Plant Hormone and Fatty Acid Screening of Nicotiana tabacum and Lilium longiflorum Stigma Exudates
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Stigma Exudate Collection
2.2. Chromato-Mass-Spectrometric Screening of Fatty Acids
2.3. Chromato-Mass-Spectrometric Screening of Phytohormones
2.4. Pollen Collection and Germination In Vitro
2.5. Data and Statistical Analyses
3. Results
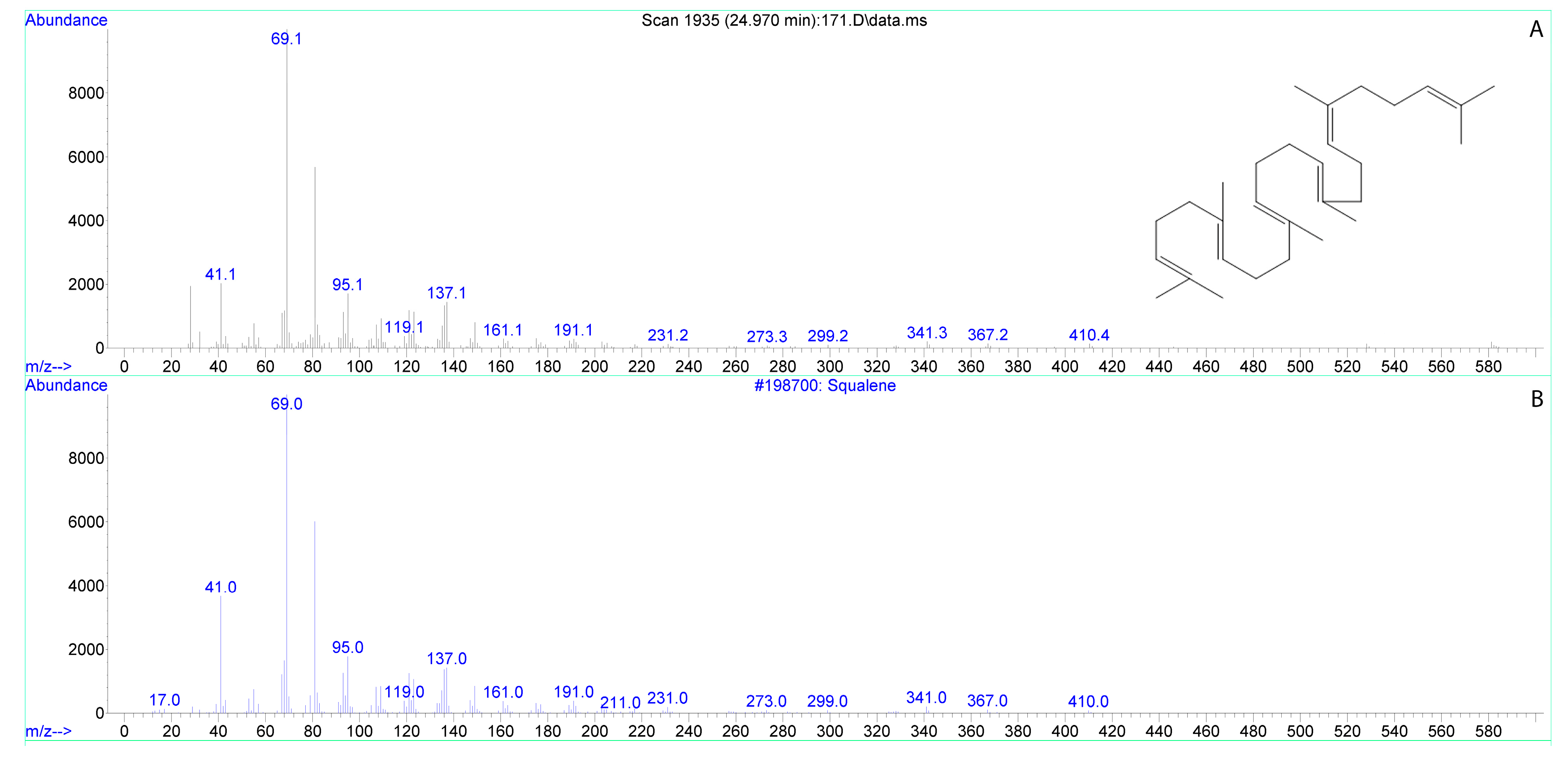
3.1. Fatty Acids of Tobacco Stigma Exudate
3.2. Fatty Acids of Lily Stigma Exudate
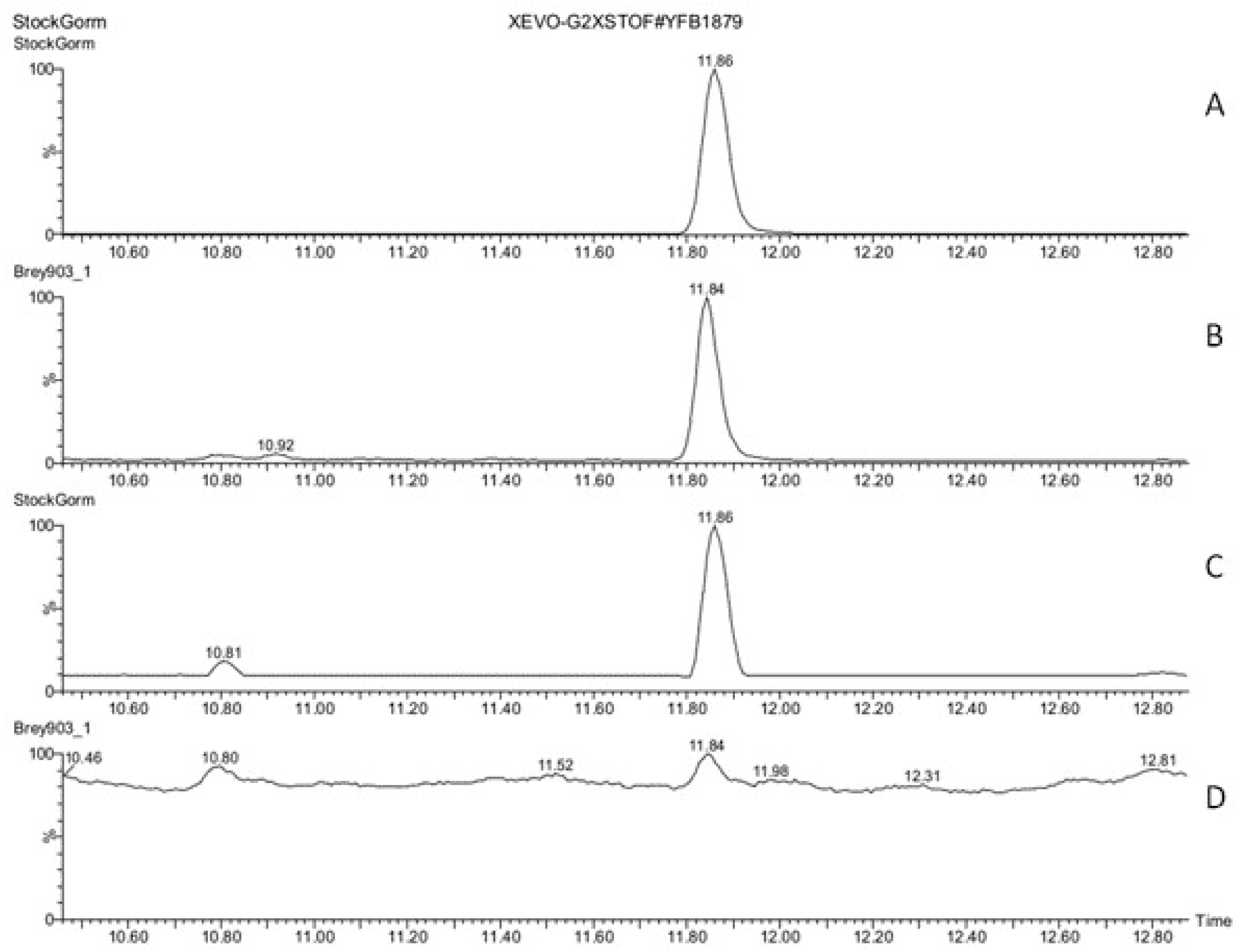
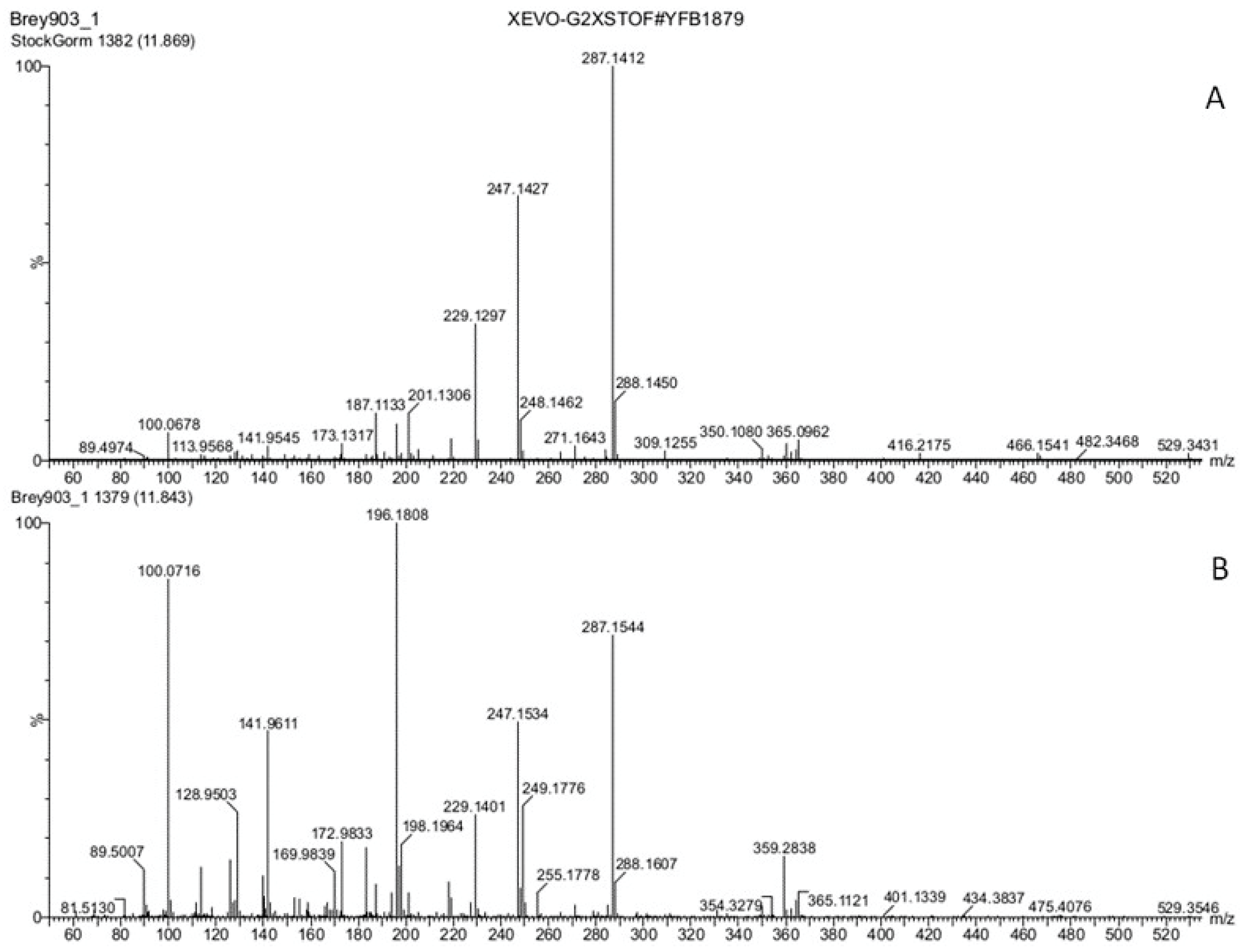
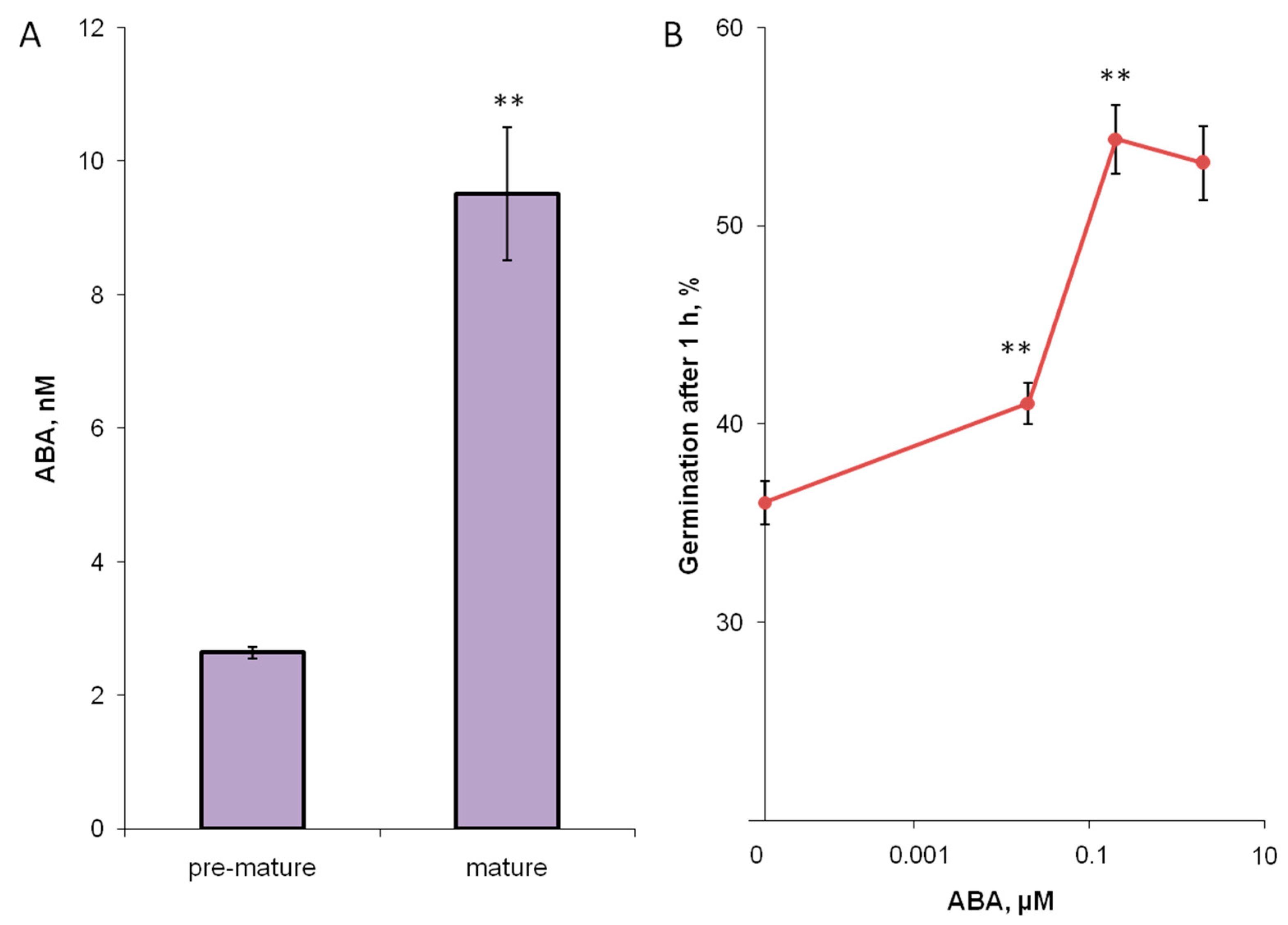
3.3. Hormones of Stigma Exudate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shivanna, K.R. The Pistil: Structure in Relation to Its Function. In Reproductive Ecology of Flowering Plants: Patterns and Processes; Springer: Berlin/Heidelberg, Germany, 2020; pp. 41–50. ISBN 9789811542107. [Google Scholar]
- Heslop-Harrison, Y.; Shivanna, K.R. The receptive surface of the angiosperm stigma. Ann. Bot. 1977, 41, 1233–1258. [Google Scholar]
- Rejón, J.D.; Delalande, F.; Schaeffer-Reiss, C.; Carapito, C.; Zienkiewicz, K.; Alché, J.d.D.; Rodríguez-García, M.I.; Van Dorsselaer, A.; Castro, A.J. The plant stigma exudate: A biochemically active extracellular environment for pollen germination? Plant Signal. Behav. 2014, 9, e28274. [Google Scholar] [CrossRef]
- Knox, R.B. Pollen-pistil interactions. In Cellular Interactions; Springer: Berlin/Heidelberg, Germany, 1984; pp. 508–608. [Google Scholar]
- Martin, F.W. Compounds from the stigmas of ten species. Am. J. Bot. 1969, 56, 1023–1027. [Google Scholar]
- Konar, R.N.; Linskens, H.F. Physiology and biochemistry of the stigmatic fluid of Petunia hybrida. Planta 1966, 71, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Tiezzi, A.; Focardi, S.; Ciampolini, F.; Oresti, M. La componentelipidicadell’essudatostigmatico di citrus limon (L.) burm. G. Bot. Ital. 1981, 115, 95–101. [Google Scholar] [CrossRef]
- Dumas, C. Lipochemistry of the progamic stage of a self-incompatible species: Neutral lipids and fatty acids of the secretory stigma during its glandular activity, and of the solid style, the ovary and the anther in Forsythia intermedia Zab. (Heterostylic species). Planta 1977, 137, 177–184. [Google Scholar] [CrossRef]
- Knox, R.B.; Kenrick, J.; Jobson, S.; Dumas, C. Reproductive Function in the Mimosoid Legume Acacia retinodes: Ultrastructural and Cytochemical Characteristics of Stigma Receptivity. Aust. J. Bot. 1989, 37, 103–124. [Google Scholar] [CrossRef]
- Hawker, J.S.; Sedgley, M.; Loveys, B.R. Composition of Stigmatic Exudate, Nectar and Pistil of Watermelon, Citrulluslanatus (Thunb.) Matsum. &Nakai, Before and After Pollination. Funct. Plant Biol. 1983, 10, 257–264. [Google Scholar]
- Cresti, M.; Keijzer, C.J.; Tiezzi, A.; Ciampolini, F.; Focardi, S. Stigma of Nicotiana: Ultrastructural and biochemical studies. Am. J. Bot. 1986, 73, 1713–1722. [Google Scholar]
- Vasil, I.K. Histology and physiology of pollen germination and pollen tube growth on the stigma and in the style. In Proceedings of the International Symposium on Fertilization in Higher Plants, Nijmegen, The Netherlands, 28–30 August 1974. [Google Scholar]
- Wolters-Arts, M.; Lush, W.M.; Mariani, C. Lipids are required for directional pollen-tube growth. Nature 1998, 392, 818–821. [Google Scholar] [CrossRef]
- Labarca, C.; Kroh, M.; Loewus, F. The composition of stigmatic exudate from Lilium longiflorum. Plant Physiol. 1970, 46, 150–156. [Google Scholar] [CrossRef]
- Labarca, C.; Loewus, F. The Nutritional Role of Pistil Exudate in Pollen Tube Wall Formation in Lilium longiflorum: I. Utilization of Injected Stigmatic Exudate 1. Plant Physiol. 1972, 50, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Miki-Hirosige, H.; Hoek, I.H.S.; Nakamura, S. Secretions from the pistil of Lilium longiflorum. Am. J. Bot. 1987, 74, 1709–1715. [Google Scholar] [CrossRef]
- Rejón, J.D.; Delalande, F.; Schaeffer-Reiss, C.; Carapito, C.; Zienkiewicz, K.; de Dios Alché, J.; Rodríguez-García, M.I.; Van Dorsselaer, A.; Castro, A.J. Proteomics profiling reveals novel proteins and functions of the plant stigma exudate. J. Exp. Bot. 2013, 64, 5695–5705. [Google Scholar] [CrossRef] [PubMed]
- Breygina, M.; Klimenko, E. ROS and ions in cell signaling during sexual plant reproduction. Int. J. Mol. Sci. 2020, 21, 9476. [Google Scholar] [CrossRef]
- Breygina, M.; Luneva, O.; Schekaleva, O.; Lazareva, N.; Babushkina, K.; Kirilyuk, I.A. Pattern of ROS generation and interconversion on wet stigmas in basal and divergent angiosperms. Plant Growth Regul. 2023. [Google Scholar] [CrossRef]
- Breygina, M.; Schekaleva, O.; Klimenko, E.; Luneva, O. The balance between different ROS on tobacco stigma during flowering and its role in pollen germination. Plants 2022, 11, 993. [Google Scholar]
- Kovaleva, L.V.; Zakharova, E.V.; Minkina, Y.V.; Timofeeva, G.V.; Andreev, I.M. Germination and In Vitro growth of petunia male gametophyte are affected by exogenous hormones and involve the changes in the endogenous hormone level. Russ. J. Plant Physiol. 2005, 52, 521–526. [Google Scholar] [CrossRef]
- Zakharova, E.V.; Khaliluev, M.R.; Kovaleva, L.V. Hormonal Signaling in the Progamic Phase of Fertilization in Plants. Horticulturae 2022, 8, 365. [Google Scholar] [CrossRef]
- Shuel, R.W. Influence of reproductive organs on secretion of sugars in flowers of Streptosolenjamesonii, Miers. Plant Physiol. 1961, 36, 265–271. [Google Scholar] [CrossRef]
- Dathe, W.; Sembdner, G. Endogenous Plant Hormones of the Broad Bean, Viciafaba L. III. Distribution of Abscisic Acid and Gibberellins in the Pistil at Anthesis. Biochem. Physiol. Pflanz. 1981, 176, 590–594. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Koiwai, A. Germination inhibition in stigma extracts of tobacco and identification of MeABA, ABA, and ABA-γ-d-Glucopyranoside. Agric. Biol. Chem. 1986, 50, 2193–2199. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, J. Free IAA in stigmas and styles during pollen germination and pollen tube growth of Nicotiana tabacum. Physiol. Plant. 2008, 134, 202–215. [Google Scholar] [CrossRef]
- Yang, M.; Yarra, R.; Zhang, R.; Zhou, L.; Jin, L.; Martin, J.J.J.; Cao, H. Transcriptome analysis of oil palm pistil during pollination and fertilization to unravel the role of phytohormone biosynthesis and signaling genes. Funct. Integr. Genomics 2022, 22, 261–278. [Google Scholar] [CrossRef]
- Ren, M.J.; Zhou, X.Y. Change of cucumber seed yield lines endogenous hormone during the process of double fertilization. J. Northeast Agric. Univ. 2017, 48, 24–32. [Google Scholar]
- Nitsch, J.P. Deux espacesphotoperiodiques de jours courts: Plumbago indica L. et P. zeyelanica L. Bull. Bot. Fr. 1965, 112, 517–522. [Google Scholar] [CrossRef]
- Ivanova, T.V.; Voronkov, A.S.; Kumakhova, T.K.; Tsydendambaev, V.D. Distinguishing Features of Fatty Acid Content and Composition in Total Lipids of Malus orientalisUglitzk. Pericarp. Russ. J. Plant Physiol. 2020, 67, 463–471. [Google Scholar] [CrossRef]
- Voronkov, A.S.; Ivanova, T.V.; Kumachova, T.K. The features of the fatty acid composition of Pyrus L. total lipids are determined by mountain ecosystem conditions. Plant Physiol. Biochem. 2022, 170, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Budge, S.M.; Barry, C. Determination of squalene in edible oils by transmethylation and GC analysis. MethodsX 2019, 6, 15–21. [Google Scholar] [CrossRef]
- Lyons, J.M.; Wheaton, T.A.; Pratt, H.K. Relationship between the Physical Nature of Mitochondrial Membranes and Chilling Sensitivity in Plants. Plant Physiol. 1964, 39, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Janson, J.; Reinders, M.C.; Valkering, A.G.M.; Van Tuyl, J.M.; Keijzer, C.J. Pistil Exudate Production and Pollen Tube Growth in Lilium longiflorum Thunb. Ann. Bot. 1994, 73, 437–446. [Google Scholar] [CrossRef]
- Reszczyńska, E.; Hanaka, A. Lipids Composition in Plant Membranes. Cell Biochem. Biophys. 2020, 78, 401–414. [Google Scholar] [CrossRef]
- Zhukov, A. V On Qualitative Composition of Membrane Lipids in Plant Cells. Russ. J. Plant Physiol. 2021, 68, 367–383. [Google Scholar] [CrossRef]
- Breygina, M.; Voronkov, A.; Ivanova, T.; Babushkina, K. Fatty acid composition of dry and germinating pollen of Gymnosperm and Angiosperm plants. Int. J. Mol. Sci. 2023, 24, 9717. [Google Scholar] [CrossRef]
- Wolters-Arts, M.; Van Der Weerd, L.; Van Aelst, A.C.; Van Der Weerd, J.; Van As, H.; Mariani, C. Water-conducting properties of lipids during pollen hydration. Plant. Cell Environ. 2002, 25, 513–519. [Google Scholar] [CrossRef]
- Okolo, E.C.; Abigor, R.D. Components Of The Stigmatic Exudate Of Raphia hookeri, Mann And Wendl. J. Agric. For. Soc. Sci. 2006, 4, 83–88. [Google Scholar] [CrossRef]
- Kandasamy, M.K.; Vivekanandan, M. Biochemical composition of stigmatic exudate of Eichhorniacrassipes (Mart.) solms. Aquat. Bot. 1983, 16, 41–47. [Google Scholar] [CrossRef]
- Vogel, S. Flowers offering fatty oil instead of nectar. In Proceedings of the Abstracts XIth International Botany Congress, Seattle, WA, USA., 24 August–2 September 1969. [Google Scholar]
- Baker, H.G.; Baker, I.; Opler, P.A. Stigmatic exudates and pollination. In Pollination and Dispersal; Brantjes, N.B.M., Ed.; Department of Botany Nijmegen: Nijmegen, The Netherland, 1974; pp. 47–60. [Google Scholar]
- Lord, E.M.; Webster, B.D. The stigmatic exudate of Phaseolus vulgaris L. Bot. Gaz. 1979, 140, 266–271. [Google Scholar] [CrossRef]
- Stevenson, P.C.; Nicolson, S.W.; Wright, G.A. Plant secondary metabolites in nectar: Impacts on pollinators and ecological functions. Funct. Ecol. 2017, 31, 65–75. [Google Scholar] [CrossRef]
- Goldman, M.H.; Goldberg, R.B.; Mariani, C. Female sterile tobacco plants are produced by stigma-specific cell ablation. EMBO J. 1994, 13, 2976–2984. [Google Scholar] [CrossRef]
- Lush, W.M.; Grieser, F.; Wolters-Arts, M. Directional guidance of Nicotiana alata pollen tubes in vitro and on the stigma. Plant Physiol. 1998, 118, 733–741. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lipp, J. Detection and origin of abscisic acid and proline in honey. Apidologie 1990, 21, 249–259. [Google Scholar] [CrossRef]
- Davis, A.R.; Gunning, B.E.S. The modified stomata of the floral nectary of Viciafaba L. 3. Physiological aspects, including comparisons with foliar stomata. Bot. Acta 1993, 106, 241–253. [Google Scholar] [CrossRef]
- Kovaleva, L.V.; Zakharova, E.V.; Minkina, Y.V.; Vvoronkov, A.S. Effects of flavonols and phytohormones on germination and growth of petunia male gametophyte. Allelopath. J. 2009, 23, 51–61. [Google Scholar]
- Guan, Y.; Guo, J.; Li, H.; Yang, Z. Signaling in pollen tube growth: Crosstalk, feedback, and missing links. Mol. Plant 2013, 6, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Breygina, M.; Voronkov, A.; Galin, I.; Akhiyarova, G.; Polevova, S.; Klimenko, E.; Ivanov, I.; Kudoyarova, G. Dynamics of endogenous levels and subcellular localization of ABA and cytokinins during pollen germination in spruce and tobacco. Protoplasma 2022, 260, 237–248. [Google Scholar] [CrossRef]
- Kovaleva, L.; Voronkov, A.; Zakharova, E.; Minkina, Y.; Timofeeva, G.; Andreev, I. Regulation of Petunia pollen tube growth by phytohormones: Identification of their potential targets. J. Agric. Sci. Technol. A 2016, 6, 239–254. [Google Scholar] [CrossRef][Green Version]
- Shi, P.; Hua, W.; Htwe, Y.M.; Zhang, D.; Li, J.; Wang, Y. Abscisic Acid Improves Linoleic Acid Accumulation Possibly by Promoting Expression of EgFAD2 and Other Fatty Acid Biosynthesis Genes in Oil Palm Mesocarp. Front. Plant Sci. 2021, 12, 748130. [Google Scholar] [CrossRef]
- Huo, K.; Shui, L.; Mai, Y.; Zhou, N.; Liu, Y.; Zhang, C.; Niu, J. Effects of exogenous abscisic acid on oil content, fatty acid composition, biodiesel properties and lipid components in developing Siberian apricot (Prunus sibirica) seeds. Plant Physiol. Biochem. 2020, 154, 260–267. [Google Scholar] [CrossRef]
- Melo-Espinosa, E.A.; Sánchez-Borroto, Y.; Errasti, M.; Piloto-Rodríguez, R.; Sierens, R.; Roger-Riba, J.; Christopher-Hansen, A. Surface Tension Prediction of Vegetable Oils Using Artificial Neural Networks and Multiple Linear Regression. Energy Procedia 2014, 57, 886–895. [Google Scholar] [CrossRef]
- Corbet, S.A.; Willmer, P.G.; Beament, J.W.L.; Unwin, D.M.; Prŷs-Jones, O.E. Post-secretory determinants of sugar concentration in nectar. Plant. Cell Environ. 1979, 2, 293–308. [Google Scholar] [CrossRef]
- Post-Beittenmiller, D. Biochemistry and molecular biology of wax production in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 405–430. [Google Scholar] [CrossRef]
- Posé, D.; Castanedo, I.; Borsani, O.; Nieto, B.; Rosado, A.; Taconnat, L.; Ferrer, A.; Dolan, L.; Valpuesta, V.; Botella, M.A. Identification of the Arabidopsis dry2/sqe1-5 mutant reveals a central role for sterols in drought tolerance and regulation of reactive oxygen species. Plant J. 2009, 59, 63–76. [Google Scholar] [CrossRef]
- Balusamy, S.R.; Rahimi, S.; Yang, D.-C. Characterization of squalene-induced PgCYP736B involved in salt tolerance by modulating key genes of abscisic acid biosynthesis. Int. J. Biol. Macromol. 2019, 121, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Cowan, A.K.; Railton, I.D. The biosynthesis of abscisic acid in a cell-free system from embryos of Hordeum vulgare. J. Plant Physiol. 1987, 131, 423–431. [Google Scholar] [CrossRef]
- Hata, S.; Sanmiya, K.; Kouchi, H.; Matsuoka, M.; Yamamoto, N.; Izui, K. cDNA cloning of squalene synthase genes from mono-and dicotyledonous plants, and expression of the gene in rice. Plant Cell Physiol. 1997, 38, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, S.; Yin, J.; Li, C.; Zhan, Y.; Xiao, J.; Liang, T.; Li, X. Molecular cloning and promoter analysis of squalene synthase and squalene epoxidase genes from Betula platyphylla. Protoplasma 2016, 253, 1347–1363. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, H.; Zhang, Y.; Lin, L.; Cui, M.; Long, Y.; Xing, Z. DNA methylation of farnesyl pyrophosphate synthase, squalene synthase, and squalene epoxidase gene promoters and effect on the saponin content of Eleutherococcus senticosus. Forests 2019, 10, 1053. [Google Scholar] [CrossRef]
- Yin, J.; Li, X.; Zhan, Y.; Li, Y.; Qu, Z.; Sun, L.; Wang, S.; Yang, J.; Xiao, J. Cloning and expression of BpMYC4 and BpbHLH9 genes and the role of BpbHLH9 in triterpenoid synthesis in birch. BMC Plant Biol. 2017, 17, 214. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yang, L.; Lan, Y.; Zhao, Y.; Han, M.; Yang, L. Exogenous abscisic acid reduces saikosaponin accumulation by inhibiting saikosaponin synthesis pathway gene expression under drought stress in Bupleurum chinense DC. Ind. Crops Prod. 2020, 154, 112686. [Google Scholar] [CrossRef]
- Romero, P.; Lafuente, M.T. The combination of abscisic acid (ABA) and water stress regulates the epicuticular wax metabolism and cuticle properties of detached citrus fruit. Int. J. Mol. Sci. 2021, 22, 10242. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, H.; Asrar, Z.; Szopa, J. Effects of ABA on primary terpenoids and Δ9-tetrahydrocannabinol in Cannabis sativa L. at flowering stage. Plant Growth Regul. 2009, 58, 269–277. [Google Scholar] [CrossRef]
- Mansouri, H.; Asrar, Z. Effects of abscisic acid on content and biosynthesis of terpenoids in Cannabis sativa at vegetative stage. Biol. Plant. 2012, 56, 153–156. [Google Scholar] [CrossRef]
- Micera, M.; Botto, A.; Geddo, F.; Antoniotti, S.; Bertea, C.M.; Levi, R.; Gallo, M.P.; Querio, G. Squalene: More than a Step toward Sterols. Antioxidants 2020, 9, 688. [Google Scholar] [CrossRef] [PubMed]
| Fatty Acid | Exudate | |
|---|---|---|
| Lily (Lilium longiflorum L.) | Tobacco (Nicotiana tabacum L.) | |
| 14:0 | 1.90 ± 0.07 | 3.03 ± 0.23 * |
| 15:0 | 3.63 ± 0.26 | 2.79 ± 0.56 |
| 16:0 | 27.36 ± 3.43 | 24.08 ± 1.80 |
| 7–16:1 | 8.42 ± 0.69 | – (not detected) |
| 9–16:1 | – | 4.44 ± 0.44 |
| 14–16:1 | 1.78 ± 0.07 | 0.93 ± 0.02 * |
| 10–17:1 | 1.78 ± 0.07 | – |
| 18:0 | 16.86 ± 0.68 | 15.33 ± 1.97 |
| 9–18:1 | 21.34 ± 0.97 | 38.47 ± 1.59 ** |
| 11–18:1 | 0.56 ± 0.13 | – |
| 9,12–18:2 | 3.01 ± 1.71 | 6.47 ± 0.56 * |
| 9,12,15–18:3 | 0.81 ± 0.55 | – |
| 20:0 | 2.69 ± 0.47 | 1.04 ± 0.10 * |
| 11–20:1 | 3.01 ± 0.50 | – |
| 22:0 | 1.88 ± 0.93 | 1.35 ± 0.83 |
| 24:0 | 3.22 ± 1.69 | 2.08 ± 0.02 |
| 25:0 | 0.58 ± 0.68 | – |
| 26:0 | 1.15 ± 0.47 | – |
| UI | 0.453 ± 0.010 | 0.568 ± 0.030 * |
| ∑VLCFAs, % | 12.53 ± 3.25 | 4.47 ± 1.91 * |
| ngsqualeneon 1 stigma | 17.66 ± 1.19 | 0.51 ± 0.03 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breygina, M.; Kochkin, D.; Voronkov, A.; Ivanova, T.; Babushkina, K.; Klimenko, E. Plant Hormone and Fatty Acid Screening of Nicotiana tabacum and Lilium longiflorum Stigma Exudates. Biomolecules 2023, 13, 1313. https://doi.org/10.3390/biom13091313
Breygina M, Kochkin D, Voronkov A, Ivanova T, Babushkina K, Klimenko E. Plant Hormone and Fatty Acid Screening of Nicotiana tabacum and Lilium longiflorum Stigma Exudates. Biomolecules. 2023; 13(9):1313. https://doi.org/10.3390/biom13091313
Chicago/Turabian StyleBreygina, Maria, Dmitry Kochkin, Alexander Voronkov, Tatiana Ivanova, Ksenia Babushkina, and Ekaterina Klimenko. 2023. "Plant Hormone and Fatty Acid Screening of Nicotiana tabacum and Lilium longiflorum Stigma Exudates" Biomolecules 13, no. 9: 1313. https://doi.org/10.3390/biom13091313
APA StyleBreygina, M., Kochkin, D., Voronkov, A., Ivanova, T., Babushkina, K., & Klimenko, E. (2023). Plant Hormone and Fatty Acid Screening of Nicotiana tabacum and Lilium longiflorum Stigma Exudates. Biomolecules, 13(9), 1313. https://doi.org/10.3390/biom13091313








