Current Clinical Trial Status and Future Prospects of PPAR-Targeted Drugs for Treating Nonalcoholic Fatty Liver Disease
Abstract
:1. Introduction
2. PPAR Agonists in the Past and Current Clinical Trials against NASH
2.1. Lanifibranor (PPAR Pan Agonist)—Under Consideration for Treating NAFLD/NASH
2.2. Chiglitazar (PPAR Pan Agonist)—Under Consideration in China
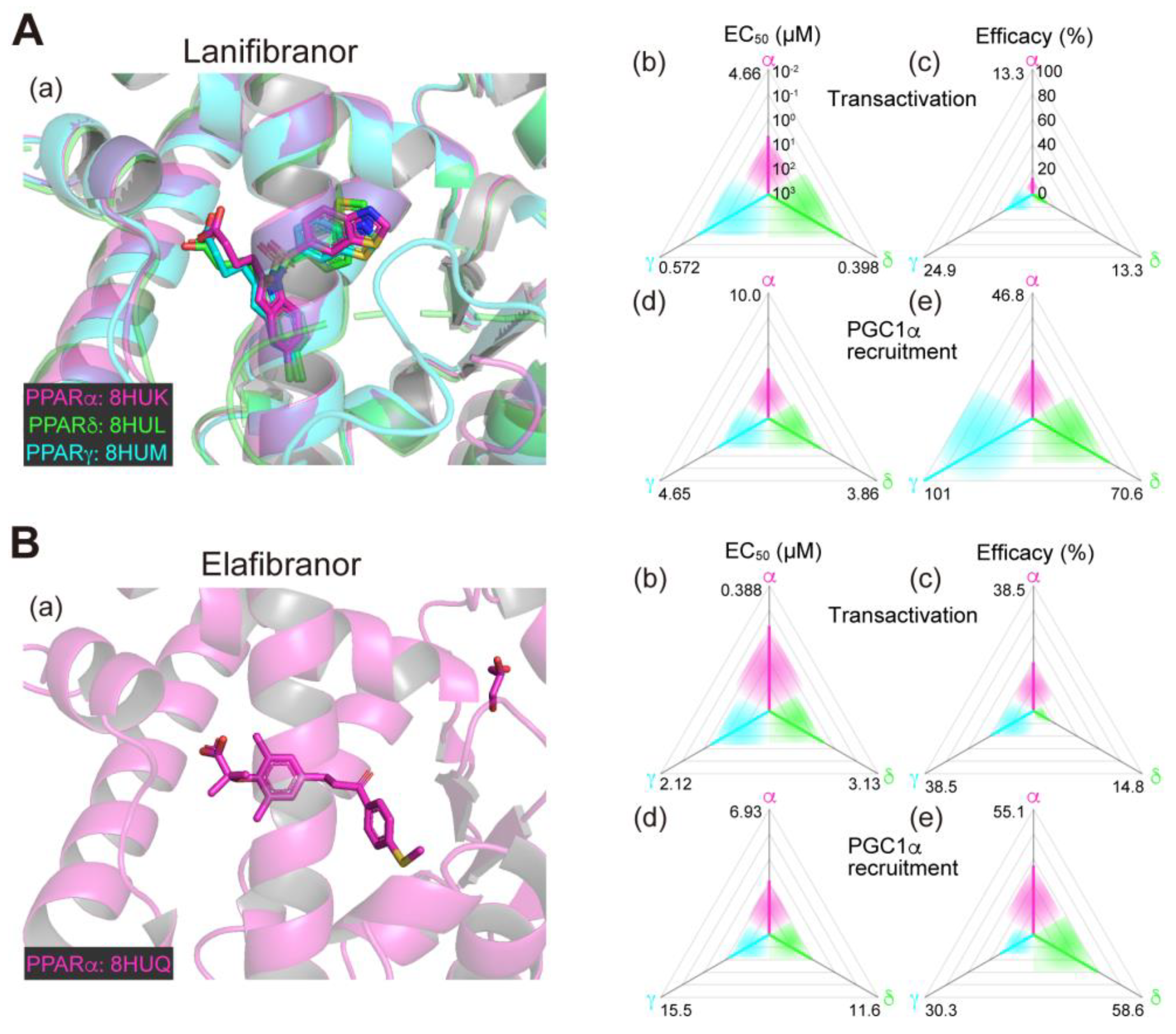
2.3. Elafibranor (PPARα/δ Dual Agonist)—Discontinued
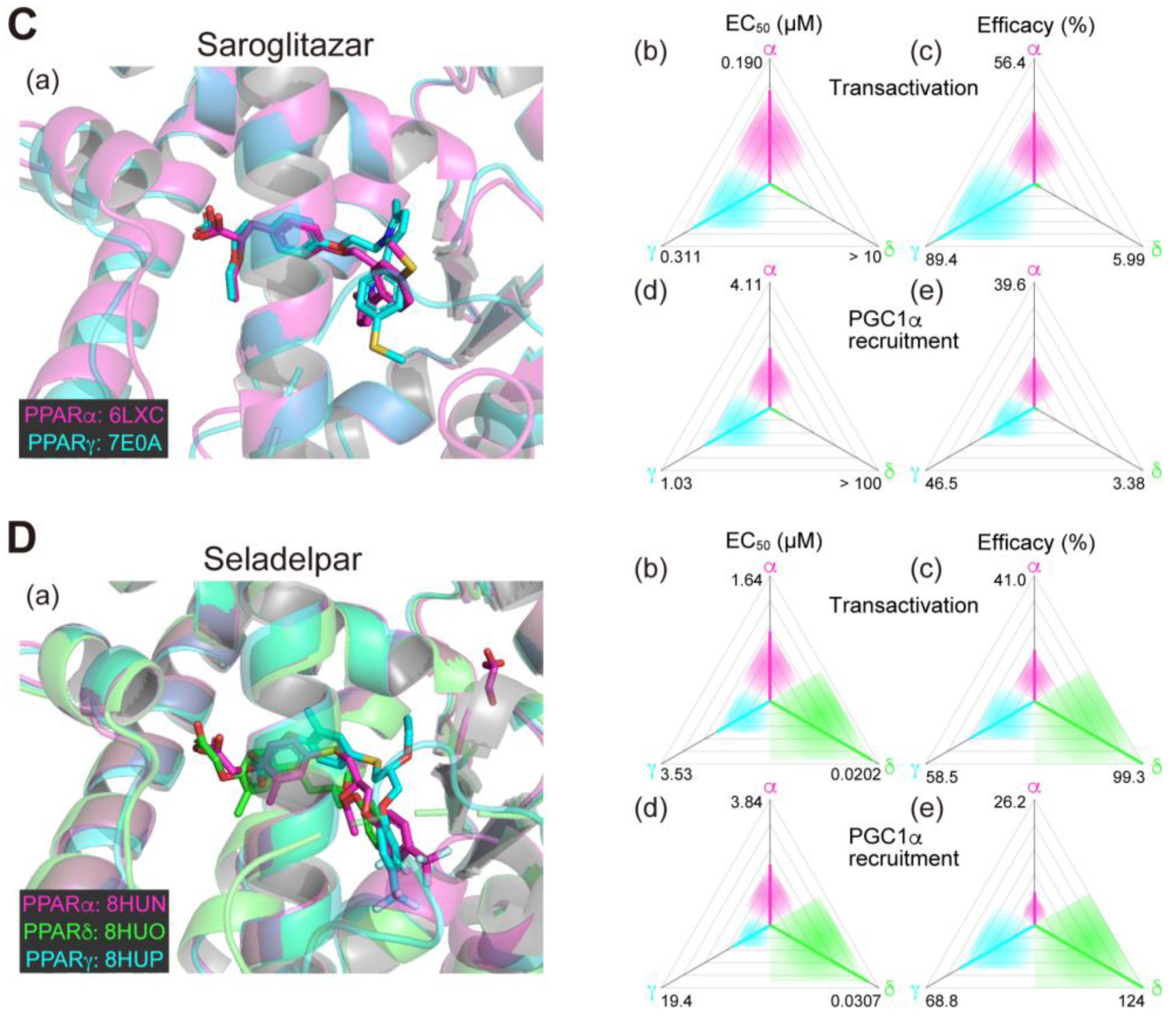
2.4. Saroglitazar (PPARα/γ Dual Agonist)—Under Consideration
2.5. Seladelpar (PPARδ-Selective Agonist)—Interrupted
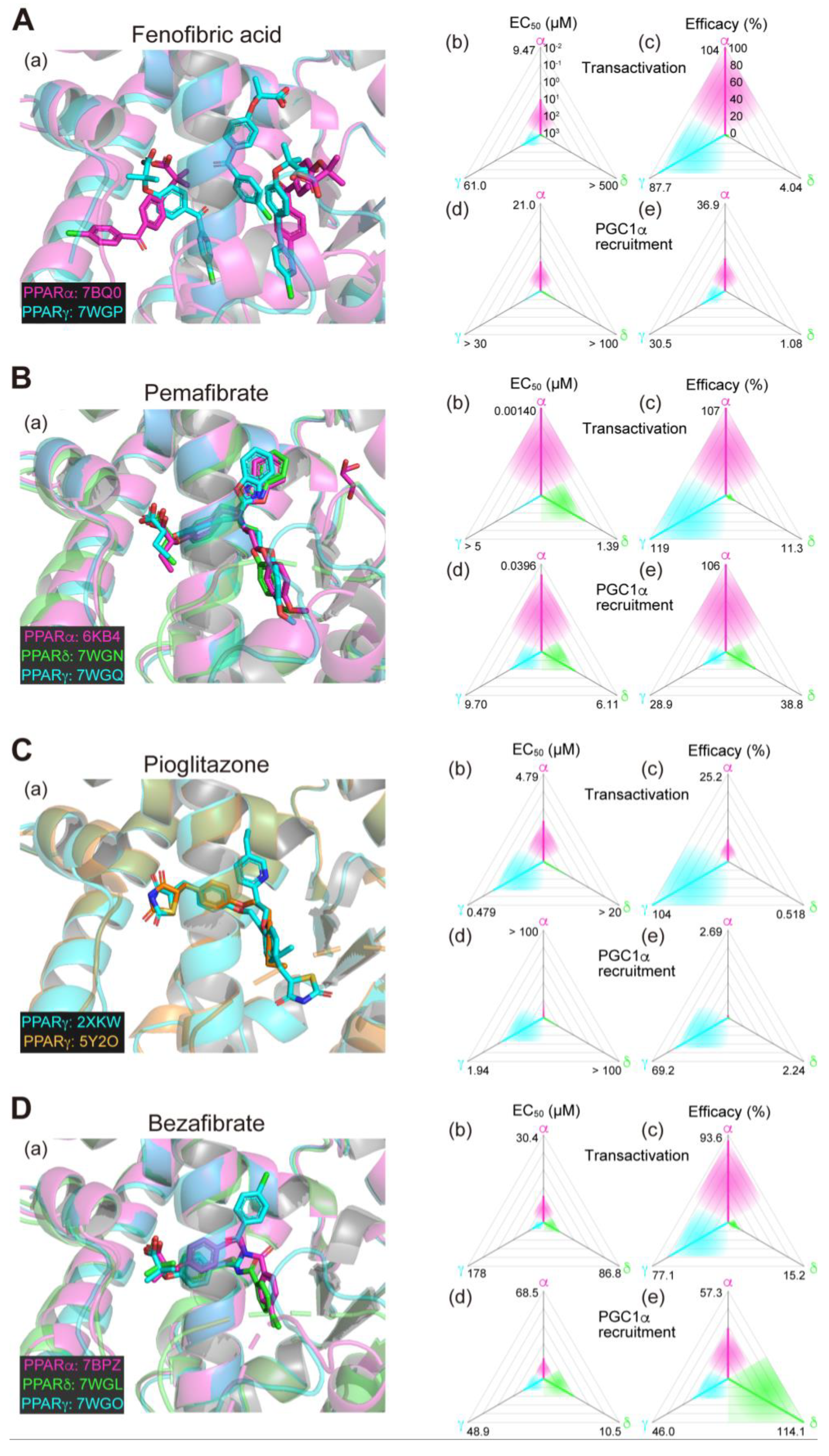
2.6. Fenofibrate (PPARα(/γ Dual) Agonist)—Discontinued
2.7. Pemafibrate (PPARα-Selective Agonist)—Under Consideration in Japan
2.8. Pioglitazone (PPARγ-Selective Agonist)—Under Consideration
2.9. Rosiglitazone (PPARγ-Selective Agonist)—Discontinued
2.10. Lobeglitazone (PPARγ Agonist)—Under Consideration in Korea
3. Future Prospects of PPAR Agonists for NAFLD/NASH
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 2019, 92, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef]
- Sheka, A.C.; Adeyi, O.; Thompson, J.; Hameed, B.; Crawford, P.A.; Ikramuddin, S. Nonalcoholic steatohepatitis: A review. JAMA 2020, 323, 1175–1183. [Google Scholar] [CrossRef]
- Ginès, P.; Krag, A.; Abraldes, J.G.; Solà, E.; Fabrellas, N.; Kamath, P.S. Liver cirrhosis. Lancet 2021, 398, 1359–1376. [Google Scholar] [CrossRef]
- Vuppalanchi, R.; Noureddin, M.; Alkhouri, N.; Sanyal, A.J. Therapeutic pipeline in nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 373–392. [Google Scholar] [CrossRef]
- Puengel, T.; Liu, H.; Guillot, A.; Heymann, F.; Tacke, F.; Peiseler, M. Nuclear receptors linking metabolism, inflammation, and fibrosis in nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2022, 23, 2668. [Google Scholar] [CrossRef]
- Cheng, H.S.; Tan, W.R.; Low, Z.S.; Marvalim, C.; Lee, J.Y.H.; Tan, N.S. Exploration and development of PPAR modulators in health and disease: An update of clinical evidence. Int. J. Mol. Sci. 2019, 20, 5055. [Google Scholar] [CrossRef]
- CymaBay Therapeutics Press Release (25 November 2019). CymaBay Therapeutics Halts Clinical Development of Seladelpar. Available online: https://ir.cymabay.com/press-releases/detail/476/cymabay-therapeutics-halts-clinical-development-of-seladelpar (accessed on 25 July 2023).
- Honda, A.; Kamata, S.; Akahane, M.; Machida, Y.; Uchii, K.; Shiiyama, Y.; Habu, Y.; Miyawaki, S.; Kaneko, C.; Oyama, T.; et al. Functional and structural insights into human PPARα/δ/γ subtype selectivity of bezafibrate, fenofibric acid, and pemafibrate. Int. J. Mol. Sci. 2022, 23, 4726. [Google Scholar] [CrossRef]
- Honda, A.; Kamata, S.; Satta, C.; Machida, Y.; Uchii, K.; Terasawa, K.; Nemoto, A.; Oyama, T.; Ishii, I. Structural basis for anti-non-alcoholic fatty liver disease and diabetic dyslipidemia drug saroglitazar as a PPAR α/γ dual agonist. Biol. Pharm. Bull. 2021, 44, 1210–1219. [Google Scholar] [CrossRef]
- Kamata, S.; Honda, A.; Ishii, I. Functional and structural insights into the human PPARα/δ/γ targeting preferences of anti-NASH investigational drugs, lanifibranor, seladelpar, and elafibranor. Antioxidants 2023, 12, 1523. [Google Scholar] [CrossRef]
- Kamata, S.; Oyama, T.; Saito, K.; Honda, A.; Yamamoto, Y.; Suda, K.; Ishikawa, R.; Itoh, T.; Watanabe, Y.; Shibata, T.; et al. PPARα ligand-binding domain structures with endogenous fatty acids and fibrates. iScience 2020, 23, 101727. [Google Scholar] [CrossRef] [PubMed]
- Wettstein, G.; Luccarini, J.M.; Poekes, L.; Faye, P.; Kupkowski, F.; Adarbes, V.; Defrêne, E.; Estivalet, C.; Gawronski, X.; Jantzen, I.; et al. The new-generation pan-peroxisome proliferator-activated receptor agonist IVA337 protects the liver from metabolic disorders and fibrosis. Hepatol. Commun. 2017, 1, 524–537. [Google Scholar] [CrossRef]
- Boubia, B.; Poupardin, O.; Barth, M.; Binet, J.; Peralba, P.; Mounier, L.; Jacquier, E.; Gauthier, E.; Lepais, V.; Chatar, M.; et al. Design, synthesis, and evaluation of a novel series of indole sulfonamide peroxisome proliferator activated receptor (PPAR) α/γ/δ triple activators: Discovery of lanifibranor, a new antifibrotic clinical candidate. J. Med. Chem. 2018, 61, 2246–2265. [Google Scholar] [CrossRef] [PubMed]
- Sven, M.F.; Pierre, B.; Manal, F.A.; Quentin, M.A.; Elisabetta, B.; Vlad, R.; Philippe, H.M.; Bruno, S.; Jean-Louis, J.; Pierre, B.; et al. A randomised, double-blind, placebo-controlled, multi-centre, dose-range, proof-of-concept, 24-week treatment study of lanifibranor in adult subjects with non-alcoholic steatohepatitis: Design of the NATIVE study. Contemp. Clin. Trials 2020, 98, 106170. [Google Scholar] [CrossRef]
- Francque, S.M.; Bedossa, P.; Ratziu, V.; Anstee, Q.M.; Bugianesi, E.; Sanyal, A.J.; Loomba, R.; Harrison, S.A.; Balabanska, R.; Mateva, L.; et al. A randomized, controlled trial of the pan-PPAR agonist lanifibranor in NASH. N. Engl. J. Med. 2021, 385, 1547–1558. [Google Scholar] [CrossRef]
- Deeks, E.D. Chiglitazar: First approval. Drugs 2022, 82, 87–92. [Google Scholar] [CrossRef]
- Kamata, S.; Oyama, T.; Ishii, I. Preparation of co-crystals of human PPARα-LBD and ligand for high-resolution X-ray crystallography. STAR Protoc. 2021, 2, 100364. [Google Scholar] [CrossRef]
- Staels, B.; Rubenstrunk, A.; Noel, B.; Rigou, G.; Delataille, P.; Millatt, L.J.; Baron, M.; Lucas, A.; Tailleux, A.; Hum, D.W.; et al. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 2013, 58, 1941–1952. [Google Scholar] [CrossRef]
- Tølbøl, K.S.; Kristiansen, M.N.; Hansen, H.H.; Veidal, S.S.; Rigbolt, K.T.; Gillum, M.P.; Jelsing, J.; Vrang, N.; Feigh, M. Metabolic and hepatic effects of liraglutide, obeticholic acid and elafibranor in diet-induced obese mouse models of biopsy-confirmed nonalcoholic steatohepatitis. World J. Gastroenterol. 2018, 24, 179–194. [Google Scholar] [CrossRef]
- Ratziu, V.; Harrison, S.A.; Francque, S.; Bedossa, P.; Lehert, P.; Serfaty, L.; Romero-Gomez, M.; Boursier, J.; Abdelmalek, M.; Caldwell, S.; et al. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-α and -δ, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology 2016, 150, 1147–1159.e5. [Google Scholar] [CrossRef]
- GENFIT Press Release (11 May 2020). GENFIT: Announces Results from Interim Analysis of RESOLVE-IT Phase 3 Trial of Elafibranor in Adults with NASH and Fibrosis. Available online: https://ir.genfit.com/news-releases/news-release-details/genfit-announces-results-interim-analysis-resolve-it-phase-3/ (accessed on 25 July 2023).
- Jani, R.H.; Kansagra, K.; Jain, M.R.; Patel, H. Pharmacokinetics, safety, and tolerability of saroglitazar (ZYH1), a predominantly PPARα agonist with moderate PPARγ agonist activity in healthy human subjects. Clin. Drug Investig. 2013, 33, 809–816. [Google Scholar] [CrossRef]
- Kaul, U.; Parmar, D.; Manjunath, K.; Shah, M.; Parmar, K.; Patil, K.P.; Jaiswal, A. New dual peroxisome proliferator activated receptor agonist–Saroglitazar in diabetic dyslipidemia and non-alcoholic fatty liver disease: Integrated analysis of the real world evidence. Cardiovasc. Diabetol. 2019, 18, 80. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A. An observational study of reduction in glycemic parameters and liver stiffness by saroglitazar 4 mg in patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Cureus 2020, 12, e9065. [Google Scholar] [CrossRef] [PubMed]
- Roy, S. Clinical case series of decrease in shear wave elastography values in ten diabetic dyslipidemia patients having NAFLD with saroglitazar 4 mg: An Indian experience. Case Rep. Med. 2020, 2020, 4287075. [Google Scholar] [CrossRef]
- Gawrieh, S.; Noureddin, M.; Loo, N.; Mohseni, R.; Awasty, V.; Cusi, K.; Kowdley, K.V.; Lai, M.; Schiff, E.; Parmar, D.; et al. Saroglitazar, a PPAR-α/γ agonist, for treatment of NAFLD: A randomized controlled double-blind phase 2 trial. Hepatology 2021, 74, 1809–1824. [Google Scholar] [CrossRef] [PubMed]
- CymaBay Therapeutics Press Release (23 July 2020). FDA Lifts All Clinical Holds on Seladelpar. Available online: https://ir.cymabay.com/press-releases/detail/485/fda-lifts-all-clinical-holds-on-seladelpar (accessed on 25 July 2023).
- Oscarsson, J.; Önnerhag, K.; Risérus, U.; Sundén, M.; Johansson, L.; Jansson, P.A.; Moris, L.; Nilsson, P.M.; Eriksson, J.W.; Lind, L. Effects of free omega-3 carboxylic acids and fenofibrate on liver fat content in patients with hypertriglyceridemia and non-alcoholic fatty liver disease: A double-blind, randomized, placebo-controlled study. J. Clin. Lipidol. 2018, 12, 1390–1403.e4. [Google Scholar] [CrossRef]
- Yamashita, S.; Masuda, D.; Matsuzawa, Y. Pemafibrate, a new selective PPARα modulator: Drug concept and its clinical applications for dyslipidemia and metabolic diseases. Curr. Atheroscler. Rep. 2020, 22, 5. [Google Scholar] [CrossRef]
- Honda, Y.; Kessoku, T.; Ogawa, Y.; Tomeno, W.; Imajo, K.; Fujita, K.; Yoneda, M.; Takizawa, T.; Saito, S.; Nagashima, Y.; et al. Pemafibrate, a novel selective peroxisome proliferator-activated receptor alpha modulator, improves the pathogenesis in a rodent model of nonalcoholic steatohepatitis. Sci. Rep. 2017, 7, 42477. [Google Scholar] [CrossRef]
- Sasaki, Y.; Asahiyama, M.; Tanaka, T.; Yamamoto, S.; Murakami, K.; Kamiya, W.; Matsumura, Y.; Osawa, T.; Anai, M.; Fruchart, J.C.; et al. Pemafibrate, a selective PPARα modulator, prevents non-alcoholic steatohepatitis development without reducing the hepatic triglyceride content. Sci. Rep. 2020, 10, 7818. [Google Scholar] [CrossRef]
- Nakajima, A.; Eguchi, Y.; Yoneda, M.; Imajo, K.; Tamaki, N.; Suganami, H.; Nojima, T.; Tanigawa, R.; Iizuka, M.; Iida, Y.; et al. Randomised clinical trial: Pemafibrate, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), versus placebo in patients with non-alcoholic fatty liver disease. Aliment Pharmacol. Ther. 2021, 54, 1263–1277. [Google Scholar] [CrossRef]
- Fruchart, J.C.; Hermans, M.P.; Fruchart-Najib, J.; Kodama, T. Selective peroxisome proliferator-activated receptor alpha modulators (SPPARMα) in the metabolic syndrome: Is pemafibrate light at the end of the tunnel? Curr. Atheroscler. Rep. 2021, 23, 3. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Yamashita, S.; Yokote, K.; Araki, E.; Suganami, H.; Ishibashi, S. Efficacy and safety of K-877, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), in combination with statin treatment: Two randomised, double-blind, placebo-controlled clinical trials in patients with dyslipidaemia. Atherosclerosis 2017, 261, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Kusunoki, M.; Sakazaki, T.; Tsutsumi, K.; Miyata, T.; Oshida, Y. The effects of pemafibrate in Japanese patients with type 2 diabetes receiving HMG-CoA reductase inhibitors. Endocr. Metab. Immune. Disord. Drug Targets 2021, 21, 919–924. [Google Scholar] [CrossRef]
- Lebovitz, H.E. Thiazolidinediones: The forgotten diabetes medications. Curr. Diab. Rep. 2019, 19, 151. [Google Scholar] [CrossRef]
- Lee, M.A.; Tan, L.; Yang, H.; Im, Y.G.; Im, Y.J. Structures of PPARγ complexed with lobeglitazone and pioglitazone reveal key determinants for the recognition of antidiabetic drugs. Sci. Rep. 2017, 7, 16837. [Google Scholar] [CrossRef] [PubMed]
- Tokushige, K.; Ikejima, K.; Ono, M.; Eguchi, Y.; Kamada, Y.; Itoh, Y.; Akuta, N.; Yoneda, M.; Iwasa, M.; Yoneda, M.; et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. J. Gastroenterol. 2021, 56, 951–963. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef]
- Huang, J.F.; Dai, C.Y.; Huang, C.F.; Tsai, P.C.; Yeh, M.L.; Hsu, P.Y.; Huang, S.F.; Bair, M.J.; Hou, N.J.; Huang, C.I.; et al. First-in-Asian double-blind randomized trial to assess the efficacy and safety of insulin sensitizer in nonalcoholic steatohepatitis patients. Hepatol. Int. 2021, 15, 1136–1147. [Google Scholar] [CrossRef]
- Jacques, V.; Bolze, S.; Hallakou-Bozec, S.; Czarnik, A.W.; Divakaruni, A.S.; Fouqueray, P.; Murphy, A.N.; Van der Ploeg, L.H.T.; DeWitt, S. Deuterium-stabilized (R)-pioglitazone (PXL065) is responsible for pioglitazone efficacy in NASH yet exhibits little to no PPARγ activity. Hepatol. Commun. 2021, 5, 1412–1425. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Wolski, K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 2007, 356, 2457–2471. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Loke, Y.K.; Furberg, C.D. Long-term risk of cardiovascular events with rosiglitazone: A meta-analysis. JAMA 2007, 298, 1189–1195. [Google Scholar] [CrossRef]
- Ratziu, V.; Giral, P.; Jacqueminet, S.; Charlotte, F.; Hartemann-Heurtier, A.; Serfaty, L.; Podevin, P.; Lacorte, J.M.; Bernhardt, C.; Bruckert, E.; et al. Rosiglitazone for nonalcoholic steatohepatitis: One-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology 2008, 135, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Charlotte, F.; Bernhardt, C.; Giral, P.; Halbron, M.; Lenaour, G.; Hartmann-Heurtier, A.; Bruckert, E.; Poynard, T. Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: Results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology 2010, 51, 445–453. [Google Scholar] [CrossRef]
- Bae, J.; Park, T.; Kim, H.; Lee, M.; Cha, B.S. Lobeglitazone: A novel thiazolidinedione for the management of type 2 diabetes mellitus. Diabetes Metab. J. 2021, 45, 326–336. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, J.H.; Kim, S.R.; Jin, H.Y.; Rhee, E.J.; Cho, Y.M.; Lee, B.W. Lobeglitazone, a novel thiazolidinedione, improves non-alcoholic fatty liver disease in type 2 diabetes: Its efficacy and predictive factors related to responsiveness. J. Korean Med. Sci. 2017, 32, 60–69. [Google Scholar] [CrossRef]
- Lefere, S.; Puengel, T.; Hundertmark, J.; Penners, C.; Frank, A.K.; Guillot, A.; de Muynck, K.; Heymann, F.; Adarbes, V.; Defrêne, E.; et al. Differential effects of selective- and pan-PPAR agonists on experimental steatohepatitis and hepatic macrophages. J. Hepatol. 2020, 73, 757–770. [Google Scholar] [CrossRef]
- Pennisi, G.; Celsa, C.; Enea, M.; Vaccaro, M.; Di Marco, V.; Ciccioli, C.; Infantino, G.; La Mantia, C.; Parisi, S.; Vernuccio, F.; et al. Effect of pharmacological interventions and placebo on liver histology in nonalcoholic steatohepatitis: A network meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2279–2288. [Google Scholar] [CrossRef]
- Agrawal, R. The first approved agent in the Glitazar’s Class: Saroglitazar. Curr. Drug Targets 2014, 15, 151–155. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Samajdar, S.S.; Das, S. Effects of saroglitazar in the treatment of non-alcoholic fatty liver disease or non-alcoholic steatohepatitis: A systematic review and meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2023, 47, 102174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Yan, Q.; Wu, W.H.; Zhao, Y.; Zhang, H.; Li, J. PPAR-alpha/gamma agonists, glucagon-like peptide-1 receptor agonists and metformin for non-alcoholic fatty liver disease: A network meta-analysis. J. Int. Med. Res. 2023, 51, 3000605231177191. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Murata, Y.; Saibara, T.; Nishioka, A.; Kariya, S.; Yoshida, S. Follow-up CT findings of tamoxifen-induced non-alcoholic steatohepatitis (NASH) of breast cancer patients treated with bezafibrate. Oncol. Rep. 2003, 10, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Hamada, N.; Ogawa, Y.; Saibara, T.; Murata, Y.; Kariya, S.; Nishioka, A.; Terashima, M.; Inomata, T.; Yoshida, S. Toremifene-induced fatty liver and NASH in breast cancer patients with breast-conservation treatment. Int. J. Oncol. 2000, 17, 1119–1123. [Google Scholar] [CrossRef]
- Franko, A.; Neschen, S.; Rozman, J.; Rathkolb, B.; Aichler, M.; Feuchtinger, A.; Brachthäuser, L.; Neff, F.; Kovarova, M.; Wolf, E.; et al. Bezafibrate ameliorates diabetes via reduced steatosis and improved hepatic insulin sensitivity in diabetic TallyHo mice. Mol. Metab. 2017, 6, 256–266. [Google Scholar] [CrossRef]
- Schmilovitz-Weiss, H.; Hochhauser, E.; Cohen, M.; Chepurko, Y.; Yitzhaki, S.; Grossman, E.; Leibowitz, A.; Ackerman, Z.; Ben-Ari, Z. Rosiglitazone and bezafibrate modulate gene expression in a rat model of non-alcoholic fatty liver disease—A historical prospective. Lipids Health Dis. 2013, 12, 41. [Google Scholar] [CrossRef]
- Nakagami, H.; Shimamura, M.; Miyake, T.; Shimosato, T.; Minobe, N.; Moritani, T.; Kiomy Osako, M.; Nakagami, F.; Koriyama, H.; Kyutoku, M.; et al. Nifedipine prevents hepatic fibrosis in a non-alcoholic steatohepatitis model induced by an L-methionine-and choline-deficient diet. Mol. Med. Rep. 2012, 5, 37–40. [Google Scholar] [CrossRef]
- Nakano, S.; Nagasawa, T.; Ijiro, T.; Inada, Y.; Tamura, T.; Maruyama, K.; Kuroda, J.; Yamazaki, Y.; Kusama, H.; Shibata, N. Bezafibrate prevents hepatic stellate cell activation and fibrogenesis in a murine steatohepatitis model, and suppresses fibrogenic response induced by transforming growth factor-beta1 in a cultured stellate cell line. Hepatol. Res. 2008, 38, 1026–1039. [Google Scholar] [CrossRef]
- Takahashi, Y.; Seko, Y.; Yamaguchi, K.; Takeuchi, K.; Yano, K.; Kataoka, S.; Moriguchi, M.; Itoh, Y. Gamma-glutamyl transferase predicts pemafibrate treatment response in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2023. [Google Scholar] [CrossRef]
- Morishita, A.; Oura, K.; Takuma, K.; Nakahara, M.; Tadokoro, T.; Fujita, K.; Tani, J.; Shi, T.; Himoto, T.; Tatsuta, M.; et al. Pemafibrate improves liver dysfunction and non-invasive surrogates for liver fibrosis in patients with non-alcoholic fatty liver disease with hypertriglyceridemia: A multicenter study. Hepatol. Int. 2023, 17, 606–614. [Google Scholar] [CrossRef]
- Hatanaka, T.; Kosone, T.; Saito, N.; Takakusagi, S.; Tojima, H.; Naganuma, A.; Takagi, H.; Uraoka, T.; Kakizaki, S. Effect of 48-week pemafibrate on non-alcoholic fatty liver disease with hypertriglyceridemia, as evaluated by the FibroScan-aspartate aminotransferase score. JGH Open 2021, 5, 1183–1189. [Google Scholar] [CrossRef]
- Sugimoto, R.; Iwasa, M.; Eguchi, A.; Tamai, Y.; Shigefuku, R.; Fujiwara, N.; Tanaka, H.; Kobayashi, Y.; Ikoma, J.; Kaito, M.; et al. Effect of pemafibrate on liver enzymes and shear wave velocity in non-alcoholic fatty liver disease patients. Front. Med. 2023, 10, 1073025. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Sugihara, T.; Kihara, T.; Matsuki, Y.; Nagahara, T.; Takata, T.; Kitao, S.; Okura, T.; Yamamoto, K.; Isomoto, H. Pemafibrate ameliorates liver dysfunction and fatty liver in patients with non-alcoholic fatty liver disease with hypertriglyceridemia: A retrospective study with the outcome after a mid-term follow-up. Diagnostics 2021, 11, 2316. [Google Scholar] [CrossRef]
- Boyer-Diaz, Z.; Aristu-Zabalza, P.; Andrés-Rozas, M.; Robert, C.; Ortega-Ribera, M.; Fernández-Iglesias, A.; Broqua, P.; Junien, J.L.; Wettstein, G.; Bosch, J.; et al. Pan-PPAR agonist lanifibranor improves portal hypertension and hepatic fibrosis in experimental advanced chronic liver disease. J. Hepatol. 2021, 74, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, S.; Dutta, A.; Chakraborty, S.B.D. Efficacy and safety of saroglitazar in real-world patients of non-alcoholic fatty liver disease with or without diabetes including compensated cirrhosis: A tertiary care center experience. JGH Open 2023, 7, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.R.; Bhoi, B.; Trivedi, C.; Rath, A.; Rathod, R.; Sharma, A.; Ranvir, R.; Kadam, S.; Ingale, K.; Patel, H.; et al. Saroglitazar suppresses the hepatocellular carcinoma induced by intraperitoneal injection of diethylnitrosamine in C57BL/6 mice fed on choline deficient, l-amino acid- defined, high-fat diet. BMC Cancer 2023, 23, 59. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Sakaida, I.; Tsuchiya, M.; Omori, K.; Takami, T.; Okita, K. Pioglitazone prevents hepatic steatosis, fibrosis, and enzyme-altered lesions in rat liver cirrhosis induced by a choline-deficient L-amino acid-defined diet. Biochem. Biophys. Res. Commun. 2004, 315, 187–195. [Google Scholar] [CrossRef]
- Huang, D.Q.; Singal, A.G.; Kono, Y.; Tan, D.J.H.; El-Serag, H.B.; Loomba, R. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab. 2022, 34, 969–977.e2. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef]
- Targher, G.; Tilg, H.; Byrne, C.D. Non-alcoholic fatty liver disease: A multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol. Hepatol. 2021, 6, 578–588. [Google Scholar] [CrossRef]
- Pipitone, R.M.; Ciccioli, C.; Infantino, G.; La Mantia, C.; Parisi, S.; Tulone, A.; Pennisi, G.; Grimaudo, S.; Petta, S. MAFLD: A multisystem disease. Ther. Adv. Endocrinol. Metab. 2023, 14, 20420188221145549. [Google Scholar] [CrossRef] [PubMed]
- Staels, B.; Butruille, L.; Francque, S. Treating NASH by targeting peroxisome proliferator-activated receptors. J. Hepatol. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
| Drug | NCT Number | Phase | Status | Participants | End Date | Liver Histology | Non-Invasive Tests | Blood Tests | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Steatosis | Ballooning | Inflammation | Fibrosis | Total Score | Fat Content | Stiffness | ALT | AST | ||||||
| PPARα/δ/γ pan agonist | ||||||||||||||
| Lanifibranor | NCT03008070 (https://classic.clinicaltrials.gov/ct2/show/NCT03008070) | 2 | Completed | 247 | 2020/3/16 | ↓ | ↓ | NS | ↓ | ↓ | ↓ | ↓ | ||
| PPARα/δ dual agonist | ||||||||||||||
| Elafibranor | NCT01694849 (https://classic.clinicaltrials.gov/ct2/show/NCT01694849) | 2 | Completed | 275 | 2015/12 | ↓ | ↓ | NS | ↓ | ↓ | ↓ | |||
| NCT02704403 (https://classic.clinicaltrials.gov/ct2/show/NCT02704403) | 3 | Terminated | 2157 | 2020/10/28 | NS | |||||||||
| NCT03883607 (https://classic.clinicaltrials.gov/ct2/show/NCT03883607) | 2 | Terminated | 10 | 2020/6/16 | No description (pharmacokinetics, pharmacodynamics, safety, and tolerability data only) | |||||||||
| NCT03953456 (https://classic.clinicaltrials.gov/ct2/show/NCT03953456) | 2 | Terminated | 17 | 2020/7/14 | No description | |||||||||
| PPARα/γ dual agonist | ||||||||||||||
| Saroglitazar | NCT02265276 (https://classic.clinicaltrials.gov/ct2/show/NCT02265276) | 3 | Unknown | 100 | 2015/9 | No description | ||||||||
| NCT03061721 (https://classic.clinicaltrials.gov/ct2/show/NCT03061721) | 2 | Completed | 106 | 2020/12/15 | ↓ | ↓ | ||||||||
| NCT03863574 (https://classic.clinicaltrials.gov/ct2/show/NCT03863574) | 2 | Completed | 16 | 2020/10/30 | No description | |||||||||
| NCT04193982 (https://classic.clinicaltrials.gov/ct2/show/NCT04193982) | 3 | Unknown | 250 | 2021/10/31 | No description | |||||||||
| PPARα agonists | ||||||||||||||
| Fenofibrate (α/γ) | NCT00252499 (https://classic.clinicaltrials.gov/ct2/show/NCT00252499) | NA | Terminated | 13 | 2010/8 | No description (protocol drug change required new clinicaltrails.gov entry) | ||||||||
| NCT00262964 (https://classic.clinicaltrials.gov/ct2/show/NCT00262964) | NA | Completed | 51 | 2008/12 | No description | |||||||||
| NCT01289639 (https://classic.clinicaltrials.gov/ct2/show/NCT01289639) | NA | Terminated | 11 | 2014/8 | No description | |||||||||
| NCT02354976 (https://classic.clinicaltrials.gov/ct2/show/NCT02354976) | 2 | Completed | 78 | 2016/5/26 | ↑ | NS | ↑ | |||||||
| NCT02781584 (https://classic.clinicaltrials.gov/ct2/show/NCT02781584) | 2 | Completed | 220 | 2020/12/17 | No description (safety and tolerability data only) | |||||||||
| NCT02891408 (https://classic.clinicaltrials.gov/ct2/show/NCT02891408) | 1 | Completed | 74 | 2019/5/13 | No description (pharmacokinetics data only) | |||||||||
| Pemafibrate | NCT03350165 (https://classic.clinicaltrials.gov/ct2/show/NCT03350165) | 2 | Completed | 118 | 2020/6/30 | NS | ↓ | ↓ | NS | |||||
| PPARδ agonist | ||||||||||||||
| Seladelpar | NCT03551522 (https://classic.clinicaltrials.gov/ct2/show/NCT03551522) | 2 | Terminated | 181 | 2020/8/10 | No description | ||||||||
| PPARγ agonists | ||||||||||||||
| Pioglitazone | NCT00013598 (https://classic.clinicaltrials.gov/ct2/show/NCT00013598) | 2 | Completed | 30 | 2004/3 | No description | ||||||||
| NCT00062764 (https://classic.clinicaltrials.gov/ct2/show/NCT00062764) | 2 | Completed | 18 | 2009/2 | No description | |||||||||
| NCT00063622 (https://classic.clinicaltrials.gov/ct2/show/NCT00063622) | 3 | Completed | 247 | 2009/9 | ↓ | ↓ | ↓ | NS | ↓ | ↓ | ||||
| NCT00227110 (https://classic.clinicaltrials.gov/ct2/show/NCT00227110) | 4 | Completed | 55 | 2006/1 | ↓ | ↓ | ↓ | NS | ↓ | ↓ | ↓ | |||
| NCT00441272 (https://classic.clinicaltrials.gov/ct2/show/NCT00441272) | 2 | Completed | 100 | NP | No description | |||||||||
| NCT00633282 (https://classic.clinicaltrials.gov/ct2/show/NCT00633282) | 2 | Completed | 184 | 2011/8 | No description (only in combination with lifestyle intervention; PMID: 26252777) | |||||||||
| NCT00994682 (https://classic.clinicaltrials.gov/ct2/show/NCT00994682) | 4 | Completed | 176 | 2014/12 | ↓ | ↓ | ↓ | NS | ↓ | ↓ | ↓ | ↓ | ||
| NCT01289639 (https://classic.clinicaltrials.gov/ct2/show/NCT01289639) | NA | Terminated | 11 | 2014/8 | No description (only baseline data published; PMID: 24360972, 24740208) | |||||||||
| NCT01002547 (https://classic.clinicaltrials.gov/ct2/show/NCT01002547) | 4 | Completed | 105 | 2016/12/31 | No description (only in combination with vitamin E; PMID: 31332029) | |||||||||
| NCT01068444 (https://classic.clinicaltrials.gov/ct2/show/NCT01068444) | 2 | Completed | 90 | 2020/7 | ↓ | NS | ↓ | NS | ↓ | ↓ | ↓ | ↓ | ||
| NCT01431521 (https://classic.clinicaltrials.gov/ct2/show/NCT01431521) | 1 | Completed | 31 | 2012/10/1 | No description | |||||||||
| NCT01703260 (https://classic.clinicaltrials.gov/ct2/show/NCT01703260) | 2 | Terminated | 20 | 2014/9 | No description | |||||||||
| NCT02265276 (https://classic.clinicaltrials.gov/ct2/show/NCT02265276) | 3 | Unknown | 100 | 2015/9 | No description | |||||||||
| NCT02365233 (https://classic.clinicaltrials.gov/ct2/show/NCT02365233) | 4 | Terminated | 5 | 2016/12/31 | No description | |||||||||
| NCT02875821 (https://classic.clinicaltrials.gov/ct2/show/NCT02875821) | 4 | Completed | 44 | 2017/6/7 | No description | |||||||||
| NCT03646292 (https://classic.clinicaltrials.gov/ct2/show/NCT03646292) | 4 | Unknown | 60 | 2021/2 | No description | |||||||||
| NCT03796975 (https://classic.clinicaltrials.gov/ct2/show/NCT03796975) | 4 | Completed | 120 | 2019/11/20 | No description | |||||||||
| NCT03910361 (https://classic.clinicaltrials.gov/ct2/show/NCT03910361) | 4 | Completed | 51 | 2020/7/2 | No description | |||||||||
| NCT03950505 (https://classic.clinicaltrials.gov/ct2/show/NCT03950505) | 4 | Unknown | 60 | 2020/12 | No description | |||||||||
| NCT05521633 (https://classic.clinicaltrials.gov/ct2/show/NCT05521633) | 3 | Completed | 96 | 2022/5/24 | No description | |||||||||
| Rosiglitazone | NCT00252499 (https://classic.clinicaltrials.gov/ct2/show/NCT00252499) | NA | Terminated | 13 | 2010/8 | No description (protocol drug change required new clinicaltrails.gov entry) | ||||||||
| NCT00492700 (https://classic.clinicaltrials.gov/ct2/show/NCT00492700) | 2 | Completed | 63 | NP | ↓ | NS | NS | NS | NS | ↓ | ↓ | |||
| NCT00699036 (https://classic.clinicaltrials.gov/ct2/show/NCT00699036) | 2 | Unknown | 165 | 2009/8 | No description | |||||||||
| NCT01406704 (https://classic.clinicaltrials.gov/ct2/show/NCT01406704) | 4 | Terminated | 26 | 2013/12 | No description | |||||||||
| Lobeglitazone | NCT02285205 (https://classic.clinicaltrials.gov/ct2/show/NCT02285205) | 4 | Completed | 38 | 2015/11 | ↓ | NS | ↓ | ↓ | |||||
| Drug | NCT Number | Phase | Status | Participants | Subject | Start Date | ESC Date |
|---|---|---|---|---|---|---|---|
| PPARα/δ/γ pan agonists | |||||||
| Chiglitazar | NCT05193916 (https://classic.clinicaltrials.gov/ct2/show/NCT05193916) | 2 | Recruiting | 100 | NASH with elevated triglyceride and insulin resistance | 2022/3/21 | (2023/11) |
| Lanifibranor | NCT03459079 (https://classic.clinicaltrials.gov/ct2/show/NCT03459079) | 2 | Recruiting | 54 | T2DM and NAFLD | 2018/8/14 | (2024/4/14) |
| NCT04849728 (https://classic.clinicaltrials.gov/ct2/show/NCT04849728) | 3 | Recruiting | 1000 | NASH with F2/F3 stage of liver fibrosis | 2021/8/19 | (2026/9/30) | |
| NCT05232071 (https://classic.clinicaltrials.gov/ct2/show/NCT05232071) | 2 | Recruiting | 63 | T2DM and NASH | 2022/6/29 | (2023/12/31) | |
| PPARα/γ dual agonist | |||||||
| Saroglitazar | NCT03617263 (https://classic.clinicaltrials.gov/ct2/show/NCT03617263) | 2 | Recruiting | 90 | NAFLD in women with polycystic ovarian syndrome | 2018/12/4 | (2024/7) |
| NCT03639623 (https://classic.clinicaltrials.gov/ct2/show/NCT03639623) | 2 | Recruiting | 15 | Liver transplant recipients with NAFLD | 2019/2/25 | 2023/6 | |
| NCT04469920 (https://classic.clinicaltrials.gov/ct2/show/NCT04469920) | 1 | Recruiting | 100 | NASH with advanced fibrosis | 2020/7/16 | (2024/10) | |
| NCT05011305 (https://classic.clinicaltrials.gov/ct2/show/NCT05011305) | 2 | Recruiting | 240 | NASH | 2021/8/18 | (2023/12) | |
| NCT05211284 (https://classic.clinicaltrials.gov/ct2/show/NCT05211284) | 2 | Recruiting | 160 | NASH with human immunodeficiency virus | 2022/9/26 | (2025/3/1) | |
| NCT05872269 (https://classic.clinicaltrials.gov/ct2/show/NCT05872269) | 4 | Not yet | 1500 | NAFLD with comorbidities (obesity, T2DM, dyslipidemia, or metabolic syndrome) | 2023/7/20 | (2025/6/10) | |
| PPARγ agonist | |||||||
| Pioglitazone | NCT04501406 (https://classic.clinicaltrials.gov/ct2/show/NCT04501406) | 2 | Recruiting | 166 | NASH in T2DM | 2020/12/15 | (2027/8/31) |
| NCT04976283 (https://classic.clinicaltrials.gov/ct2/show/NCT04976283) | 4 | Recruiting | 123 | Liver fat in T2DM and NAFLD | 2021/9/15 | (2023/11/15) | |
| NCT05254626 (https://classic.clinicaltrials.gov/ct2/show/NCT05254626) | 4 | Recruiting | 100 | NASH | 2022/8/1 | (2025/8) | |
| NCT05305287 (https://classic.clinicaltrials.gov/ct2/show/NCT05305287) | 4 | Recruiting | 60 | NAFLD in T2DM | 2022/11/1 | (2027/3/31) | |
| NCT05422092 (https://classic.clinicaltrials.gov/ct2/show/NCT05422092) | NA | Not yet | 80 | NAFLD in T2DM | 2022/9/20 | (2023/12) | |
| NCT05513729 (https://classic.clinicaltrials.gov/ct2/show/NCT05513729) | NA | Recruiting | 80 | NAFLD in T2DM | 2022/8/18 | (2024/3/1) | |
| NCT05605158 (https://classic.clinicaltrials.gov/ct2/show/NCT05605158) | 3 | Not yet | 56 | NASH | 2022/11 | (2024/11) | |
| NCT05813249 (https://classic.clinicaltrials.gov/ct2/show/NCT05813249) | 4 | Recruiting | 180 | NAFLD in obesity and/or T2DM | 2023/2/15 | (2024/8/15) | |
| NCT05942963 (https://classic.clinicaltrials.gov/ct2/show/NCT05942963) | 4 | Not yet | 240 | NAFLD and T2DM | (2023/10) | (2024/4) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamata, S.; Honda, A.; Ishii, I. Current Clinical Trial Status and Future Prospects of PPAR-Targeted Drugs for Treating Nonalcoholic Fatty Liver Disease. Biomolecules 2023, 13, 1264. https://doi.org/10.3390/biom13081264
Kamata S, Honda A, Ishii I. Current Clinical Trial Status and Future Prospects of PPAR-Targeted Drugs for Treating Nonalcoholic Fatty Liver Disease. Biomolecules. 2023; 13(8):1264. https://doi.org/10.3390/biom13081264
Chicago/Turabian StyleKamata, Shotaro, Akihiro Honda, and Isao Ishii. 2023. "Current Clinical Trial Status and Future Prospects of PPAR-Targeted Drugs for Treating Nonalcoholic Fatty Liver Disease" Biomolecules 13, no. 8: 1264. https://doi.org/10.3390/biom13081264
APA StyleKamata, S., Honda, A., & Ishii, I. (2023). Current Clinical Trial Status and Future Prospects of PPAR-Targeted Drugs for Treating Nonalcoholic Fatty Liver Disease. Biomolecules, 13(8), 1264. https://doi.org/10.3390/biom13081264






