Improving the Stability and Effectiveness of Immunotropic Squalene Nanoemulsion by Adding Turpentine Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation Procedure and Characterization
2.2.1. Preparation of the Emulsions
2.2.2. Analysis of Dispersed Phase
2.2.3. PH Measurements
2.2.4. Dynamic Viscosity
2.2.5. Dynamic Light Scattering (DLS) and ζ Potential
2.2.6. Nuclear Magnetic Resonance (NMR)
2.2.7. Thiobarbituric Acid-Reactive Substance Assay
2.2.8. Emulsion Stability Measurement
2.3. In Vitro Studies
2.3.1. Cell Cultures
2.3.2. Cytotoxicity Assay
2.3.3. Genotoxicity Assay
2.3.4. Hemolysis Assay
2.3.5. Phagocytosis Assay
2.3.6. Mitogen Stimulation Assays (MSAs) on Proliferation Mononuclear Cells
2.4. In Vivo Studies
2.4.1. Animals
2.4.2. Cytokine Measurement by ELISA
2.4.3. Immunoglobulin Production Assay by ELISA
2.4.4. Histamine Production Assay by ELISA
2.4.5. Maximum Injectable Dose (MID) Studies
2.4.6. Xenospecific Antibody Measurement Using ELISA
2.5. Statistical Analysis
3. Results
3.1. Results of Preparation and Physicochemical Characterization of Emulsions
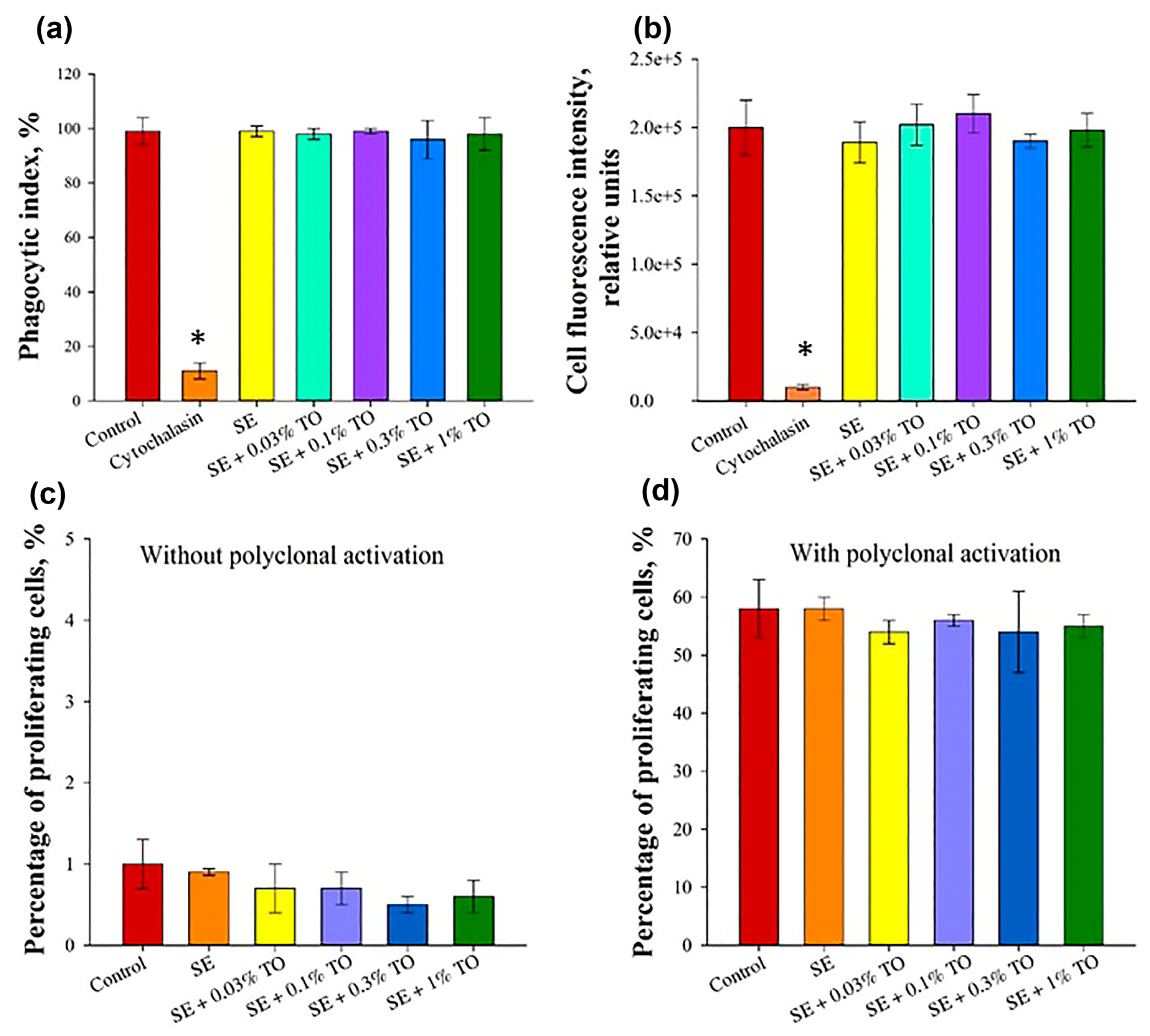
3.2. Results of Safety Studies of Emulsions
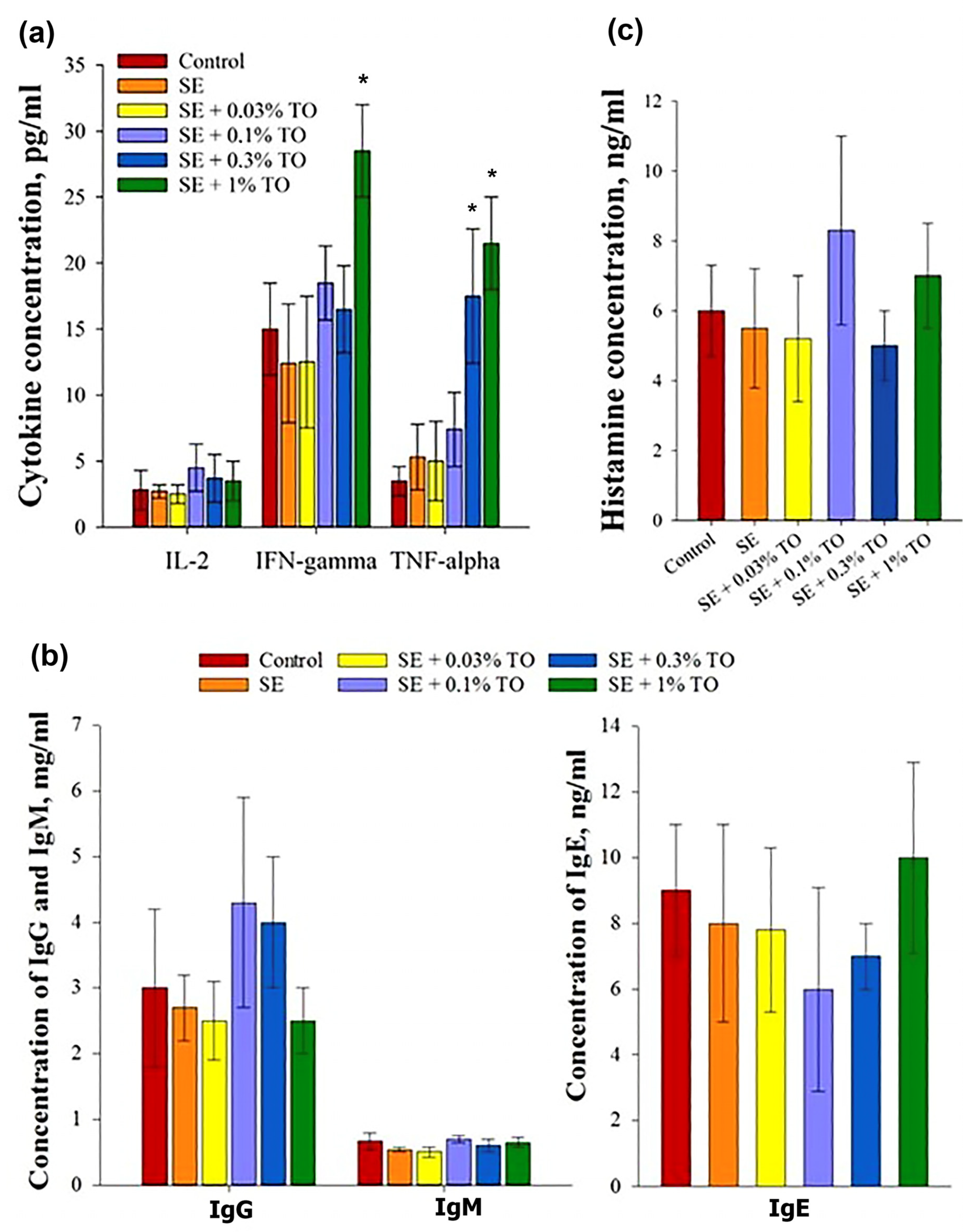
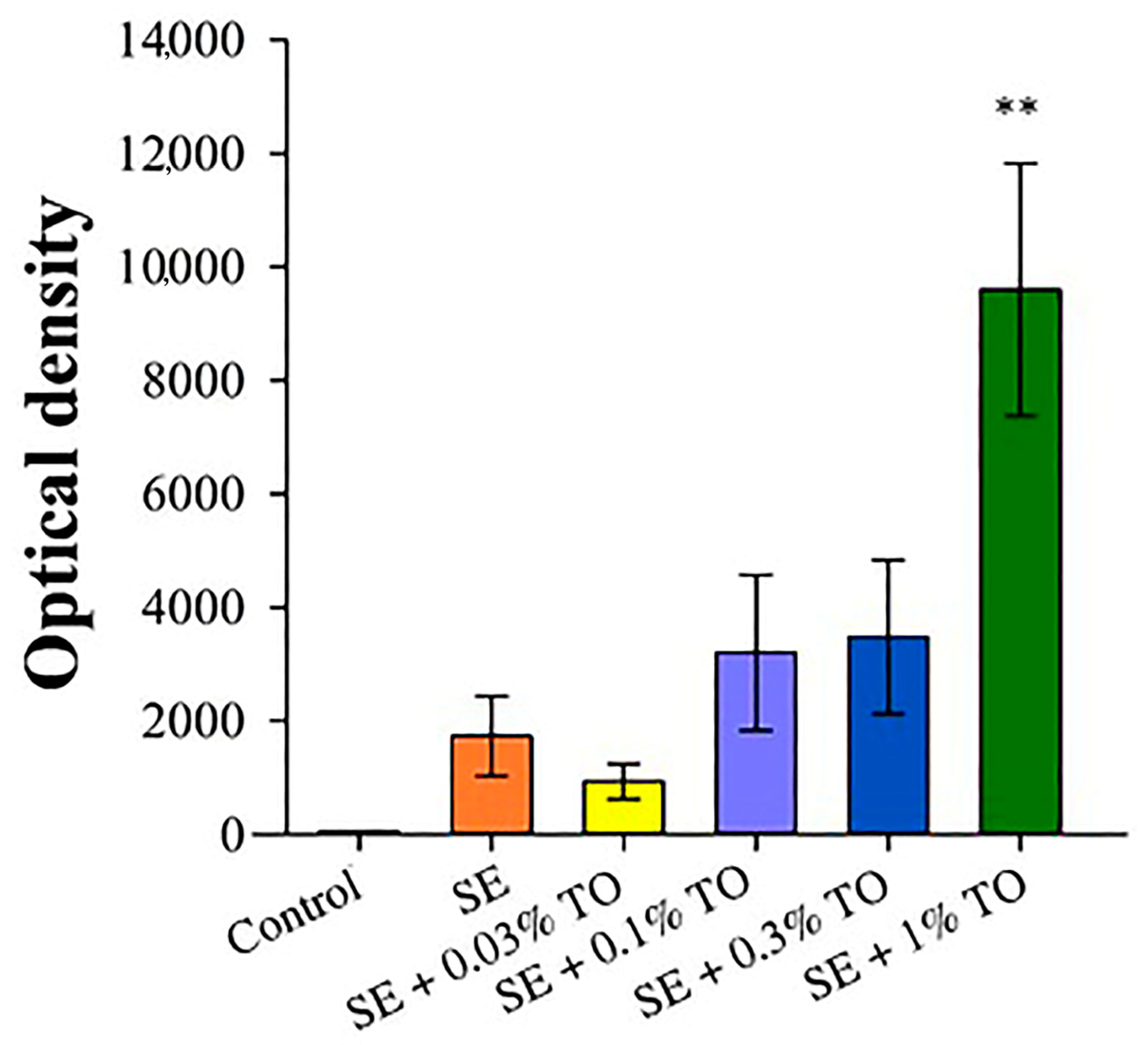
3.3. Results of Emulsion Effectiveness Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feyaerts, A.F.; Luyten, W.; Van Dijck, P. Striking essential oil: Tapping into a largely unexplored source for drug discovery. Sci. Rep. 2020, 10, 2867. [Google Scholar] [CrossRef]
- Salakhutdinov, N.F.; Volcho, K.P.; Yarovaya, O.I. Monoterpenes as a renewable source of biologically active compounds. Pure Appl. Chem. 2017, 89, 1105–1117. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Mendes, A.; Azevedo-Silva, J.; Fernandes, J.C. From Sharks to Yeasts: Squalene in the Development of Vaccine Adjuvants. Pharmaceuticals 2022, 15, 265. [Google Scholar] [CrossRef]
- Micera, M.; Botto, A.; Geddo, F.; Antoniotti, S.; Bertea, C.M.; Levi, R.; Gallo, M.P.; Querio, G. Squalene: More than a Step toward Sterols. Antioxidants 2020, 9, 688. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Karadeniz, F. Biological Importance and Applications of Squalene and Squalane. Adv. Food Nutr. Res. 2012, 65, 223–233. [Google Scholar] [PubMed]
- Cárdeno, A.; Aparicio-Soto, M.; Montserrat-de la Paz, S.; Bermudez, B.; Muriana, F.J.G.; Alarcón-de-la-Lastra, C. Squalene Targets Pro- and Anti-Inflammatory Mediators and Pathways to Modulate over-Activation of Neutrophils, Monocytes and Macrophages. J. Funct. Foods 2015, 14, 779–790. [Google Scholar] [CrossRef]
- Dormont, F.; Brusini, R.; Cailleau, C.; Reynaud, F.; Peramo, A.; Gendron, A.; Mougin, J.; Gaudin, F.; Varna, M.; Couvreur, P. Squalene-based multidrug nanoparticles for improved mitigation of uncontrolled inflammation in rodents. Sci. Adv. 2020, 6, eaaz5466. [Google Scholar] [CrossRef]
- Sobot, D.; Mura, S.; Yesylevskyy, S.O.; Dalbin, L.; Cayre, F.; Bort, G.; Mougin, J.; Desmaële, D.; Lepetre-Mouelhi, S.; Pieters, G.; et al. Conjugation of Squalene to Gemcitabine as Unique Approach Exploiting Endogenous Lipoproteins for Drug Delivery. Nat. Commun. 2017, 8, 15678. [Google Scholar] [CrossRef]
- Nguyen-Contant, P.; Sangster, M.Y.; Topham, D.J. Squalene-Based Influenza Vaccine Adjuvants and Their Impact on the Hemagglutinin-Specific B Cell Response. Pathogens 2021, 10, 355. [Google Scholar] [CrossRef] [PubMed]
- Billiau, A.; Matthys, P. Modes of action of Freund’s adjuvants in experimental models of autoimmune diseases. J. Leukoc. Biol. 2001, 70, 849–860. [Google Scholar] [CrossRef]
- Lattanzi, M. Non-recent history of influenza pandemics, vaccines, and adjuvants. In Influenza Vaccines for the Future; Rappuoli, R., Del Giudice, G., Eds.; Birkhäuser: Basel, Switzerland, 2008; pp. 245–259. [Google Scholar]
- Shi, S.; Zhu, H.; Xia, X.; Liang, Z.; Ma, X.; Sun, B. Vaccine adjuvants: Understanding the structure and mechanism of adjuvanticity. Vaccine 2019, 37, 3167–3178. [Google Scholar] [CrossRef]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef]
- Garcon, N.; Vaughn, D.W.; Didierlaurent, A.M. Development and evaluation of AS03, an Adjuvant System containing alphatocopherol and squalene in an oil-in-water emulsion. Expert. Rev. Vaccines 2012, 11, 349–366. [Google Scholar] [CrossRef]
- Markets and Markets. Squalene Market by Source Type (Animal Source (Shark Liver Oil), Vegetable Source (Olive Oil, Palm Oil, Amaranth Oil), Biosynthetic (GM Yeast]), End-Use Industry (Cosmetics, Food, and Pharmaceuticals), and Region—Global Forecast to 2025; Markets and Markets: Hong Kong, China, 2020. [Google Scholar]
- Tsujimoto, M. About Kuroko-Zame Shark Oil. J. Soc. Chem. Ind. 1916, 9, 953–958. [Google Scholar]
- Gohil, N.; Bhattacharjee, G.; Khambhati, K.; Braddick, D.; Singh, V. Engineering Strategies in Microorganisms for the Enhanced Production of Squalene: Advances, Challenges and Opportunities. Front. Bioeng. Biotechnol. 2019, 7, 50. [Google Scholar] [CrossRef]
- Paramasivan, K.; Rajagopal, K.; Mutturi, S. Studies on Squalene Biosynthesis and the Standardization of Its Extraction Methodology from Saccharomyces Cerevisiae. Appl. Biochem. Biotechnol. 2019, 187, 691–707. [Google Scholar] [CrossRef]
- McPhee, D.; Pin, A.; Kizer, L.; Perelman, L. Deriving Renewable Squalane from Sugarcane. Cosmet. Toilet. Mag. 2014, 129, 1–6. [Google Scholar]
- Meadows, A.L.; Hawkins, K.M.; Tsegaye, Y.; Antipov, E.; Kim, Y.; Raetz, L.; Dahl, R.H.; Tai, A.; Mahatdejkul-Meadows, T.; Xu, L.; et al. Rewriting Yeast Central Carbon Metabolism for Industrial Isoprenoid Production. Nature 2016, 537, 694–697. [Google Scholar] [CrossRef]
- Paramasivan, K.; Gupta, N.; Mutturi, S. Adaptive Evolution of Engineered Yeast for Squalene Production Improvement and Its Genome-Wide Analysis. Yeast 2021, 38, 424–437. [Google Scholar] [CrossRef]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef] [PubMed]
- Loparev, V.I.; Yurov, K.P. Adjuvant for Viral Vaccines. USSR Patent No 1620108, 15 January 1991. [Google Scholar]
- Tynyo, Y.Y.; Novikov, V.E.; Yarygina, E.I.; Ustinova, V.A. Method and Device for Obtaining Stable Ultra-Dispersed Aqueous Lyosols of Turpentine Oil with Specified Dispersion Parameters. RF Patent No 2566068, 20 October 2015. [Google Scholar]
- Tynyo, Y.Y.; Kochish, I.I.; Novikov, V.E. Obtaining aqueous lyosols of triterpenes for use as adjuvants for vaccines. Rep. Rus. Acad. Agric. Sci. 2015, 6, 57–60. [Google Scholar]
- Tynyo, Y.Y.; Yarygina, E.I.; Prokhorova, L.G.; Morozova, G.V.; Novikova, A.V. History and prospects for the development of new adjuvants. Veterinary 2016, 3, 23–27. [Google Scholar]
- Tynyo, Y.Y.; Yarygina, E.I.; Morozova, G.V.; Ustinova, V.A.; Vidrashko, M. Immunogenic activity of the rabies vaccine with an adjuvant based on nanoparticles of triterpene compounds. Int. Bull. Vet. Med. 2016, 2, 11–14. [Google Scholar]
- Hartwig, A. Turpentine Oil. MAK-Collect. Occup. Health Saf. 2019, 4, 128–147. [Google Scholar]
- Sagunski, H.; Heinzow, B. Richtwerte fur die Innenraumluft: Bicyclische Terpene (Leitsubstanz a-Pinen). Bundesgesundheitsblatt-Gesundheitsforsch.-Gesundh. 2003, 46, 346–352. [Google Scholar] [CrossRef]
- Lukowicz, T.; Company Maldonado, R.; Molinier, V.; Aubry, J.-M.; Nardello-Rataj, V. Fragrance solubilization in temperature insensitive aqueous microemulsions based on synergistic mixtures of nonionic and anionic surfactants. Colloids Surf. 2014, 458, 85–95. [Google Scholar] [CrossRef]
- GOST 1571-82; Spirits of Turpentine. Specifications; Approved 7 Jaunary 1983. Standards Publishing: Moscow, Russia, 1983.
- Utegenova, G.A.; Pallister, K.B.; Kushnarenko, S.V.; Özek, G.; Özek, T.; Abidkulova, K.T.; Kirpotina, L.N.; Schepetkin, I.A.; Quinn, M.T.; Voyich, J.M. Chemical Composition and Antibacterial Activity of Essential Oils from Ferula L. Species against Methicillin-Resistant Staphylococcus aureus. Molecules 2018, 23, 1679. [Google Scholar] [CrossRef]
- Yang, C.; Hu, D.H.; Feng, Y. Antibacterial activity and mode of action of the Artemisia capillaris essential oil and its constituents against respiratory tract infection-causing pathogens. Mol. Med. Rep. 2015, 11, 2852–2860. [Google Scholar] [CrossRef]
- Nikitina, L.E.; Startseva, V.A.; Vakulenko, I.A.; Krishmatulina, I.M.; Lisovskaya, S.A.; Glushko, N.P.; Fassakov, R.S. Synthesis and antifungal activity of compounds of the pinane series. Pharm. Chem. J. 2009, 43, 251–254. [Google Scholar] [CrossRef]
- Bouzenna, H.; Hfaiedh, N.; Giroux-Metges, M.A.; Elfeki, A.; Talarmin, H. Potential protective effects of alpha-pinene against cytotoxicity caused by aspirin in the IEC-6 cells. Biomed Pharm. 2017, 93, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Lee, H.J.; Jeon, Y.D.; Han, Y.H.; Kee, J.Y.; Kim, H.J.; Shin, H.J.; Kang, J.; Lee, B.S.; Kim, S.H.; et al. Alpha-Pinene Exhibits Anti-Inflammatory Activity through the Suppression of MAPKs and the NF-κB Pathway in Mouse Peritoneal Macrophages. Am. J. Chin. Med. 2015, 43, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, R.; Kanimozhi, G.; Prasad, N.R.; Agilan, B.; Ganesan, M.; Srithar, G. Alpha pinene modulates UVA-induced oxidative stress, DNA damage and apoptosis in human skin epidermal keratinocytes. Life Sci. 2018, 212, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Porres-Martínez, M.; González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. In vitro neuroprotective potential of the monoterpenes α-pinene and 1,8-cineole against H2O2-induced oxidative stress in PC12 cells. Z. Nat. C J. Biosci. 2016, 71, 191–199. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, Y.; Zhu, Y.; Zhou, B.; Ren, C.; Liang, S.; Guo, Y. α-Pinene Induces Apoptotic Cell Death via Caspase Activation in Human Ovarian Cancer Cells. Med. Sci. Monit. 2019, 25, 6631–6638. [Google Scholar] [CrossRef]
- de Sousa Eduardo, L.; Farias, T.C.; Ferreira, S.B.; Ferreira, P.B.; Lima, Z.N.; Ferreira, S.B. Antibacterial Activity and Time-kill Kinetics of Positive Enantiomer of α-pinene against Strains of Staphylococcus aureus and Escherichia coli. Curr. Top. Med. Chem. 2018, 18, 917–924. [Google Scholar] [CrossRef]
- Rivas da Silva, A.C.; Lopes, P.M.; Barros de Azevedo, M.M.; Costa, D.C.; Alviano, C.S.; Alviano, D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef]
- de Macedo Andrade, A.C.; Rosalen, P.L.; Freires, I.A.; Scotti, L.; Scotti, M.T.; Aquino, S.G.; de Castro, R.D. Antifungal Activity, Mode of Action, Docking Prediction and Anti-biofilm Effects of (+)-beta-pinene Enantiomers against Candida spp. Curr. Top. Med. Chem. 2018, 18, 2481–2490. [Google Scholar] [CrossRef]
- Ložienė, K.; Švedienė, J.; Paškevičius, A.; Raudonienė, V.; Sytar, O.; Kosyan, A. Influence of plant origin natural α-pinene with different enantiomeric composition on bacteria, yeasts and fungi. Fitoterapia 2018, 27, 20–24. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, N.; Zu, Y.; Fu, Y. Comparative anti-infectious bronchitis virus (IBV) activity of (−)-pinene: Effect on nucleocapsid (N) protein. Molecules 2011, 16, 1044–1054. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- van Zyl, R.L.; Seatlholo, S.T.; van Vuuren, S.F.; Viljoen, A.M. The Biological Activities of 20 Nature Identical Essential Oil Constituents. J. Essent. Oil Res. 2006, 18, 129–133. [Google Scholar] [CrossRef]
- Baik, J.S.; Kim, S.S.; Lee, J.A.; Oh, T.H.; Kim, J.Y.; Lee, N.H.; Hyun, C.G. Chemical composition and biological activities of essential oils extracted from Korean endemic citrus species. J. Microbiol. Biotechnol. 2008, 18, 74–79. [Google Scholar] [PubMed]
- Schepetkin, I.A.; Kushnarenko, S.V.; Özek, G.; Kirpotina, L.N.; Sinharoy, P.; Utegenova, G.A.; Abidkulova, K.T.; Özek, T.; Başer, K.H.; Kovrizhina, A.R.; et al. Modulation of Human Neutrophil Responses by the Essential Oils from Ferula akitschkensis and Their Constituents. J. Agric. Food Chem. 2016, 64, 7156–7170. [Google Scholar] [CrossRef]
- Shu, H.; Chen, H.; Wang, X.; Hu, Y.; Yun, Y.; Zhong, Q.; Chen, W.; Chen, W. Antimicrobial Activity and Proposed Action Mechanism of 3-Carene against Brochothrix thermosphacta and Pseudomonas fluorescens. Molecules 2019, 24, 3246. [Google Scholar] [CrossRef]
- Yang, J.; Choi, W.-S.; Kim, K.-J.; Eom, C.-D.; Park, M.-J. Investigation of Active Anti-Inflammatory Constituents of Essential Oil from Pinus koraiensis (Sieb. et Zucc.) Wood in LPS-Stimulated RBL-2H3 Cells. Biomolecules 2021, 11, 817. [Google Scholar] [CrossRef]
- Barkin, R.L. The Pharmacology of Topical Analgesics. Postgrad. Med. 2013, 125, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Van Nest, G.; Ott, G.; Barchfeld, G. Adjuvant Formulation Comprising a Submicron Oil Droplet Emulsion. U.S. Patent 6,299,884 B1, 9 October 2001. [Google Scholar]
- The Ministry of Health. Labour and Welfare (厚生労働省). Available online: https://www.mhlw.go.jp/shingi/2010/01/dl/s0115-7z.pdf (accessed on 21 December 2021).
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Shubina, V.S.; Kozina, V.I.; Shatalin, Y.V. Comparison of Antioxidant Properties of a Conjugate of Taxifolin with Glyoxylic Acid and Selected Flavonoids. Antioxidants 2021, 10, 1262. [Google Scholar] [CrossRef]
- United States Pharmacopoeia, 40 NF 35; The United States Pharmacopoeial Convention Inc.: Rockville, MD, USA, 2017; p. 6066.
- European Pharmacopoeia 8.0; Council of Europe: Strasbourg, France, 2014; p. 3181.
- The Russian Federation Pharmacopoeia. OFS.1.4.1.0007.15 Dosage Forms for Parenteral Use [Electronic Resource]. Available online: https://pharmacopoeia.ru/ofs-1-4-1-0007-15-lekarstvennye-formy-dlya-parenteralnogo-primeneniya/?amp=1 (accessed on 7 March 2023).
- The Russian Federation Pharmacopoeia. OFS.1.4.1.0017.15 Emulsions [Electronic Resource]. Available online: https://pharmacopoeia.ru/ofs-1-4-1-0017-15-ofs-1-4-1-0017-15/?amp=1 (accessed on 7 March 2023).
- Brosseron, F.; May, C.; Schoenebeck, B.; Tippler, B.; Woitalla, D.; Kauth, M.; Brockmann, K.; Meyer, H.E.; Berg, D.; Bufe, A.; et al. Stepwise isolation of human peripheral erythrocytes, T lymphocytes, and monocytes for blood cell proteomics. Proteomics Clin. Appl. 2012, 6, 497–501. [Google Scholar] [CrossRef]
- Houthuys, E.; Movahedi, K.; De Baetselier, P.; Van Ginderachter, J.A.; Brouckaert, P. A method for the isolation and purification of mouse peripheral blood monocytes. J. Immunol. Methods 2010, 359, 1–10. [Google Scholar] [CrossRef]
- García-Rodríguez, A.; Rubio, L.; Vila, L.; Xamena, N.; Velázquez, A.; Marcos, R.; Hernández, A. The Comet Assay as a Tool to Detect the Genotoxic Potential of Nanomaterials. Nanomaterials 2019, 9, 1385. [Google Scholar] [CrossRef]
- Lomovskaya, Y.V.; Kobyakova, M.I.; Senotov, A.S.; Lomovsky, A.I.; Minaychev, V.V.; Fadeeva, I.S.; Shtatnova, D.Y.; Krasnov, K.S.; Zvyagina, A.I.; Akatov, V.S.; et al. Macrophage-like THP-1 Cells Derived from High-Density Cell Culture Are Resistant to TRAIL-Induced Cell Death via Down-Regulation of Death-Receptors DR4 and DR5. Biomolecules 2022, 12, 150. [Google Scholar] [CrossRef]
- Teterina, A.Y.; Smirnov, I.V.; Fadeeva, I.S.; Fadeev, R.S.; Smirnova, P.V.; Minaychev, V.V.; Kobyakova, M.I.; Fedotov, A.Y.; Barinov, S.M.; Komlev, V.S. Octacalcium Phosphate for Bone Tissue Engineering: Synthesis, Modification, and In Vitro Biocompatibility Assessment. Int. J. Mol. Sci. 2021, 22, 12747. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Meiselman, H.J. Blood rheology and hemodynamics. Semin. Thromb. Hemost. 2003, 29, 435–450. [Google Scholar]
- Levy, G.; Jusko, W.J. Effect of Viscosity on Drug Absorption. J. Pharm. Sci. 1965, 54, 219–225. [Google Scholar] [CrossRef]
- Shimomura, T.; Sekiguchi, M.; Honda, R.; Yamazaki, M.; Yokoyama, M.; Uchiyama, S. Estimation of the Viscosity of an Antibody Solution from the Diffusion Interaction Parameter. Biol. Pharm. Bull. 2022, 45, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Haensler, J. Manufacture of Oil-in-Water Emulsion Adjuvants. Methods Mol. Biol. 2017, 1494, 165–180. [Google Scholar]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.W.; Gao, P.C. 3—Emulsions and Microemulsions for Topical and Transdermal Drug Delivery. In Handbook of Non-Invasive Drug Delivery Systems; Kulkarni, V.S., Ed.; William Andrew Publishing: Norwich, NY, USA, 2010; pp. 59–94. ISBN 9780815520252. [Google Scholar] [CrossRef]
- Fisher, K.J.; Kinsey, R.; Mohamath, R.; Phan, T.; Liang, H.; Orr, M.T.; Lykins, W.R.; Guderian, J.A.; Bakken, J.; Argilla, D.; et al. Semi-synthetic terpenoids with differential adjuvant properties as sustainable replacements for shark squalene in vaccine emulsions. NPJ Vaccines 2023, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Cai, W.; Jin, F. A novel oil-in-water emulsion as a potential adjuvant for influenza vaccine: Development, characterization, stability and in vivo evaluation. Int. J. Pharm. 2014, 468, 187–195. [Google Scholar] [CrossRef]
- Shimizu, N.; Ito, J.; Kato, S.; Otoki, Y.; Goto, M.; Eitsuka, T.; Miyazawa, T.; Nakagawa, K. Oxidation of squalene by singlet oxygen and free radicals results in different compositions of squalene monohydroperoxide isomers. Sci. Rep. 2018, 8, 9116. [Google Scholar] [CrossRef]
- Fooshee, D.R.; Aiona, P.K.; Laskin, A.; Laskin, J.; Nizkorodov, S.A.; Baldi, P.F. Atmospheric Oxidation of Squalene: Molecular Study Using COBRA Modeling and High-Resolution Mass Spectrometry. Environ. Sci. Technol. 2015, 49, 13304–13313. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.; Seaman, M.; Tonks, P.; Wegmann, F.; Seilly, D.; Frost, S.; LaBranche, C.C.; Montefiori, D.C.; Dey, A.K.; Srivastava, I.K.; et al. Mixed adjuvant formulations reveal a new combination that elicit antibody response comparable to Freund’s adjuvants. PLoS ONE 2012, 7, e35083. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.B.; Kramer, R.M.; Barnes, L.V.; Dowling, Q.M.; Vedvick, T.S. Working together: Interactions between vaccine antigens and adjuvants. Ther. Adv. Vaccines 2013, 1, 7–20. [Google Scholar] [CrossRef] [PubMed]
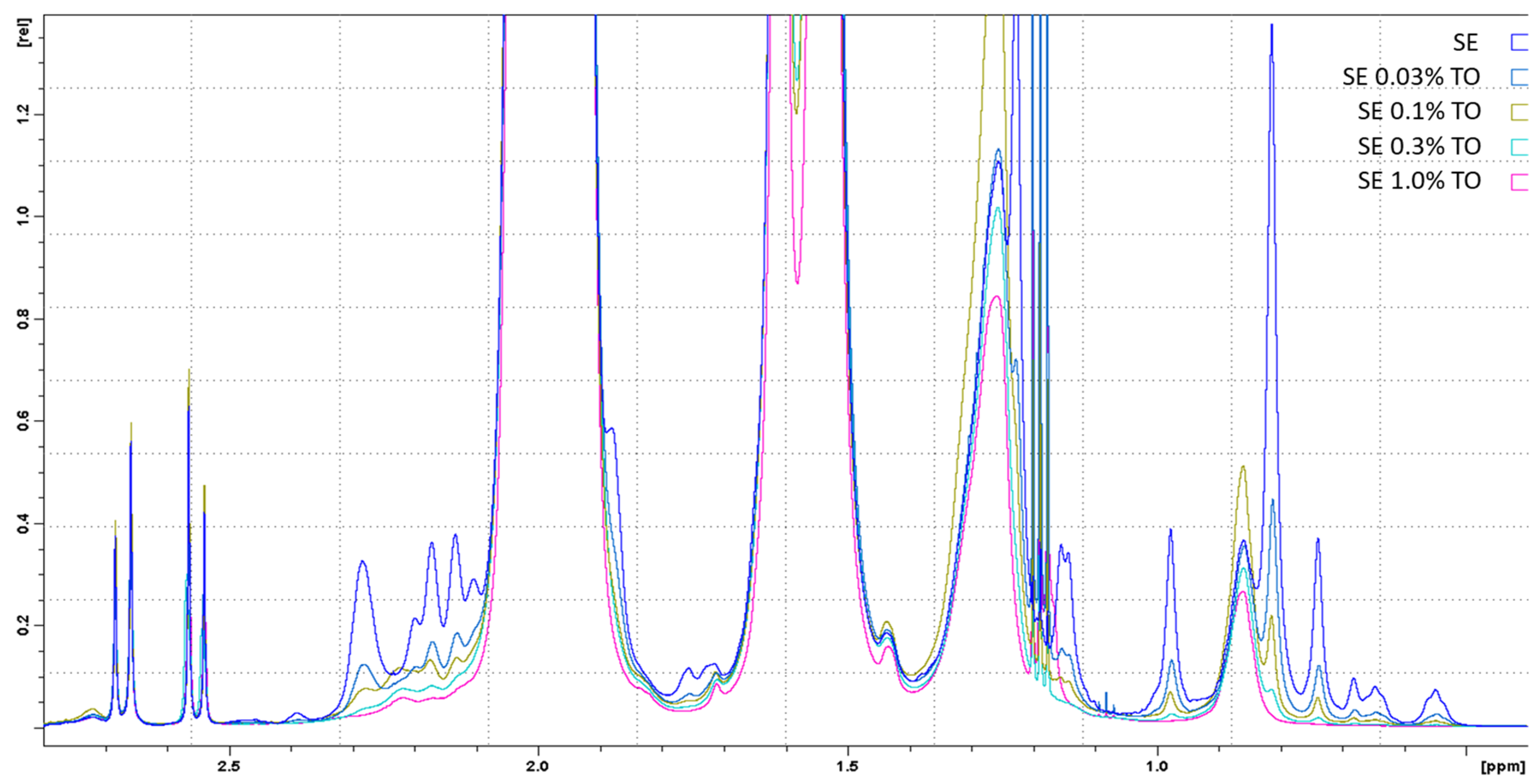
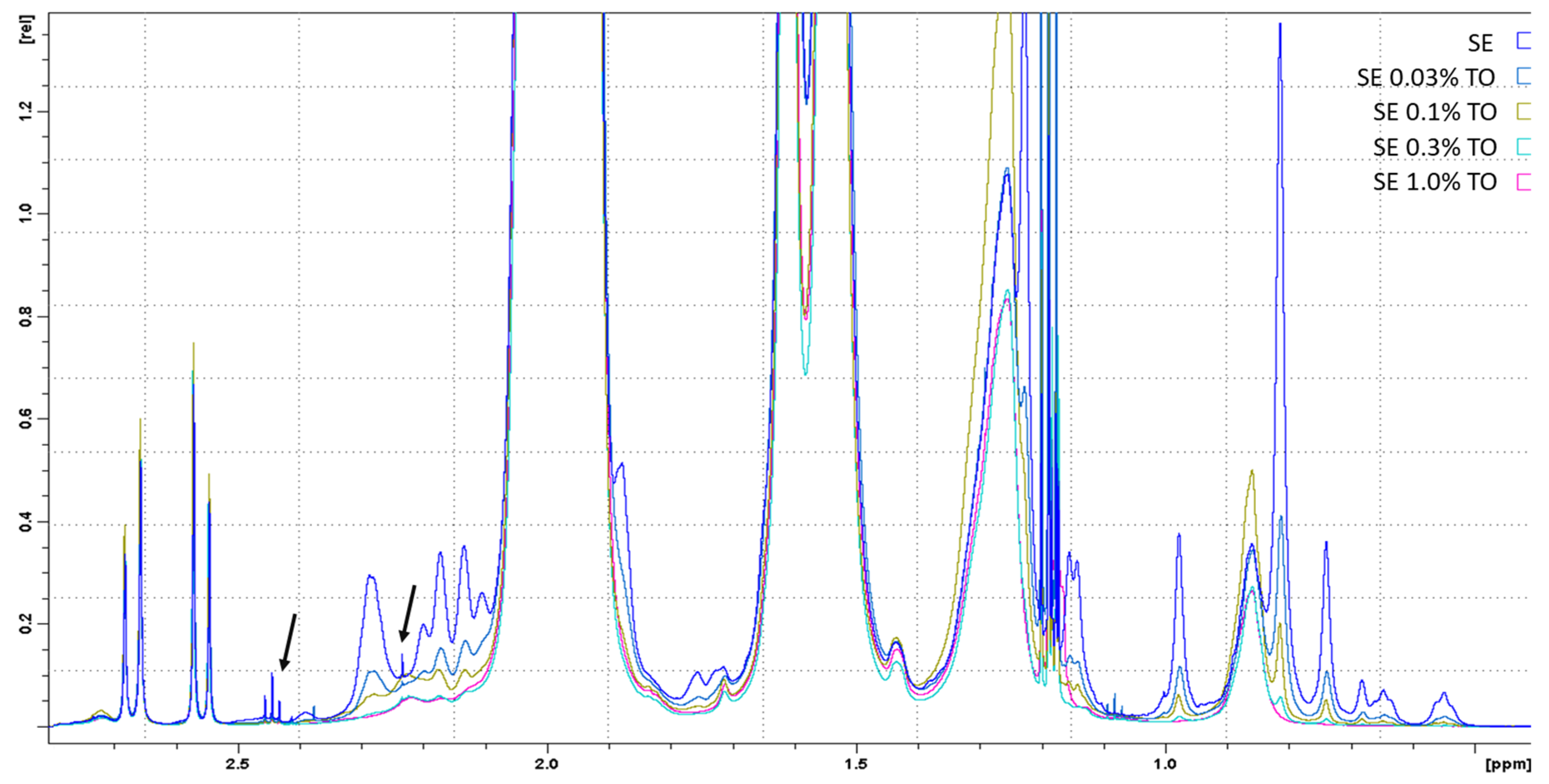
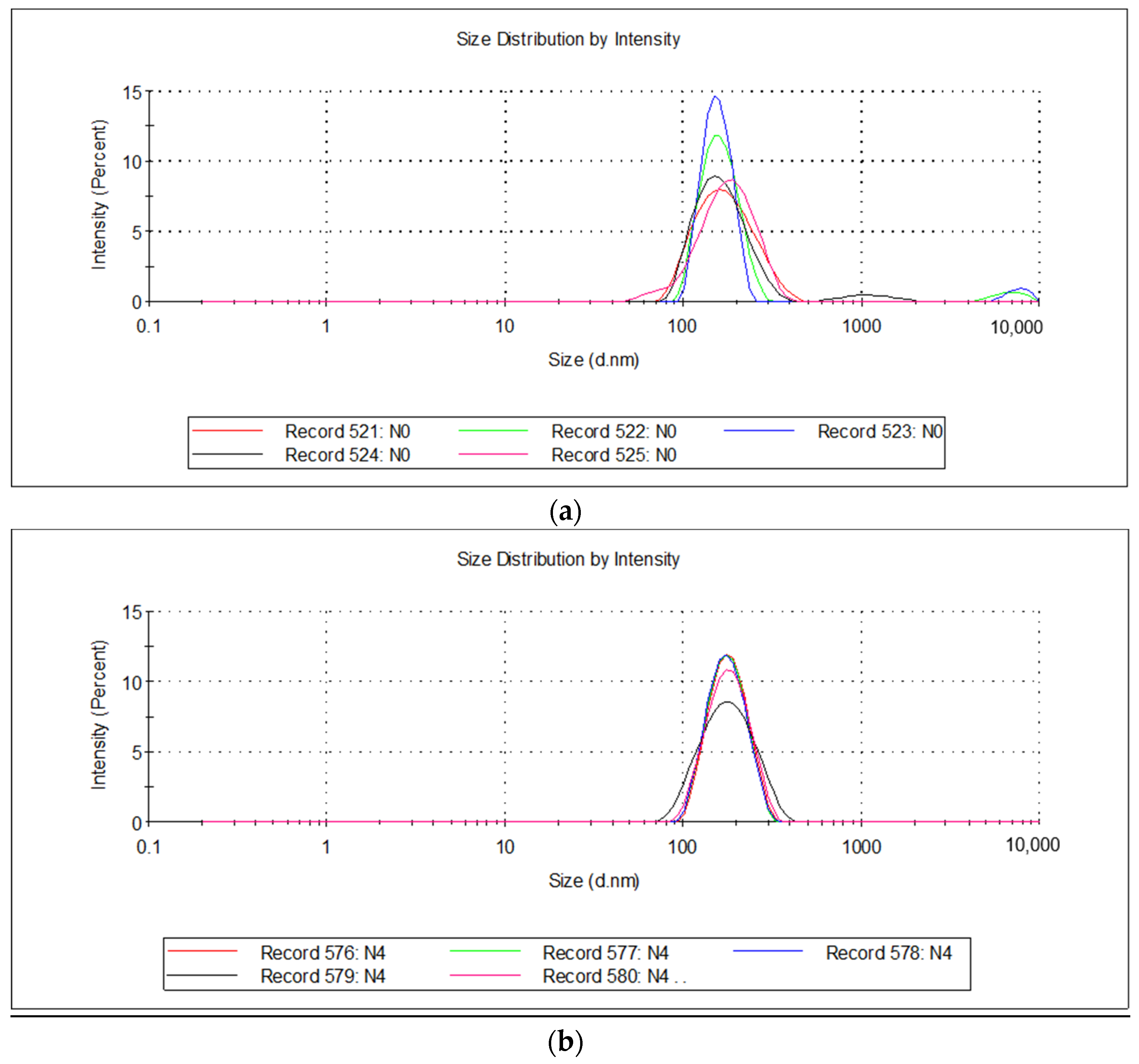
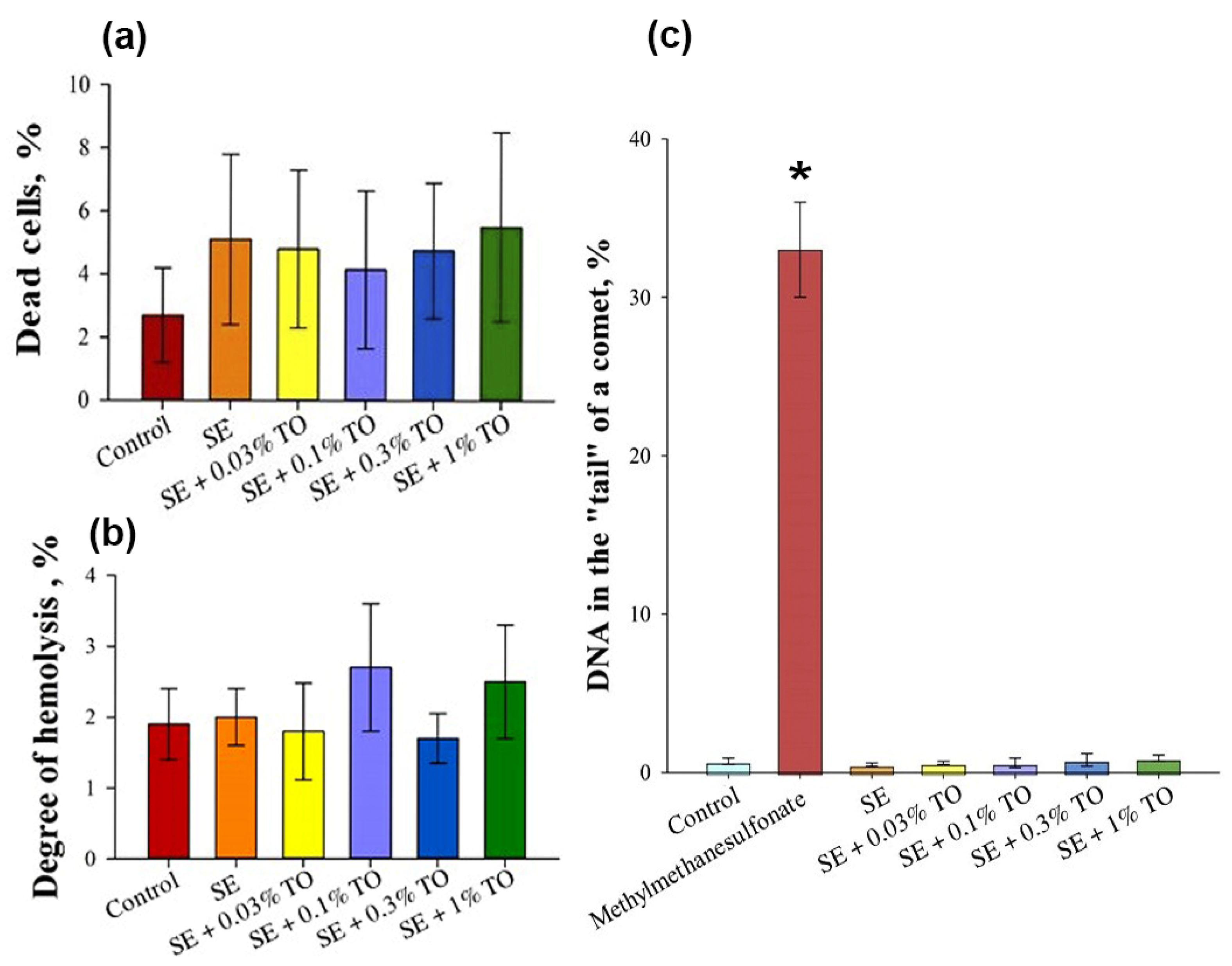
| Emulsion | % Water/Oil | PH | Dynamic Viscosity, cPs | Particle Size, nm | ζ-Potential, mV |
|---|---|---|---|---|---|
| Squalene emulsion (SE) | 95/5 | 6.01 ± 0.01 | 1.194 ± 0.009 | 175.4 ± 54.57 | −3.34 ± 1.16 |
| SE + 0.03% turpentine oil (TO) | 95/5 | 5.99 ± 0.01 | 1.148 ± 0.008 | 181.5 ± 51.97 | −3.19 ± 1.43 |
| SE + 0.1% TO | 94/6 | 5.97 ± 0.01 | 1.194 ± 0.005 | 169.1 ± 43.49 | −3.01 ± 0.6 |
| SE + 0.3% TO | 94/6 | 5.98 ± 0.01 | 1.176 ± 0.005 | 174.4 ± 58.84 | −3.36 ± 0.69 |
| SE + 1.0% TO | 95/5 | 5.98 ± 0.01 | 1.186 ± 0.005 | 178.4 ± 49.41 | −2.55 ± 0.88 |
| Emulsion | t0min | t30min | t24h |
|---|---|---|---|
| SE | 1.15 ± 0.14 | 8.28 ± 0.44 | 16.45 ± 2.07 *** |
| SE + 0.03% TO | 1.34 ± 0.22 | 11.31 ± 0.30 | 12.56 ± 1.14 |
| SE + 0.1% TO | 1.82 ± 0.19 | 12.90 ± 0.56 | 15.82 ± 1.05 * |
| SE + 0.3% TO | 1.37 ± 0.13 | 27.39 ± 0.80 | 29.06 ± 2.54 |
| SE + 1.0% TO | 1.95 ± 0.21 | 23.55 ± 1.10 | 29.13 ± 3.17 * |
| 10% lecithin emulsion | 1.28 ± 0.10 | 67.91 ± 2.52 | 78.15 ± 5.11 * |
| Emulsion | PH | Dynamic Viscosity, cPs | Particle Size, nm | ζ-Potential, mV * | ||
|---|---|---|---|---|---|---|
| 12 Month | 24 Month | 12 Month | 24 Month | |||
| SE | 6.00 ± 0.01 | 1.193 ± 0.009 | 169.8 ± 14.9 | 162.2 ± 16.1 | −4.05 ± 0.42 | −5.46 ± 0.75 |
| SE + 0.03% TO | 5.99 ± 0.01 | 1.147 ± 0.009 | 175.3 ± 16.1 | 172.0 ± 5.9 | −4.82 ± 0.66 | −7.23 ± 0.54 |
| SE + 0.1% TO | 5.98 ± 0.01 | 1.196 ± 0.007 | 154.5 ± 10.4 | 159.3 ± 10.4 | −4.18 ± 0.52 | −6.44 ± 0.39 |
| SE + 0.3% TO | 5.97 ± 0.02 | 1.177 ± 0.008 | 174.8 ± 9.7 | 180.3 ± 7.8 | −4.34 ± 1.08 | −6.35 ± 0.78 |
| SE + 1.0% TO | 5.99 ± 0.01 | 1.19 ± 0.008 | 164.1 ± 5.9 | 174.8 ± 9.7 | −4.1 ± 0.86 | −5.18 ± 0.91 |
| Control (Saline) | SE + 1% TO | |||
|---|---|---|---|---|
| n | n | |||
| Day 1 | 30.4 ± 0.6 | 3 | 30.0 ± 1.3 | 8 |
| Day 2 | 30.3 ± 0.8 | 3 | 29.9 ± 1.2 | 8 |
| Day 3 | 30.4 ± 0.4 | 3 | 30.0 ± 0.9 | 8 |
| Day 2 | −0.2 ± 0.8 | 3 | −0.2 ± 0.7 | 8 |
| Day 3 | 0.0 ± 0.6 | 3 | 0.2 ± 1.3 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krasnova, O.A.; Minaychev, V.V.; Akatov, V.S.; Fadeev, R.S.; Senotov, A.S.; Kobyakova, M.I.; Lomovskaya, Y.V.; Lomovskiy, A.I.; Zvyagina, A.I.; Krasnov, K.S.; et al. Improving the Stability and Effectiveness of Immunotropic Squalene Nanoemulsion by Adding Turpentine Oil. Biomolecules 2023, 13, 1053. https://doi.org/10.3390/biom13071053
Krasnova OA, Minaychev VV, Akatov VS, Fadeev RS, Senotov AS, Kobyakova MI, Lomovskaya YV, Lomovskiy AI, Zvyagina AI, Krasnov KS, et al. Improving the Stability and Effectiveness of Immunotropic Squalene Nanoemulsion by Adding Turpentine Oil. Biomolecules. 2023; 13(7):1053. https://doi.org/10.3390/biom13071053
Chicago/Turabian StyleKrasnova, Olga A., Vladislav V. Minaychev, Vladimir S. Akatov, Roman S. Fadeev, Anatoly S. Senotov, Margarita I. Kobyakova, Yana V. Lomovskaya, Alexey I. Lomovskiy, Alyona I. Zvyagina, Kirill S. Krasnov, and et al. 2023. "Improving the Stability and Effectiveness of Immunotropic Squalene Nanoemulsion by Adding Turpentine Oil" Biomolecules 13, no. 7: 1053. https://doi.org/10.3390/biom13071053
APA StyleKrasnova, O. A., Minaychev, V. V., Akatov, V. S., Fadeev, R. S., Senotov, A. S., Kobyakova, M. I., Lomovskaya, Y. V., Lomovskiy, A. I., Zvyagina, A. I., Krasnov, K. S., Shatalin, Y. V., Penkov, N. V., Zhalimov, V. K., Molchanov, M. V., Palikova, Y. A., Murashev, A. N., Maevsky, E. I., & Fadeeva, I. S. (2023). Improving the Stability and Effectiveness of Immunotropic Squalene Nanoemulsion by Adding Turpentine Oil. Biomolecules, 13(7), 1053. https://doi.org/10.3390/biom13071053









