Candidate Molecular Biomarkers of Traumatic Brain Injury: A Systematic Review
Abstract
1. Introduction
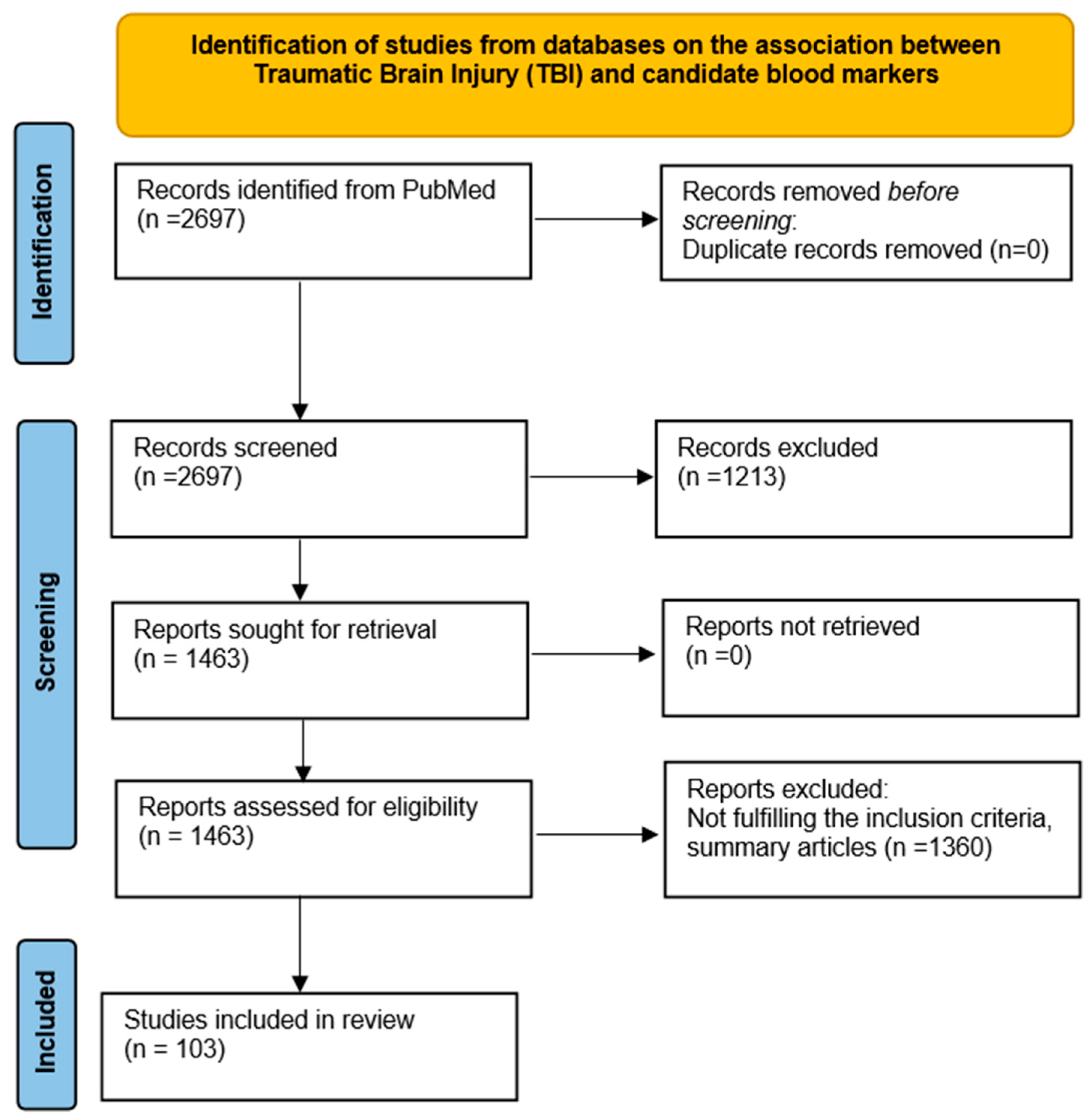
2. Methodology
3. Candidate Blood Biomarkers of TBI
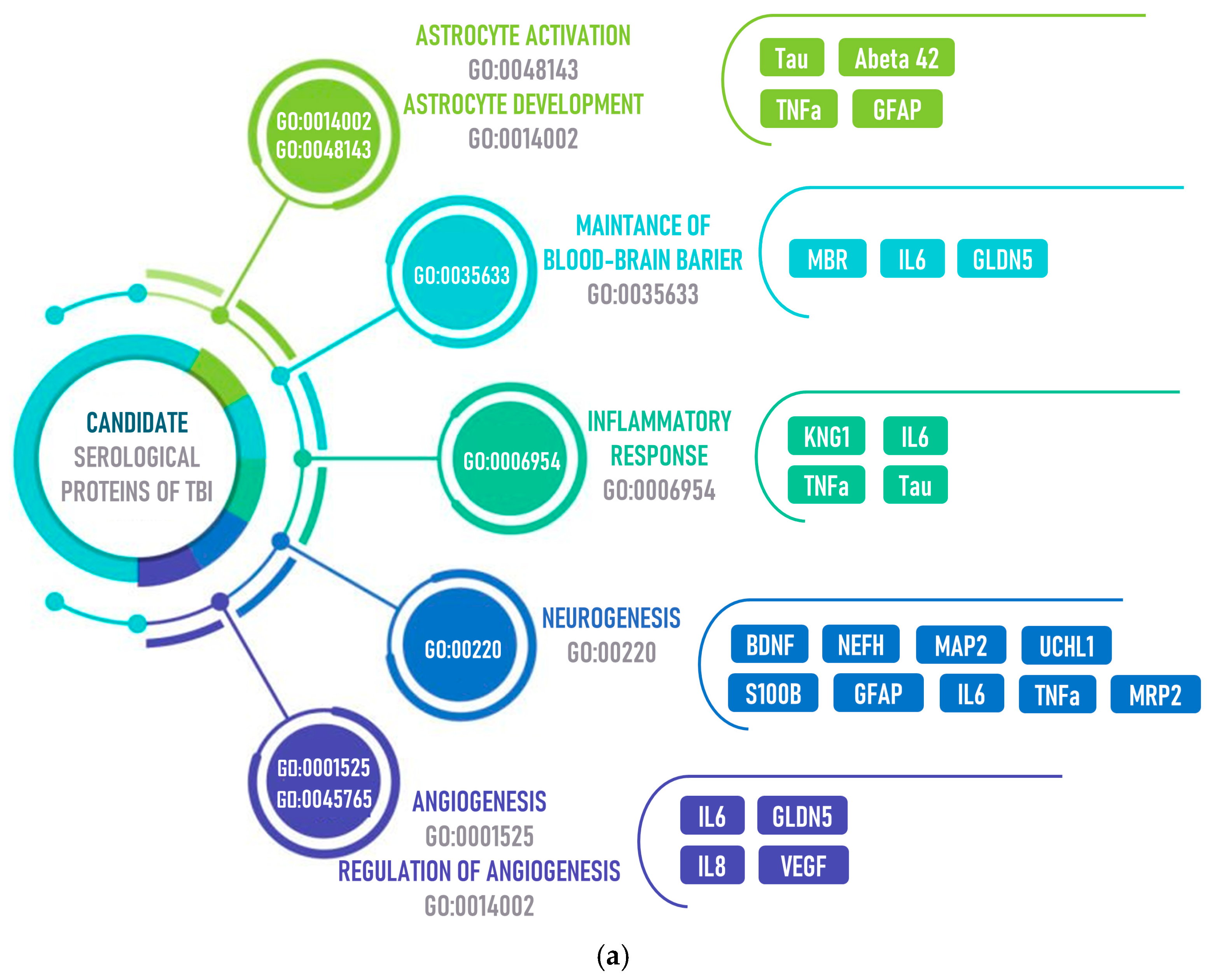
3.1. Changes in the Blood Levels of Candidate Proteins after TBI
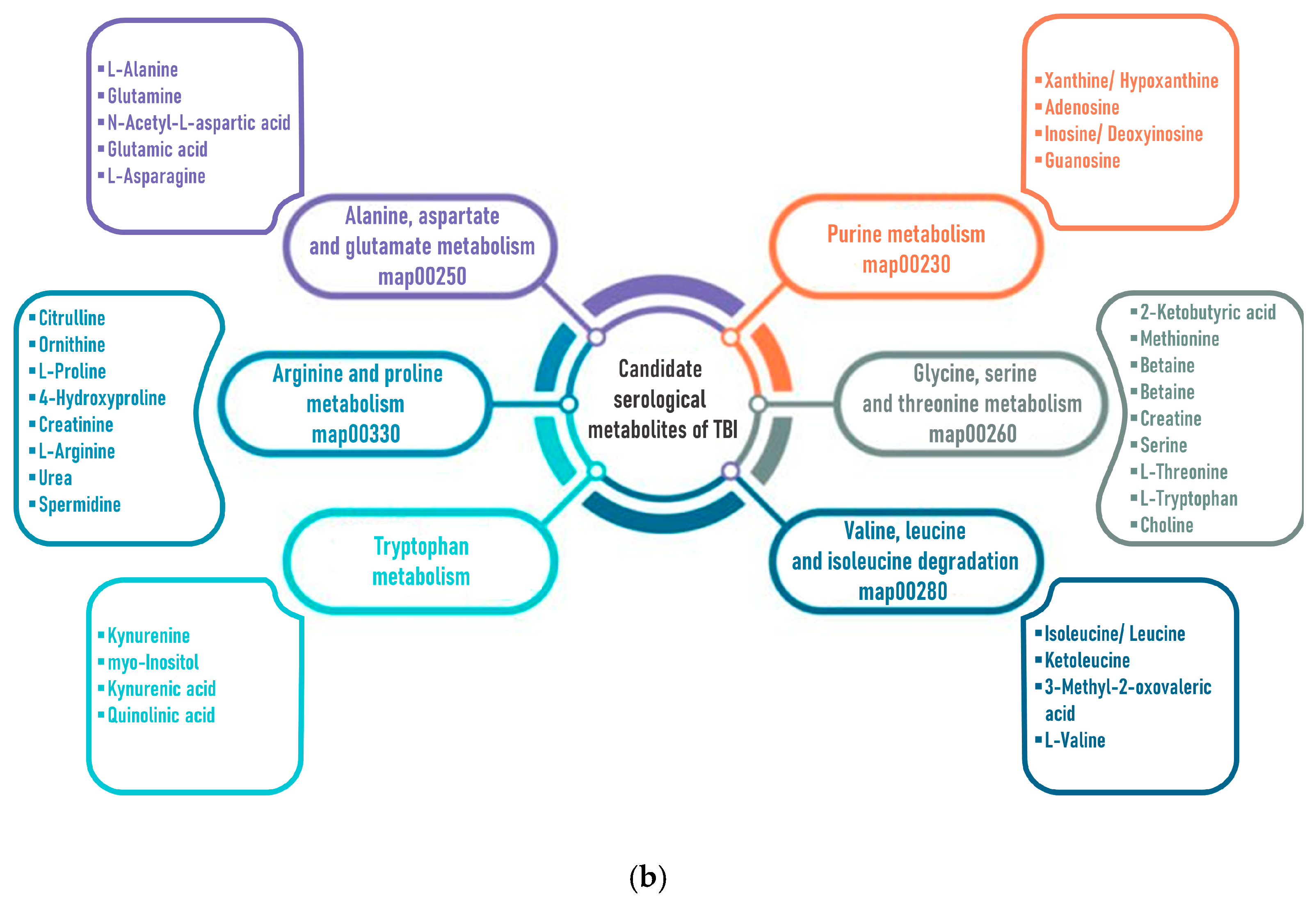
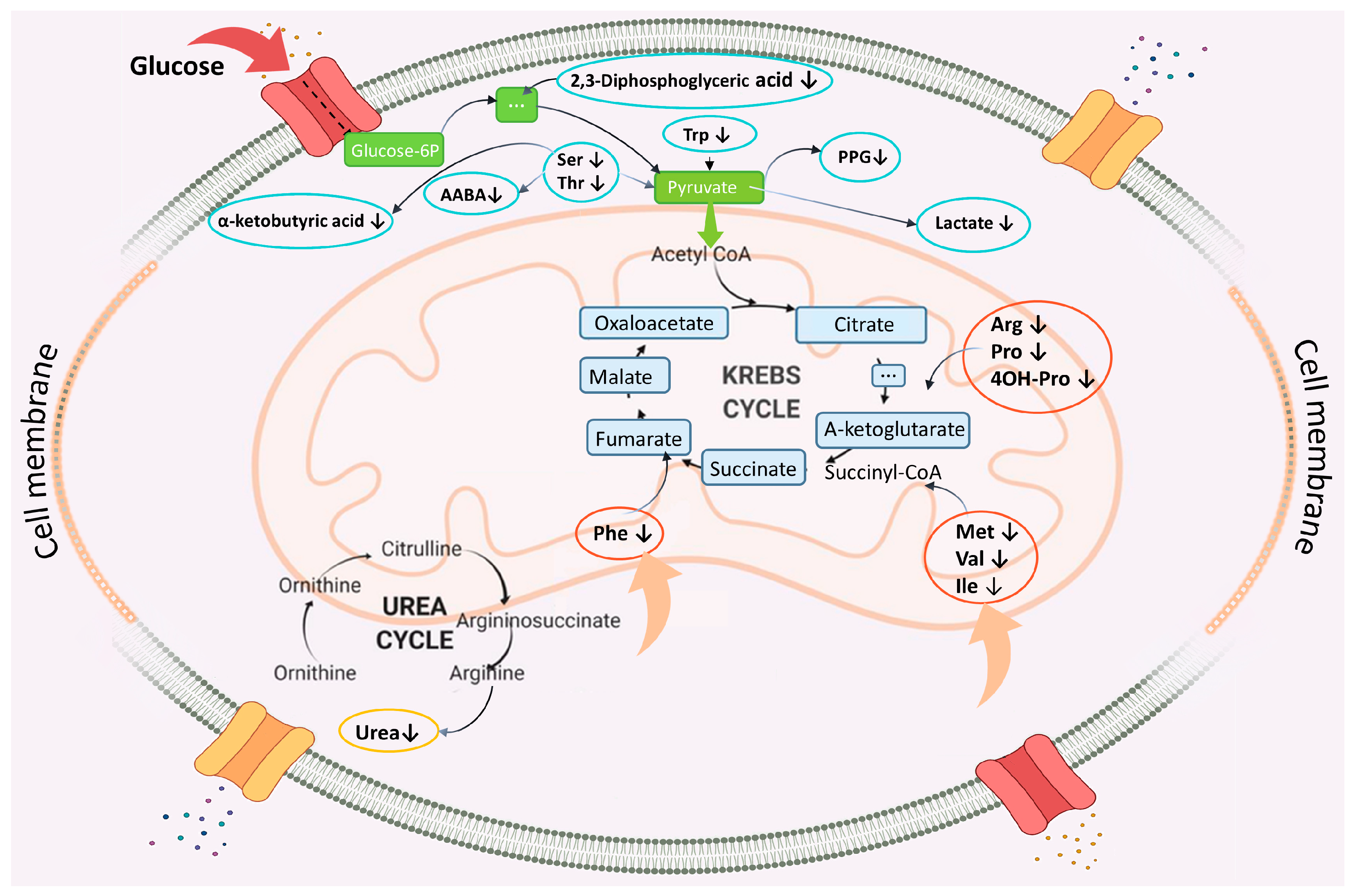
3.2. Changes in the Blood Levels of Endogenous Metabolites after TBI
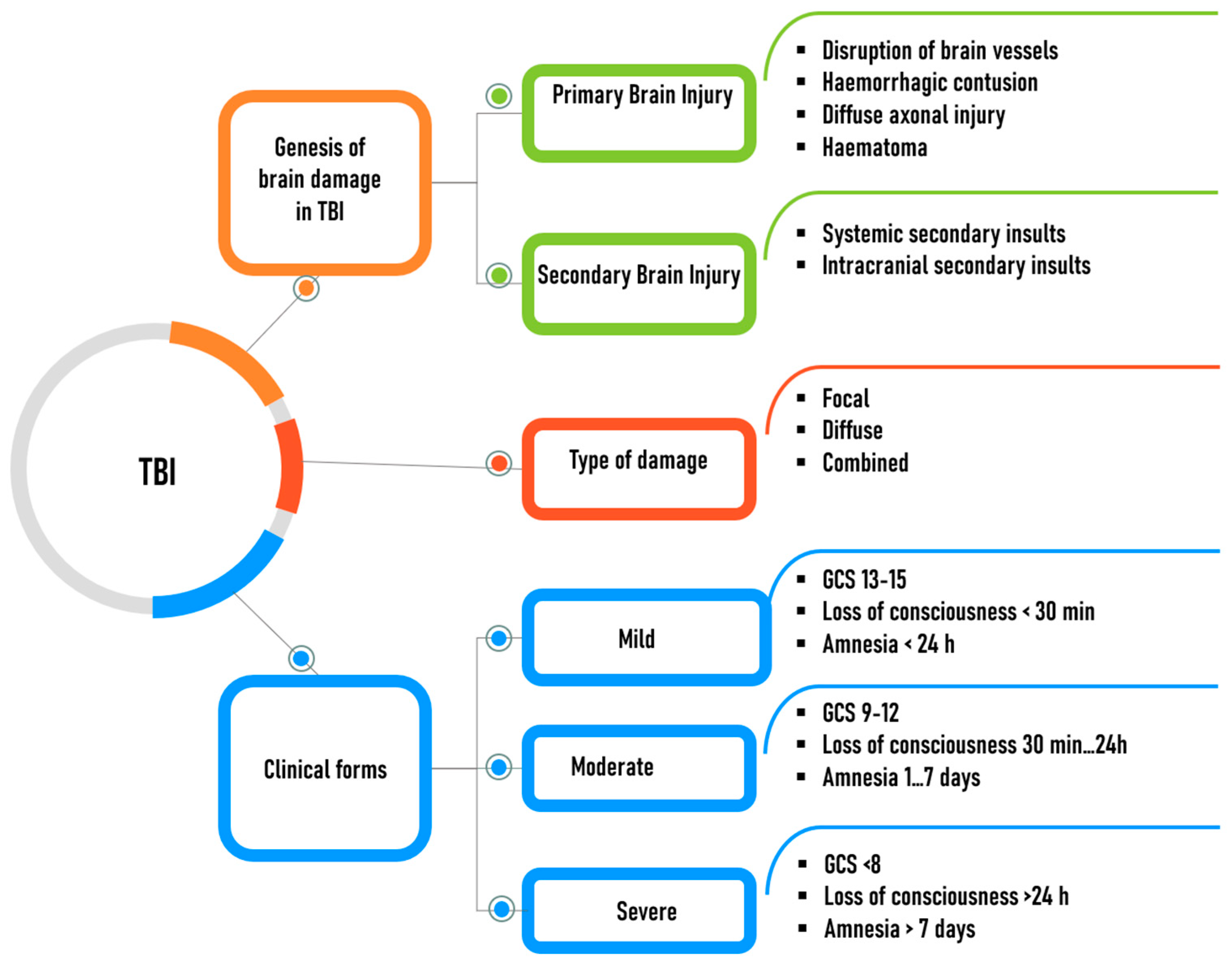
4. The Key Mechanisms of Brain Damage
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hier, D.B.; Obafemi-Ajayi, T.; Thimgan, M.S.; Olbricht, G.R.; Azizi, S.; Allen, B.; Hadi, B.A.; Wunsch, D.C. Blood Biomarkers for Mild Traumatic Brain Injury: A Selective Review of Unresolved Issues. Biomark. Res. 2021, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- CDC. Let’s Prevent Traumatic Brain Injury. Available online: https://www.cpha.info/news/671673/CDC-Lets-Prevent-Traumatic-Brain-Injury.html (accessed on 13 May 2024).
- Yang, L.-Y.; Greig, N.H.; Tweedie, D.; Jung, Y.J.; Chiang, Y.-H.; Hoffer, B.J.; Miller, J.P.; Chang, K.-H.; Wang, J.-Y. The P53 Inactivators Pifithrin-μ and Pifithrin-α Mitigate TBI-Induced Neuronal Damage through Regulation of Oxidative Stress, Neuroinflammation, Autophagy and Mitophagy. Exp. Neurol. 2020, 324, 113135. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.; Miller, G.F.; Barnett, S.B.L.; Florence, C. Economic Cost of Injury—United States, 2019. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1655–1659. [Google Scholar] [CrossRef] [PubMed]
- Baracaldo-Santamaría, D.; Ariza-Salamanca, D.F.; Corrales-Hernández, M.G.; Pachón-Londoño, M.J.; Hernandez-Duarte, I.; Calderon-Ospina, C.-A. Revisiting Excitotoxicity in Traumatic Brain Injury: From Bench to Bedside. Pharmaceutics 2022, 14, 152. [Google Scholar] [CrossRef] [PubMed]
- Cash, A.; Theus, M.H. Mechanisms of Blood–Brain Barrier Dysfunction in Traumatic Brain Injury. Int. J. Mol. Sci. 2020, 21, 3344. [Google Scholar] [CrossRef]
- Berwick, D.; Bowman, K.; Matney, C. (Eds.) Traumatic Brain Injury: A Roadmap for Accelerating Progress; National Academies Press: Washington, DC, USA, 2022; ISBN 978-0-309-49043-6. [Google Scholar]
- Parfenov, V.A. [E.I. Gusev, A.N. Konovalova, V.I. Skvortsova “Neurology and neurosurgery”. Textbook, 4th Ed]. Zhurnal Nevrol. I Psikhiatrii Im. S.S. Korsakova 2015, 115, 130–131. [Google Scholar] [CrossRef]
- Pearn, M.L.; Niesman, I.R.; Egawa, J.; Sawada, A.; Almenar-Queralt, A.; Shah, S.B.; Duckworth, J.L.; Head, B.P. Pathophysiology Associated with Traumatic Brain Injury: Current Treatments and Potential Novel Therapeutics. Cell Mol. Neurobiol. 2017, 37, 571–585. [Google Scholar] [CrossRef]
- Thapa, K.; Khan, H.; Singh, T.G.; Kaur, A. Traumatic Brain Injury: Mechanistic Insight on Pathophysiology and Potential Therapeutic Targets. J. Mol. Neurosci. 2021, 71, 1725–1742. [Google Scholar] [CrossRef]
- Abu Hamdeh, S.; Shevchenko, G.; Mi, J.; Musunuri, S.; Bergquist, J.; Marklund, N. Proteomic Differences between Focal and Diffuse Traumatic Brain Injury in Human Brain Tissue. Sci. Rep. 2018, 8, 6807. [Google Scholar] [CrossRef]
- Mohammed, F.S.; Omay, S.B.; Sheth, K.N.; Zhou, J. Nanoparticle-Based Drug Delivery for the Treatment of Traumatic Brain Injury. Expert. Opin. Drug Deliv. 2023, 20, 55–73. [Google Scholar] [CrossRef]
- Obasa, A.A.; Olopade, F.E.; Juliano, S.L.; Olopade, J.O. Traumatic Brain Injury or Traumatic Brain Disease: A Scientific Commentary. Brain Multiphysics 2024, 6, 100092. [Google Scholar] [CrossRef]
- Rauchman, S.H.; Albert, J.; Pinkhasov, A.; Reiss, A.B. Mild-to-Moderate Traumatic Brain Injury: A Review with Focus on the Visual System. Neurol. Int. 2022, 14, 453–470. [Google Scholar] [CrossRef] [PubMed]
- Traumatic Brain Injury (TBI). National Institute of Neurological Disorders and Stroke. Available online: https://www.ninds.nih.gov/health-information/disorders/traumatic-brain-injury-tbi (accessed on 13 May 2024).
- Maas, A.I.R.; Menon, D.K.; Manley, G.T.; Abrams, M.; Åkerlund, C.; Andelic, N.; Aries, M.; Bashford, T.; Bell, M.J.; Bodien, Y.G.; et al. Traumatic Brain Injury: Progress and Challenges in Prevention, Clinical Care, and Research. Lancet Neurol. 2022, 21, 1004–1060. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Statistics 2023: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2023; ISBN 978-92-4-007432-3. [Google Scholar]
- Abboud, T.; Rohde, V.; Mielke, D. Mini Review: Current Status and Perspective of S100B Protein as a Biomarker in Daily Clinical Practice for Diagnosis and Prognosticating of Clinical Outcome in Patients with Neurological Diseases with Focus on Acute Brain Injury. BMC Neurosci. 2023, 24, 38. [Google Scholar] [CrossRef] [PubMed]
- Chamoun, R.; Suki, D.; Gopinath, S.P.; Goodman, J.C.; Robertson, C. Role of Extracellular Glutamate Measured by Cerebral Microdialysis in Severe Traumatic Brain Injury. J. Neurosurg. 2010, 113, 564–570. [Google Scholar] [CrossRef]
- Guilfoyle, M.R.; Helmy, A.; Donnelly, J.; Stovell, M.G.; Timofeev, I.; Pickard, J.D.; Czosnyka, M.; Smielewski, P.; Menon, D.K.; Carpenter, K.L.H.; et al. Characterising the dynamics of cerebral metabolic dysfunction following traumatic brain injury: A microdialysis study in 619 patients. PLoS ONE 2021, 16, e0260291. [Google Scholar] [CrossRef]
- Alqahtani, F.; Chowdhury, E.A.; Bhattacharya, R.; Noorani, B.; Mehvar, R.; Bickel, U. Brain Uptake of [13C] and [14C] Sucrose Quantified by Microdialysis and Whole Tissue Analysis in Mice. Drug Metab. Dispos. 2018, 46, 1514–1518. [Google Scholar] [CrossRef]
- Jarrahi, A.; Braun, M.; Ahluwalia, M.; Gupta, R.V.; Wilson, M.; Munie, S.; Ahluwalia, P.; Vender, J.R.; Vale, F.L.; Dhandapani, K.M.; et al. Revisiting Traumatic Brain Injury: From Molecular Mechanisms to Therapeutic Interventions. Biomedicines 2020, 8, 389. [Google Scholar] [CrossRef]
- Kumar Sahel, D.; Kaira, M.; Raj, K.; Sharma, S.; Singh, S. Mitochondrial Dysfunctioning and Neuroinflammation: Recent Highlights on the Possible Mechanisms Involved in Traumatic Brain Injury. Neurosci. Lett. 2019, 710, 134347. [Google Scholar] [CrossRef]
- Kempuraj, D.; Ahmed, M.E.; Selvakumar, G.P.; Thangavel, R.; Raikwar, S.P.; Zaheer, S.A.; Iyer, S.S.; Govindarajan, R.; Nattanmai Chandrasekaran, P.; Burton, C.; et al. Acute Traumatic Brain Injury-Induced Neuroinflammatory Response and Neurovascular Disorders in the Brain. Neurotox. Res. 2021, 39, 359–368. [Google Scholar] [CrossRef]
- Yuan, M.; Wu, H. Astrocytes in the Traumatic Brain Injury: The Good and the Bad. Exp. Neurol. 2022, 348, 113943. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shao, A.; Yao, Y.; Tu, S.; Deng, Y.; Zhang, J. Dual Roles of Astrocytes in Plasticity and Reconstruction after Traumatic Brain Injury. Cell Commun. Signal. 2020, 18, 62. [Google Scholar] [CrossRef] [PubMed]
- Chukhlovina, M.L.; Chukhlovin, A.A. Features of the Patient Management with Traumatic Brain Injury. Zhurnal Nevrol. I Psikhiatrii Im. S.S. Korsakova 2021, 121, 145. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hou, Y.; Zhang, L.; Liu, M.; Zhao, J.; Zhang, Z.; Ma, Y.; Hou, W. Estrogen Attenuates Traumatic Brain Injury by Inhibiting the Activation of Microglia and Astrocyte-Mediated Neuroinflammatory Responses. Mol. Neurobiol. 2021, 58, 1052–1061. [Google Scholar] [CrossRef]
- Burda, J.E.; Bernstein, A.M.; Sofroniew, M.V. Astrocyte Roles in Traumatic Brain Injury. Exp. Neurol. 2016, 275, 305–315. [Google Scholar] [CrossRef]
- Sorokina, E.G.; Semenova, Z.B.; Averianova, N.S.; Karaseva, O.V.; Arsenieva, E.N.; Luk’yanov, V.I.; Reutov, V.P.; Asanov, A.Y.; Roshal, L.M.; Pinelis, V.G. [APOΕ gene polymorphism and markers of brain damage in the outcomes of severe traumatic brain injury in children]. Zhurnal Nevrol. I Psikhiatrii Im. S.S. Korsakova 2020, 120, 72–80. [Google Scholar] [CrossRef]
- Sivandzade, F.; Alqahtani, F.; Cucullo, L. Traumatic Brain Injury and Blood-Brain Barrier (BBB): Underlying Pathophysiological Mechanisms and the Influence of Cigarette Smoking as a Premorbid Condition. Int. J. Mol. Sci. 2020, 21, 2721. [Google Scholar] [CrossRef]
- Bolden, C.T.; Skibber, M.A.; Olson, S.D.; Zamorano Rojas, M.; Milewicz, S.; Gill, B.S.; Cox, C.S. Validation and Characterization of a Novel Blood–Brain Barrier Platform for Investigating Traumatic Brain Injury. Sci. Rep. 2023, 13, 16150. [Google Scholar] [CrossRef]
- Kokiko-Cochran, O.N.; Godbout, J.P. The Inflammatory Continuum of Traumatic Brain Injury and Alzheimer’s Disease. Front. Immunol. 2018, 9, 672. [Google Scholar] [CrossRef]
- van Erp, I.A.M.; Michailidou, I.; van Essen, T.A.; van der Jagt, M.; Moojen, W.; Peul, W.C.; Baas, F.; Fluiter, K. Tackling Neuroinflammation After Traumatic Brain Injury: Complement Inhibition as a Therapy for Secondary Injury. Neurotherapeutics 2023, 20, 284–303. [Google Scholar] [CrossRef]
- Krishnamurthy, K.; Laskowitz, D.T. Cellular and Molecular Mechanisms of Secondary Neuronal Injury. In Translational Research in Traumatic Brain Injury; Laskowitz, D., Grant, G., Eds.; Frontiers in Neuroscience; CRC Press/Taylor and Francis Group: Boca Raton, FL, USA, 2016; ISBN 978-1-4665-8491-4. [Google Scholar]
- Ngwenya, L.B.; Danzer, S.C. Impact of Traumatic Brain Injury on Neurogenesis. Front. Neurosci. 2019, 12, 1014. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, S.; Marei, O.; Elalfy, O.; Zaben, M. Neurogenesis after Traumatic Brain Injury—The Complex Role of HMGB1 and Neuroinflammation. Neuropharmacology 2021, 183, 108400. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.; Hanley, N.; Reschke, C.R.; Reddy, A.; Mäe, M.A.; Connolly, R.; Behan, C.; O’Keeffe, E.; Bolger, I.; Hudson, N.; et al. Microvascular Stabilization via Blood-Brain Barrier Regulation Prevents Seizure Activity. Nat. Commun. 2022, 13, 2003. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Greene, C.; Munnich, A.; Campbell, M. The CLDN5 Gene at the Blood-Brain Barrier in Health and Disease. Fluids Barriers CNS 2023, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Frenguelli, B.G.; Dale, N. Purines: From Diagnostic Biomarkers to Therapeutic Agents in Brain Injury. Neurosci. Bull. 2020, 36, 1315–1326. [Google Scholar] [CrossRef]
- Hajiaghamemar, M.; Kilbaugh, T.; Arbogast, K.B.; Master, C.L.; Margulies, S.S. Using Serum Amino Acids to Predict Traumatic Brain Injury: A Systematic Approach to Utilize Multiple Biomarkers. Int. J. Mol. Sci. 2020, 21, 1786. [Google Scholar] [CrossRef]
- Yilmaz, A.; Liraz-Zaltsman, S.; Shohami, E.; Gordevičius, J.; Kerševičiūtė, I.; Sherman, E.; Bahado-Singh, R.O.; Graham, S.F. The Longitudinal Biochemical Profiling of TBI in a Drop Weight Model of TBI. Sci. Rep. 2023, 13, 22260. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, S.; Liu, B.; Hu, W.; Zhu, Y. Host–Microbiome Interactions: Tryptophan Metabolism and Aromatic Hydrocarbon Receptors after Traumatic Brain Injury. Int. J. Mol. Sci. 2023, 24, 10820. [Google Scholar] [CrossRef]
- Baker, E.W.; Henderson, W.M.; Kinder, H.A.; Hutcheson, J.M.; Platt, S.R.; West, F.D. Scaled Traumatic Brain Injury Results in Unique Metabolomic Signatures between Gray Matter, White Matter, and Serum in a Piglet Model. PLoS ONE 2018, 13, e0206481. [Google Scholar] [CrossRef]
- Sowers, J.L.; Sowers, M.L.; Shavkunov, A.S.; Hawkins, B.E.; Wu, P.; DeWitt, D.S.; Prough, D.S.; Zhang, K. Traumatic Brain Injury Induces Region-Specific Glutamate Metabolism Changes as Measured by Multiple Mass Spectrometry Methods. iScience 2021, 24, 103108. [Google Scholar] [CrossRef]
- Puangmalai, N.; Bhatt, N.; Bittar, A.; Jerez, C.; Shchankin, N.; Kayed, R. Traumatic Brain Injury Derived Pathological Tau Polymorphs Induce the Distinct Propagation Pattern and Neuroinflammatory Response in Wild Type Mice. Prog. Neurobiol. 2024, 232, 102562. [Google Scholar] [CrossRef] [PubMed]
- Targeting Tau in Traumatic Brain Injury. Available online: https://www.nature.com/articles/d41573-021-00070-2 (accessed on 13 May 2024).
- Michinaga, S.; Koyama, Y. Pathophysiological Responses and Roles of Astrocytes in Traumatic Brain Injury. Int. J. Mol. Sci. 2021, 22, 6418. [Google Scholar] [CrossRef] [PubMed]
- Plasma Biomarkers in Chronic Single Moderate–Severe Traumatic Brain Injury|Brain|Oxford Academic. Available online: https://academic.oup.com/brain/advance-article/doi/10.1093/brain/awae255/7766056?searchresult=1 (accessed on 25 September 2024).
- Streubel-Gallasch, L.; Zyśk, M.; Beretta, C.; Erlandsson, A. Traumatic Brain Injury in the Presence of Aβ Pathology Affects Neuronal Survival, Glial Activation and Autophagy. Sci. Rep. 2021, 11, 22982. [Google Scholar] [CrossRef] [PubMed]
- Mondello, S.; Buki, A.; Barzo, P.; Randall, J.; Provuncher, G.; Hanlon, D.; Wilson, D.; Kobeissy, F.; Jeromin, A. CSF and Plasma Amyloid-β Temporal Profiles and Relationships with Neurological Status and Mortality after Severe Traumatic Brain Injury. Sci. Rep. 2014, 4, 6446. [Google Scholar] [CrossRef]
- Komoltsev, I.G.; Tret’yakova, L.V.; Frankevich, S.O.; Shirobokova, N.I.; Volkova, A.A.; Butuzov, A.V.; Novikova, M.R.; Kvichansky, A.A.; Moiseeva, Y.V.; Onufriev, M.V.; et al. Neuroinflammatory Cytokine Response, Neuronal Death, and Microglial Proliferation in the Hippocampus of Rats During the Early Period After Lateral Fluid Percussion-Induced Traumatic Injury of the Neocortex. Mol. Neurobiol. 2022, 59, 1151–1167. [Google Scholar] [CrossRef]
- Yue, J.K.; Kobeissy, F.H.; Jain, S.; Sun, X.; Phelps, R.R.L.; Korley, F.K.; Gardner, R.C.; Ferguson, A.R.; Huie, J.R.; Schneider, A.L.C.; et al. Neuroinflammatory Biomarkers for Traumatic Brain Injury Diagnosis and Prognosis: A TRACK-TBI Pilot Study. Neurotrauma Rep. 2023, 4, 171–183. [Google Scholar] [CrossRef]
- Ling, T.; Yin, A.; Cao, Y.; Li, J.; Li, H.; Zhou, Y.; Guo, X.; Li, J.; Zhang, R.; Wu, H.; et al. Purinergic Astrocyte Signaling Driven by TNF-α After Cannabidiol Administration Restores Normal Synaptic Remodeling Following Traumatic Brain Injury. Neuroscience 2024, 545, 31–46. [Google Scholar] [CrossRef]
- Hellewell, S.; Semple, B.D.; Morganti-Kossmann, M.C. Therapies Negating Neuroinflammation after Brain Trauma. Brain Res. 2016, 1640, 36–56. [Google Scholar] [CrossRef]
- Rodney, T.; Osier, N.; Gill, J. Pro- and Anti-Inflammatory Biomarkers and Traumatic Brain Injury Outcomes: A Review. Cytokine 2018, 110, 248–256. [Google Scholar] [CrossRef]
- Sahyouni, R.; Gutierrez, P.; Gold, E.; Robertson, R.T.; Cummings, B.J. Effects of Concussion on the Blood–Brain Barrier in Humans and Rodents. J. Concussion 2017, 1, 1–15. [Google Scholar] [CrossRef]
- Shahim, P.; Politis, A.; van der Merwe, A.; Moore, B.; Ekanayake, V.; Lippa, S.M.; Chou, Y.-Y.; Pham, D.L.; Butman, J.A.; Diaz-Arrastia, R.; et al. Time Course and Diagnostic Utility of NfL, Tau, GFAP, and UCH-L1 in Subacute and Chronic TBI. Neurology 2020, 95, e623–e636. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Wang, T.; Xu, Z.; Liu, S.; Zhang, H.; Du, Z.; Wang, J.; Wang, Y.; Wang, Z.; Yuan, S.; et al. Ectoderm-Derived Frontal Bone Mesenchymal Stem Cells Promote Traumatic Brain Injury Recovery by Alleviating Neuroinflammation and Glutamate Excitotoxicity Partially via FGF1. Stem Cell Res. Ther. 2022, 13, 341. Available online: https://stemcellres.biomedcentral.com/articles/10.1186/s13287-022-03032-6 (accessed on 13 May 2024). [CrossRef] [PubMed]
- He, L.; Zhang, R.; Yang, M.; Lu, M. The Role of Astrocyte in Neuroinflammation in Traumatic Brain Injury. Biochim. Et. Biophys. Acta (BBA) Mol. Basis Dis. 2024, 1870, 166992. [Google Scholar] [CrossRef] [PubMed]
- Trautz, F.; Franke, H.; Bohnert, S.; Hammer, N.; Müller, W.; Stassart, R.; Tse, R.; Zwirner, J.; Dreßler, J.; Ondruschka, B. Survival-Time Dependent Increase in Neuronal IL-6 and Astroglial GFAP Expression in Fatally Injured Human Brain Tissue. Sci. Rep. 2019, 9, 11771. [Google Scholar] [CrossRef] [PubMed]
- Pelinka, L.E.; Kroepfl, A.; Leixnering, M.; Buchinger, W.; Raabe, A.; Redl, H. GFAP versus S100B in Serum after Traumatic Brain Injury: Relationship to Brain Damage and Outcome. J. Neurotrauma 2004, 21, 1553–1561. [Google Scholar] [CrossRef]
- Zwirner, J.; Lier, J.; Franke, H.; Hammer, N.; Matschke, J.; Trautz, F.; Tse, R.; Ondruschka, B. GFAP Positivity in Neurons Following Traumatic Brain Injuries. Int. J. Leg. Med. 2021, 135, 2323–2333. [Google Scholar] [CrossRef]
- Sirko, S.; Schichor, C.; Vecchia, P.D.; Metzger, F.; Sonsalla, G.; Simon, T.; Burkle, M.; Kalpazidou, S.; Ninkovic, J.; Masserdotti, G.; et al. Injury-Specific Factors in the Cerebrospinal Fluid Regulate Astrocyte Plasticity in the Human Brain. Nat. Med. 2023, 29, 3149–3161. Available online: https://www.nature.com/articles/s41591-023-02644-6 (accessed on 13 May 2024). [CrossRef]
- Darrabie, M.D.; Cheeseman, J.; Limkakeng, A.T.; Borawski, J.; Sullenger, B.A.; Elster, E.A.; Kirk, A.D.; Lee, J. Toll-like Receptor Activation as a Biomarker in Traumatically Injured Patients. J. Surg. Res. 2018, 231, 270–277. [Google Scholar] [CrossRef]
- Gerzanich, V.; Stokum, J.A.; Ivanova, S.; Woo, S.K.; Tsymbalyuk, O.; Sharma, A.; Akkentli, F.; Imran, Z.; Aarabi, B.; Sahuquillo, J.; et al. Sulfonylurea Receptor 1, Transient Receptor Potential Cation Channel Subfamily M Member 4, and KIR6.2:Role in Hemorrhagic Progression of Contusion. J. Neurotrauma 2019, 36, 1060–1079. [Google Scholar] [CrossRef]
- Zhuge, C.-J.; Zhan, C.-P.; Wang, K.-W.; Yan, X.-J.; Yu, G.-F. Serum Sulfonylurea Receptor-1 Levels After Acute Supratentorial Intracerebral Hemorrhage: Implication for Prognosis. Neuropsychiatr. Dis. Treat. 2022, 18, 1117–1126. [Google Scholar] [CrossRef]
- Gustafsson, D.; Klang, A.; Thams, S.; Rostami, E. The Role of BDNF in Experimental and Clinical Traumatic Brain Injury. Int. J. Mol. Sci. 2021, 22, 3582. [Google Scholar] [CrossRef] [PubMed]
- Wurzelmann, M.; Romeika, J.; Sun, D. Therapeutic Potential of Brain-Derived Neurotrophic Factor (BDNF) and a Small Molecular Mimics of BDNF for Traumatic Brain Injury. Neural Regen. Res. 2017, 12, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; do Nascimento, R.I.M.; Filho, E.M.R.; Bencke, J.; Regner, A. Plasma Brain-Derived Neurotrophic Factor Levels after Severe Traumatic Brain Injury. Brain Inj. 2016, 30, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Ghaith, H.S.; Nawar, A.A.; Gabra, M.D.; Abdelrahman, M.E.; Nafady, M.H.; Bahbah, E.I.; Ebada, M.A.; Ashraf, G.M.; Negida, A.; Barreto, G.E. A Literature Review of Traumatic Brain Injury Biomarkers. Mol. Neurobiol. 2022, 59, 4141–4158. [Google Scholar] [CrossRef]
- Wang, K.K.; Yang, Z.; Zhu, T.; Shi, Y.; Rubenstein, R.; Tyndall, J.A.; Manley, G.T. An Update on Diagnostic and Prognostic Biomarkers for Traumatic Brain Injury. Expert. Rev. Mol. Diagn. 2018, 18, 165–180. [Google Scholar] [CrossRef]
- Ooi, S.Z.Y.; Spencer, R.J.; Hodgson, M.; Mehta, S.; Phillips, N.L.; Preest, G.; Manivannan, S.; Wise, M.P.; Galea, J.; Zaben, M. Interleukin-6 as a Prognostic Biomarker of Clinical Outcomes after Traumatic Brain Injury: A Systematic Review. Neurosurg. Rev. 2022, 45, 3035–3054. [Google Scholar] [CrossRef]
- Rowland, B.; Savarraj, J.P.J.; Karri, J.; Zhang, X.; Cardenas, J.; Choi, H.A.; Holcomb, J.B.; Wade, C.E. Acute Inflammation in Traumatic Brain Injury and Polytrauma Patients Using Network Analysis. Shock 2020, 53, 24–34. [Google Scholar] [CrossRef]
- Xiong, Y.; Mahmood, A.; Chopp, M. Current Understanding of Neuroinflammation after Traumatic Brain Injury and Cell-Based Therapeutic Opportunities. Chin. J. Traumatol. 2018, 21, 137–151. [Google Scholar] [CrossRef]
- Aisiku, I.P.; Yamal, J.-M.; Doshi, P.; Benoit, J.S.; Gopinath, S.; Goodman, J.C.; Robertson, C.S. Plasma Cytokines IL-6, IL-8, and IL-10 Are Associated with the Development of Acute Respiratory Distress Syndrome in Patients with Severe Traumatic Brain Injury. Crit. Care 2016, 20, 288. [Google Scholar] [CrossRef]
- Eagle, S.R.; Puccio, A.M.; Agoston, D.V.; Soose, R.; Mancinelli, M.; Nwafo, R.; McIntyre, P.; Agnone, A.; Tollefson, S.; Collins, M.; et al. Evaluating Targeted Therapeutic Response with Predictive Blood-Based Biomarkers in Patients With Chronic Mild Traumatic Brain Injury. Neurotrauma Rep. 2023, 4, 404–409. [Google Scholar] [CrossRef]
- Popiolek-Barczyk, K.; Ciechanowska, A.; Ciapała, K.; Pawlik, K.; Oggioni, M.; Mercurio, D.; De Simoni, M.-G.; Mika, J. The CCL2/CCL7/CCL12/CCR2 Pathway Is Substantially and Persistently Upregulated in Mice after Traumatic Brain Injury, and CCL2 Modulates the Complement System in Microglia. Mol. Cell. Probes 2020, 54, 101671. [Google Scholar] [CrossRef] [PubMed]
- Huie, J.R.; Diaz-Arrastia, R.; Yue, J.K.; Sorani, M.D.; Puccio, A.M.; Okonkwo, D.O.; Manley, G.T.; Ferguson, A.R. Testing a Multivariate Proteomic Panel for Traumatic Brain Injury Biomarker Discovery: A TRACK-TBI Pilot Study. J. Neurotrauma 2019, 36, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Loane, D.J.; Kumar, A. Microglia in the TBI Brain: The Good, the Bad, and the Dysregulated. Exp. Neurol. 2016, 275 Pt 3, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Koivikko, P.; Posti, J.P.; Mohammadian, M.; Lagerstedt, L.; Azurmendi, L.; Hossain, I.; Katila, A.J.; Menon, D.; Newcombe, V.F.J.; Hutchinson, P.J.; et al. Potential of Heart Fatty-Acid Binding Protein, Neurofilament Light, Interleukin-10 and S100 Calcium-Binding Protein B in the Acute Diagnostics and Severity Assessment of Traumatic Brain Injury. Emerg. Med. J. 2022, 39, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.G.; Balasubramanian, N.; Manjrekar, R.; Banerjee, T.; Sakharkar, A. DNA Methylation-Mediated Mfn2 Gene Regulation in the Brain: A Role in Brain Trauma-Induced Mitochondrial Dysfunction and Memory Deficits. Cell Mol. Neurobiol. 2023, 43, 3479–3495. [Google Scholar] [CrossRef]
- Masi, M.; Biundo, F.; Fiou, A.; Racchi, M.; Pascale, A.; Buoso, E. The Labyrinthine Landscape of APP Processing: State of the Art and Possible Novel Soluble APP-Related Molecular Players in Traumatic Brain Injury and Neurodegeneration. Int. J. Mol. Sci. 2023, 24, 6639. [Google Scholar] [CrossRef]
- Abu Hamdeh, S.; Ciuculete, D.-M.; Sarkisyan, D.; Bakalkin, G.; Ingelsson, M.; Schiöth, H.B.; Marklund, N. Differential DNA Methylation of the Genes for Amyloid Precursor Protein, Tau, and Neurofilaments in Human Traumatic Brain Injury. J. Neurotrauma 2021, 38, 1679–1688. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Son, M.; Lee, H.H.; Kang, M.-G.; Yun, S.J.; Seo, H.G.; Kim, Y.; Oh, B.-M. Proteomic Discovery of Prognostic Protein Biomarkers for Persisting Problems after Mild Traumatic Brain Injury. Sci. Rep. 2023, 13, 19786. [Google Scholar] [CrossRef]
- Chohan, M.O.; Bragina, O.; Kazim, S.F.; Statom, G.; Baazaoui, N.; Bragin, D.; Iqbal, K.; Nemoto, E.; Yonas, H. Enhancement of Neurogenesis and Memory by a Neurotrophic Peptide in Mild to Moderate Traumatic Brain Injury. Neurosurgery 2015, 76, 201–214; discussion 214–215. [Google Scholar] [CrossRef]
- Quintard, H.; Lorivel, T.; Gandin, C.; Lazdunski, M.; Heurteaux, C. MLC901, a Traditional Chinese Medicine Induces Neuroprotective and Neuroregenerative Benefits after Traumatic Brain Injury in Rats. Neuroscience 2014, 277, 72–86. [Google Scholar] [CrossRef]
- Mondello, S.; Gabrielli, A.; Catani, S.; D’Ippolito, M.; Jeromin, A.; Ciaramella, A.; Bossù, P.; Schmid, K.; Tortella, F.; Wang, K.K.W.; et al. Increased Levels of Serum MAP-2 at 6-Months Correlate with Improved Outcome in Survivors of Severe Traumatic Brain Injury. Brain Inj. 2012, 26, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- Mi, Z.; Graham, S.H. Role of UCHL1 in the Pathogenesis of Neurodegenerative Diseases and Brain Injury. Ageing Res. Rev. 2023, 86, 101856. [Google Scholar] [CrossRef] [PubMed]
- Oris, C.; Kahouadji, S.; Durif, J.; Bouvier, D.; Sapin, V. S100B, Actor and Biomarker of Mild Traumatic Brain Injury. Int. J. Mol. Sci. 2023, 24, 6602. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, E.-S.; Vlachodimitropoulou, L.; Alexiou, G.A. S100B As a Biomarker in Traumatic Brain Injury. In Biomarkers in Trauma, Injury and Critical Care; Springer: Cham, Switzerland, 2022; Available online: https://link.springer.com/referenceworkentry/10.1007/978-3-030-87302-8_39-1 (accessed on 13 May 2024).
- Bhowmick, S.; D’Mello, V.; Caruso, D.; Abdul-Muneer, P.M. Traumatic Brain Injury-Induced Downregulation of Nrf2 Activates Inflammatory Response and Apoptotic Cell Death. J. Mol. Med. 2019, 97, 1627–1641. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Baker, T.L.; Wilson, C.T.; Brady, R.D.; Mychasiuk, R.; Yamakawa, G.R.; Vo, A.; Wilson, T.; McDonald, S.J.; Shultz, S.R. Treatment with Vascular Endothelial Growth Factor-A Worsens Cognitive Recovery in a Rat Model of Mild Traumatic Brain Injury. Front. Mol. Neurosci. 2022, 15, 937350. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, Y.; Mahmood, A.; Zhsng, Z.G.; Chopp, M. Angiogenesis and Functional Recovery After Traumatic Brain Injury. In Vascular Mechanisms in CNS Trauma; Springer: New York, NY, USA, 2013; Available online: https://link.springer.com/chapter/10.1007/978-1-4614-8690-9_8 (accessed on 13 May 2024).
- Li, M.; Jia, Q.; Chen, T.; Zhao, Z.; Chen, J.; Zhang, J. The Role of Vascular Endothelial Growth Factor and Vascular Endothelial Growth Inhibitor in Clinical Outcome of Traumatic Brain Injury. Clin. Neurol. Neurosurg. 2016, 144, 7–13. [Google Scholar] [CrossRef]
- Edwards, K.A.; Pattinson, C.L.; Guedes, V.A.; Peyer, J.; Moore, C.; Davis, T.; Devoto, C.; Turtzo, L.C.; Latour, L.; Gill, J.M. Inflammatory Cytokines Associate with Neuroimaging After Acute Mild Traumatic Brain Injury. Front. Neurol. 2020, 11, 348. [Google Scholar] [CrossRef]
- Jacob, M.; Lopata, A.L.; Dasouki, M.; Abdel Rahman, A.M. Metabolomics toward Personalized Medicine. Mass. Spectrom. Rev. 2019, 38, 221–238. [Google Scholar] [CrossRef]
- Dickens, A.M.; Posti, J.P.; Takala, R.S.K.; Ala-Seppälä, H.; Mattila, I.; Coles, J.P.; Frantzén, J.; Hutchinson, P.J.; Katila, A.J.; Kyllönen, A.; et al. Serum Metabolites Associated with Computed Tomography Findings after Traumatic Brain Injury. J. Neurotrauma 2018, 35, 2673–2683. [Google Scholar] [CrossRef]
- Orešič, M.; Posti, J.P.; Kamstrup-Nielsen, M.H.; Takala, R.S.K.; Lingsma, H.F.; Mattila, I.; Jäntti, S.; Katila, A.J.; Carpenter, K.L.H.; Ala-Seppälä, H.; et al. Human Serum Metabolites Associate with Severity and Patient Outcomes in Traumatic Brain Injury. EBioMedicine 2016, 12, 118–126. [Google Scholar] [CrossRef]
- Wolahan, S.M.; Lebby, E.; Mao, H.C.; McArthur, D.; Real, C.; Vespa, P.; Braas, D.; Glenn, T.C. Novel Metabolomic Comparison of Arterial and Jugular Venous Blood in Severe Adult Traumatic Brain Injury Patients and the Impact of Pentobarbital Infusion. J. Neurotrauma 2019, 36, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Thomas, I.; Dickens, A.M.; Posti, J.P.; Czeiter, E.; Duberg, D.; Sinioja, T.; Kråkström, M.; Retel Helmrich, I.R.A.; Wang, K.K.W.; Maas, A.I.R.; et al. Serum Metabolome Associated with Severity of Acute Traumatic Brain Injury. Nat. Commun. 2022, 13, 2545. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.K.; Hergenroeder, G.W.; Jeter, C.B.; Choi, H.A.; Kobori, N.; Moore, A.N. Traumatic Brain Injury Alters Methionine Metabolism: Implications for Pathophysiology. Front. Syst. Neurosci. 2016, 10, 00036. [Google Scholar] [CrossRef] [PubMed]
- Jeter, C.B.; Hergenroeder, G.W.; Ward, N.H.; Moore, A.N.; Dash, P.K. Human Traumatic Brain Injury Alters Circulating L-Arginine and Its Metabolite Levels: Possible Link to Cerebral Blood Flow, Extracellular Matrix Remodeling, and Energy Status. J. Neurotrauma 2012, 29, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Vuille-Dit-Bille, R.N.; Ha-Huy, R.; Stover, J.F. Changes in Plasma Phenylalanine, Isoleucine, Leucine, and Valine Are Associated with Significant Changes in Intracranial Pressure and Jugular Venous Oxygen Saturation in Patients with Severe Traumatic Brain Injury. Amino Acids 2012, 43, 1287–1296. [Google Scholar] [CrossRef]
- Jeter, C.B.; Hergenroeder, G.W.; Ward, N.H.; Moore, A.N.; Dash, P.K. Human Mild Traumatic Brain Injury Decreases Circulating Branched-Chain Amino Acids and Their Metabolite Levels. J. Neurotrauma 2013, 30, 671–679. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, H.; Zhang, J.; Yu, H.; Lin, Z.; Cai, Y. Spermidine Exhibits Protective Effects Against Traumatic Brain Injury. Cell Mol. Neurobiol. 2020, 40, 927–937. [Google Scholar] [CrossRef]
- Wang, H.-C.; Lin, Y.-J.; Shih, F.-Y.; Chang, H.-W.; Su, Y.-J.; Cheng, B.-C.; Su, C.-M.; Tsai, N.-W.; Chang, Y.-T.; Kwan, A.-L.; et al. The Role of Serial Oxidative Stress Levels in Acute Traumatic Brain Injury and as Predictors of Outcome. World Neurosurg. 2016, 87, 463–470. [Google Scholar] [CrossRef]
- Bahado-Singh, R.O.; Graham, S.F.; Han, B.; Turkoglu, O.; Ziadeh, J.; Mandal, R.; Er, A.; Wishart, D.S.; Stahel, P.L. Serum Metabolomic Markers for Traumatic Brain Injury: A Mouse Model. Metabolomics 2016, 12, 100. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Ishiguro-Watanabe, M.; Tanabe, M. KEGG: Integrating Viruses and Cellular Organisms. Nucleic Acids Res. 2021, 49, D545–D551. [Google Scholar] [CrossRef]
- Lai, J.; Shi, Y.-C.; Lin, S.; Chen, X.-R. Metabolic Disorders on Cognitive Dysfunction after Traumatic Brain Injury. Trends Endocrinol. Metab. 2022, 33, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.E.; Weber, M.T.; Xiao, R.; Cullen, D.K.; Meaney, D.F.; Stewart, W.; Smith, D.H. Mechanical Disruption of the Blood–Brain Barrier Following Experimental Concussion. Acta Neuropathol. 2018, 135, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-A.; Yan, L.; Wen, J.; Satyanarayanan, S.K.; Yu, F.; Lu, J.; Liu, Y.U.; Su, H. Cellular and Molecular Mechanisms in Vascular Repair after Traumatic Brain Injury: A Narrative Review. Burn. Trauma. 2023, 11, tkad033. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Paoletti, P. Allosteric Modulators of NMDA Receptors: Multiple Sites and Mechanisms. Curr. Opin. Pharmacol. 2015, 20, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.L.; Kulbe, J.R.; Singh, I.N.; Wang, J.A.; Hall, E.D. Synaptic Mitochondria Are More Susceptible to Traumatic Brain Injury-Induced Oxidative Damage and Respiratory Dysfunction than Non-Synaptic Mitochondria. Neuroscience 2018, 386, 265–283. [Google Scholar] [CrossRef]
- Lazzarino, G.; Amorini, A.M.; Signoretti, S.; Musumeci, G.; Lazzarino, G.; Caruso, G.; Pastore, F.S.; Di Pietro, V.; Tavazzi, B.; Belli, A. Pyruvate Dehydrogenase and Tricarboxylic Acid Cycle Enzymes Are Sensitive Targets of Traumatic Brain Injury Induced Metabolic Derangement. Int. J. Mol. Sci. 2019, 20, 5774. [Google Scholar] [CrossRef]
- Hu, Y.; Tao, W. Microenvironmental Variations After Blood-Brain Barrier Breakdown in Traumatic Brain Injury. Front. Mol. Neurosci. 2021, 14, 750810. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Gradisnik, L.; Velnar, T. Astrocytes in the Central Nervous System and Their Functions in Health and Disease: A Review. World J. Clin. Cases 2023, 11, 3385–3394. [Google Scholar] [CrossRef]
- Orr, T.J.; Lesha, E.; Kramer, A.H.; Cecia, A.; Dugan, J.E.; Schwartz, B.; Einhaus, S.L. Traumatic Brain Injury: A Comprehensive Review of Biomechanics and Molecular Pathophysiology. World Neurosurg. 2024, 185, 74–88. [Google Scholar] [CrossRef]
| Candidate Biomarkers | Event | Change | Diagnosis/Prognosis | Pathogenesis | Biosample | Participants | Method Detection | Limit of Detection | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| Tau | Astrocyte activation GO:0048143 Astrocyte development GO:0014002 Inflammatory response GO:0006954 | ↑ | Marker of moderate/severe TBI (AUC 0.63) | oxidative stress | blood | 122 | bead-based immunoassay | – | [46,47,48,49] |
| Abeta 42 | Astrocyte activation GO:0048143 Astrocyte development GO:0014002 | ↑ | Marker of TBI, predictor of neurological status and mortality after TBI | oxidative stress | plasma | 12 | immunoassay | 0.02 ng/L | [50,51] |
| TNFa | Astrocyte activation GO:0048143 Astrocyte development GO:0014002 Inflammatory response GO:0006954 | ↑ | Marker of TBI, predictor of chronic phases | oxidative stress | plasma | 160 | electrochemiluminescent assay | 0.04 ng/L | [28,52,53,54,55,56] |
| GFAP | Astrocyte activation GO:0048143 Astrocyte development GO:0014002 Neurogenesis GO:00220 | ↑ | prediction of mortality after TBI (AUC 0.84 ± 0.05, <12 h) | neurogenesis synaptogenesis | serum | 92 | immunoluminometric assay | 0.03 μg/L | [28,57,58,59,60,61,62,63,64] |
| TLR | Astrocyte activation GO:0048143 | ↑ | therapeutic targets for cerebrovascular disorders | inflammatory response | blood | 146 | flow cytometry | – | [60,65] |
| SUR1 | Astrocyte activation GO:0048143 | ↑ | Prognose of severity, poor outcome after hemorrhagic stroke (~0.8 AUC) | – | serum | 262 | immunoassay | <1 μg/L | [66,67] |
| BDNF | Astrocyte activation GO:0048143 Neurogenesis GO:00220 | ↑ | Marker of severe TBI, predictor of mortality after TBI | neurogenesis | plasma | 120 | immunoassay | 16 ng/L | [57,60,68,69,70] |
| MBP | Maintenance of blood–brain barrier GO:0035633 | ↑ | Marker of severe TBI, predictor of mortality after TBI (AUC 0.83) | – | plasma | 127 | – | – | [1,57,71,72] |
| IL6 | Maintenance of blood–brain barrier GO:0035633 Inflammatory response GO:0006954 Angiogenesis GO:0001525 | ↑ | Clinical Diagnosis and Severity (AUC 0.92) | inflammatory response | plasma | 160 | electrochemiluminescent assay | 0.06 ng/L | [28,53,55,56,61,71,73,74,75,76] |
| CLDN5 | Maintenance of blood–brain barrier GO:0035633 | ↓ | Marker of chronic mild TBI | inflammatory response | plasma | 84 | fluorescent immunoassay | – | [38,39,77] |
| IL8 | Inflammatory response GO:0006954 Angiogenesis GO:0001525 | ↑ | Clinical Diagnosis and Severity (AUC 0.76) | inflammatory response | plasma | 160 | electrochemiluminescent assay | 0.07 ng/L | [28,53,55,56,74,75,76] |
| CCL2 | Inflammatory response GO:0006954 | ↑ | Marker of TBI (AUC > 0.62) | inflammatory response | plasma | 130 | fluorescent immunoassay | ~60 ng/L | [74,78,79] |
| IL-10 | Inflammatory response GO:0006954 | ↓ | Clinical Diagnosis and Severity (AUC 0.86) | inflammatory response | plasma | 160 | electrochemiluminescent assay | 0.04 ng/L | [55,56,75,76,80,81] |
| NEFH | Neurogenesis GO:00220 | ↑ | Marker of mild TBI (AUC 0.83) | neurogenesis | plasma | 42 | MRM-MS | – | [82,83,84,85] |
| MAP2 | Neurogenesis GO:00220 | ↓ | Prognosis of severe TBI | neurogenesis | serum | 32 | immunoassay | <0.01 μg/L | [59,86,87,88] |
| UCHL1 | Neurogenesis GO:00220 | ↑ | Marker of moderate/severe TBI (AUC 0.61) | neurogenesis | blood | 122 | bead-based immunoassay | 1.90 ng/L | [49,57,58,89] |
| S100B | Neurogenesis GO:00220 | ↑ | prediction of mortality after TBI, brain injury marker of circulatory arrest, st, TBI (AUC 0.82 ± 0.06, 85–108 h) | neurogenesissynaptogenesis | serum | 92 | immunoluminometric assay | 0.02 μg/L | [62,90,91] |
| NRF2 | Neurogenesis GO:00220 | ↑ | Marker of TBI, prognose of early neurologic deterioration (AUC 0.77–0.8, 6–24 h) | neurogenesis | serum | 230 | immunoassay | – | [92] |
| VEGF | Regulation of angiogenesis GO:0045765 | ↑ | Marker of mild TBI (AUC 0.86, 24 h) | angiogenesis | plasma | 250 | immunoassay | – | [93,94,95,96] |
| Metabolite | Pathway Name | Pathway ID | Change | Blood | Refs. |
|---|---|---|---|---|---|
| L-alpha-Aminobutyric acid | Cysteine and methionine metabolism | map00270 | ↓ | Serum | [98] |
| Methionine sulfoxide | ↑ | Serum | [99] | ||
| 3-Hydroxybutyric acid | Glycolysis/Gluconeogenesis | map00010 | ↑ | Serum | [99] |
| 2,3-Diphosphoglyceric acid | ↓ | Plasma | [100] | ||
| Glycerol | Pentose and glucuronate interconversions | map00040 | ↑ | Serum | [99,101] |
| Caprylic acid | Fatty acid biosynthesis | map00061 | ↑ | Serum | [83,85,86] |
| Capric acid | ↓ | Plasma | |||
| Glycine | Primary bile acid biosynthesis | map00120 | ↑ | Plasma | [100] |
| Thymine | Pyrimidine metabolism | map00240 | ↓ | Serum | [99,101] |
| Glutamic acid | Alanine, aspartate and glutamate metabolism | map00250 | ↑ | Plasma | [45] |
| L-Asparagine | ↑ | Plasma | [102] | ||
| 2-Ketobutyric acid | Glycine, serine and threonine metabolism | map00260 | ↓ | Plasma | [102] |
| Methionine | ↓ | Plasma | [102] | ||
| Betaine | ↑ | Plasma | [103] | ||
| Creatine | ↓ | Serum | [99,100,101] | ||
| Serine | ↓ | Serum | [101] | ||
| L-Threonine | ↓ | Plasma | [104] | ||
| L-Tryptophan | ↓ and ↑ | Plasma | [100,102] | ||
| Choline | ↓ | Plasma | [102] | ||
| Dimethylglycine | ↓ | Plasma | [104,105] | ||
| Isoleucine | Valine, leucine and isoleucine degradation | map00280 | ↓ | Plasma | [105] |
| Ketoleucine | ↓ | Plasma | [104,105] | ||
| 3-Methyl-2-oxovaleric acid | ↓ | Plasma | [104,105] | ||
| L-Valine | ↓ | Plasma | [104,105] | ||
| Leucine | ↓ | Plasma | [105] | ||
| Isoleucine | ↓ | Plasma | [100,103] | ||
| Citrulline | Arginine and proline metabolism | map00330 | ↓ | Plasma | [103] |
| Ornithine | ↓ | Plasma | [100,102,103] | ||
| L-Proline | ↓ | Plasma | [103] | ||
| 4-Hydroxyproline | ↓ | Plasma | [103] | ||
| Creatinine | ↓ | Plasma | [103] | ||
| L-Arginine | ↓ | Plasma | [87] | ||
| Urea | ↓ | Serum | [106] | ||
| Proline | ↑ | Serum | [100,101] | ||
| Kynurenic acid | Tryptophan metabolism | map00380 | ↑ | Plasma | [104] |
| Quinolinic acid | ↑ | Plasma | [104] | ||
| Phenylalanine | Phenylalanine, tyrosine and tryptophan biosynthesis | map00400 | ↓ | Plasma | [102] |
| L-Tyrosine | ↑ | Plasma/Serum | [99,102] | ||
| 2-Hydroxybutyric acid | Propanoate metabolism | map00640 | ↑ | Plasma | [100] |
| Lactic acid | Butanoate metabolism | map00650 | ↓ | Plasma | [100] |
| Niacinamide | Nicotinate and nicotinamide metabolism | map00760 | ↓ | Plasma | [105] |
| Glucose 6-phosphate | Insulin secretion | map04911 | ↓ | Plasma | [105] |
| Isobutyryl-L-carnitine | n/d* | n/d | ↓ | Plasma | [105] |
| Propionylcarnitine | n/d | n/d | ↓ | Plasma | [105] |
| Succinylcarnitine | n/d | n/d | ↓ | Plasma | [105] |
| 2-Hydroxyisovalerylcarnitine | n/d | n/d | ↓ | Plasma | [105] |
| 2-Methylbutyroylcarnitine | n/d | n/d | ↑ | Plasma | [105] |
| Isovalerylcarnitine | n/d | n/d | ↓ | Plasma | [102] |
| Methylglutarylcarnitine | n/d | n/d | ↓ | Plasma | [102] |
| Cysteine hydrochloride | n/d | n/d | ↓ | Plasma | [102] |
| gamma-Glutamylvaline | n/d | n/d | ↓ | Plasma | [102] |
| gamma-Glutamylleucine | n/d | n/d | ↓ | Plasma | [102] |
| gamma-Glutamylisoleucine | n/d | n/d | ↓ | Plasma | [102] |
| gamma-Glutamyltyrosine | n/d | n/d | ↑ | Serum | [98] |
| epi-Inositol | n/d | n/d | ↑ | Serum | [98] |
| Propylene glycol | n/d | n/d | ↓ | Serum | [99] |
| Ribonic acid | n/d | n/d | ↓ | Plasma | [105] |
| Indole-3-propionic acid | n/d | n/d | ↓ | Plasma | [105] |
| 2-methylbutyrylcarnitine | n/d | n/d | ↓ | Plasma | [100] |
| Isobutyryl-L-carnitine | n/d | n/d | ↓ | Plasma | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butkova, T.V.; Malsagova, K.A.; Nakhod, V.I.; Petrovskiy, D.V.; Izotov, A.A.; Balakin, E.I.; Yurku, K.A.; Umnikov, A.S.; Pustovoyt, V.I.; Kaysheva, A.L. Candidate Molecular Biomarkers of Traumatic Brain Injury: A Systematic Review. Biomolecules 2024, 14, 1283. https://doi.org/10.3390/biom14101283
Butkova TV, Malsagova KA, Nakhod VI, Petrovskiy DV, Izotov AA, Balakin EI, Yurku KA, Umnikov AS, Pustovoyt VI, Kaysheva AL. Candidate Molecular Biomarkers of Traumatic Brain Injury: A Systematic Review. Biomolecules. 2024; 14(10):1283. https://doi.org/10.3390/biom14101283
Chicago/Turabian StyleButkova, Tatiana V., Kristina A. Malsagova, Valeriya I. Nakhod, Denis V. Petrovskiy, Alexander A. Izotov, Evgenii I. Balakin, Ksenia A. Yurku, Alexey S. Umnikov, Vasiliy I. Pustovoyt, and Anna L. Kaysheva. 2024. "Candidate Molecular Biomarkers of Traumatic Brain Injury: A Systematic Review" Biomolecules 14, no. 10: 1283. https://doi.org/10.3390/biom14101283
APA StyleButkova, T. V., Malsagova, K. A., Nakhod, V. I., Petrovskiy, D. V., Izotov, A. A., Balakin, E. I., Yurku, K. A., Umnikov, A. S., Pustovoyt, V. I., & Kaysheva, A. L. (2024). Candidate Molecular Biomarkers of Traumatic Brain Injury: A Systematic Review. Biomolecules, 14(10), 1283. https://doi.org/10.3390/biom14101283







