Space Flight Enhances Stress Pathways in Human Neural Stem Cells
Abstract
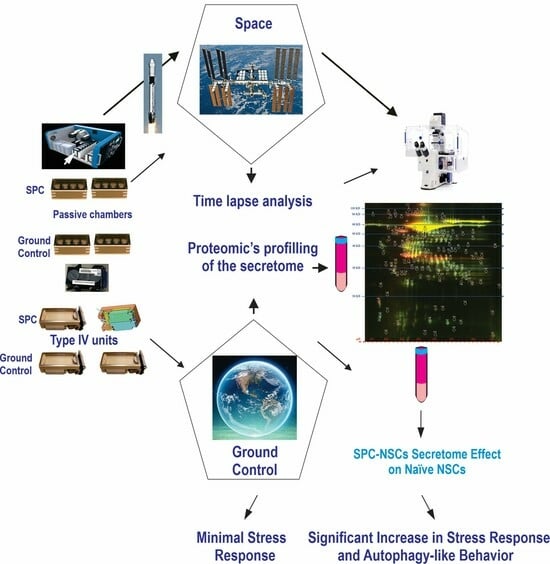
1. Introduction
2. Materials and Methods
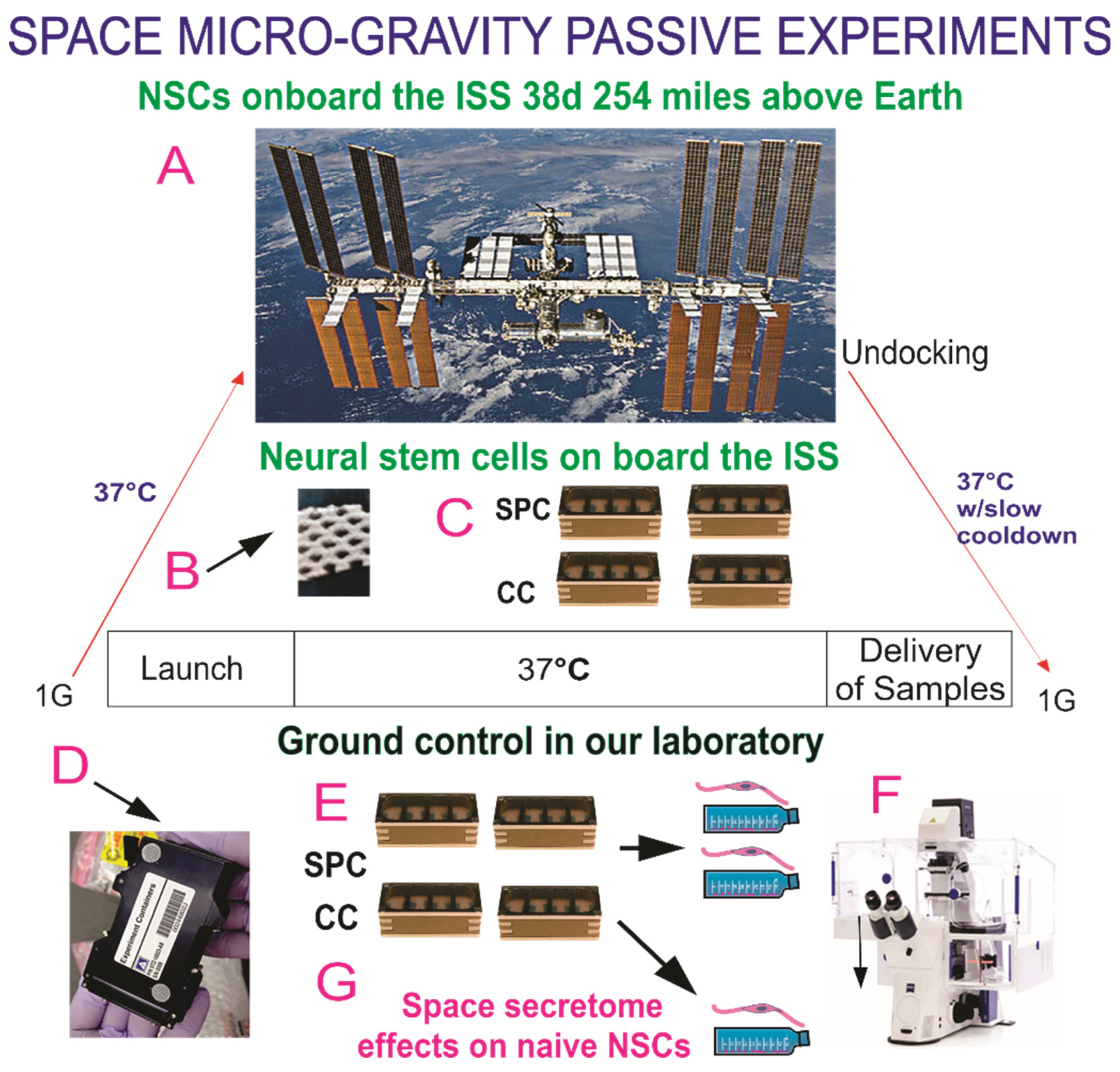
2.1. Cells and Culture System
2.2. Cultures of NSCs
2.3. Space Flight
2.4. Recovery of the Hardware and Harvesting of Samples
2.5. Time-Lapse Microscopy
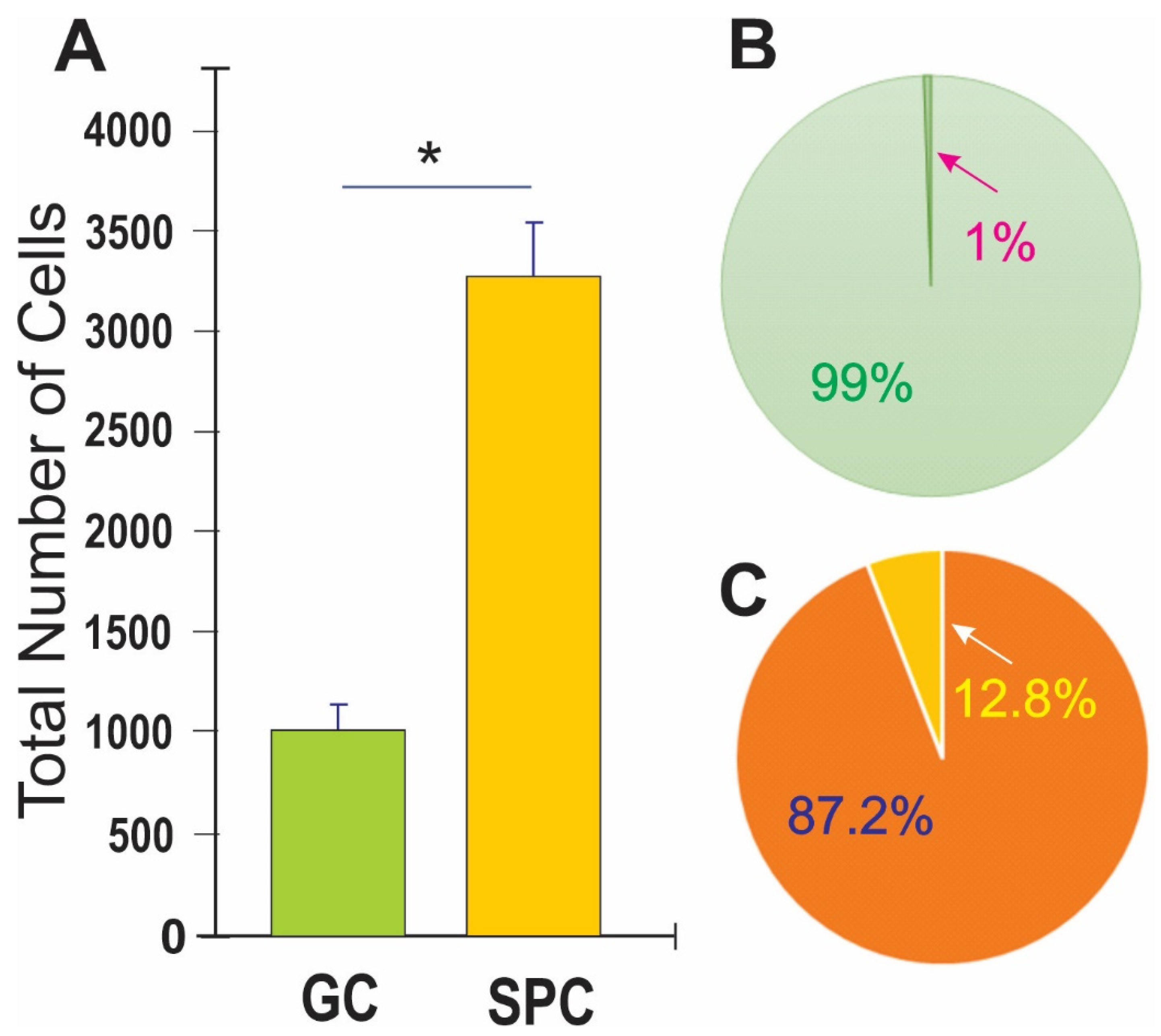
2.6. Cell Counts and Statistical Analysis
2.7. Secretome Collection and Proteomics Analysis
Two-Dimensional DIGE Preparation of Samples and Proteomic Analysis
3. Results
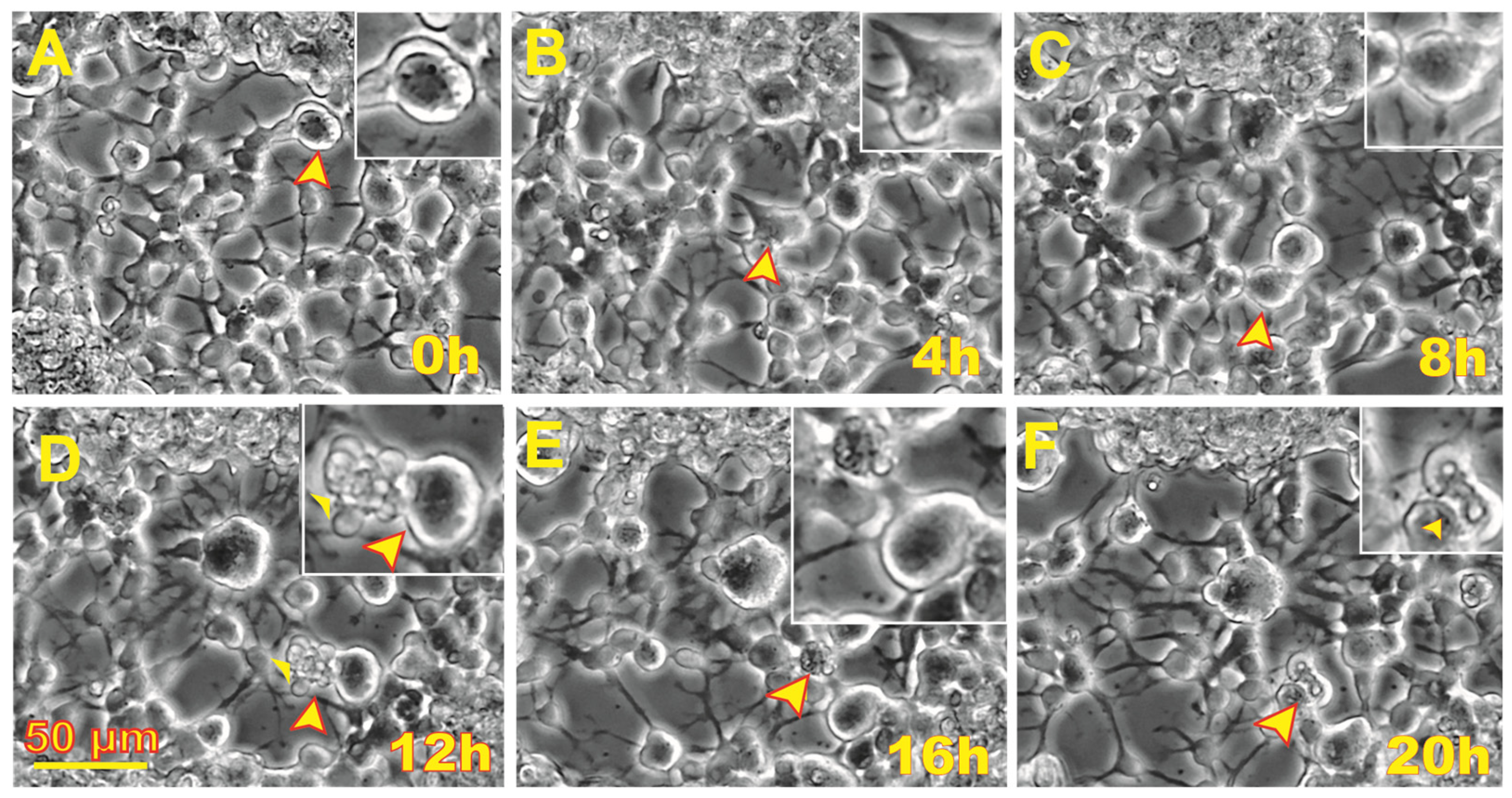
3.1. Post-Space Flight Observations of NSCs
3.2. Space Flight Activates Stress Responses and ALB in NSCs
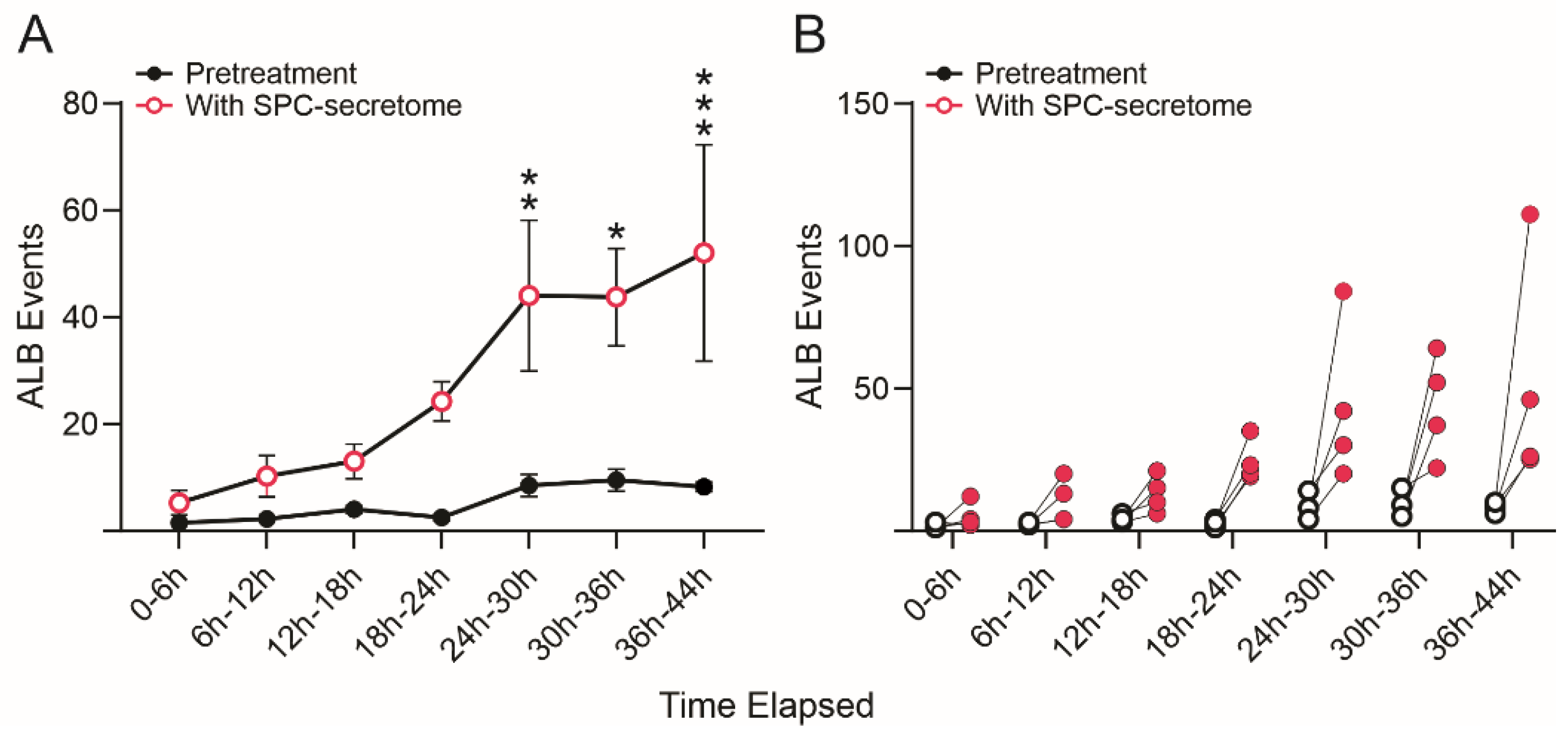
3.3. SPC-Flown NSCs’ Secretome Increases Naïve NSCs ALB
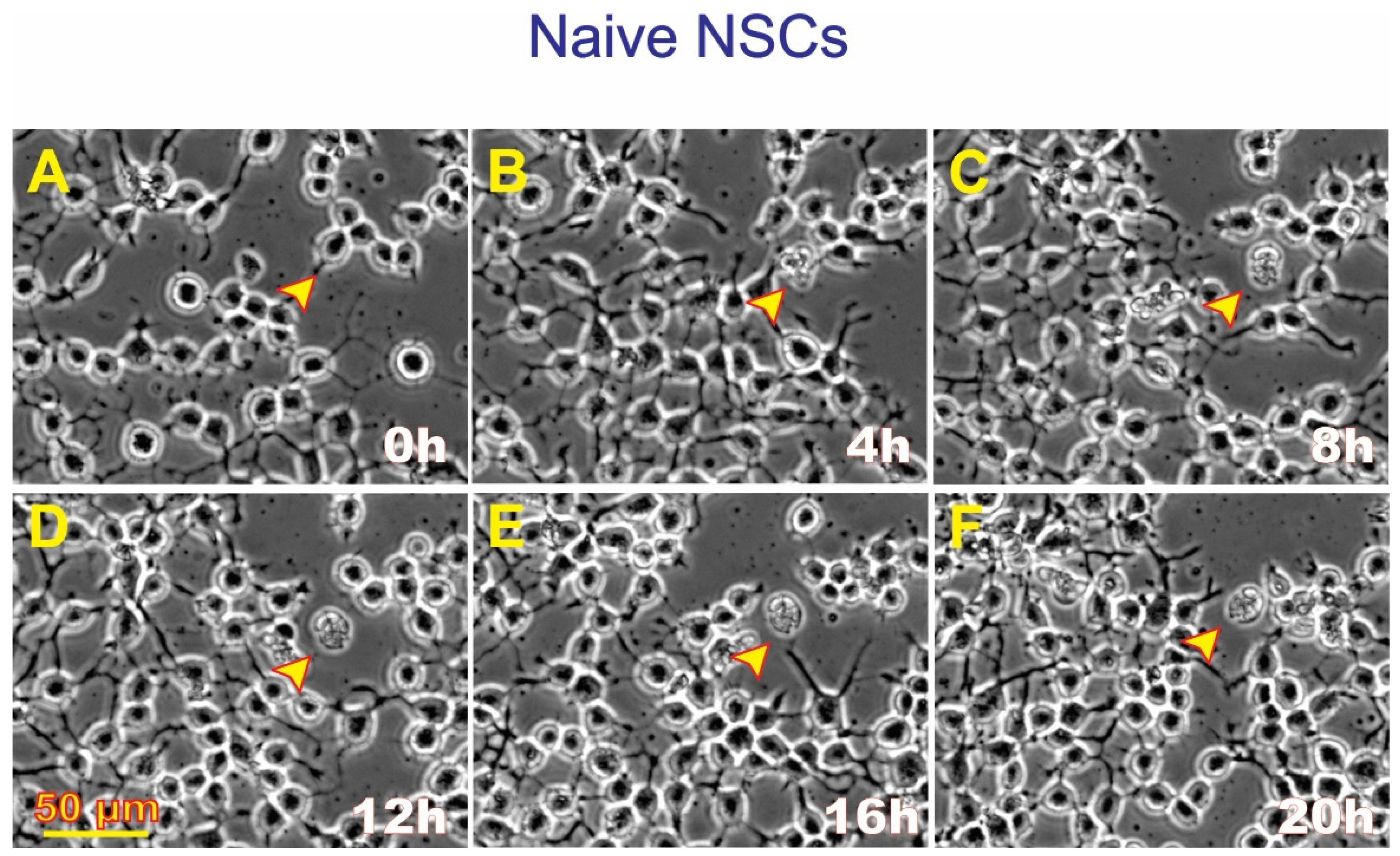
3.4. ALB also Occurs in Untreated, Naïve NSCs although to a Much Lesser Extent
3.5. Secretome Analysis
4. Discussion
4.1. Microgravity as a Potential Modulator of ALB
4.2. Microgravity as a Modulator of ALB Proteins
4.3. Space Flight and Radiation during SpX-16
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kramer, L.A.; Hasan, K.M.; Stenger, M.B.; Sargsyan, A.; Laurie, S.S.; Otto, C.; Ploutz-Snyder, R.J.; Marshall-Goebel, K.; Riascos, R.F.; Macias, B.R. Intracranial Effects of Microgravity: A Prospective Longitudinal MRI study. Radiology 2020, 295, 640–648. [Google Scholar] [CrossRef]
- Lee, J.K.; Koppelmans, V.; Riascos, R.F.; Hasan, K.M.; Pasternak, O.; Mulavara, A.P.; Bloomberg, J.J.; Seidler, R.D. Spaceflight-Associated Brain White Matter Microstructural Changes and Intracranial Fluid Redistribution. JAMA Neurol. 2019, 76, 412–419. [Google Scholar] [CrossRef]
- Cepeda, C.; Vergnes, L.; Carpo, N.; Schibler, M.J.; Bentolila, L.A.; Karouia, F.; Espinosa-Jeffrey, A. Human Neural Stem Cells Flown into Space Proliferate and Generate Young Neurons. Appl. Sci. 2019, 9, 4042. [Google Scholar] [CrossRef]
- Shaka, S.; Carpo, N.; Tran, V.; Ma, Y.-Y.; Karouia, F.; Espinosa-Jeffrey, A. Human Neural Stem Cells in Space Proliferate More than Ground Control Cells: Implications for Long-Term Space Travel. Stem Cell Res. 2021, 7, 69. [Google Scholar] [CrossRef]
- Kroemer, G.; Levine, B. Autophagic Cell Death: The Story of a Misnomer. Nat. Rev. Mol. Cell Biol. 2008, 9, 1004–1010. [Google Scholar] [CrossRef]
- Alkabie, S.; Basivireddy, J.; Zhou, L.; Roskams, J.; Rieckmann, P.; Quandt, J.A. SPARC Expression by Cerebral Microvascular Endothelial Cells in Vitro and Its Influence on Blood-Brain Barrier Properties. J. Neuroinflamm. 2016, 13, 225. [Google Scholar] [CrossRef]
- Bradshaw, A.D. The Role of SPARC in Extracellular Matrix Assembly. J. Cell Commun. Signal. 2009, 3, 239–246. [Google Scholar] [CrossRef]
- Denton, D.; Kumar, S. Autophagy-Dependent Cell Death. Cell Death Differ. 2019, 26, 605–616. [Google Scholar] [CrossRef]
- Nagaraju, G.P.; Dontula, R.; El-Rayes, B.F.; Lakka, S.S. Molecular Mechanisms Underlying the Divergent Roles of SPARC in Human Carcinogenesis. Carcinogenesis 2014, 35, 967–973. [Google Scholar] [CrossRef]
- Espinosa-Jeffrey, A.; Becker-Catania, S.; Zhao, P.M.; Cole, R.; de Vellis, J. Phenotype Specification and Development of Oligodendrocytes and Neurons from Rat Stem Cell Cultures Using Two Chemically Defined Media. J. Neurosci. Res. 2002, 69, 810–825. [Google Scholar] [PubMed]
- Espinosa-Jeffrey, A.; Wakeman, D.R.; Kim, S.U.; Snyder, E.Y.; de Vellis, J. Culture System for Rodent and Human Oligodendrocyte Specification, Lineage Progression, and Maturation. Curr. Protoc. Stem Cell Biol. 2009, 10, 2D.4.1–2D.4.26. [Google Scholar] [CrossRef]
- Espinosa-Jeffrey, A.; Blanchi, B.; Biancotti, J.C.; Kumar, S.; Hirose, M.; Mandefro, B.; Talavera-Adame, D.; Benvenisty, N.; de Vellis, J. Efficient Generation of Viral and Integration-Free Human Induced Pluripotent Stem Cell-Derived Oligodendrocytes. Curr. Protoc. Stem Cell Biol. 2016, 38, 2D.18.1–2D.18.27. [Google Scholar] [CrossRef]
- Shaka, S.; Carpo, N.; Tran, V.; Espinosa-Jeffrey, A. Behavior of Astrocytes Derived from Human Neural Stem Cells Flown onto Space and Their Progenies. Appl. Sci. 2021, 11, 41. [Google Scholar] [CrossRef]
- Tran, V.; Carpo, N.; Shaka, S.; Zamudio, J.; Choi, S.; Cepeda, C.; Espinosa-Jeffrey, A. Delayed Maturation of Oligodendrocyte Progenitors by Microgravity: Implications for Multiple Sclerosis and Space Flight. Life 2022, 12, 797. [Google Scholar] [CrossRef]
- Biancotti, J.C.; Carpo, N.; Zamudio, J.; Vergnes, L.; Espinosa-Jeffrey, A. Profiling the Secretome of Space Traveler Human Neural Stem Cells. HSOA J. Stem Cells Res. Dev. 2022, 8, 1–12. [Google Scholar] [CrossRef]
- Laggner, M.; Pollreisz, A.; Schmidinger, G.; Schmidt-Erfurth, U.; Chen, Y.-T. Autophagy Mediates Cell Cycle Response by Regulating Nucleocytoplasmic Transport of PAX6 in Limbal Stem Cells under Ultraviolet-A Stress. PLoS ONE 2017, 12, e0180868. [Google Scholar] [CrossRef]
- Wang, M.-M.; Feng, Y.-S.; Yang, S.-D.; Xing, Y.; Zhang, J.; Dong, F.; Zhang, F. The Relationship Between Autophagy and Brain Plasticity in Neurological Diseases. Front. Cell. Neurosci. 2019, 13, 228. [Google Scholar] [CrossRef]
- Ohsumi, Y. Molecular Dissection of Autophagy: Two Ubiquitin-like Systems. Nat. Rev. Mol. Cell Biol. 2001, 2, 211–216. [Google Scholar] [CrossRef]
- Ohsumi, T. Yoshinori Ohsumi: Autophagy from Beginning to End. Interview by Caitlin Sedwick. J. Cell Biol. 2012, 197, 164–165. [Google Scholar] [CrossRef]
- Noda, T.; Ohsumi, Y. Tor, a Phosphatidylinositol Kinase Homologue, Controls Autophagy in Yeast. J. Biol. Chem. 1998, 273, 3963–3966. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.; Kaushik, S.; Clement, C.C.; Cannizzo, E.S.; Scharf, B.; Follenzi, A.; Potolicchio, I.; Nieves, E.; Cuervo, A.M.; Santambrogio, L. Microautophagy of Cytosolic Proteins by Late Endosomes. Dev. Cell 2011, 20, 131–139. [Google Scholar] [CrossRef]
- Li, W.-W.; Li, J.; Bao, J.-K. Microautophagy: Lesser-known Self-eating. Cell Mol. Life Sci. 2012, 69, 1125–1136. [Google Scholar] [CrossRef]
- Vincent, J.A.; Kwong, T.J.; Tsukiyama, T. ATP-Dependent Chromatin Remodeling Shapes the DNA Replication Landscape. Nat. Struct. Mol. Biol. 2008, 15, 477–484. [Google Scholar] [CrossRef]
- Funk, S.E.; Sage, E.H. The Ca2+-Binding Glycoprotein SPARC Modulates Cell Cycle Progression in Bovine Aortic Endothelial Cells. Proc. Natl. Acad. Sci. USA 1991, 88, 2648–2652. [Google Scholar] [CrossRef]
- Funk, S.E.; Sage, E.H. Differential Effects of SPARC and Cationic SPARC Peptides on DNA Synthesis by Endothelial Cells and Fibroblasts. J. Cell. Physiol. 1993, 154, 53–63. [Google Scholar] [CrossRef]
- Sage, E.H. Terms of Attachment: SPARC and Tumorigenesis. Nat. Med. 1997, 3, 144–146. [Google Scholar] [CrossRef]
- Vadlamuri, S.V.; Media, J.; Sankey, S.S.; Nakeff, A.; Divine, G.; Rempel, S.A. SPARC Affects Glioma Cell Growth Differently When Grown on Brain ECM Proteins in Vitro under Standard versus Reduced-Serum Stress Conditions. Neuro-Oncol. 2003, 5, 244–254. [Google Scholar] [CrossRef]
- Carrara, M.; Prischi, F.; Nowak, P.R.; Kopp, M.C.; Ali, M.M. Noncanonical Binding of BiP ATPase Domain to Ire1 and Perk Is Dissociated by Unfolded Protein CH1 to Initiate ER Stress Signaling. eLife 2015, 4, e03522. [Google Scholar] [CrossRef]
- Sailaja, G.S.; Bhoopathi, P.; Gorantla, B.; Chetty, C.; Gogineni, V.R.; Velpula, K.K.; Gondi, C.S.; Rao, J.S. The Secreted Protein Acidic and Rich in Cysteine (SPARC) Induces Endoplasmic Reticulum Stress Leading to Autophagy-Mediated Apoptosis in Neuroblastoma. Int. J. Oncol. 2013, 42, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zou, Q.; Guo, Q.; Chen, Y.; Kuang, X.; Zhang, Y.; Liu, Y.; Wu, W.; Li, G.; Tu, L.; et al. SPARC Knockdown Reduces Glutamate-Induced HT22 Hippocampal Nerve Cell Damage by Regulating Autophagy. Front. Neurosci. 2021, 14, 581441. [Google Scholar] [CrossRef] [PubMed]
- Bhoopathi, P.; Chetty, C.; Gujrati, M.; Dinh, D.H.; Rao, J.S.; Lakka, S. Cathepsin B Facilitates Autophagy-Mediated Apoptosis in SPARC Overexpressed Primitive Neuroectodermal Tumor Cells. Cell Death Differ. 2010, 17, 1529–1539. [Google Scholar] [CrossRef]
- Pannuru, P.; Dontula, R.; Khan, A.A.; Herbert, E.; Ozer, H.; Chetty, C.; Lakka, S.S. MiR-Let-7f-1 Regulates SPARC Mediated Cisplatin Resistance in Medulloblastoma Cells. Cell. Signal. 2014, 26, 2193–2201. [Google Scholar] [CrossRef]
- Biskou, O.; Casanova, V.; Hooper, K.M.; Kemp, S.; Wright, G.P.; Satsangi, J.; Barlow, P.G.; Stevens, C. The Type III Intermediate Filament Vimentin Regulates Organelle Distribution and Modulates Autophagy. PLoS ONE 2019, 14, e0209665. [Google Scholar] [CrossRef]
- Kast, D.J.; Dominguez, R. The Cytoskeleton–Autophagy Connection. Curr. Biol. 2017, 27, R318–R326. [Google Scholar] [CrossRef]
- Shigyo, M.; Kuboyama, T.; Sawai, Y.; Tada-Umezaki, M.; Tohda, C. Extracellular vimentin interacts with insulin-like growth factor 1 receptor to promote axonal growth. Sci. Rep. 2015, 5, 12055. [Google Scholar] [CrossRef]
- van Beijnum, J.R.; Huijbers, E.J.; van Loon, K.; Blanas, A.; Akbari, P.; Roos, A.; Wong, T.J.; Denisov, S.S.; Hackeng, T.M.; Jimenez, C.R.; et al. Extracellular vimentin mimics VEGF and is a target for anti-angiogenic immunotherapy. Nat. Commun. 2022, 13, 2842. [Google Scholar] [CrossRef]
- Thalla, D.G.; Jung, P.; Bischoff, M.; Lautenschläger, F. Role of extracellular vimentin in cancer-cell functionality and its influence on cell monolayer permeability changes induced by SARS-CoV-2 receptor binding domain. Int. J. Mol. Sci. 2021, 22, 7469. [Google Scholar] [CrossRef]
- Chen, K.Z.; Liu, S.X.; Li, Y.W.; He, T.; Zhao, J.; Wang, T.; Qiu, X.X.; Wu, H.F. Vimentin as a potential target for diverse nervous system diseases. Neural Regen. Res. 2023, 18, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ma, Y.; Chen, S.; Guo, S.; Hu, J.; Yue, T.; Zhang, J.; Zhu, J.; Wang, P.; Chen, G.; et al. SPARC Enhances 5-FU Chemosensitivity in Gastric Cancer by Modulating Epithelial-Mesenchymal Transition and Apoptosis. Biochem. Biophys. Res. Commun. 2021, 558, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Fenouille, N.; Robert, G.; Tichet, M.; Puissant, A.; Dufies, M.; Rocchi, S.; Ortonne, J.-P.; Deckert, M.; Ballotti, R.; Tartare-Deckert, S. The P53/P21Cip1/Waf1 Pathway Mediates the Effects of SPARC on Melanoma Cell Cycle Progression. Pigment Cell Melanoma Res. 2011, 24, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.Y.; Wu, V.W.C.; Law, H.K.W. Influence of Autophagy on the Efficacy of Radiotherapy. Radiat. Oncol. 2017, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, S.; Pirtoli, L.; Tini, P.; Cevenini, G.; Calderaro, F.; Toscano, M.; Miracco, C.; Comincini, S. Different Involvement of Autophagy in Human Malignant Glioma Cell Lines Undergoing Irradiation and Temozolomide Combined Treatments. J. Cell. Biochem. 2012, 113, 2308–2318. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, S.M.; Moretti, L.; Varki, V.; Lu, B. Progress in the Unraveling of the Endoplasmic Reticulum Stress/Autophagy Pathway and Cancer: Implications for Future Therapeutic Approaches. Drug Resist. Updates 2010, 13, 79–86. [Google Scholar] [CrossRef]
- Nagelkerke, A.; Bussink, J.; Geurts-Moespot, A.; Sweep, F.C.G.J.; Span, P.N. Therapeutic Targeting of Autophagy in Cancer. Part II: Pharmacological Modulation of Treatment-Induced Autophagy. Semin. Cancer Biol. 2015, 31, 99–105. [Google Scholar] [CrossRef]
- Wickman, G.; Julian, L.; Olson, M.F. How Apoptotic Cells Aid in the Removal of Their Own Cold Dead Bodies. Cell Death Differ. 2012, 19, 735–742. [Google Scholar] [CrossRef]
- Tran, V.; Carpo, N.; Cepeda, C.; Espinosa-Jeffrey, A. Oligodendrocyte Progenitors Display Enhanced Proliferation and Autophagy after Space Flight. Biomolecules 2023, 13, 201. [Google Scholar] [CrossRef]

| Proteomic Profile of SPC-NSCs-Produced Secretome | |||
|---|---|---|---|
| SPC/1G | Accession No. | Gene | Top Ranked Protein (Species) |
| 12.84 | HUMAN | SPARC | SPARC OS = Homo Sapiens OX = 9606 GN = SPARC PE = 1 SV = 1 |
| 7.3 | HUMAN | P90AB1 | Heat shock protein HSP 90-beta OS = Homo sapiens OX = 9606 GN = HSP90AB1 PE = 1 SV = 4 |
| 5.2 | HUMAN | CALR | Calreticulin OS = Homo sapiens OX = 9606 GN = CALR PE = 1 SV = 1 |
| 3.7 | HUMAN | HSPA8 | Heat shock cognate 71 kDa protein OS = Homo sapiens OX = 9606 GN = HSPA8 PE = 1 SV = 1 |
| 3.3 | HUMAN | ENPL | Endoplasmin OS = Homo sapiens OX-9606 GN = HSP90B1 PE = 1 SV = 1 |
| 3.0 | HUMAN | Vimentin OS = Homo sapiens OX = 9606 GN = VIM PE = 1 SV = 4 | |
| Mission GCR Dose (mGy) | Mission SAA Dose (mGy) | Mission Total Dose (mGy) | Maximum Daily Total Dose (mGy) | Minimum Daily Total Dose (mGy) | Average Daily Total Dose (mGy) | |
|---|---|---|---|---|---|---|
| SpX-16 | 5.197 | 8.022 | 13.219 | 0.417 | 0.322 | 0.357 (0.026) |
| (12/17/18) | (1/12/19) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carpo, N.; Tran, V.; Biancotti, J.C.; Cepeda, C.; Espinosa-Jeffrey, A. Space Flight Enhances Stress Pathways in Human Neural Stem Cells. Biomolecules 2024, 14, 65. https://doi.org/10.3390/biom14010065
Carpo N, Tran V, Biancotti JC, Cepeda C, Espinosa-Jeffrey A. Space Flight Enhances Stress Pathways in Human Neural Stem Cells. Biomolecules. 2024; 14(1):65. https://doi.org/10.3390/biom14010065
Chicago/Turabian StyleCarpo, Nicholas, Victoria Tran, Juan Carlos Biancotti, Carlos Cepeda, and Araceli Espinosa-Jeffrey. 2024. "Space Flight Enhances Stress Pathways in Human Neural Stem Cells" Biomolecules 14, no. 1: 65. https://doi.org/10.3390/biom14010065
APA StyleCarpo, N., Tran, V., Biancotti, J. C., Cepeda, C., & Espinosa-Jeffrey, A. (2024). Space Flight Enhances Stress Pathways in Human Neural Stem Cells. Biomolecules, 14(1), 65. https://doi.org/10.3390/biom14010065