; Rozengurt, E. Biphasic regulation of Yes-associated Protein (YAP) cellular localization, phosphorylation, and activity by G proteincoupled receptor agonists in intestinal epithelial cells: A novel role for Protein Kinase D (PKD). J. Biol. Chem. 2016, 291, 17988–18005. [Google Scholar] [CrossRef]
- Shreberk-Shaked, M.; Oren, M. New insights into YAP/TAZ nucleo-cytoplasmic shuttling: New cancer therapeutic opportunities? Mol. Oncol. 2019, 13, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Fischer, R.S.; Pan, D.; Waterman, C.M. YAP Nuclear Localization in the Absence of Cell-Cell Contact Is Mediated by a Filamentous Actin-dependent, Myosin II- and Phospho-YAP-independent Pathway during Extracellular Matrix Mechanosensing. J. Biol. Chem. 2016, 291, 6096–6110. [Google Scholar] [CrossRef] [PubMed]
- Effendi, W.I.; Nagano, T. Connective Tissue Growth Factor in Idiopathic Pulmonary Fibrosis: Breaking the Bridge. Int. J. Mol. Sci. 2022, 23, 6064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Wei, C.Y.; Wang, Q.; Wang, L.; Lu, L.; Qi, F.Z. M2-polarized macrophages mediate wound healing by regulating connective tissue growth factor via AKT, ERK1/2, and STAT3 signaling pathways. Mol. Biol. Rep. 2021, 48, 6443–6456. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.C.; Chuang, S.M.; Hsu, C.J.; Tsai, C.H.; Wang, S.W.; Tang, C.H. CTGF increases vascular endothelial growth factor-dependent angiogenesis in human synovial fibroblasts by increasing miR-210 expression. Cell Death Dis. 2014, 5, e1485. [Google Scholar] [CrossRef]
- Moon, S.; Lee, S.; Caesar, J.A.; Pruchenko, S.; Leask, A.; Knowles, J.A.; Sinon, J.; Chaqour, B. A CTGF-YAP Regulatory Pathway Is Essential for Angiogenesis and Barriergenesis in the Retina. iScience 2020, 23, 101184. [Google Scholar] [CrossRef]
- Sharifi-Sanjani, M.; Berman, M.; Goncharov, D.; Alhamaydeh, M.; Avolio, T.G.; Baust, J.; Chang, B.; Kobir, A.; Ross, M.; St. Croix, C.; et al. Yes-associated protein (Yap) is up-regulated in heart failure and promotes cardiac fibroblast proliferation. Int. J. Mol. Sci. 2021, 22, 6164. [Google Scholar] [CrossRef]
- Garoffolo, G.; Casaburo, M.; Amadeo, F.; Salvi, M.; Bernava, G.; Piacentini, L.; Chimenti, I.; Zaccagnini, G.; Milcovich, G.; Zuccolo, E.; et al. Reduction of Cardiac Fibrosis by Interference with YAP-Dependent Transactivation. Circ. Res. 2022, 131, 239–257. [Google Scholar] [CrossRef]
- Vainio, L.E.; Szabó, Z.; Lin, R.; Ulvila, J.; Yrjölä, R.; Alakoski, T.; Piuhola, J.; Koch, W.J.; Ruskoaho, H.; Fouse, S.D.; et al. Connective Tissue Growth Factor Inhibition Enhances Cardiac Repair and Limits Fibrosis After Myocardial Infarction. JACC Basic Transl. Sci. 2019, 4, 83–94. [Google Scholar] [CrossRef]
- Matsui, Y.; Sadoshima, J. Rapid upregulation of CTGF in cardiac myocytes by hypertrophic stimuli: Implication for cardiac fibrosis and hypertrophy. J. Mol. Cell. Cardiol. 2004, 37, 477–481. [Google Scholar] [CrossRef]
- Du, X.; You, H.; Li, Y.; Wang, Y.; Hui, P.; Qiao, B.; Lu, J.; Zhang, W.; Zhou, S.; Zheng, Y.; et al. Relationships between circulating branched chain amino acid concentrations and risk of adverse cardiovascular events in patients with STEMI treated with PC. Sci. Rep. 2018, 8, 15809. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, F.; Xia, Y.; Zhao, S.; Yan, W.; Wang, H.; Lee, Y.; Li, C.; Zhang, L.; Lian, K.; et al. Defective branched chain amino acid catabolism contributes to cardiac dysfunction and remodeling following myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1160–H1169. [Google Scholar] [CrossRef] [PubMed]
- Wendel, U. Metabolism of branched-chain amino acids in maple syrup urine disease. Eur. J. Pediatr. Suppl. 1997, 156, S62–S66. [Google Scholar] [CrossRef]
- Gannon, N.P.; Schnuck, J.K.; Vaughan, R.A. BCAA Metabolism and Insulin Sensitivity—Dysregulated by Metabolic Status? Mol. Nutr. Food Res. 2018, 62, 1700756. [Google Scholar] [CrossRef]
- Najumudeen, A.K.; Ceteci, F.; Fey, S.K.; Hamm, G.; Steven, R.T.; Hall, H.; Nikula, C.J.; Dexter, A.; Murta, T.; Race, A.M.; et al. The amino acid transporter SLC7A5 is required for efficient growth of KRAS-mutant colorectal cancer. Nat. Genet. 2021, 53, 16–26. [Google Scholar] [CrossRef]
- Ong, Y.T.; Andrade, J.; Armbruster, M.; Shi, C.; Castro, M.; Costa, A.S.H.; Sugino, T.; Eelen, G.; Zimmermann, B.; Wilhelm, K.; et al. A YAP/TAZ-TEAD signalling module links endothelial nutrient acquisition to angiogenic growth. Nat. Metab. 2022, 4, 672–682. [Google Scholar] [CrossRef]

| Target | Primer Sequence (5′-3′) | |
|---|---|---|
| RtAng2 | Forward | TCAGCACTATGATGCCAAGCC |
| Reverse | TTGATGCTGCCCTTATTGCCAT | |
| RtAnkrd1 | Forward | GGCCAGCTCCAGGGGTTCAGC |
| Reverse | GCTGAACCCCTGGAGCTGGCC | |
| RtBirc5 | Forward | TTCCTTACAGTCAAGAAGCAGGT |
| Reverse | TTCTTGGCTCTTTGTTTGTCCA | |
| RtB2m | Forward | ACATCCTGGCTCACATGAA |
| Reverse | ATGTCTTCGGTCCCAGGTG | |
| RtCcl24 | Forward | CTCTAAGAAGCAGTTCAAGGCTA |
| Reverse | ACCTCAAATTTTCTATGTGGCTA | |
| RtCtgf | Forward | CGCTGACATTCTGATTCCAGT |
| Reverse | CTGATCCATTGCTTTACCGTCT | |
| RtGapdh | Forward | GGCCGAAGGGCCCACTA |
| Reverse | TGTTGAAGTCACAGGAGACAACCT | |
| RtHprt1 | Forward | CCCAGCGTCGTGATTAGTGATG |
| Reverse | TTCAGTCCTGTCCATAATCAGTC | |
| RtIgf2bp3 | Forward | ATCCCCTTGAAGATTTTAGCTC |
| Reverse | ATTTTAGTGTCCGTGTCTTGC | |
| RtMlc2 | Forward | TCAAAGTCTGTTCCGTCCCT |
| Reverse | AACTTGGCGTCCATAATTGCT | |
| RtNppa | Forward | CGGACAAAGGCTGAGAGAGAA |
| Reverse | TTCTCTCTCAGCCTTTGTCCG | |
| RtU6 | Forward | GCTTCGGCAGCACATATACTAA |
| Reverse | AACGCTTCACGAATTTGCGT | |
| RtVegfα | Forward | TGGTGCTACTGTTTATCCGTA |
| Reverse | ATTATCTCGGAAAACTGCTCT | |
| Rtβ-actin | Forward | CGAGTACAACCTTCTTGCAGC |
| Reverse | ACCCATACCCACCATCACAC | |
| Rt18s | Forward | GAGCTGGAATTACCGCGGCT |
| Reverse | AAACGGCTACCACATCCAAG | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelson, V.L.; Eadie, A.L.; Perez, L.; Madhu, M.; Platt, M.; Mercer, A.; Pulinilkunnil, T.; Kienesberger, P.; Simpson, J.A.; Brunt, K.R. Yap Is a Nutrient Sensor Sensitive to the Amino Acid L-Isoleucine and Regulates the Expression of Ctgf in Cardiomyocytes. Biomolecules 2024, 14, 1299. https://doi.org/10.3390/biom14101299
Nelson VL, Eadie AL, Perez L, Madhu M, Platt M, Mercer A, Pulinilkunnil T, Kienesberger P, Simpson JA, Brunt KR. Yap Is a Nutrient Sensor Sensitive to the Amino Acid L-Isoleucine and Regulates the Expression of Ctgf in Cardiomyocytes. Biomolecules. 2024; 14(10):1299. https://doi.org/10.3390/biom14101299
Chicago/Turabian StyleNelson, Victoria L., Ashley L. Eadie, Lester Perez, Malav Madhu, Mathew Platt, Angella Mercer, Thomas Pulinilkunnil, Petra Kienesberger, Jeremy A. Simpson, and Keith R. Brunt. 2024. "Yap Is a Nutrient Sensor Sensitive to the Amino Acid L-Isoleucine and Regulates the Expression of Ctgf in Cardiomyocytes" Biomolecules 14, no. 10: 1299. https://doi.org/10.3390/biom14101299
APA StyleNelson, V. L., Eadie, A. L., Perez, L., Madhu, M., Platt, M., Mercer, A., Pulinilkunnil, T., Kienesberger, P., Simpson, J. A., & Brunt, K. R. (2024). Yap Is a Nutrient Sensor Sensitive to the Amino Acid L-Isoleucine and Regulates the Expression of Ctgf in Cardiomyocytes. Biomolecules, 14(10), 1299. https://doi.org/10.3390/biom14101299









