Myokines May Be the Answer to the Beneficial Immunomodulation of Tailored Exercise—A Narrative Review
Abstract
1. Introduction
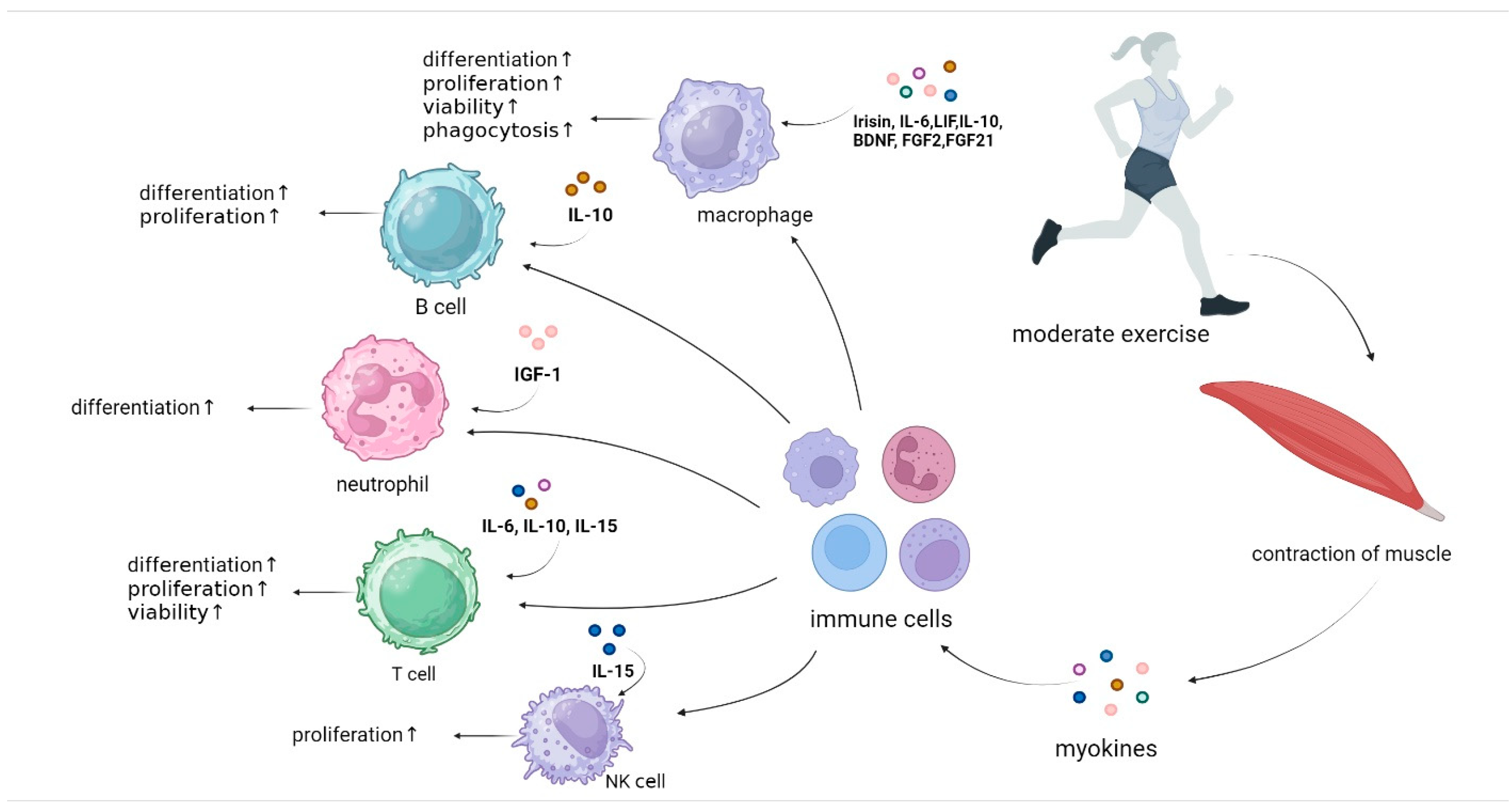
2. Potential Immune Cells Impacted by Exercise
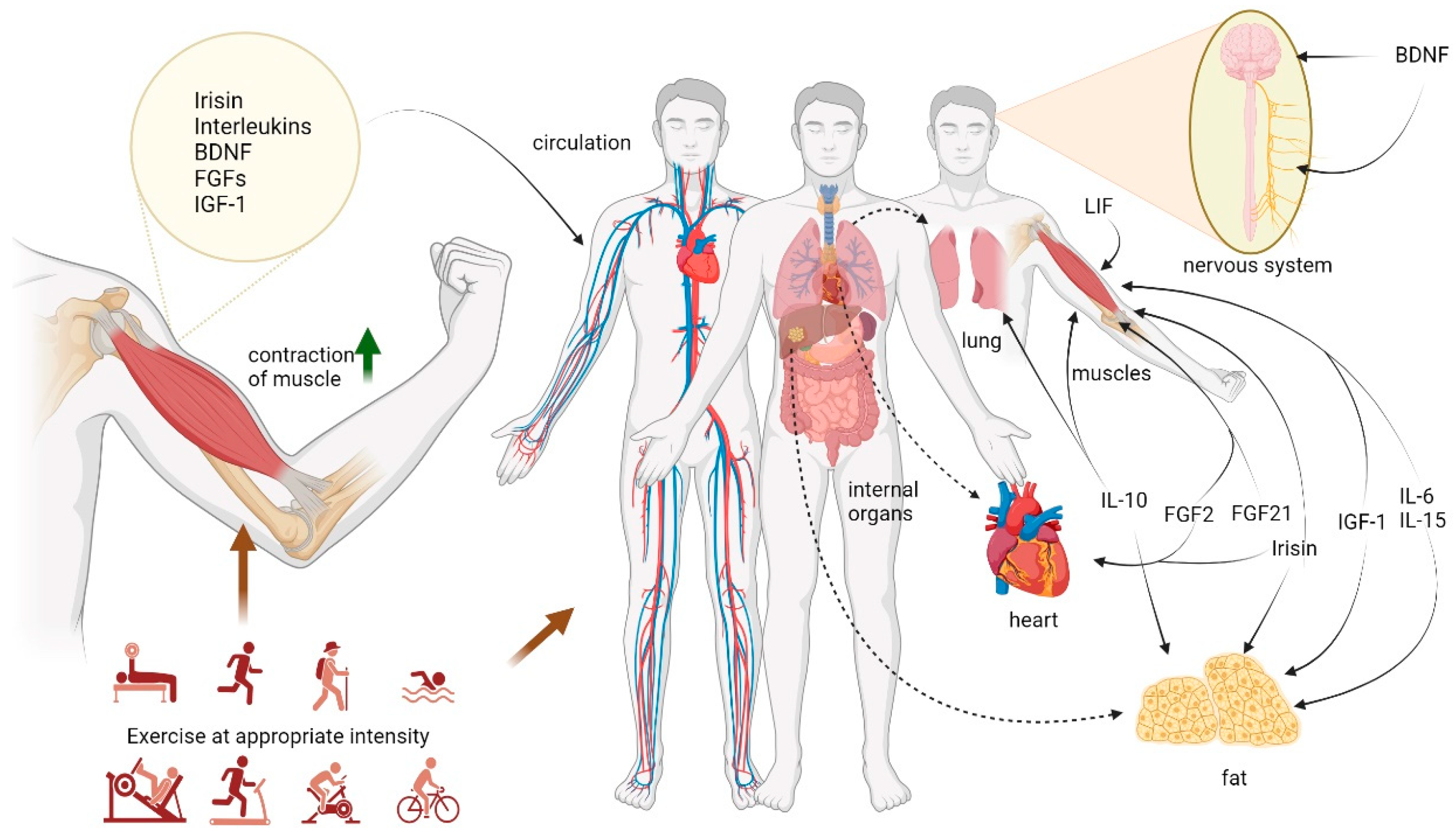
3. Exercise and Myokines
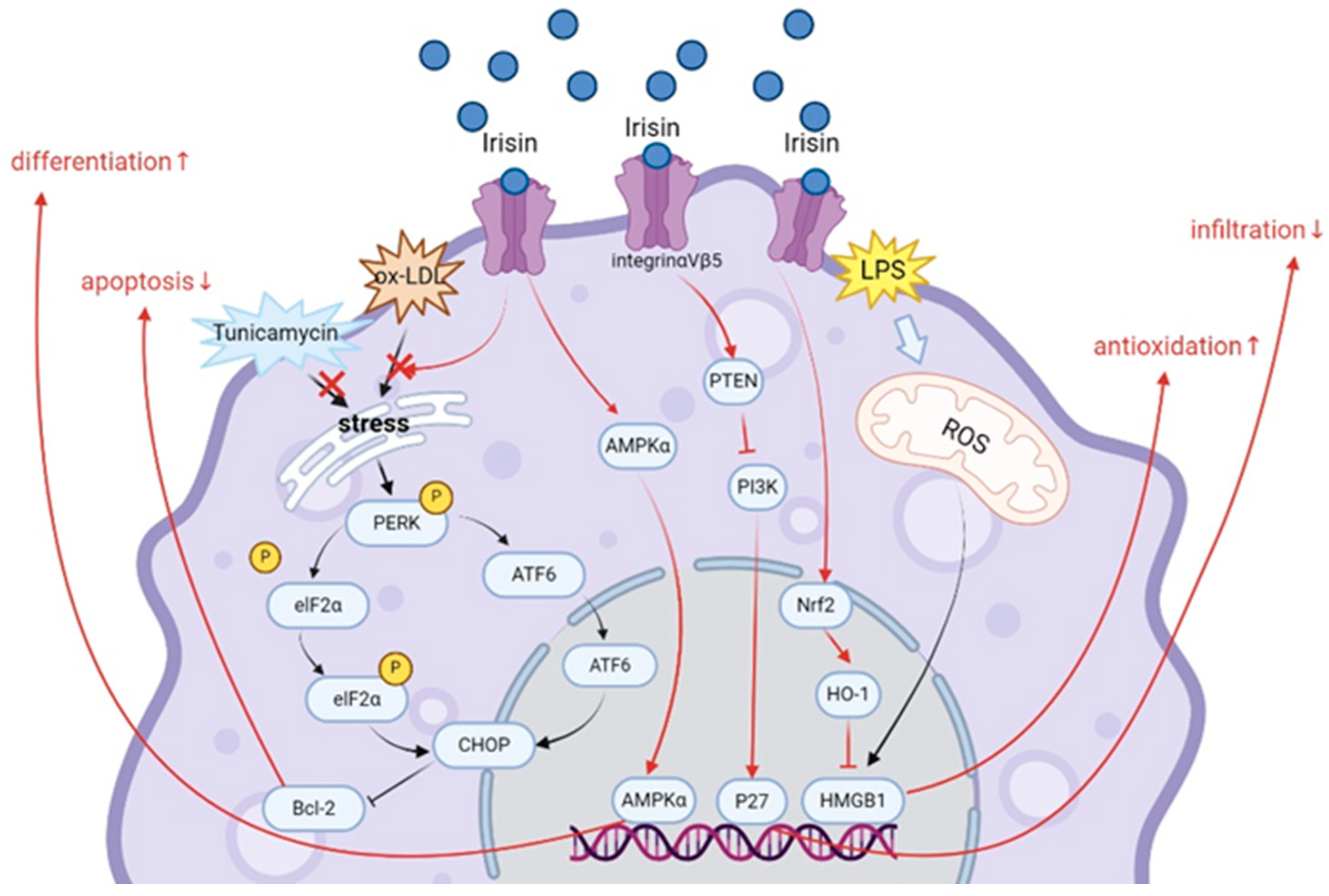
3.1. Irisin
3.2. Interleukins
3.3. Brain-Derived Neurotrophic Factor (BDNF)
3.4. Fibroblast Growth Factors (FGFs)
3.5. Insulin-like Growth Factor-1 (IGF-1)
4. Myokines and Immune Cells
4.1. Irisin
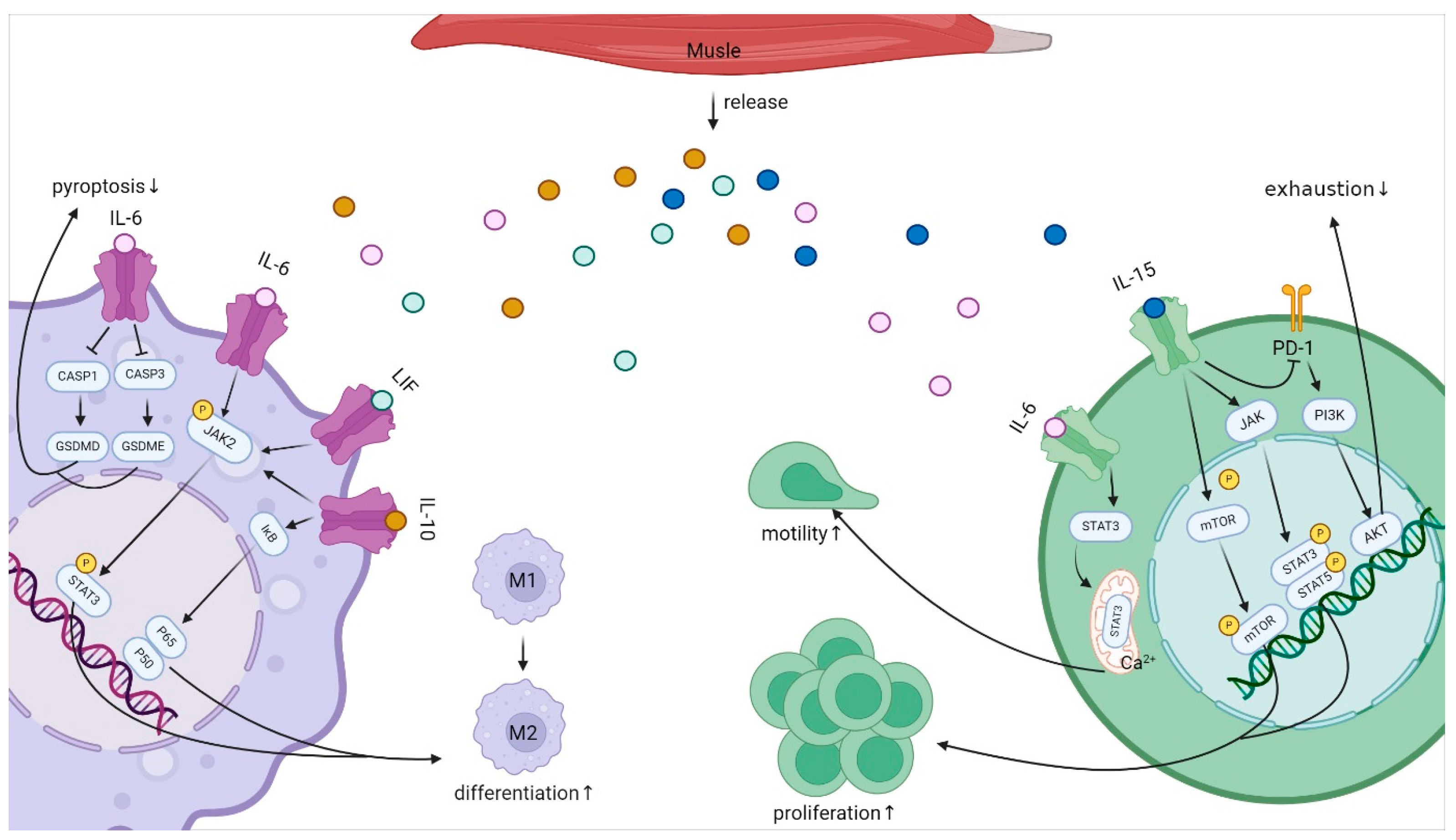
4.2. Interleukins
4.2.1. IL-6 Family
4.2.2. IL-10
4.2.3. IL-15
4.3. BDNF
4.4. FGF Family
4.4.1. FGF2
4.4.2. FGF21
4.5. IGF-1
5. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar] [PubMed]
- Wang, J.; Liu, S.; Li, G.; Xiao, J. Exercise Regulates the Immune System. Adv. Exp. Med. Biol. 2020, 1228, 395–408. [Google Scholar] [PubMed]
- Crescioli, C. Vitamin D, exercise, and immune health in athletes: A narrative review. Front. Immunol. 2022, 13, 954994. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Campbell, J.P.; Gleeson, M.; Krüger, K.; Nieman, D.C.; Pyne, D.B.; Turner, J.E.; Walsh, N.P. Can exercise affect immune function to increase susceptibility to infection? Exerc. Immunol. Rev. 2020, 26, 8–22. [Google Scholar]
- Kwon, J.H.; Moon, K.M.; Min, K.-W. Exercise-Induced Myokines can Explain the Importance of Physical Activity in the Elderly: An Overview. Healthcare 2020, 8, 378. [Google Scholar] [CrossRef]
- Pal, M.; Febbraio, M.A.; Whitham, M. From cytokine to myokine: The emerging role of interleukin-6 in metabolic regulation. Immunol. Cell Biol. 2014, 92, 331–339. [Google Scholar] [CrossRef]
- Alizadeh Pahlavani, H. Exercise Therapy for People with Sarcopenic Obesity: Myokines and Adipokines as Effective Actors. Front. Endocrinol. 2022, 13, 811751. [Google Scholar] [CrossRef]
- Domin, R.; Dadej, D.; Pytka, M.; Zybek-Kocik, A.; Ruchała, M.; Guzik, P. Effect of Various Exercise Regimens on Selected Exercise-Induced Cytokines in Healthy People. Int. J. Env. Res. Public Health 2021, 18, 1261. [Google Scholar] [CrossRef]
- Pedersen, B.K. Muscle as a secretory organ. Compr. Physiol. 2013, 3, 1337–1362. [Google Scholar]
- Hojman, P. Exercise protects from cancer through regulation of immune function and inflammation. Biochem. Soc. Trans. 2017, 45, 905–911. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Borkoles, E.; Polman, R.; Stojanovska, L. Physical and immunological aspects of exercise in chronic diseases. Immunotherapy 2014, 6, 1145–1157. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, G.; Rudensky, A.Y. Interactions between innate and adaptive lymphocytes. Nat. Rev. Immunol. 2014, 14, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Okabe, Y.; Medzhitov, R. Tissue biology perspective on macrophages. Nat. Immunol. 2016, 17, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Murdoch, C.; Lewis, C.E. Macrophage migration and gene expression in response to tumor hypoxia. Int. J. Cancer 2005, 117, 701–708. [Google Scholar] [CrossRef]
- Bashir, S.; Sharma, Y.; Elahi, A.; Khan, F. Macrophage polarization: The link between inflammation and related diseases. Inflamm. Res. 2016, 65, 1–11. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.-A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Porta, C.; Riboldi, E.; Ippolito, A.; Sica, A. Molecular and epigenetic basis of macrophage polarized activation. Semin. Immunol. 2015, 27, 237–248. [Google Scholar] [CrossRef]
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef] [PubMed]
- Braga, T.T.; Agudelo, J.S.H.; Camara, N.O.S. Macrophages During the Fibrotic Process: M2 as Friend and Foe. Front. Immunol. 2015, 6, 602. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Obstacles and opportunities for understanding macrophage polarization. J. Leukoc. Biol. 2011, 89, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, M.R.; Muenchow, M.; Wallig, M.A.; Horn, P.L.; Woods, J.A. Exercise delays allogeneic tumor growth and reduces intratumoral inflammation and vascularization. J. Appl. Physiol. 2004, 96, 2249–2256. [Google Scholar] [CrossRef]
- Okutsu, M.; Suzuki, K.; Ishijima, T.; Peake, J.; Higuchi, M. The effects of acute exercise-induced cortisol on CCR2 expression on human monocytes. Brain Behav. Immun. 2008, 22, 1066–1071. [Google Scholar] [CrossRef]
- Ortega, E.; Forner, M.A.; Barriga, C. Exercise-induced stimulation of murine macrophage chemotaxis: Role of corticosterone and prolactin as mediators. J. Physiol. 1997, 498 Pt 3, 729–734. [Google Scholar] [CrossRef]
- Woods, J.A.; Davis, J.M. Exercise, monocyte/macrophage function, and cancer. Med. Sci. Sports Exerc. 1994, 26, 147–156. [Google Scholar] [CrossRef]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014, 9, 181–218. [Google Scholar] [CrossRef]
- Liew, P.X.; Kubes, P. The Neutrophil’s Role During Health and Disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef]
- Hulmi, J.J.; Myllymäki, T.; Tenhumäki, M.; Mutanen, N.; Puurtinen, R.; Paulsen, G.; Mero, A.A. Effects of resistance exercise and protein ingestion on blood leukocytes and platelets in young and older men. Eur. J. Appl. Physiol. 2010, 109, 343–353. [Google Scholar] [CrossRef]
- Grégoire, C.; Chasson, L.; Luci, C.; Tomasello, E.; Geissmann, F.; Vivier, E.; Walzer, T. The trafficking of natural killer cells. Immunol. Rev. 2007, 220, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.C.; Lanier, L.L. NK cell development, homeostasis and function: Parallels with CD8+ T cells. Nat. Rev. Immunol. 2011, 11, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, F.; Imani, S.; Tao, L.; Deng, Y.; Cai, Y. Natural Killer Cells: Friend or Foe in Metabolic Diseases? Front. Immunol. 2021, 12, 614429. [Google Scholar] [CrossRef] [PubMed]
- Ramel, A.; Wagner, K.-H.; Elmadfa, I. Acute impact of submaximal resistance exercise on immunological and hormonal parameters in young men. J. Sports Sci. 2003, 21, 1001–1008. [Google Scholar] [CrossRef]
- Simonson, S.R.; Jackson, C.G.R. Leukocytosis occurs in response to resistance exercise in men. J. Strength. Cond. Res. 2004, 18, 266–271. [Google Scholar]
- Hussaarts, L.; Yazdanbakhsh, M.; Guigas, B. Priming dendritic cells for th2 polarization: Lessons learned from helminths and implications for metabolic disorders. Front. Immunol. 2014, 5, 499. [Google Scholar] [CrossRef]
- Chiang, L.M.; Chen, Y.J.; Chiang, J.; Lai, L.Y.; Chen, Y.Y.; Liao, H.F. Modulation of dendritic cells by endurance training. Int. J. Sports Med. 2007, 28, 798–803. [Google Scholar] [CrossRef]
- Bosteels, V.; Janssens, S. Striking a balance: New perspectives on homeostatic dendritic cell maturation. Nat. Rev. Immunol. 2024. [Google Scholar] [CrossRef]
- Eibel, H.; Kraus, H.; Sic, H.; Kienzler, A.-K.; Rizzi, M. B cell biology: An overview. Curr. Allergy Asthma Rep. 2014, 14, 434. [Google Scholar] [CrossRef]
- Dohi, K.; Mastro, A.M.; Miles, M.P.; Bush, J.A.; Grove, D.S.; Leach, S.K.; Volek, J.S.; Nindl, B.C.; Marx, J.O.; Gotshalk, L.A.; et al. Lymphocyte proliferation in response to acute heavy resistance exercise in women: Influence of muscle strength and total work. Eur. J. Appl. Physiol. 2001, 85, 367–373. [Google Scholar] [CrossRef]
- Miles, M.P.; Kraemer, W.J.; Nindl, B.C.; Grove, D.S.; Leach, S.K.; Dohi, K.; Marx, J.O.; Volek, J.S.; Mastro, A.M. Strength, workload, anaerobic intensity and the immune response to resistance exercise in women. Acta Physiol. Scand. 2003, 178, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Natale, V.M.; Brenner, I.K.; Moldoveanu, A.I.; Vasiliou, P.; Shek, P.; Shephard, R.J. Effects of three different types of exercise on blood leukocyte count during and following exercise. Sao Paulo Med. J. Rev. Paul. De. Med. 2003, 121, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Szlezak, A.M.; Szlezak, S.L.; Keane, J.; Tajouri, L.; Minahan, C. Establishing a dose-response relationship between acute resistance-exercise and the immune system: Protocol for a systematic review. Immunol. Lett. 2016, 180, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. T cells and their immunometabolism: A novel way to understanding sepsis immunopathogenesis and future therapeutics. Eur. J. Cell Biol. 2018, 97, 379–392. [Google Scholar] [CrossRef]
- Mathur, N.; Pedersen, B.K. Exercise as a mean to control low-grade systemic inflammation. Mediat. Inflamm. 2008, 2008, 109502. [Google Scholar] [CrossRef]
- Kawao, N.; Iemura, S.; Kawaguchi, M.; Mizukami, Y.; Takafuji, Y.; Kaji, H. Role of irisin in effects of chronic exercise on muscle and bone in ovariectomized mice. J. Bone Miner. Metab. 2021, 39, 547–557. [Google Scholar] [CrossRef]
- Tsourdi, E.; Anastasilakis, A.D.; Hofbauer, L.C.; Rauner, M.; Lademann, F. Irisin and Bone in Sickness and in Health: A Narrative Review of the Literature. J. Clin. Med. 2022, 11, 6863. [Google Scholar] [CrossRef]
- Arıkan, S.; Alaca, N.; Özbeyli, D.; Elmas, M.A.; Arbak, S.; Suyen, G. Effects of moderate aerobic exercise, low-level laser therapy, or their combination on muscles pathology, oxidative stress and irisin levels in the mdx mouse model of Duchenne muscular dystrophy. Lasers Med. Sci. 2022, 37, 2925–2936. [Google Scholar] [CrossRef]
- Kang, Y.-S.; Kim, J.-C.; Kim, J.-S.; Kim, S.H. Effects of Swimming Exercise on Serum Irisin and Bone FNDC5 in Rat Models of High-Fat Diet-Induced Osteoporosis. J. Sports Sci. Med. 2019, 18, 596–603. [Google Scholar]
- Li, H.; Qin, S.; Liang, Q.; Xi, Y.; Bo, W.; Cai, M.; Tian, Z. Exercise Training Enhances Myocardial Mitophagy and Improves Cardiac Function via Irisin/FNDC5-PINK1/Parkin Pathway in MI Mice. Biomedicines 2021, 9, 701. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Brenmoehl, J.; Albrecht, E.; Komolka, K.; Schering, L.; Langhammer, M.; Hoeflich, A.; Maak, S. Irisin is elevated in skeletal muscle and serum of mice immediately after acute exercise. Int. J. Biol. Sci. 2014, 10, 338–349. [Google Scholar] [CrossRef]
- Norheim, F.; Langleite, T.M.; Hjorth, M.; Holen, T.; Kielland, A.; Stadheim, H.K.; Gulseth, H.L.; Birkeland, K.I.; Jensen, J.; Drevon, C.A. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014, 281, 739–749. [Google Scholar] [CrossRef] [PubMed]
- McCormick, J.J.; King, K.E.; Notley, S.R.; Fujii, N.; Boulay, P.; Sigal, R.J.; Kenny, G.P. Exercise in the heat induces similar elevations in serum irisin in young and older men despite lower resting irisin concentrations in older adults. J. Therm. Biol. 2022, 104, 103189. [Google Scholar] [CrossRef] [PubMed]
- Raschke, S.; Eckel, J. Adipo-myokines: Two sides of the same coin-mediators of inflammation and mediators of exercise. Mediat. Inflamm. 2013, 2013, 320724. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, D.S.; McFarlin, B.; Bois, C. Interleukin-6 expression after repeated bouts of eccentric exercise. Int. J. Sports Med. 2003, 24, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Ahn, N.; Kim, K. Effects of Aerobic and Resistance Exercise on Myokines in High Fat Diet-Induced Middle-Aged Obese Rats. Int. J. Environ. Res. Public Health 2020, 17, 2685. [Google Scholar] [CrossRef]
- Jia, D.; Cai, M.; Xi, Y.; Du, S.; Tian, Z. Interval exercise training increases LIF expression and prevents myocardial infarction-induced skeletal muscle atrophy in rats. Life Sci. 2018, 193, 77–86. [Google Scholar] [CrossRef]
- Broholm, C.; Laye, M.J.; Brandt, C.; Vadalasetty, R.; Pilegaard, H.; Pedersen, B.K.; Scheele, C. LIF is a contraction-induced myokine stimulating human myocyte proliferation. J. Appl. Physiol. 2011, 111, 251–259. [Google Scholar] [CrossRef]
- Saberi, S.; Askaripour, M.; Khaksari, M.; Amin Rajizadeh, M.; Abbas Bejeshk, M.; Akhbari, M.; Jafari, E.; Khoramipour, K. Exercise training improves diabetic renal injury by reducing fetuin-A, oxidative stress and inflammation in type 2 diabetic rats. Heliyon 2024, 10, e27749. [Google Scholar] [CrossRef]
- Lira, F.S.; Dos Santos, T.; Caldeira, R.S.; Inoue, D.S.; Panissa, V.L.G.; Cabral-Santos, C.; Campos, E.Z.; Rodrigues, B.; Monteiro, P.A. Short-Term High- and Moderate-Intensity Training Modifies Inflammatory and Metabolic Factors in Response to Acute Exercise. Front. Physiol. 2017, 8, 856. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.A.d.S.; Lira, F.S.; Pimentel, G.D.; Oliveira de Souza, C.; Batatinha, H.; Biondo, L.A.; Yamashita, A.S.; Junior, E.A.L.; Neto, J.C.R. Aerobic Exercise Modulates the Free Fatty Acids and Inflammatory Response During Obesity and Cancer Cachexia. Crit. Rev. Eukaryot. Gene Expr. 2016, 26, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, A.; McKendry, J.; Martin-Rincon, M.; Morales-Alamo, D.; Pérez-Köhler, B.; Valadés, D.; Buján, J.; Calbet, J.A.L.; Breen, L. Skeletal muscle IL-15/IL-15Rα and myofibrillar protein synthesis after resistance exercise. Scand. J. Med. Sci. Sports 2018, 28, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Micielska, K.; Gmiat, A.; Zychowska, M.; Kozlowska, M.; Walentukiewicz, A.; Lysak-Radomska, A.; Jaworska, J.; Rodziewicz, E.; Duda-Biernacka, B.; Ziemann, E. The beneficial effects of 15 units of high-intensity circuit training in women is modified by age, baseline insulin resistance and physical capacity. Diabetes Res. Clin. Pract. 2019, 152, 156–165. [Google Scholar] [CrossRef]
- Wang, L.; Bian, X.; Liu, L.; He, Q.; Xu, J.; Chen, X.; Ye, H.; Yang, J.; Jiang, L. Association between cognitive function and skeletal muscle in patients undergoing maintenance hemodialysis. Front. Endocrinol. 2024, 15, 1324867. [Google Scholar] [CrossRef]
- Cheng, S.-M.; Lee, S.-D. Exercise Training Enhances BDNF/TrkB Signaling Pathway and Inhibits Apoptosis in Diabetic Cerebral Cortex. Int. J. Mol. Sci. 2022, 23, 6740. [Google Scholar] [CrossRef]
- Bastioli, G.; Arnold, J.C.; Mancini, M.; Mar, A.C.; Gamallo-Lana, B.; Saadipour, K.; Chao, M.V.; Rice, M.E. Voluntary Exercise Boosts Striatal Dopamine Release: Evidence for the Necessary and Sufficient Role of BDNF. J. Neurosci. 2022, 42, 4725–4736. [Google Scholar] [CrossRef]
- Choi, S.H.; Bylykbashi, E.; Chatila, Z.K.; Lee, S.W.; Pulli, B.; Clemenson, G.D.; Kim, E.; Rompala, A.; Oram, M.K.; Asselin, C.; et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 2018, 361, eaan8821. [Google Scholar] [CrossRef]
- Rahmani, F.; Saghazadeh, A.; Rahmani, M.; Teixeira, A.L.; Rezaei, N.; Aghamollaii, V.; Ardebili, H.E. Plasma levels of brain-derived neurotrophic factor in patients with Parkinson disease: A systematic review and meta-analysis. Brain Res. 2019, 1704, 127–136. [Google Scholar] [CrossRef]
- Eliakim, A.; Oh, Y.; Cooper, D.M. Effect of single wrist exercise on fibroblast growth factor-2, insulin-like growth factor, and growth hormone. Am. J. Physiology. Regul. Integr. Comp. Physiol. 2000, 279, R548–R553. [Google Scholar] [CrossRef]
- Soori, R.; Amini, A.A.; Choobineh, S.; Eskandari, A.; Behjat, A.; Ghram, A.; Voltarelli, F.A. Exercise attenuates myocardial fibrosis and increases angiogenesis-related molecules in the myocardium of aged rats. Arch. Physiol. Biochem. 2022, 128, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, W.; Zeng, L.-Q.; Bai, H.; Li, J.; Zhou, J.; Zhou, G.-Y.; Fang, C.-W.; Wang, F.; Qin, X.-J. Exercise and dietary intervention ameliorate high-fat diet-induced NAFLD and liver aging by inducing lipophagy. Redox Biol. 2020, 36, 101635. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Song, W. Resistance training increases fibroblast growth factor-21 and irisin levels in the skeletal muscle of Zucker diabetic fatty rats. J. Exerc. Nutr. Biochem. 2017, 21, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Khalafi, M.; Alamdari, K.A.; Symonds, M.E.; Nobari, H.; Carlos-Vivas, J. Impact of acute exercise on immediate and following early post-exercise FGF-21 concentration in adults: Systematic review and meta-analysis. Hormones 2021, 20, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Ren, H.; Gao, S. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins: Roles in skeletal muscle growth and differentiation. Gen. Comp. Endocrinol. 2010, 167, 344–351. [Google Scholar] [CrossRef]
- Matheny, R.W.; Merritt, E.; Zannikos, S.V.; Farrar, R.P.; Adamo, M.L. Serum IGF-I-deficiency does not prevent compensatory skeletal muscle hypertrophy in resistance exercise. Exp. Biol. Med. 2009, 234, 164–170. [Google Scholar] [CrossRef]
- Ning, K.; Wang, Z.; Zhang, X.-A. Exercise-induced modulation of myokine irisin in bone and cartilage tissue-Positive effects on osteoarthritis: A narrative review. Front. Aging Neurosci. 2022, 14, 934406. [Google Scholar] [CrossRef]
- Jedrychowski, M.P.; Wrann, C.D.; Paulo, J.A.; Gerber, K.K.; Szpyt, J.; Robinson, M.M.; Nair, K.S.; Gygi, S.P.; Spiegelman, B.M. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab. 2015, 22, 734–740. [Google Scholar] [CrossRef]
- Guo, A.; Li, K.; Xiao, Q. Sarcopenic obesity: Myokines as potential diagnostic biomarkers and therapeutic targets? Exp. Gerontol. 2020, 139, 111022. [Google Scholar] [CrossRef]
- Leger, C.; Quirié, A.; Méloux, A.; Fontanier, E.; Chaney, R.; Basset, C.; Lemaire, S.; Garnier, P.; Prigent-Tessier, A. Impact of Exercise Intensity on Cerebral BDNF Levels: Role of FNDC5/Irisin. Int. J. Mol. Sci. 2024, 25, 1213. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Anastasilakis, A.D.; Efstathiadou, Z.A.; Makras, P.; Perakakis, N.; Kountouras, J.; Mantzoros, C.S. Irisin in metabolic diseases. Endocrine 2018, 59, 260–274. [Google Scholar] [CrossRef]
- Pérez-Sotelo, D.; Roca-Rivada, A.; Baamonde, I.; Baltar, J.; Castro, A.I.; Domínguez, E.; Collado, M.; Casanueva, F.F.; Pardo, M. Lack of Adipocyte-Fndc5/Irisin Expression and Secretion Reduces Thermogenesis and Enhances Adipogenesis. Sci. Rep. 2017, 7, 16289. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; So, B.; Choi, M.; Kang, D.; Song, W. Resistance exercise training increases the expression of irisin concomitant with improvement of muscle function in aging mice and humans. Exp. Gerontol. 2015, 70, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Mu, S.; Ding, D.; Ji, C.; Wu, Q.; Xia, Y.; Zhou, L.; Yang, L.; Ba, G.; Chang, Q.; Fu, Q.; et al. Relationships Between Circulating Irisin Response to Ice Swimming and Body Composition in People with Regular Exercise Experience. Front. Physiol. 2020, 11, 596896. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Valverde, P.; Zhu, X.; Murray, D.; Wu, Y.; Yu, L.; Jiang, H.; Dard, M.M.; Huang, J.; Xu, Z.; et al. Exercise-induced irisin in bone and systemic irisin administration reveal new regulatory mechanisms of bone metabolism. Bone Res. 2017, 5, 16056. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Linderman, J.D.; Smith, S.; Brychta, R.J.; Wang, J.; Idelson, C.; Perron, R.M.; Werner, C.D.; Phan, G.Q.; Kammula, U.S.; et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014, 19, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, L.; Zhao, H.; Yan, Y.; Lu, J. The Role of Interleukins in Colorectal Cancer. Int. J. Biol. Sci. 2020, 16, 2323–2339. [Google Scholar] [CrossRef]
- Kirk, B.; Feehan, J.; Lombardi, G.; Duque, G. Muscle, Bone, and Fat Crosstalk: The Biological Role of Myokines, Osteokines, and Adipokines. Curr. Osteoporos. Rep. 2020, 18, 388–400. [Google Scholar] [CrossRef]
- Northoff, H.; Berg, A. Immunologic mediators as parameters of the reaction to strenuous exercise. Int. J. Sports Med. 1991, 12 (Suppl. S1), S9–S15. [Google Scholar] [CrossRef]
- Ostrowski, K.; Rohde, T.; Zacho, M.; Asp, S.; Pedersen, B.K. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J. Physiol. 1998, 508 Pt 3, 949–953. [Google Scholar] [CrossRef]
- Steensberg, A.; Keller, C.; Starkie, R.L.; Osada, T.; Febbraio, M.A.; Pedersen, B.K. IL-6 and TNF-alpha expression in, and release from, contracting human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E1272–E1278. [Google Scholar] [CrossRef] [PubMed]
- Mathers, J.L.; Farnfield, M.M.; Garnham, A.P.; Caldow, M.K.; Cameron-Smith, D.; Peake, J.M. Early inflammatory and myogenic responses to resistance exercise in the elderly. Muscle Nerve 2012, 46, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Schindler, R.; Mancilla, J.; Endres, S.; Ghorbani, R.; Clark, S.C.; Dinarello, C.A. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood 1990, 75, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.L.; Baeza-Raja, B.; Perdiguero, E.; Jardí, M.; Muñoz-Cánoves, P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008, 7, 33–44. [Google Scholar] [CrossRef]
- Jung, S.H.; Park, H.S.; Kim, K.-S.; Choi, W.H.; Ahn, C.W.; Kim, B.T.; Kim, S.M.; Lee, S.Y.; Ahn, S.M.; Kim, Y.K.; et al. Effect of weight loss on some serum cytokines in human obesity: Increase in IL-10 after weight loss. J. Nutr. Biochem. 2008, 19, 371–375. [Google Scholar] [CrossRef]
- Oliver, J.M.; Jenke, S.C.; Mata, J.D.; Kreutzer, A.; Jones, M.T. Acute Effect of Cluster and Traditional Set Configurations on Myokines Associated with Hypertrophy. Int. J. Sports Med. 2016, 37, 1019–1024. [Google Scholar] [CrossRef]
- Park, K.M.; Park, S.C.; Kang, S. Effects of resistance exercise on adipokine factors and body composition in pre- and postmenopausal women. J. Exerc. Rehabil. 2019, 15, 676–682. [Google Scholar] [CrossRef]
- Binder, D.K.; Scharfman, H.E. Brain-derived neurotrophic factor. Growth Factors 2004, 22, 123–131. [Google Scholar] [CrossRef]
- Foltran, R.B.; Diaz, S.L. BDNF isoforms: A round trip ticket between neurogenesis and serotonin? J. Neurochem. 2016, 138, 204–221. [Google Scholar] [CrossRef]
- Tsimpolis, A.; Kalafatakis, K.; Charalampopoulos, I. Recent advances in the crosstalk between the brain-derived neurotrophic factor and glucocorticoids. Front. Endocrinol. 2024, 15, 1362573. [Google Scholar] [CrossRef]
- Sakuma, K.; Watanabe, K.; Sano, M.; Uramoto, I.; Nakano, H.; Li, Y.J.; Kaneda, S.; Sorimachi, Y.; Yoshimoto, K.; Yasuhara, M.; et al. A possible role for BDNF, NT-4 and TrkB in the spinal cord and muscle of rat subjected to mechanical overload, bupivacaine injection and axotomy. Brain Res. 2001, 907, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, X.; Yan, J.; Cheng, F.; Ma, X.; Chen, C.; Wang, W.; Wang, Q. Cholic Acid Protects In Vitro Neurovascular Units against Oxygen and Glucose Deprivation-Induced Injury through the BDNF-TrkB Signaling Pathway. Oxid. Med. Cell Longev. 2020, 2020, 1201624. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Dhapola, R.; Reddy, D.H. Apoptosis in Alzheimer’s disease: Insight into the signaling pathways and therapeutic avenues. Apoptosis 2023, 28, 943–957. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Pedersen, M.; Krabbe, K.S.; Bruunsgaard, H.; Matthews, V.B.; Febbraio, M.A. Role of exercise-induced brain-derived neurotrophic factor production in the regulation of energy homeostasis in mammals. Exp. Physiol. 2009, 94, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Banks, W.A.; Fasold, M.B.; Bluth, J.; Kastin, A.J. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology 1998, 37, 1553–1561. [Google Scholar] [CrossRef]
- Sartorius, A.; Hellweg, R.; Litzke, J.; Vogt, M.; Dormann, C.; Vollmayr, B.; Danker-Hopfe, H.; Gass, P. Correlations and discrepancies between serum and brain tissue levels of neurotrophins after electroconvulsive treatment in rats. Pharmacopsychiatry 2009, 42, 270–276. [Google Scholar] [CrossRef]
- Amidfar, M.; de Oliveira, J.; Kucharska, E.; Budni, J.; Kim, Y.-K. The role of CREB and BDNF in neurobiology and treatment of Alzheimer’s disease. Life Sci. 2020, 257, 118020. [Google Scholar] [CrossRef]
- Edman, S.; Horwath, O.; Van der Stede, T.; Blackwood, S.J.; Moberg, I.; Strömlind, H.; Nordström, F.; Ekblom, M.; Katz, A.; Apró, W.; et al. Pro-Brain-Derived Neurotrophic Factor (BDNF), but Not Mature BDNF, Is Expressed in Human Skeletal Muscle: Implications for Exercise-Induced Neuroplasticity. Function 2024, 5, zqae005. [Google Scholar] [CrossRef]
- Håkansson, K.; Ledreux, A.; Daffner, K.; Terjestam, Y.; Bergman, P.; Carlsson, R.; Kivipelto, M.; Winblad, B.; Granholm, A.-C.; Mohammed, A.K.H. BDNF Responses in Healthy Older Persons to 35 Minutes of Physical Exercise, Cognitive Training, and Mindfulness: Associations with Working Memory Function. J. Alzheimer’s Dis. 2017, 55, 645–657. [Google Scholar] [CrossRef]
- Azevedo, L.V.D.S.; Pereira, J.R.; Silva Santos, R.M.; Rocha, N.P.; Teixeira, A.L.; Christo, P.P.; Santos, V.R.; Scalzo, P.L. Acute exercise increases BDNF serum levels in patients with Parkinson’s disease regardless of depression or fatigue. Eur. J. Sport. Sci. 2022, 22, 1296–1303. [Google Scholar] [CrossRef]
- Wagner, G.; Herbsleb, M.; de la Cruz, F.; Schumann, A.; Brünner, F.; Schachtzabel, C.; Gussew, A.; Puta, C.; Smesny, S.; Gabriel, H.W.; et al. Hippocampal structure, metabolism, and inflammatory response after a 6-week intense aerobic exercise in healthy young adults: A controlled trial. J. Cereb. Blood Flow. Metab. 2015, 35, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ibrahimi, O.A.; Olsen, S.K.; Umemori, H.; Mohammadi, M.; Ornitz, D.M. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J. Biol. Chem. 2006, 281, 15694–15700. [Google Scholar] [CrossRef] [PubMed]
- Oulion, S.; Bertrand, S.; Escriva, H. Evolution of the FGF Gene Family. Int. J. Evol. Biol. 2012, 2012, 298147. [Google Scholar] [CrossRef]
- Hamrick, M.W.; McNeil, P.L.; Patterson, S.L. Role of muscle-derived growth factors in bone formation. J. Musculoskelet. Neuronal Interact. 2010, 10, 64–70. [Google Scholar] [PubMed]
- Brenner, D.R.; Ruan, Y.; Adams, S.C.; Courneya, K.S.; Friedenreich, C.M. The impact of exercise on growth factors (VEGF and FGF2): Results from a 12-month randomized intervention trial. Eur. Rev. Aging Phys. Act. 2019, 16, 8. [Google Scholar] [CrossRef]
- Romanello, V. The Interplay between Mitochondrial Morphology and Myomitokines in Aging Sarcopenia. Int. J. Mol. Sci. 2020, 22, 91. [Google Scholar] [CrossRef]
- Garneau, L.; Aguer, C. Role of myokines in the development of skeletal muscle insulin resistance and related metabolic defects in type 2 diabetes. Diabetes Metab. 2019, 45, 505–516. [Google Scholar] [CrossRef]
- Ji, M.; Cho, C.; Lee, S. Acute effect of exercise intensity on circulating FGF-21, FSTL-1, cathepsin B, and BDNF in young men. J. Exerc. Sci. Fit. 2024, 22, 51–58. [Google Scholar] [CrossRef]
- Cuevas-Ramos, D.; Almeda-Valdés, P.; Meza-Arana, C.E.; Brito-Córdova, G.; Gómez-Pérez, F.J.; Mehta, R.; Oseguera-Moguel, J.; Aguilar-Salinas, C.A. Exercise increases serum fibroblast growth factor 21 (FGF21) levels. PLoS ONE 2012, 7, e38022. [Google Scholar] [CrossRef]
- Taniguchi, H.; Tanisawa, K.; Sun, X.; Kubo, T.; Higuchi, M. Endurance Exercise Reduces Hepatic Fat Content and Serum Fibroblast Growth Factor 21 Levels in Elderly Men. J. Clin. Endocrinol. Metab. 2016, 101, 191–198. [Google Scholar] [CrossRef]
- Chen, H.-T.; Chung, Y.-C.; Chen, Y.-J.; Ho, S.-Y.; Wu, H.-J. Effects of Different Types of Exercise on Body Composition, Muscle Strength, and IGF-1 in the Elderly with Sarcopenic Obesity. J. Am. Geriatr. Soc. 2017, 65, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Gannon, N.P.; Vaughan, R.A.; Garcia-Smith, R.; Bisoffi, M.; Trujillo, K.A. Effects of the exercise-inducible myokine irisin on malignant and non-malignant breast epithelial cell behavior in vitro. Int. J. Cancer 2015, 136, E197–E202. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xiang, G.; Liu, M.; Mei, W.; Xiang, L.; Dong, J. Irisin protects against endothelial injury and ameliorates atherosclerosis in apolipoprotein E-Null diabetic mice. Atherosclerosis 2015, 243, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Dong, Y.; Dong, Y.; Chen, F.; Mitch, W.E.; Zhang, L. Inhibition of myostatin in mice improves insulin sensitivity via irisin-mediated cross talk between muscle and adipose tissues. Int. J. Obes. 2016, 40, 434–442. [Google Scholar] [CrossRef]
- Bozinovski, S.; Seow, H.J.; Crack, P.J.; Anderson, G.P.; Vlahos, R. Glutathione peroxidase-1 primes pro-inflammatory cytokine production after LPS challenge in vivo. PLoS ONE 2012, 7, e33172. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I. Irisin acts as a regulator of macrophages host defense. Life Sci. 2017, 176, 21–25. [Google Scholar] [CrossRef]
- Wang, J.-S.; Ho, F.-M.; Kang, H.-C.; Lin, W.-W.; Huang, K.-C. Celecoxib induces heme oxygenase-1 expression in macrophages and vascular smooth muscle cells via ROS-dependent signaling pathway. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 159–168. [Google Scholar] [CrossRef]
- Borgia, F.; Li Pomi, F.; Vaccaro, M.; Alessandrello, C.; Papa, V.; Gangemi, S. Oxidative Stress and Phototherapy in Atopic Dermatitis: Mechanisms, Role, and Future Perspectives. Biomolecules 2022, 12, 1904. [Google Scholar] [CrossRef]
- Thirupathi, A.; Gu, Y.; Pinho, R.A. Exercise Cuts Both Ways with ROS in Remodifying Innate and Adaptive Responses: Rewiring the Redox Mechanism of the Immune System during Exercise. Antioxidants 2021, 10, 1846. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I.; Kozlowska, K.; Pochec, E.; Bilski, J.; Brzozowski, T. Myokine irisin-induced protection against oxidative stress in vitro. Involvement of heme oxygenase-1 and antioxidazing enzymes superoxide dismutase-2 and glutathione peroxidase. J. Physiol. Pharmacol. 2018, 69, 117–125. [Google Scholar]
- Mazur-Bialy, A.I.; Pocheć, E. The Time-Course of Antioxidant Irisin Activity: Role of the Nrf2/HO-1/HMGB1 Axis. Antioxidants 2021, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Wang, J.; Lin, D.; Ding, Z. The immunomodulatory role of irisin on osteogenesis via AMPK-mediated macrophage polarization. Int. J. Biol. Macromol. 2020, 146, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Li, H.; Zhang, T.; Yang, L.; Yao, S.; Chen, S.; Zheng, M.; Zhao, Q.; Tian, H. Irisin protects macrophages from oxidized low density lipoprotein-induced apoptosis by inhibiting the endoplasmic reticulum stress pathway. Saudi J. Biol. Sci. 2018, 25, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, J.; Wang, C.; Shao, J.; Lai, Z.; Yu, X.; Du, F.; Gao, R.; Wang, J.; Liu, B. Irisin ameliorates nicotine-mediated atherosclerosis via inhibition of the PI3K pathway. Ann. Transl. Med. 2021, 9, 805. [Google Scholar] [CrossRef]
- Bustamante, M.; Fernández-Verdejo, R.; Jaimovich, E.; Buvinic, S. Electrical stimulation induces IL-6 in skeletal muscle through extracellular ATP by activating Ca(2+) signals and an IL-6 autocrine loop. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E869–E882. [Google Scholar] [CrossRef]
- Kawanishi, N.; Mizokami, T.; Niihara, H.; Yada, K.; Suzuki, K. Macrophage depletion by clodronate liposome attenuates muscle injury and inflammation following exhaustive exercise. Biochem. Biophys. Rep. 2016, 5, 146–151. [Google Scholar] [CrossRef]
- Tominaga, T.; Ma, S.; Saitou, K.; Suzuki, K. Glucose Ingestion Inhibits Endurance Exercise-Induced IL-6 Producing Macrophage Infiltration in Mice Muscle. Nutrients 2019, 11, 1496. [Google Scholar] [CrossRef]
- Carson, J.A.; Baltgalvis, K.A. Interleukin 6 as a key regulator of muscle mass during cachexia. Exerc. Sport. Sci. Rev. 2010, 38, 168–176. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Behrmann, I.; Müller-Newen, G.; Schaper, F.; Graeve, L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem. J. 1998, 334 Pt 2, 297–314. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rane, M. Interleukin-6 Signaling and Anti-Interleukin-6 Therapeutics in Cardiovascular Disease. Circ. Res. 2021, 128, 1728–1746. [Google Scholar] [CrossRef]
- Peng, L.; Zhong, J.; Xiao, Y.; Wang, B.; Li, S.; Deng, Y.; He, D.; Yuan, J. Therapeutic effects of an anti-IL-6 antibody in fungal keratitis: Macrophage inhibition and T cell subset regulation. Int. Immunopharmacol. 2020, 85, 106649. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Guo, P.; Wang, P.; Wang, W.; Liu, J. IL-6/ERK signaling pathway participates in type I IFN-programmed, unconventional M2-like macrophage polarization. Sci. Rep. 2023, 13, 1827. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Chang, P.-Y.; Chen, J.-Y.; Wu, B.-S.; Yang, A.-H.; Lee, O.K.-S. Adipose-derived mesenchymal stem cells attenuate dialysis-induced peritoneal fibrosis by modulating macrophage polarization via interleukin-6. Stem Cell Res. Ther. 2021, 12, 193. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Yang, C.; Cheng, Y.; Wang, J.; Zhang, S.; Yan, S.; He, F.; Yin, T.; Yang, J. Trophoblast-derived IL-6 serves as an important factor for normal pregnancy by activating Stat3-mediated M2 macrophages polarization. Int. Immunopharmacol. 2021, 90, 106788. [Google Scholar] [CrossRef] [PubMed]
- Gou, X.; Xu, W.; Liu, Y.; Peng, Y.; Xu, W.; Yin, Y.; Zhang, X. IL-6 Prevents Lung Macrophage Death and Lung Inflammation Injury by Inhibiting GSDME- and GSDMD-Mediated Pyroptosis during Pneumococcal Pneumosepsis. Microbiol. Spectr. 2022, 10, e0204921. [Google Scholar] [CrossRef] [PubMed]
- Valença-Pereira, F.; Fang, Q.; Marié, I.J.; Giddings, E.L.; Fortner, K.A.; Yang, R.; Villarino, A.V.; Huang, Y.H.; Frank, D.A.; Wen, H.; et al. IL-6 enhances CD4 cell motility by sustaining mitochondrial Ca2+ through the noncanonical STAT3 pathway. Proc. Natl. Acad. Sci. USA 2021, 118, e2103444118. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Jones, L.L.; Geiger, T.L. IL-6 Promotes T Cell Proliferation and Expansion under Inflammatory Conditions in Association with Low-Level RORγt Expression. J. Immunol. 2018, 201, 2934–2946. [Google Scholar] [CrossRef] [PubMed]
- Hop, H.T.; Huy, T.X.N.; Reyes, A.W.B.; Arayan, L.T.; Vu, S.H.; Min, W.; Lee, H.J.; Kang, C.K.; Kim, D.H.; Tark, D.S.; et al. Interleukin 6 Promotes Brucella abortus Clearance by Controlling Bactericidal Activity of Macrophages and CD8+ T Cell Differentiation. Infect. Immun. 2019, 87, e00431-19. [Google Scholar] [CrossRef]
- Liu, K.; He, K.; Xue, T.; Liu, P.; Xu, L.X. The cryo-thermal therapy-induced IL-6-rich acute pro-inflammatory response promoted DCs phenotypic maturation as the prerequisite to CD4+ T cell differentiation. Int. J. Hyperth. 2018, 34, 261–272. [Google Scholar] [CrossRef]
- Yu, S.; Li, Q.; Wang, Y.; Cui, Y.; Yu, Y.; Li, W.; Liu, F.; Liu, T. Tumor-derived LIF promotes chemoresistance via activating tumor-associated macrophages in gastric cancers. Exp. Cell Res. 2021, 406, 112734. [Google Scholar] [CrossRef]
- Jung, M.; Ma, Y.; Iyer, R.P.; DeLeon-Pennell, K.Y.; Yabluchanskiy, A.; Garrett, M.R.; Lindsey, M.L. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic. Res. Cardiol. 2017, 112, 33. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Wehling-Henricks, M.; Villalta, S.A.; Wang, Y.; Tidball, J.G. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J. Immunol. 2012, 189, 3669–3680. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Yang, K.; Kim, M.; Kim, H.-S.; Kang, J.L. Apoptosis inhibitor of macrophage (AIM) contributes to IL-10-induced anti-inflammatory response through inhibition of inflammasome activation. Cell Death Dis. 2021, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zhao, K.; Zhu, J.; Wang, Y.; Sun, P.; Yang, Q.; Zhang, T.; Han, W.; Hu, W.; Yang, W.; et al. Sarsasapogenin Suppresses RANKL-Induced Osteoclastogenesis in vitro and Prevents Lipopolysaccharide-Induced Bone Loss in vivo. Drug Des. Dev. Ther. 2020, 14, 3435–3447. [Google Scholar] [CrossRef]
- Piao, H.; Fu, L.; Wang, Y.; Liu, Y.; Wang, Y.; Meng, X.; Yang, D.; Xiao, X.; Zhang, J. A positive feedback loop between gastric cancer cells and tumor-associated macrophage induces malignancy progression. J. Exp. Clin. Cancer Res. 2022, 41, 174. [Google Scholar] [CrossRef]
- Rousset, F.; Garcia, E.; Defrance, T.; Péronne, C.; Vezzio, N.; Hsu, D.H.; Kastelein, R.; Moore, K.W.; Banchereau, J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc. Natl. Acad. Sci. USA 1992, 89, 1890–1893. [Google Scholar] [CrossRef]
- Victora, G.D.; Nussenzweig, M.C. Germinal Centers. Annu. Rev. Immunol. 2022, 40, 413–442. [Google Scholar] [CrossRef]
- Choe, J.; Choi, Y.S. IL-10 interrupts memory B cell expansion in the germinal center by inducing differentiation into plasma cells. Eur. J. Immunol. 1998, 28, 508–515. [Google Scholar] [CrossRef]
- Singh, K.; Chang, C.; Gershwin, M.E. IgA deficiency and autoimmunity. Autoimmun. Rev. 2014, 13, 163–177. [Google Scholar] [CrossRef]
- Burdin, N.; Van Kooten, C.; Galibert, L.; Abrams, J.S.; Wijdenes, J.; Banchereau, J.; Rousset, F. Endogenous IL-6 and IL-10 contribute to the differentiation of CD40-activated human B lymphocytes. J. Immunol. 1995, 154, 2533–2544. [Google Scholar] [CrossRef]
- Brière, F.; Bridon, J.M.; Chevet, D.; Souillet, G.; Bienvenu, F.; Guret, C.; Martinez-Valdez, H.; Banchereau, J. Interleukin 10 induces B lymphocytes from IgA-deficient patients to secrete IgA. J. Clin. Investig. 1994, 94, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Ma, R.; Yang, S.; Fan, T.; Cao, J.; Wang, Y.; Ma, W.; Yang, W.; Wang, F.; et al. IL-10 enhances T cell survival and is associated with faster relapse in patients with inactive ulcerative colitis. Mol. Immunol. 2020, 121, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Yogev, N.; Bedke, T.; Kobayashi, Y.; Brockmann, L.; Lukas, D.; Regen, T.; Croxford, A.L.; Nikolav, A.; Hövelmeyer, N.; von Stebut, E.; et al. CD4+ T-cell-derived IL-10 promotes CNS inflammation in mice by sustaining effector T cell survival. Cell Rep. 2022, 38, 110565. [Google Scholar] [CrossRef] [PubMed]
- Judge, S.J.; Darrow, M.A.; Thorpe, S.W.; Gingrich, A.A.; O’Donnell, E.F.; Bellini, A.R.; Sturgill, I.R.; Vick, L.V.; Dunai, C.; Stoffel, K.M.; et al. Analysis of tumor-infiltrating NK and T cells highlights IL-15 stimulation and TIGIT blockade as a combination immunotherapy strategy for soft tissue sarcomas. J. Immunother. Cancer 2020, 8, e001355. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, H.; Zhou, J.; Xu, S.; Gao, Y. Treatment with Recombinant Interleukin-15 (IL-15) Increases the Number of T Cells and Natural Killer (NK) Cells and Levels of Interferon-γ (IFN-γ) in a Rat Model of Sepsis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 4450–4456. [Google Scholar] [CrossRef]
- Watkinson, F.; Nayar, S.K.; Rani, A.; Sakellariou, C.A.; Elhage, O.; Papaevangelou, E.; Dasgupta, P.; Galustian, C. IL-15 Upregulates Telomerase Expression and Potently Increases Proliferative Capacity of NK, NKT-Like, and CD8 T Cells. Front. Immunol. 2020, 11, 594620. [Google Scholar] [CrossRef]
- Dubois, S.P.; Miljkovic, M.D.; Fleisher, T.A.; Pittaluga, S.; Hsu-Albert, J.; Bryant, B.R.; Petrus, M.N.; Perera, L.P.; Müller, J.R.; Shih, J.H.; et al. Short-course IL-15 given as a continuous infusion led to a massive expansion of effective NK cells: Implications for combination therapy with antitumor antibodies. J. Immunother. Cancer 2021, 9, e002193. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, H.; Kim, J.H.; Kim, S.-Y.; Koh, J.-Y.; Sa, M.; Park, S.-H.; Shin, E.-C. CD5 Suppresses IL-15-Induced Proliferation of Human Memory CD8 T Cells by Inhibiting mTOR Pathways. J. Immunol. 2022, 209, 1108–1117. [Google Scholar] [CrossRef]
- Chen, X.; Guo, W.; Chang, Y.; Chen, J.; Kang, P.; Yi, X.; Cui, T.; Guo, S.; Xiao, Q.; Jian, Z.; et al. Oxidative stress-induced IL-15 trans-presentation in keratinocytes contributes to CD8 T cells activation via JAK-STAT pathway in vitiligo. Free Radic. Biol. Med. 2019, 139, 80–91. [Google Scholar] [CrossRef]
- Berrien-Elliott, M.M.; Becker-Hapak, M.; Cashen, A.F.; Jacobs, M.; Wong, P.; Foster, M.; McClain, E.; Desai, S.; Pence, P.; Cooley, S.; et al. Systemic IL-15 promotes allogeneic cell rejection in patients treated with natural killer cell adoptive therapy. Blood 2022, 139, 1177–1183. [Google Scholar] [CrossRef]
- Patsoukis, N.; Brown, J.; Petkova, V.; Liu, F.; Li, L.; Boussiotis, V.A. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci. Signal. 2012, 5, ra46. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Inoue, S.; Yamashita, K.; Kakeji, Y.; Fukumoto, T.; Kotani, J. IL-15 Improves Aging-Induced Persistent T Cell Exhaustion in Mouse Models of Repeated Sepsis. Shock 2020, 53, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Takeda, K.; Ouhara, K.; Shirawachi, S.; Kajiya, M.; Matsuda, S.; Kono, S.; Shiba, H.; Kurihara, H.; Mizuno, N. Involvement of Rac1 in macrophage activation by brain-derived neurotrophic factor. Mol. Biol. Rep. 2021, 48, 5249–5257. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.-C.; Dang, Y.-Y.; Gao, H.-Y.; Wang, Z.-T.; Gao, M.; Yang, Y.; Zhang, H.-T.; Xu, R.-X. Local Injection of Lenti-BDNF at the Lesion Site Promotes M2 Macrophage Polarization and Inhibits Inflammatory Response After Spinal Cord Injury in Mice. Cell. Mol. Neurobiol. 2015, 35, 881–890. [Google Scholar] [CrossRef]
- Bi, C.; Fu, Y.; Li, B. Brain-derived neurotrophic factor alleviates diabetes mellitus-accelerated atherosclerosis by promoting M2 polarization of macrophages through repressing the STAT3 pathway. Cell. Signal. 2020, 70, 109569. [Google Scholar] [CrossRef]
- Bi, C.; Fu, Y.; Zhang, Z.; Li, B. Prostaglandin E2 confers protection against diabetic coronary atherosclerosis by stimulating M2 macrophage polarization via the activation of the CREB/BDNF/TrkB signaling pathway. FASEB J. 2020, 34, 7360–7371. [Google Scholar] [CrossRef]
- Chen, L.; Fu, L.; Sun, J.; Huang, Z.; Fang, M.; Zinkle, A.; Liu, X.; Lu, J.; Pan, Z.; Wang, Y.; et al. Structural basis for FGF hormone signalling. Nature 2023, 618, 862–870. [Google Scholar] [CrossRef]
- Ruan, R.; Li, L.; Li, X.; Huang, C.; Zhang, Z.; Zhong, H.; Zeng, S.; Shi, Q.; Xia, Y.; Zeng, Q.; et al. Unleashing the potential of combining FGFR inhibitor and immune checkpoint blockade for FGF/FGFR signaling in tumor microenvironment. Mol. Cancer 2023, 22, 60. [Google Scholar] [CrossRef]
- Im, J.H.; Buzzelli, J.N.; Jones, K.; Franchini, F.; Gordon-Weeks, A.; Markelc, B.; Chen, J.; Kim, J.; Cao, Y.; Muschel, R.J. FGF2 alters macrophage polarization, tumour immunity and growth and can be targeted during radiotherapy. Nat. Commun. 2020, 11, 4064. [Google Scholar] [CrossRef]
- Qin, S.; Wang, Z.; Huang, C.; Huang, P.; Li, D. Serine protease PRSS23 drives gastric cancer by enhancing tumor associated macrophage infiltration FGF2. Front. Immunol. 2022, 13, 955841. [Google Scholar] [CrossRef]
- Yu, Y.; He, J.; Li, S.; Song, L.; Guo, X.; Yao, W.; Zou, D.; Gao, X.; Liu, Y.; Bai, F.; et al. Fibroblast growth factor 21 (FGF21) inhibits macrophage-mediated inflammation by activating Nrf2 and suppressing the NF-κB signaling pathway. Int. Immunopharmacol. 2016, 38, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, F.; Zhu, L.; Lin, P.; Han, F.; Wang, X.; Tan, X.; Lin, L.; Xiong, Y. FGF21 alleviates neuroinflammation following ischemic stroke by modulating the temporal and spatial dynamics of microglia/macrophages. J. Neuroinflam. 2020, 17, 257. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhao, T.-T.; Li, S.-M.; Li, Y.-H.; Wang, Y.-J.; Li, D.-S.; Wang, W.-F. Fibroblast growth factor 21 ameliorates pancreatic fibrogenesis via regulating polarization of macrophages. Exp. Cell Res. 2019, 382, 111457. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Xia, A.; Meng, F.; Chunyu, J.; Sun, X.; Ren, G.; Yu, D.; Jiang, X.; Tang, L.; Xiao, W.; et al. FGF21 alleviates chronic inflammatory injury in the aging process through modulating polarization of macrophages. Int. Immunopharmacol. 2021, 96, 107634. [Google Scholar] [CrossRef]
- Nederlof, R.; Reidel, S.; Spychala, A.; Gödecke, S.; Heinen, A.; Lautwein, T.; Petzsch, P.; Köhrer, K.; Gödecke, A. Insulin-Like Growth Factor 1 Attenuates the Pro-Inflammatory Phenotype of Neutrophils in Myocardial Infarction. Front. Immunol. 2022, 13, 908023. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- O’Riordan, C.; Clifford, A.; Van De Ven, P.; Nelson, J. Chronic neck pain and exercise interventions: Frequency, intensity, time, and type principle. Arch. Phys. Med. Rehabil. 2014, 95, 770–783. [Google Scholar] [CrossRef]
- Rao, J.; Wang, H.; Ni, M.; Wang, Z.; Wang, Z.; Wei, S.; Liu, M.; Wang, P.; Qiu, J.; Zhang, L.; et al. FSTL1 promotes liver fibrosis by reprogramming macrophage function through modulating the intracellular function of PKM2. Gut 2022, 71, 2539–2550. [Google Scholar] [CrossRef]
- Li, G.; Ren, H.; Wu, X.; Hu, Q.; Hong, Z.; Wang, G.; Gu, G.; Ren, J.; Li, J. Follistatin like protein-1 modulates macrophage polarization and aggravates dextran sodium sulfate-induced colitis. Int. Immunopharmacol. 2020, 83, 106456. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Luo, J.; Xie, T.; Mor, G.; Liao, A. Decorin promotes decidual M1-like macrophage polarization via mitochondrial dysfunction resulting in recurrent pregnancy loss. Theranostics 2022, 12, 7216–7236. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Vachliotis, I.D.; Mantzoros, C.S. Sarcopenia, sarcopenic obesity and nonalcoholic fatty liver disease. Metabolism 2023, 147, 155676. [Google Scholar] [CrossRef]
- Ragland, T.J.; Malin, S.K. Exercise increases TCA intermediate concentrations during low-calorie diet independent of insulin resistance among women with obesity. Physiol. Rep. 2024, 12, e15987. [Google Scholar] [CrossRef]
| Myokines | Model | Stimulation | Myokine Alteration | Physical Signs | Ref. |
|---|---|---|---|---|---|
| Irisin | Mice | Swimming training (30 min/day, for 3 days/week for 8 weeks) | Irisin ↑ in muscle | Reduced the oxidative stress index (OSI), degeneration in the heart muscle, inflammation and cardiopathy | [46] |
| Mice | Treadmill exercise with moderate intensity (5 days/week for 8 weeks) | Irisin ↑ in muscle | Increased bone mineral density of trabecular bone in mice | [47] | |
| Rat | Swimming training (60 min/day, for 7 days/week for 8 weeks) | Irisin ↑ in serum | Effectively improved bone health caused by obesity | [48] | |
| Mice | Aerobic exercise, Resistance exercise, Vibration exercise, The Electrical stimulation (60 min/day, for 5 days/week for 4 weeks) | Irisin/FNDC5 ↑ in muscle | Promoted mitochondrial autophagy, improving heart function and resisting exercise. | [49] | |
| Human | Cycling on stationary bikes (20–35 min/week for 10 weeks) | Irisin ↑ in plasma | Improved glucose homeostasis and caused a small weight loss | [50] | |
| Human | Progressive resistance training (1 h/day, for 2 days/week for 12 weeks) | Irisin ↑ in serum | Increased grip strengthand leg strength | [51] | |
| Human | Strength and endurance training intervention (60 min/day, for 2 days/week for 12 weeks) | Irisin ↑ in serum | Not described | [52] | |
| Human | Moderate-intensity treadmill walking (180 min, 21.9 °C and 41.1 °C) | Irisin ↑ in serum | Reduced oxidative stress and inflammation | [53] | |
| Human | Winter swimming | Irisin ↓ in serum | Not described | [54] | |
| IL-6 | Human | Marathon | IL-6 ↑ in plasma | Not described | [55] |
| Rat | Treadmill running and ladder climbing (75 min/day, for 3 days/week for 12 weeks) | IL-6 ↑ in muscle | Helped reduce inflammation | [56] | |
| LIF | Rat | Interval exercise training (60 min/day, for 5 days/week for 8 weeks) | LIF ↑ in muscle | Reduced apoptosis and promoted proliferation in gastrocnemius muscle | [57] |
| Human | Heavy resistance exercise of 6–8 repetitions | LIF ↑ in muscle | Not described | [58] | |
| IL-10 | Rat | HIIT (Running with 8 m/min, 10 min/day for 5 days) | IL-10 ↑ in serum | The expression of pro-inflammatory factors was inhibited | [59] |
| Human | Physical activity of moderate intensity (12 weeks) | IL-10 ↑ in serum | Improvement of metabolic risk factors | [60] | |
| Human | Running 5 km intermittently; running 5 km continuously at 70% of MAS (determined in the incremental test) on the treadmill | IL-10 ↑ in serum | It effectively inhibits the progression of inflammatory response | [61] | |
| IL-15 | Human | A bilateral leg resistance exercise | IL-15 ↑ in muscle serum | Improved myofibrillar fractional synthetic rate | [62] |
| Human | High-intensity circuit training (HICT) (3 days/week for 5 weeks) | IL-15 ↑ in serum | Improved an insulin sensitivity | [63] | |
| Human | Moderate intensity exercise (60 min/day, for 3 days/week for 12 weeks) | IL-15 ↑ in serum | Decreased body fat | [64] | |
| BDNF | Mice | Swimming training (30 min/day, for 7 days/week for 12 weeks) | BDNF ↑ in cerebral cortex | Provided neuroprotective effects | [65] |
| Mice | Voluntary wheel-running exercise (30 days) | BDNF ↑ in striatum | Enhanced striatal dopamine (DA) release | [66] | |
| Mice | Rat cages equipped with running Wheels (3 h/day for 2 weeks) | BDNF ↑ in hippocampal tissues | Improved cognition in Alzheimer’s disease (AD) | [67] | |
| Human | Aerobic exercise (35 min) | BDNF ↑ in serum | Improved cognition in Alzheimer’s disease (AD) | [68] | |
| Human | Walk on a treadmill at light to moderate intensity (30 min) | mBDNF ↑ in serum | Enhancement of neuroplasticity and facilitate the improvement of motor performance | [69] | |
| FGF2 | Rat | Continuous exercise training (15 min at 65% maximal speed for 1 week, 20 min for 2 weeks, 25 min for three weeks at 70% maximal speed, and 30 min for 4, 5, and 6 weeks at 70% maximal speed) | FGF2↑ in heart tissue | Delayed age-related myocardial fibrosis | [70] |
| Human | Aerobic exercise (300 min/week for 12 months) | FGF2 ↑ in serum | Reduced postmenopausal breast cancer risk | [71] | |
| FGF21 | Mice | Resistance training (10 repetitions/day, 3 days/week for 8 weeks | FGF21 ↑ in muscle | Improved muscle strength | [72] |
| Mice | Performed treadmill exercises at 30 m/min for 60 min | FGF21 ↑ in plasma and muscle | Not described | [73] | |
| Human | A treadmill exercise test (following the Bruce’s protocol) (5 days/week for 2 weeks) | FGF21 ↑ in serum | Increased glucose intake | [74] | |
| IGF-1 | Mice | Ladder climbing (85-degree incline, 1.5 cm spacing), utilizing progressive overload (twice a day, every third day for 16–18 weeks) | IGF-1 ↑ in muscle | Compensatory growth of muscle | [75] |
| Human | Resistance training (RT), aerobic training (AT), combination training (CT) (60 min/day, for 3 days/week for 8 weeks) | IGF-1 ↑ in serum | Increased muscle mass and reduced total fat mass and visceral fat area (VFA) | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Wang, Z.; Zhang, X.-A.; Ning, K. Myokines May Be the Answer to the Beneficial Immunomodulation of Tailored Exercise—A Narrative Review. Biomolecules 2024, 14, 1205. https://doi.org/10.3390/biom14101205
Lu Z, Wang Z, Zhang X-A, Ning K. Myokines May Be the Answer to the Beneficial Immunomodulation of Tailored Exercise—A Narrative Review. Biomolecules. 2024; 14(10):1205. https://doi.org/10.3390/biom14101205
Chicago/Turabian StyleLu, Zheng, Zhuo Wang, Xin-An Zhang, and Ke Ning. 2024. "Myokines May Be the Answer to the Beneficial Immunomodulation of Tailored Exercise—A Narrative Review" Biomolecules 14, no. 10: 1205. https://doi.org/10.3390/biom14101205
APA StyleLu, Z., Wang, Z., Zhang, X.-A., & Ning, K. (2024). Myokines May Be the Answer to the Beneficial Immunomodulation of Tailored Exercise—A Narrative Review. Biomolecules, 14(10), 1205. https://doi.org/10.3390/biom14101205






