Development and Validation of an Endometriosis Diagnostic Method Based on Serum Biomarkers and Clinical Variables
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Populations
2.1.1. Development Cohort
2.1.2. External Validation Cohort
2.2. Sample Collection
2.3. ELISA Method: CA125 and BDNF Concentrations
2.4. Software Input and Score Calculation
2.5. Statistical Analysis
3. Results
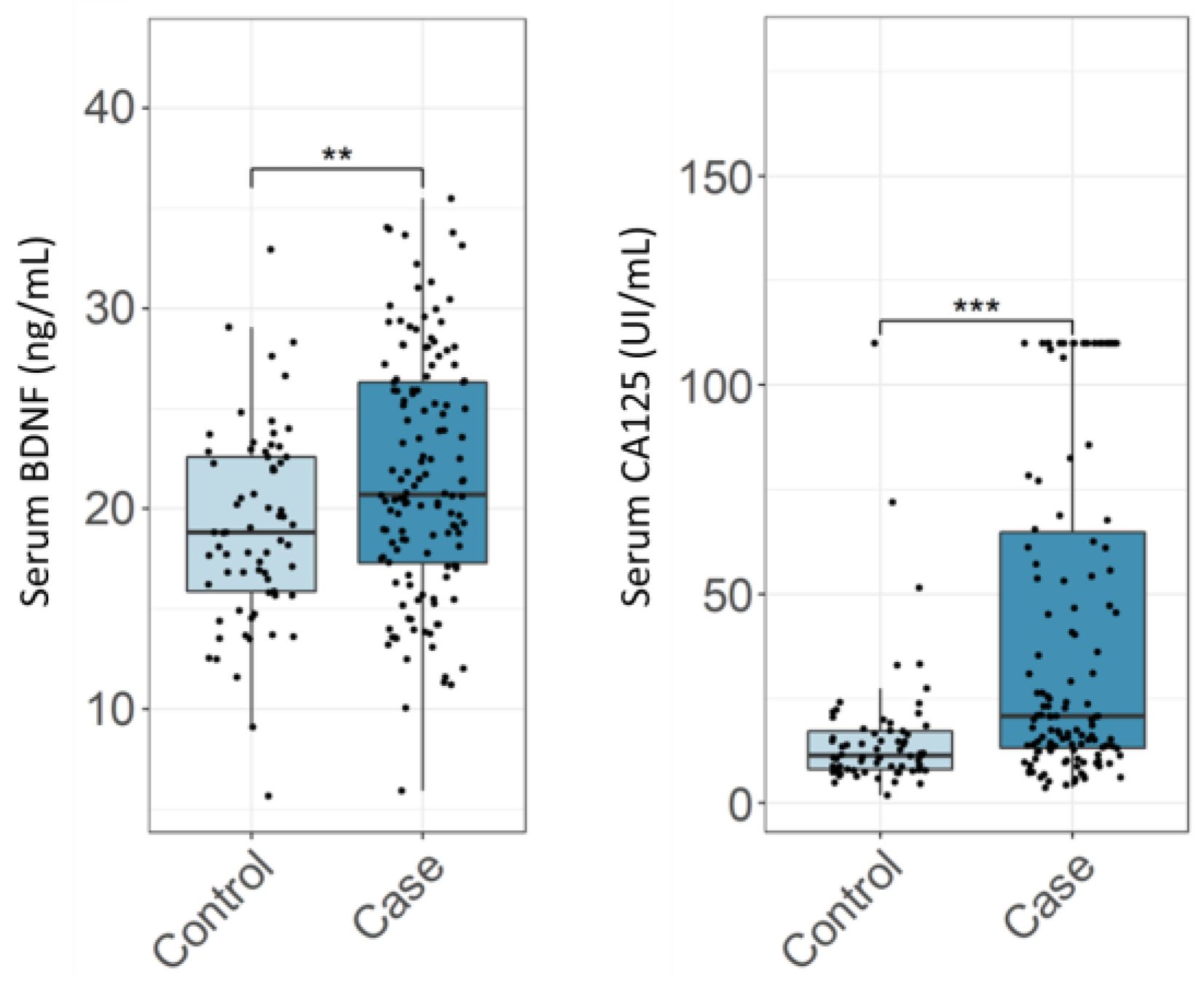
3.1. Diagnostic Performance of CA125 and BDNF in Endometriosis
3.2. Development of Prediction Model for Endometriosis
3.3. Clinical Performance Evaluation (Validation of the IVD Test)
3.4. Differential Diagnosis
3.4.1. Confounding Conditions
3.4.2. Detection of Superficial Endometriosis
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Viganò, P. Endometriosis. Nat. Rev. Dis. Prim. 2018, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Symons, L.K.; Miller, J.E.; Kay, V.R.; Marks, R.M.; Liblik, K.; Koti, M.; Tayade, C. The Immunopathophysiology of Endometriosis. Trends. Mol. Med. 2018, 24, 748–762. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis Is a Chronic Systemic Disease: Clinical Challenges and Novel Innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef]
- Rossi, H.-R.; Uimari, O.; Terho, A.; Pesonen, P.; Koivurova, S.; Piltonen, T. Increased Overall Morbidity in Women with Endometriosis: A Population-Based Follow-up Study until Age 50. Fertil. Steril. 2023, 119, 89–98. [Google Scholar] [CrossRef]
- Sinaii, N.; Cleary, S.D.; Ballweg, M.L.; Nieman, L.K.; Stratton, P. High Rates of Autoimmune and Endocrine Disorders, Fibromyalgia, Chronic Fatigue Syndrome and Atopic Diseases among Women with Endometriosis: A Survey Analysis. Hum. Reprod. 2002, 17, 2715–2724. [Google Scholar] [CrossRef]
- Somigliana, E.; Vigano’, P.; Parazzini, F.; Stoppelli, S.; Giambattista, E.; Vercellini, P. Association between Endometriosis and Cancer: A Comprehensive Review and a Critical Analysis of Clinical and Epidemiological Evidence. Gynecol. Oncol. 2006, 101, 331–341. [Google Scholar] [CrossRef]
- Hsu, A.L.; Khachikyan, I.; Stratton, P. Invasive and Non-Invasive Methods for the Diagnosis of Endometriosis. Clin. Obs. Gynecol. 2010, 53, 413–419. [Google Scholar] [CrossRef]
- Simoens, S.; Dunselman, G.; Dirksen, C.; Hummelshoj, L.; Bokor, A.; Brandes, I.; Brodszky, V.; Canis, M.; Colombo, G.L.; DeLeire, T.; et al. The Burden of Endometriosis: Costs and Quality of Life of Women with Endometriosis and Treated in Referral Centres. Hum. Reprod. 2012, 27, 1292–1299. [Google Scholar] [CrossRef]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE Guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef]
- Dunselman, G.A.J.; Vermeulen, N.; Becker, C.; Calhaz-Jorge, C.; D’Hooghe, T.; De Bie, B.; Heikinheimo, O.; Horne, A.W.; Kiesel, L.; Nap, A.; et al. ESHRE Guideline: Management of Women with Endometriosis. Hum. Reprod. 2014, 29, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.K.; Chapron, C.; Giudice, L.C.; Laufer, M.R.; Leyland, N.; Missmer, S.A.; Singh, S.S.; Taylor, H.S. Clinical Diagnosis of Endometriosis: A Call to Action. Am. J. Obs. Gynecol. 2019, 220, 354.e1–354.e12. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Singh, V.; Tayade, C. Biomarkers in Endometriosis: Challenges and Opportunities. Fertil. Steril. 2017, 107, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Irungu, S.; Mavrelos, D.; Worthington, J.; Blyuss, O.; Saridogan, E.; Timms, J.F. Discovery of Non-Invasive Biomarkers for the Diagnosis of Endometriosis. Clin. Proteom. 2019, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Nisenblat, V.; Bossuyt, P.M.M.; Farquhar, C.; Johnson, N.; Hull, M.L. Imaging Modalities for the Non-Invasive Diagnosis of Endometriosis. Cochrane Database Syst. Rev. 2016, 2, CD009591. [Google Scholar] [CrossRef] [PubMed]
- Nisenblat, V.; Bossuyt, P.M.; Shaikh, R.; Farquhar, C.; Jordan, V.; Scheffers, C.S.; Mol, B.W.J.; Johnson, N.; Hull, M.L. Blood Biomarkers for the Non-invasive Diagnosis of Endometriosis. Cochrane Database Syst. Rev. 2016, 5, CD012179. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, R.L.; Niloff, J.M.; Bast, R.C.; Schaetzl, E.; Kistner, R.W.; Knapp, R.C. Elevated Serum Concentrations of CA-125 in Patients with Advanced Endometriosis. Fertil. Steril. 1986, 45, 630–634. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, M.; Zuo, Y.; Yang, B.; Wang, S. Research Progress of CA125 in Endometriosis: Teaching an Old Dog New Tricks. Gynecol. Obstet. Clin. Med. 2022, 2, 191–198. [Google Scholar] [CrossRef]
- Nagamani, M.; Kelver, M.E.; Smith, E.R. CA 125 Levels in Monitoring Therapy for Endometriosis and in Prediction of Recurrence. Int. J. Fertil. 1992, 37, 227–231. [Google Scholar]
- Shen, A.; Xu, S.; Ma, Y.; Guo, H.; Li, C.; Yang, C.; Zou, S. Diagnostic Value of Serum CA125, CA19-9 and CA15-3 in Endometriosis: A Meta-Analysis. J. Int. Med. Res. 2015, 43, 599–609. [Google Scholar] [CrossRef]
- Kafali, H.; Artuc, H.; Demir, N. Use of CA125 Fluctuation during the Menstrual Cycle as a Tool in the Clinical Diagnosis of Endometriosis; a Preliminary Report. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 116, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Zarchi, M.; Dehshiri-Zadeh, N.; Sekhavat, L.; Nosouhi, F. Correlation of CA-125 Serum Level and Clinico-Pathological Characteristic of Patients with Endometriosis. Int. J. Reprod. Biomed. 2016, 14, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, M.; Duffy, J.; Davis, C.; Nieves Plana, M.; Khan, K.; on behalf of the International Collaboration to Harmonise Outcomes and Measures for Endometriosis. Diagnostic Accuracy of Cancer Antigen 125 for Endometriosis: A Systematic Review and Meta-Analysis. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 1761–1768. [Google Scholar] [CrossRef]
- Hirsch, M.; Duffy, J.M.N.; Deguara, C.S.; Davis, C.J.; Khan, K.S. Diagnostic Accuracy of Cancer Antigen 125 (CA125) for Endometriosis in Symptomatic Women: A Multi-Center Study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 210, 102–107. [Google Scholar] [CrossRef]
- Chao, M.V. Neurotrophins and Their Receptors: A Convergence Point for Many Signalling Pathways. Nat. Rev. Neurosci. 2003, 4, 299–309. [Google Scholar] [CrossRef]
- Wessels, J.M.; Wu, L.; Leyland, N.A.; Wang, H.; Foster, W.G. The Brain-Uterus Connection: Brain Derived Neurotrophic Factor (BDNF) and Its Receptor (Ntrk2) Are Conserved in the Mammalian Uterus. PLoS ONE 2014, 9, e94036. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Duan, H.; Li, B.; Hong, W.; Li, X.; Wang, Y.; Guo, Z.C. BDNF and TrKB Expression Levels in Patients with Endometriosis and Their Associations with Dysmenorrhoea. J. Ovarian. Res. 2022, 15, 35. [Google Scholar] [CrossRef]
- Lim, W.; Bae, H.; Bazer, F.W.; Song, G. Brain-Derived Neurotrophic Factor Improves Proliferation of Endometrial Epithelial Cells by Inhibition of Endoplasmic Reticulum Stress during Early Pregnancy. J. Cell. Physiol. 2017, 232, 3641–3651. [Google Scholar] [CrossRef]
- Dong, F.; Zhang, Q.; Kong, W.; Chen, J.; Ma, J.; Wang, L.; Wang, Y.; Liu, Y.; Li, Y.; Wen, J. Regulation of Endometrial Cell Proliferation by Estrogen-Induced BDNF Signaling Pathway. Gynecol. Endocrinol. 2017, 33, 485–489. [Google Scholar] [CrossRef]
- Godin, S.K.; Wagner, J.; Huang, P.; Bree, D. The Role of Peripheral Nerve Signaling in Endometriosis. FASEB BioAdvances 2021, 3, 802–813. [Google Scholar] [CrossRef]
- Greaves, E.; Temp, J.; Esnal-Zufiurre, A.; Mechsner, S.; Horne, A.W.; Saunders, P.T.K. Estradiol Is a Critical Mediator of Macrophage-Nerve Cross Talk in Peritoneal Endometriosis. Am. J. Pathol. 2015, 185, 2286–2297. [Google Scholar] [CrossRef] [PubMed]
- Perricos, A.; Ashjaei, K.; Husslein, H.; Proestling, K.; Kuessel, L.; Obwegeser, R.; Wenzl, R.; Yotova, I. Increased Serum Levels of MBDNF in Women with Minimal and Mild Endometriosis Have No Predictive Power for the Disease. Exp. Biol. Med. 2018, 243, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Wessels, J.M.; Kay, V.R.; Leyland, N.A.; Agarwal, S.K.; Foster, W.G. Assessing Brain-Derived Neurotrophic Factor as a Novel Clinical Marker of Endometriosis. Fertil. Steril. 2016, 105, 119–128.e5. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Zhu, T.; Tian, Y.; Xu, P.; Chen, Z.; Huang, X.; Zhang, X. Role of Brain-Derived Neurotrophic Factor in Endometriosis Pain. Reprod. Sci. 2018, 25, 1045–1057. [Google Scholar] [CrossRef]
- Rahmioglu, N.; Fassbender, A.; Vitonis, A.F.; Tworoger, S.S.; Hummelshoj, L.; D’Hooghe, T.M.; Adamson, G.D.; Giudice, L.C.; Becker, C.M.; Zondervan, K.T.; et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonization Project: III. Fluid Biospecimen Collection, Processing, and Storage in Endometriosis Research. Fertil. Steril. 2014, 102, 1233–1243. [Google Scholar] [CrossRef]
- Newcombe, R.G. Interval Estimation for the Difference between Independent Proportions: Comparison of Eleven Methods. Stat. Med. 1998, 17, 873–890. [Google Scholar] [CrossRef]
- Trevethan, R. Sensitivity, Specificity, and Predictive Values: Foundations, Pliabilities, and Pitfalls in Research and Practice. Front. Public Health 2017, 5, 307. [Google Scholar] [CrossRef]
- Giannini, A.; Bucci, F.; Luisi, S.; Cela, V.; Pluchino, N.; Merlini, S.; Casarosa, E.; Russo, M.; Cubeddu, A.; Daino, D.; et al. Brain-Derived Neurotrophic Factor in Plasma of Women with Endometriosis. J. Endometr. 2010, 2, 144–150. [Google Scholar] [CrossRef]
- Rocha, A.L.; Vieira, E.L.; Ferreira, M.C.; Maia, L.M.; Teixeira, A.L.; Reis, F.M. Plasma Brain-Derived Neurotrophic Factor in Women with Pelvic Pain: A Potential Biomarker for Endometriosis? Biomark. Med. 2017, 11, 313–317. [Google Scholar] [CrossRef]
- Liang, Y.F.; Huang, X.M.; Wen, L.L.; Kang, H.; Tao, M.H.; Ye, M.Z. [Relationship between serum brain-derived neurotrophic factor and clinical stage and dysmenorrhoea of enodmetriosis]. Zhonghua Yi Xue Za Zhi 2020, 100, 771–774. [Google Scholar] [CrossRef]
- Polacchini, A.; Metelli, G.; Francavilla, R.; Baj, G.; Florean, M.; Mascaretti, L.G.; Tongiorgi, E. A Method for Reproducible Measurements of Serum BDNF: Comparison of the Performance of Six Commercial Assays. Sci. Rep. 2015, 5, 17989. [Google Scholar] [CrossRef] [PubMed]
- Polyakova, M.; Schlögl, H.; Sacher, J.; Schmidt-Kassow, M.; Kaiser, J.; Stumvoll, M.; Kratzsch, J.; Schroeter, M.L. Stability of BDNF in Human Samples Stored Up to 6 Months and Correlations of Serum and EDTA-Plasma Concentrations. Int. J. Mol. Sci. 2017, 18, 1189. [Google Scholar] [CrossRef] [PubMed]
- Merriel, S.W.D.; Pocock, L.; Gilbert, E.; Creavin, S.; Walter, F.M.; Spencer, A.; Hamilton, W. Systematic Review and Meta-Analysis of the Diagnostic Accuracy of Prostate-Specific Antigen (PSA) for the Detection of Prostate Cancer in Symptomatic Patients. BMC Med. 2022, 20, 54. [Google Scholar] [CrossRef] [PubMed]
| Controls | Cases | |
|---|---|---|
| n = 68 | n = 136 | |
| Age years (mean ± SD) | 33.5 (5.96) | 35.6 (6.42) |
| BMI (mean ± SD) | 25.38 (4.63) | 26.46 (5.32) |
| rASRM classification | ||
| I–II | - | 68 (50%) |
| III–IV | - | 68 (50%) |
| Endometriosis classification | ||
| Superficial | - | 54 (39.7%) |
| Endometrioma | - | 26 (19.1%) |
| DIE | - | 29 (21.3%) |
| DIE + endometrioma | - | 25 (18.4%) |
| Unclassified | - | 2 (1.5%) |
| Other gynaecological conditions | ||
| Ovarian cysts | 28 | 66 |
| Ovarian cancer | 1 | 6 |
| Uterine fibroids | 7 | 25 |
| Adenomyosis | 0 | 7 |
| Controls | Cases | |
|---|---|---|
| n = 25 | n = 52 | |
| Age years (mean ± SD) | 35 (6.44) | 35 (6.47) |
| BMI (mean ± SD) | 26 (5.23) | 26 (5.14) |
| rASRM classification | ||
| I–II | - | 42 (81%) |
| III–IV | - | 7 (13%) |
| Missing information | 3 (6%) | |
| Endometriosis classification | ||
| Superficial | - | 25 (48.1%) |
| Endometrioma | - | 3 (5.8%) |
| DIE | - | 14 (26.9%) |
| DIE + endometrioma | - | 8 (15.4%) |
| Unclassified | - | 3 (5.8%) |
| Other conditions | ||
| Ovarian cysts | 11 | 16 |
| Ovarian cancer | 0 | 4 |
| Uterine fibroids | 3 | 4 |
| Adenomyosis | 1 | 1 |
| Model | Area under Curve | Youden’s Index | Accuracy | Sensitivity | Specificity |
|---|---|---|---|---|---|
| At 95% specificity | 0.867 | 47.1% | 66.2% | 51.5% | 95.6% |
| (0.819–0.915) | (37.3–56.8%) | (59.2–72.5%) | (42.8–60.1%) | (86.8–98.9%) | |
| At 95% sensitivity | 0.867 | 44.1% | 79.9% | 95.6% | 48.5% |
| (0.819–0.915) | (31.7–56.5%) | (73.6–85%) | (90.2–98.2%) | (36.4–60.9%) | |
| At maximum Youden’s index | 0.867 | 58.8% | 82.4% | 88.2% | 70.6% |
| (0.819–0.915) | (46.7–70.9%) | (76.3–87.2%) | (81.3–92.9%) | (58.1–80.7%) | |
| At maximum accuracy | 0.867 | 58.1% | 82.4% | 89% | 69.1% |
| (0.819–0.915) | (45.9–70.3%) | (76.3–87.2%) | (82.2–93.5%) | (56.6–79.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herranz-Blanco, B.; Daoud, E.; Viganò, P.; García-Velasco, J.A.; Colli, E. Development and Validation of an Endometriosis Diagnostic Method Based on Serum Biomarkers and Clinical Variables. Biomolecules 2023, 13, 1052. https://doi.org/10.3390/biom13071052
Herranz-Blanco B, Daoud E, Viganò P, García-Velasco JA, Colli E. Development and Validation of an Endometriosis Diagnostic Method Based on Serum Biomarkers and Clinical Variables. Biomolecules. 2023; 13(7):1052. https://doi.org/10.3390/biom13071052
Chicago/Turabian StyleHerranz-Blanco, Bárbara, Elza Daoud, Paola Viganò, Juan Antonio García-Velasco, and Enrico Colli. 2023. "Development and Validation of an Endometriosis Diagnostic Method Based on Serum Biomarkers and Clinical Variables" Biomolecules 13, no. 7: 1052. https://doi.org/10.3390/biom13071052
APA StyleHerranz-Blanco, B., Daoud, E., Viganò, P., García-Velasco, J. A., & Colli, E. (2023). Development and Validation of an Endometriosis Diagnostic Method Based on Serum Biomarkers and Clinical Variables. Biomolecules, 13(7), 1052. https://doi.org/10.3390/biom13071052






