Innovative Strategy for Aroma Stabilization Using Green Solvents: Supercritical CO2 Extracts of Satureja montana Dispersed in Deep Eutectic Solvents
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation and Characterization of NADES
2.3. Determination of Viscosity
2.4. Preparation of Samples
Supercritical Carbon Dioxide Extraction and Dispersion in NADES
2.5. Headspace Solid-Phase Microextraction (HS-SPME)
2.6. Gas Chromatography with Mass Spectrometry Analysis (GC-MS)
2.7. Chemometric Methods
3. Results and Discussion
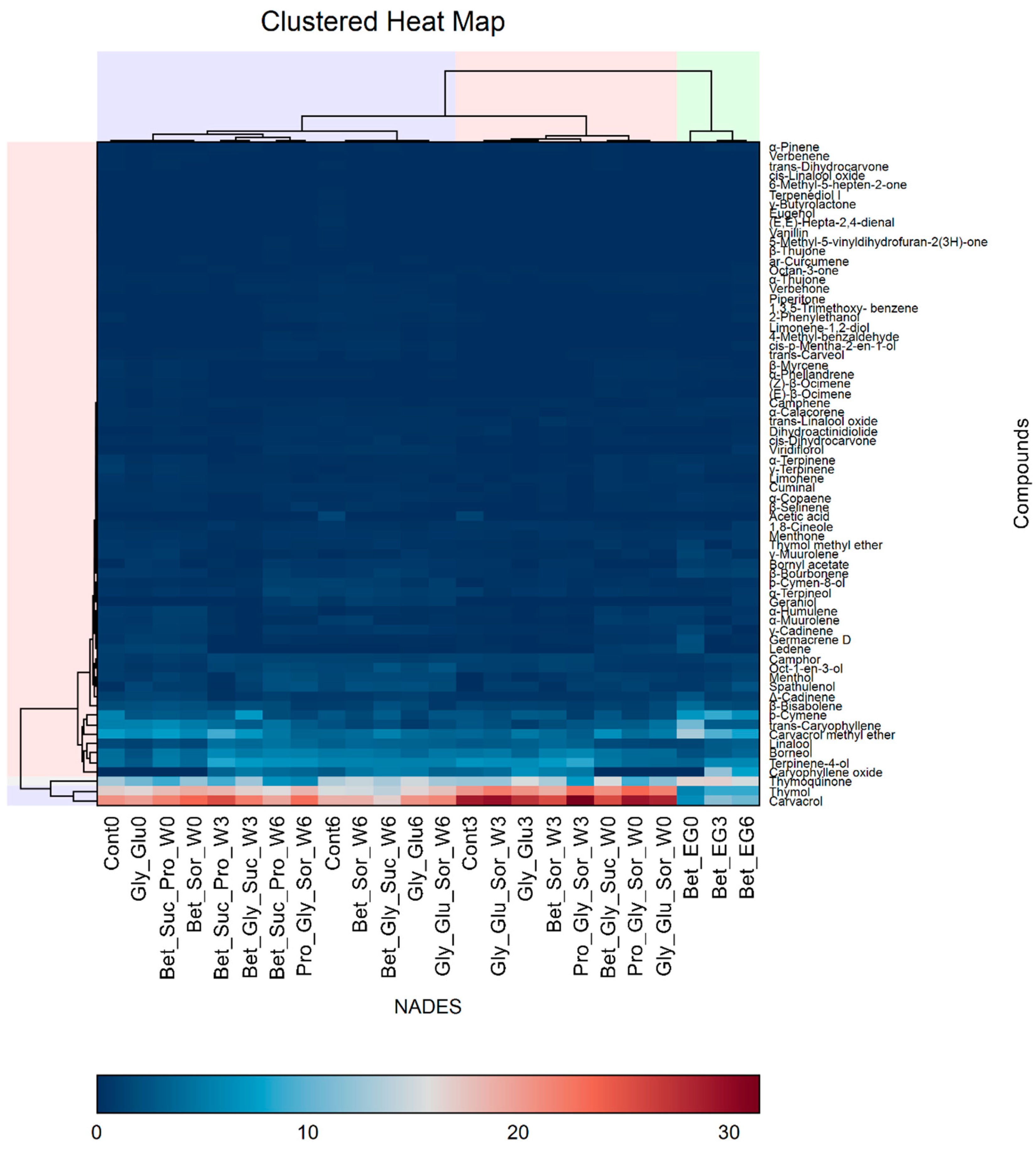
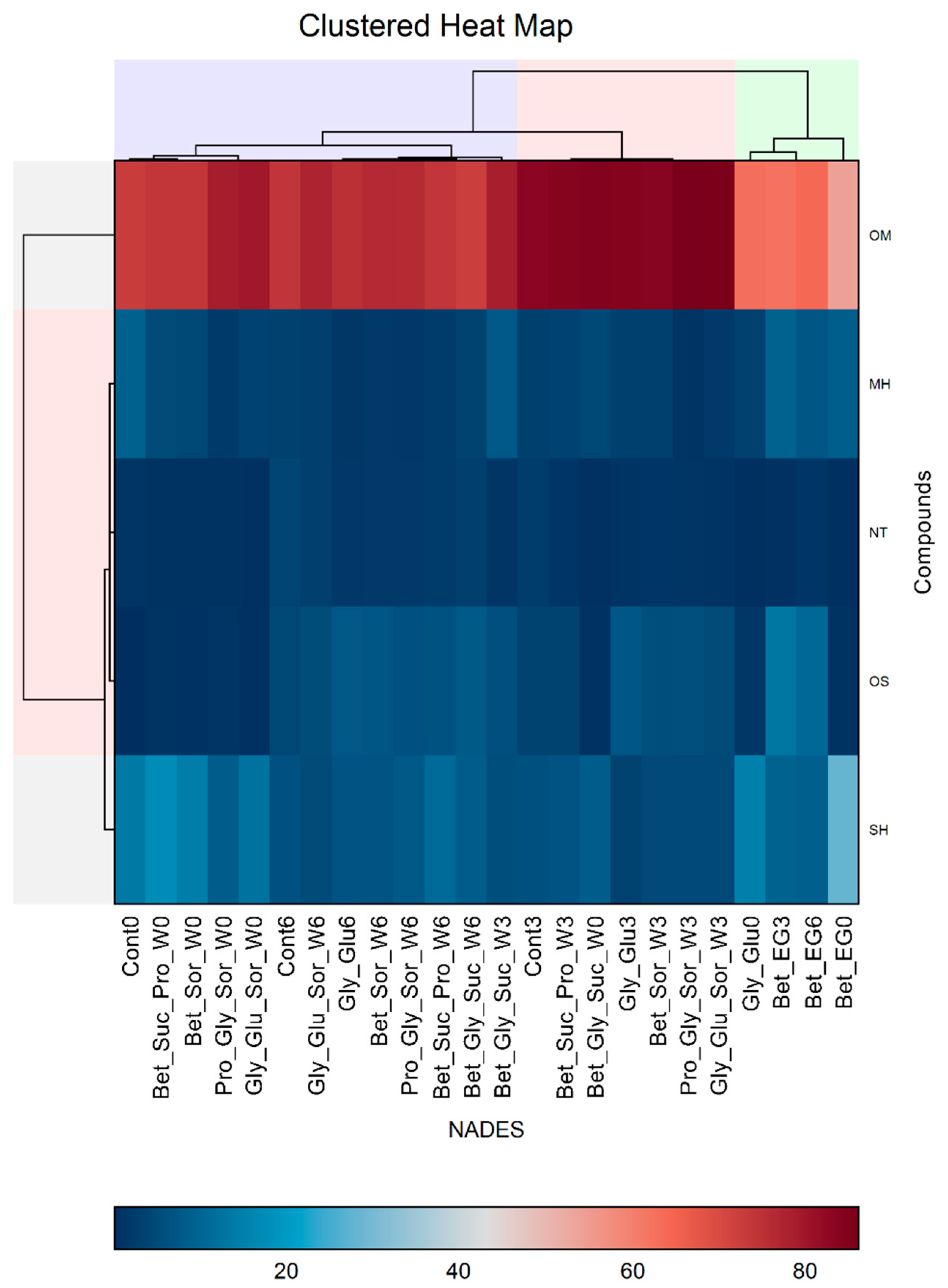
3.1. Hierarchical Cluster Analysis and Principal Component Analysis
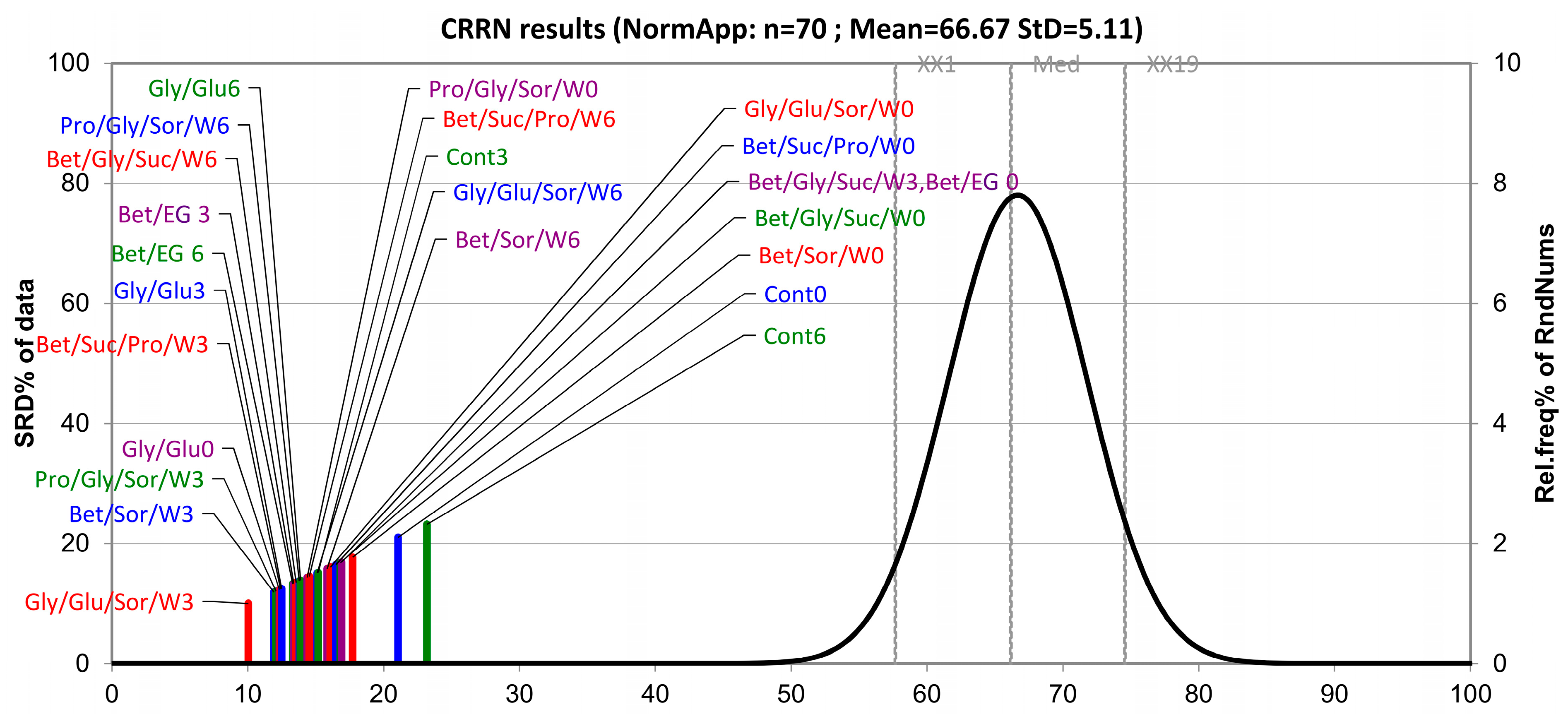
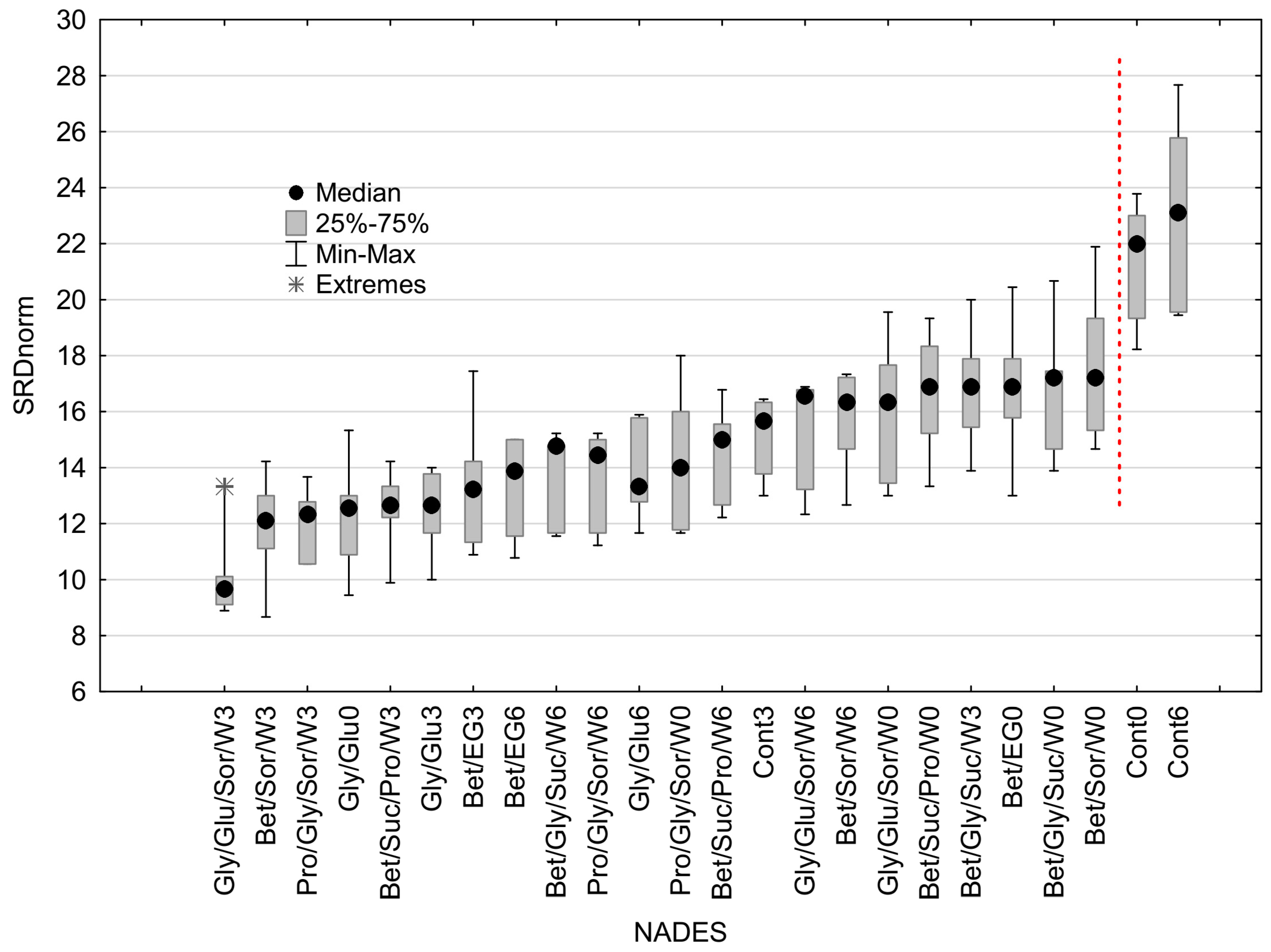
3.2. The Ranking of the Samples Based on the SRD Approach
3.3. Wilcoxon Matched Pairs Test of the NADES Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, C.; Borsotto, P. Essential oils: Market and legislation. Potential Essent. Oils 2018, 107–127. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar]
- United Nations. The UN Sustainable Development Goals; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Lopes, D.B.; Speranza, P.; Macedo, G.A. A new approach for flavor and aroma encapsulation. In Novel Approaches of Nanotechnology in Food; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 623–661. [Google Scholar]
- Plati, F.; Paraskevopoulou, A. Micro-and nano-encapsulation as tools for essential oils advantages’ exploitation in food applications: The case of oregano essential oil. Food Bioprocess Technol. 2022, 15, 949–977. [Google Scholar]
- Arumugham, T.; Rambabu, K.; Hasan, S.W.; Show, P.L.; Rinklebe, J.; Banat, F. Supercritical carbon dioxide extraction of plant phytochemicals for biological and environmental applications—A review. Chemosphere 2021, 271, 129525. [Google Scholar]
- Essien, S.O.; Young, B.; Baroutian, S. Recent advances in subcritical water and supercritical carbon dioxide extraction of bioactive compounds from plant materials. Trends Food Sci. Technol. 2020, 97, 156–169. [Google Scholar]
- Wu, K.; Ren, J.; Wang, Q.; Nuerjiang, M.; Xia, X.; Bian, C. Research progress on the preparation and action mechanism of natural deep eutectic solvents and their application in food. Foods 2022, 11, 3528. [Google Scholar] [CrossRef]
- Espino, M.; de los Ángeles Fernández, M.; Gomez, F.J.; Silva, M.F. Natural designer solvents for greening analytical chemistry. TrAC Trends Anal. Chem. 2016, 76, 126–136. [Google Scholar] [CrossRef]
- Mišan, A.; Nađpal, J.; Stupar, A.; Pojić, M.; Mandić, A.; Verpoorte, R.; Choi, Y.H. The perspectives of natural deep eutectic solvents in agri-food sector. Crit. Rev. Food Sci. Nutr. 2020, 60, 2564–2592. [Google Scholar] [CrossRef]
- Vladić, J.; Kovačević, S.; Aladić, K.; Rebocho, S.; Jokić, S.; Podunavac-Kuzmanović, S.; Duarte, A.R.; Jerković, I. Novel Insights into Recovery and Stabilization of Rosmarinus officinalis Volatile Aroma Compounds Using Green Solvents. 2023; submitted. [Google Scholar]
- Vladić, J.; Kovačević, K.; Rebocho, S.; Paiva, A.; Jokić, S.; Duarte, A.R.; Jerković, I. Towards A New Approach for Green Aroma Stabilisation: Supercritical CO2 Extracts of Lavandula stoechas Dispersed in Natural Deep Eutectic Solvents. 2023; submitted. [Google Scholar]
- Vladić, J.; Zeković, Z.; Jokić, S.; Svilović, S.; Kovačević, S.; Vidović, S. Winter savory: Supercritical carbon dioxide extraction and mathematical modeling of extraction process. J. Supercrit. Fluids 2016, 117, 89–97. [Google Scholar] [CrossRef]
- Jerković, I.; Marijanović, Z.; Roje, M.; Kuś, P.M.; Jokić, S.; Čož-Rakovac, R. Phytochemical study of the headspace volatile organic compounds of fresh algae and seagrass from the Adriatic Sea (single point collection). PLoS ONE 2018, 13, e0196462. [Google Scholar] [CrossRef]
- NCSS 2021 Statistical Software; NCSS, LLC: Kaysville, UT, USA, 2021; Available online: Ncss.com/software/ncss (accessed on 17 April 2023).
- STATISTICA (Data Analysis Software System), version 12; StatSoft, Inc.: Tulsa, OK, USA, 2014; Available online: www.statsoft.com (accessed on 17 April 2023).
- Héberger, K.; Kollár-Hunek, K. Comparison of validation variants by sum of ranking differences and ANOVA. J. Chemom. 2019, 33, e3104. [Google Scholar] [CrossRef]
- Héberger, K.; Kollár-Hunek, K. Um of ranking differences for method discrimination and its validation: Comparison of ranks with random numbers. J. Chemom. 2011, 25, 151–158. [Google Scholar] [CrossRef]
- Mehdizadeh, L.; Ghasemi Pirbalouti, A.; Moghaddam, M. Storage stability of essential oil of cumin (Cuminum cyminum L.) as a function of temperature. Int. J. Food Prop. 2017, 20, 1742–1750. [Google Scholar] [CrossRef]
- Dai, Y.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents providing enhanced stability of natural colorants from safflower (Carthamus tinctorius). Food Chem. 2014, 159, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Escobar, A.; Perez, M.; Romanelli, G.; Blustein, G. Thymol bioactivity: A review focusing on practical applications. Arab. J. Chem. 2020, 13, 9243–9269. [Google Scholar]
- Ćavar, S.; Šolić, M.E.; Maksimović, M. Chemical composition and antioxidant activity of two Satureja species from Mt. Biokovo. Bot. Serb. 2013, 37, 159–165. [Google Scholar]
- Fraternale, D.; Giamperi, L.; Bucchini, A.; Ricci, D.; Epifano, F.; Genovese, S.; Curini, M. Chemical composition and antifungal activity of the essential oil of Satureja montana from central Italy. Chem. Nat. Compd. 2007, 43, 622–624. [Google Scholar] [CrossRef]
- Goyal, S.N.; Prajapati, C.P.; Gore, P.R.; Patil, C.R.; Mahajan, U.B.; Sharma, C.; Talla, S.P.; Ojha, S.K. Therapeutic potential and pharmaceutical development of thymoquinone: A multitargeted molecule of natural origin. Front. Pharmacol. 2017, 8, 656. [Google Scholar]
- Choi, H.S.; Sawamura, M. Effects of storage conditions on the composition of Citrus tamurana Hort. Ex Tanaka (Hyuganatsu) essential oil. Biosci. Biotechnol. Biochem. 2002, 66, 439–443. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.F.; Tsetegho Sokeng, A.J.; Izadi, M.; Jonaidi Jafari, N.J.; Suntar, I.; Daglia, M.; et al. Update on monoterpenes as antimicrobial agents: A particular focus on p-cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Assessment of factors influencing antimicrobial activity of carvacrol and cymene against Vibrio cholerae in food. J. Biosci. Bioeng. 2010, 110, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Balaban-Ucar, M.; Gönültaş, O. Volatile compounds of archaeological wood from the ancient harbor Thedosius in Istanbul. Eur. J. Wood Wood Prod. 2019, 77, 475–481. [Google Scholar] [CrossRef]
- He, F.; Qian, Y.L.; Qian, M.C. Flavor and chiral stability of lemon-flavored hard tea during storage. Food Chem. 2018, 239, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Njoroge, S.M.; Ukeda, H.; Sawamura, M. Changes in the volatile composition of yuzu (Citrus junos Tanaka) cold-pressed oil during storage. J. Agric. Food Chem. 1996, 44, 550–556. [Google Scholar] [CrossRef]
- Misharina, T.A.; Polshkov, A.N. Antioxidant properties of essential oils: Autoxidation of essential oils from laurel and fennel and of their mixtures with essential oil from coriander. Appl. Biochem. Microbiol. 2005, 41, 610–618. [Google Scholar] [CrossRef]
- Sayyad, R.; Farahmandfar, R. Influence of Teucrium polium L. essential oil on the oxidative stability of canola oil during storage. J. Food Sci. Technol. 2017, 54, 3073–3081. [Google Scholar]
- Cecchi, L.; Migliorini, M.; Giambanelli, E.; Rossetti, A.; Cane, A.; Mulinacci, N. New volatile molecular markers of rancidity in virgin olive oils under nonaccelerated oxidative storage conditions. J. Agric. Food Chem. 2019, 67, 13150–13163. [Google Scholar] [CrossRef]
- Henry, T.B.; Menn, F.M.; Fleming, J.T.; Wilgus, J.; Compton, R.N.; Sayler, G.S. Attributing effects of aqueous C60 nano-aggregates to tetrahydrofuran decomposition products in larval zebrafish by assessment of gene expression. Environ. Health Perspect. 2007, 115, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Duan, H.; Barringer, S.A. Effects of buffer and temperature on formation of furan, acetic acid and formic acid from carbohydrate model systems. LWT-Food Sci. Technol. 2011, 44, 1761–1765. [Google Scholar] [CrossRef]
- Chirife, J.; Zamora, M.C.; Motto, A. The correlation between water activity and% moisture in honey: Fundamental aspects and application to Argentine honeys. J. Food Eng. 2006, 72, 287–292. [Google Scholar] [CrossRef]
- Das, D.; Rana, N.; Pramanik, G.; Sen, K. Fluorosensing of benzaldehydes by CuI-graphene: A spectroscopy, thermodynamics and docking supported phenomenon. Anal. Chim. Acta 2023, 1249, 340897. [Google Scholar] [CrossRef]
- da Silva Santos, B.R.; Minho, L.A.C.; Silva, E.F.R.; Sauthier, M.C.S.; Caldas, J.C.; Silva, E.G.P.; Santana, D.A.; Santos, W.N.L. Chemometric tools applied to evaluation of fruit bioactive compounds extraction. Food Anal. Methods 2020, 13, 1176–1189. [Google Scholar] [CrossRef]
- Ghendov-Mosanu, A.; Cojocari, D.; Balan, G.; Patras, A.; Lung, I.; Soran, M.L.; Opriş, O.; Cristea, E.; Sturza, R. Chemometric optimization of biologically active compounds extraction from grape marc: Composition and antimicrobial activity. Molecules 2022, 27, 1610. [Google Scholar] [CrossRef]
- Shojaei, S.; Shojaei, S.; Nouri, A.; Baharinikoo, L. Application of chemometrics for modeling and optimization of ultrasound-assisted dispersive liquid–liquid microextraction for the simultaneous determination of dyes. NPJ Clean Water 2021, 4, 23. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, D. Supercritical CO2 extraction of Eucalyptus leaves oil and comparison with Soxhlet extraction and hydro-distillation methods. Sep. Purif. Technol. 2014, 133, 443–451. [Google Scholar] [CrossRef]
- Jafari, F.; Ghavidel, F.; Zarshenas, M.M. A critical overview on the pharmacological and clinical aspects of popular Satureja species. J. Acupunct. Meridian Stud. 2016, 9, 118–127. [Google Scholar]
- Vladic, J.; Vidovic, S.; Acimovic, M.; Gavaric, A.; Jokic, S. Satureja montana: Cultivation, Production and Uses In Medicinal Plants: Production, Cultivation and Uses; Matthias, A., Laisné, N., Eds.; Nova Science Publishers: New York, NY, USA, 2017; pp. 27–58. [Google Scholar]

| NADES Composition | Molar Ratio | Abbreviation | Price (EUR kg−1) a | Viscosity (mPa·s) 30 °C |
|---|---|---|---|---|
| Betaine/Ethylene glycol | 1:3 | Bet/EG | 95.34 | 48.63 ± 0.42 |
| Betaine/Sorbitol/Water | 1:1:3 | Bet/Sor/W | 81.07 | 747.40 ± 14.83 |
| Betaine/Sucrose/Proline/Water | 5:2:2:21 | Bet/Suc/Pro/W | 170.09 | 853.70 ± 39.37 |
| Betaine/Glycerol/Sucrose/Water | 2:3:1:5 | Bet/Gly/Suc/W | 109.47 | 2246.77 ± 45.27 |
| Glycerol/Glucose | 4:1 | Gly/Glu | 118.13 | 5258.47 ± 322.86 |
| Glycerol/Glucose/Sorbitol/Water | 1:1:1:3 | Gly/Glu/Sorb/W | 68.95 | 1460.03 ± 53.23 |
| Glycerol/Proline/Sorbitol/Water | 1:1:1:13 | Pro/Gly/Sorb/W | 190.55 | 23.70 ± 1.44 |
| Compound | RI | Sample | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Glyc/Glu | Bet/Suc/Pro/W | Bet/Gly/Suc/W | Bet/Sor/W | Pro/Gly/Sor/W | Gly/Glu/Sor/W | Bet/EG | ||
| Monoterpene hydrocarbons | |||||||||
| α-Pinene | 940 | 0.09 ± 0.01 | 0.02 ± 0.00 | 0.06 ± 0.02 | 0.03 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.02 ± 0.01 | |
| Camphene | 955 | 0.20 ± 0.00 | 0.05 ± 0.03 | 0.03 ± 0.01 | 0.06 ± 0.02 | 0.01 ± 0.00 | 0.04 ± 0.02 | 0.11 ± 0.07 | |
| β-Myrcene | 993 | 0.38 ± 0.07 | 0.08 ± 0.01 | 0.32 ± 0.19 | 0.24 ± 0.19 | 0.25 ± 0.12 | 0.13 ± 0.07 | 0.22 ± 0.05 | 0.16 ± 0.08 |
| α-Phellandrene | 1011 | 0.26 ± 0.11 | 0.06 ± 0.02 | 0.26 ± 0.10 | 0.24 ± 0.12 | 0.20 ± 0.08 | 0.15 ± 0.05 | 0.19 ± 0.04 | 0.08 ± 0.01 |
| α-Terpinene | 1023 | 0.73 ± 0.09 | 0.16 ± 0.06 | 0.41 ± 0.29 | 0.36 ± 0.21 | 0.36 ± 0.04 | 0.21 ± 0.10 | 0.33 ± 0.13 | 0.31 ± 0.12 |
| p-Cymene | 1030 | 5.61 ± 0.42 | 2.47 ± 0.88 | 2.79 ± 0.28 | 2.56 ± 1.54 | 2.51 ± 0.88 | 1.11 ± 0.32 | 1.81 ± 0.81 | 7.00 ± 0.55 |
| Limonene | 1035 | 0.55 ± 0.05 | 0.16 ± 0.04 | 0.42 ± 0.09 | 0.35 ± 0.14 | 0.33 ± 0.09 | 0.21 ± 0.19 | 0.29 ± 0.14 | 0.29 ± 0.11 |
| (Z)-β-Ocimene | 1043 | 0.18 ± 0.06 | 0.05 ± 0.01 | 0.13 ± 0.04 | 0.11 ± 0.01 | 0.10 ± 0.01 | 0.06 ± 0.01 | 0.10 ± 0.04 | 0.08 ± 0.02 |
| (E)-β-Ocimene | 1054 | 0.22 ± 0.02 | 0.01 ± 0.00 | 0.20 ± 0.10 | 0.13 ± 0.10 | 0.16 ± 0.14 | 0.06 ± 0.02 | 0.14 ± 0.07 | 0.02 ± 0.01 |
| γ-Terpinene | 1065 | 0.88 ± 0.36 | 0.28 ± 0.08 | 0.47 ± 0.19 | 0.34 ± 0.08 | 0.38 ± 0.09 | 0.21 ± 0.09 | 0.31 ± 0.18 | 0.57 ± 0.05 |
| Oxygenated monoterpenes | |||||||||
| Verbenene | 968 | 0.08 ± 0.01 | 0.09 ± 0.01 | 0.08 ± 0.02 | 0.06 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.00 | 0.02 ± 0.01 | |
| 1,8-Cineole | 1037 | 0.33 ± 0.15 | 0.15 ± 0.04 | 0.17 ± 0.08 | 0.14 ± 0.02 | 0.15 ± 0.05 | 0.10 ± 0.00 | 0.13 ± 0.02 | 0.29 ± 0.14 |
| trans-Linalool oxide | 1078 | 0.12 ± 0.08 | 0.05 ± 0.02 | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.00 |
| Linalool | 1103 | 1.67 ± 1.03 | 1.21 ± 0.25 | 2.11 ± 0.32 | 1.81 ± 0.33 | 1.53 ± 0.22 | 1.51 ± 0.14 | 1.29 ± 0.11 | 1.50 ± 0.25 |
| α-Thujone | 1104 | 0.08 ± 0.02 | 0.06 ± 0.01 | 0.08 ± 0.02 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.05 ± 0.02 | 0.05 ± 0.00 | 0.10 ± 0.04 |
| β-Thujone | 1114 | 0.03 ± 0.00 | 0.03 ± 0.02 | ||||||
| Camphor | 1150 | 0.86 ± 0.12 | 0.57 ± 0.28 | 0.90 ± 0.19 | 0.77 ± 0.15 | 0.72 ± 0.14 | 0.64 ± 0.24 | 0.62 ± 0.28 | 0.72 ± 0.23 |
| Menthone | 1160 | 0.44 ± 0.11 | 0.35 ± 0.15 | 0.46 ± 0.02 | 0.39 ± 0.18 | 0.33 ± 0.21 | 0.30 ± 0.17 | 0.28 ± 0.08 | 0.57 ± 0.12 |
| Borneol | 1170 | 4.13 ± 1.35 | 2.19 ± 0.88 | 4.90 ± 0.41 | 4.60 ± 1.89 | 3.74 ± 0.71 | 3.86 ± 0.17 | 3.93 ± 0.41 | 2.15 ± 0.58 |
| Menthol | 1171 | 0.87 ± 0.18 | 0.66 ± 0.14 | 1.27 ± 0.28 | 1.16 ± 0.88 | 0.87 ± 0.19 | 0.99 ± 0.22 | 0.86 ± 0.14 | 0.65 ± 0.17 |
| Terpinene-4-ol | 1182 | 4.22 ± 1.23 | 2.75 ± 0.25 | 5.14 ± 0.54 | 4.87 ± 1.26 | 3.81 ± 0.88 | 3.83 ± 1.21 | 3.70 ± 0.98 | 3.33 ± 0.87 |
| p-Cymen-8-ol | 1190 | 0.27 ± 0.12 | 0.23 ± 0.8 | 0.26 ± 0.11 | 0.32 ± 0.12 | 0.25 ± 0.04 | 0.35 ± 0.17 | 0.24 ± 0.14 | 0.16 ± 0.02 |
| α-Terpineol | 1194 | 0.46 ± 0.27 | 0.32 ± 0.10 | 0.61 ± 0.28 | 0.63 ± 0.14 | 0.46 ± 0.15 | 0.47 ± 0.11 | 0.43 ± 0.05 | 0.29 ± 0.09 |
| cis-Dihydrocarvone | 1199 | 0.16 ± 0.04 | 0.09 ± 0.02 | 0.13 ± 0.04 | 0.17 ± 0.04 | 0.15 ± 0.03 | 0.17 ± 0.04 | 0.10 ± 0.07 | 0.08 ± 0.02 |
| trans-Dihydrocarvone | 1207 | 0.07 ± 0.03 | 0.04 ± 0.01 | 0.06 ± 0.01 | |||||
| Verbenone | 1224 | 0.08 ± 0.02 | 0.02 ± 0.01 | 0.09 ± 0.01 | 0.09 ± 0.03 | 0.07 ± 0.04 | 0.07 ± 0.02 | 0.05 ± 0.01 | 0.07 ± 0.03 |
| trans-Carveol | 1230 | 0.06 ± 0.01 | 0.01 ± 0.01 | 0.07 ± 0.00 | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.02 ± 0.01 |
| Thymol methyl ether | 1241 | 0.59 ± 0.21 | 0.46 ± 0.13 | 2.00 ± 0.88 | 0.47 ± 0.31 | 0.44 ± 0.11 | 0.25 ± 0.06 | 0.39 ± 0.09 | 1.16 ± 0.51 |
| Cuminal | 1246 | 0.29 ± 0.20 | 0.14 ± 0.04 | 0.36 ± 0.13 | 0.36 ± 0.18 | 0.29 ± 0.05 | 0.29 ± 0.03 | 0.24 ± 0.10 | 0.31 ± 0.28 |
| Carvacrol methyl ether | 1250 | 7.43 ± 2.69 | 6.24 ± 1.25 | 7.48 ± 2.02 | 6.51 ± 2.46 | 6.03 ± 1.04 | 4.05 ± 0.78 | 5.16 ± 1.05 | 13.15 ± 2.54 |
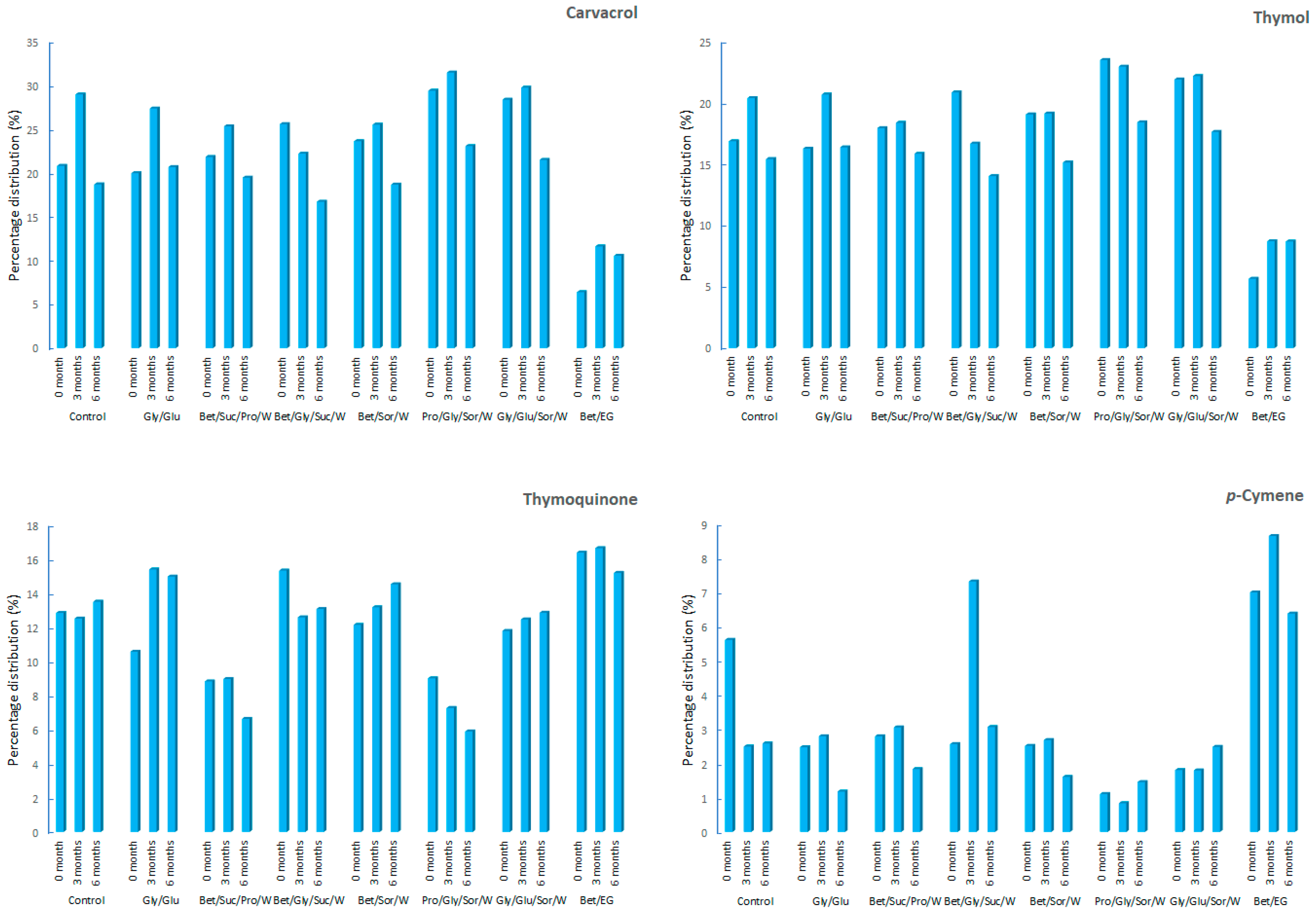
| Thymoquinone | 1258 | 12.84 ± 3.49 | 10.56 ± 1.67 | 8.82 ± 1.98 | 15.33 ± 2.06 | 12.14 ± 1.06 | 9.00 ± 2.24 | 11.78 ± 1.68 | 16.38 ± 0.92 |
| Bornyl acetate | 1297 | 0.67 ± 0.13 | 0.51 ± 0.80 | 0.96 ± 0.35 | |||||
| Thymol | 1297 | 16.86 ± 2.97 | 16.25 ± 2.57 | 17.93 ± 1.68 | 20.84 ± 2.68 | 19.05 ± 0.47 | 23.48 ± 1.77 | 21.89 ± 1.92 | 5.65 ± 0.36 |
| Carvacrol | 1307 | 20.79 ± 4.59 | 19.96 ± 3.04 | 21.81 ± 2.16 | 25.56 ± 4.22 | 23.61 ± 1.28 | 29.37 ± 3.02 | 28.35 ± 2.07 | 6.38 ± 0.97 |
| Dihydroactinidiolide | 1532 | 0.11 ± 0.02 | 0.11 ± 0.04 | ||||||
| Sesquiterpene hydrocarbons | |||||||||
| α-Copaene | 1378 | 0.32 ± 0.18 | 0.26 ± 0.04 | 0.30 ± 0.18 | 0.13 ± 0.03 | 0.25 ± 0.08 | 0.12 ± 0.04 | 0.21 ± 0.05 | 0.49 ± 0.25 |
| β-Bourbonene | 1386 | 0.68 ± 0.34 | 0.69 ± 0.12 | 0.65 ± 0.24 | 0.25 ± 0.05 | 0.52 ± 0.24 | 0.25 ± 0.13 | 0.42 ± 0.18 | 1.40 ± 0.88 |
| trans-Caryophyllene | 1419 | 5.35 ± 1.98 | 5.57 ± 1.38 | 6.12 ± 0.58 | 2.81 ± 1.52 | 5.09 ± 1.44 | 2.79 ± 0.77 | 4.42 ± 1.21 | 10.7 ± 0.69 |
| α-Humulene | 1456 | 0.77 ± 0.24 | 0.65 ± 0.14 | 1.10 ± 0.54 | 0.70 ± 0.12 | 1.06 ± 0.41 | 0.43 ± 0.18 | 0.86 ± 0.30 | 0.88 ± 0.22 |
| γ-Muurolene | 1477 | 0.58 ± 0.39 | 0.60 ± 0.23 | 0.79 ± 0.41 | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.36 ± 0.22 | 0.08 ± 0.01 | 1.31 ± 0.68 |
| Germacrene D | 1482 | 0.55 ± 0.12 | 0.91 ± 0.14 | 0.89 ± 0.49 | 0.35 ± 0.20 | 0.69 ± 0.32 | 0.46 ± 0.18 | 0.51 ± 0.14 | 2.04 ± 0.87 |
| ar-Curcumene | 1483 | 0.05 ± 0.01 | |||||||
| β-Selinene | 1486 | 0.21 ± 0.05 | 0.24 ± 0.08 | 0.27 ± 0.04 | 0.19 ± 0.10 | 0.28 ± 0.02 | 0.18 ± 0.11 | 0.28 ± 0.14 | 0.34 ± 0.14 |
| Ledene | 1493 | 0.98 ± 0.41 | 1.18 ± 0.41 | 1.34 ± 0.09 | 0.74 ± 0.04 | 1.14 ± 0.37 | 0.87 ± 0.51 | 0.97 ± 0.26 | 1.99 ± 0.63 |
| α-Muurolene | 1499 | 0.58 ± 0.29 | 0.52 ± 0.18 | 0.97 ± 0.17 | 0.83 ± 0.24 | 0.96 ± 0.18 | 0.73 ± 0.13 | 0.84 ± 0.15 | 0.73 ± 0.14 |
| β-Bisabolene | 1500 | 1.85 ± 0.46 | 2.08 ± 0.56 | 2.36 ± 0.95 | 1.15 ± 0.88 | 2.07 ± 0.56 | 0.94 ± 0.11 | 1.73 ± 0.34 | 4.06 ± 1.69 |
| γ-Cadinene | 1505 | 0.65 ± 0.18 | 0.74 ± 0.15 | 0.95 ± 0.18 | 0.50 ± 0.16 | 0.89 ± 0.12 | 0.52 ± 0.30 | 0.67 ± 0.15 | 1.37 ± 0.37 |
| Δ-Cadinene | 1525 | 1.07 ± 0.56 | 1.39 ± 0.23 | 1.44 ± 0.55 | 0.74 ± 0.22 | 1.28 ± 0.04 | 0.70 ± 0.19 | 1.07 ± 0.24 | 2.46 ± 0.84 |
| α-Calacorene | 1546 | 0.12 ± 0.08 | 0.16 ± 0.05 | 0.20 ± 0.02 | 0.11 ± 0.05 | 0.10 ± 0.02 | 0.12 ± 0.04 | 0.16 ± 0.04 | 0.26 ± 0.12 |
| Oxygenated sesquiterpenes | |||||||||
| Spathulenol | 1581 | 0.17 ± 0.03 | 1.46 ± 0.47 | 0.87 ± 0.12 | 0.84 ± 0.27 | 0.83 ± 0.62 | 1.26 ± 0.36 | 0.64 ± 0.08 | 0.57 ± 0.14 |
| Others | |||||||||
| Acetic acid | <900 | 0.01 ± 0.00 | |||||||
| Oct-1-en-3-ol | 986 | 0.80 ± 0.26 | 0.40 ± 0.12 | 0.66 ± 0.18 | 0.61 ± 0.18 | 0.51 ± 0.17 | 0.52 ± 0.14 | 0.54 ± 0.14 | 0.42 ± 0.04 |
| 6-Methyl-5-hepten-2-one | 988 | 0.03 ± 0.01 | |||||||
| Octan-3-one | 989 | 0.07 ± 0.01 | |||||||
| 2-Phenylethanol | 1112 | 0.15 ± 0.07 | 0.06 ± 0.03 | ||||||
| Compound | RI | Sample | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Glyc/Glu | Bet/Suc/Pro/W | Bet/Gly/Suc/W | Bet/Sor/W | Pro/Gly/Sor/W | Gly/Glu/Sor/W | Bet/EG | ||
| Monoterpene hydrocarbons | |||||||||
| α-Pinene | 940 | 0.04 ± 0.01 | 0.05 ± 0.02 | 0.07 ± 0.02 | 0.05 ± 0.04 | 0.07 ± 0.05 | 0.02 ± 0.01 | 0.03 ± 0.02 | 0.15 ± 0.02 |
| Camphene | 955 | 0.08 ± 0.03 | 0.09 ± 0.02 | 0.13 ± 0.06 | 0.04 ± 0.01 | 0.12 ± 0.05 | 0.05 ± 0.02 | 0.06 ± 0.02 | 0.28 ± 0.10 |
| β-Myrcene | 993 | 0.07 ± 0.01 | |||||||
| α-Phellandrene | 1011 | 0.05 ± 0.00 | |||||||
| α-Terpinene | 1023 | 0.10 ± 0.02 | 0.03 ± 0.01 | 0.05 ± 0.01 | 0.07 ± 0.02 | 0.01 ± 0.00 | 0.07 ± 0.01 | 0.14 ± 0.04 | |
| p-Cymene | 1030 | 2.49 ± 0.65 | 2.79 ± 0.41 | 3.05 ± 0.44 | 7.32 ± 3.04 | 2.68 ± 0.58 | 0.84 ± 0.17 | 1.80 ± 0.41 | 8.65 ± 2.58 |
| Limonene | 1035 | 0.22 ± 0.04 | 0.15 ± 0.03 | 0.14 ± 0.02 | 0.12 ± 0.03 | 0.19 ± 0.03 | |||
| γ-Terpinene | 1065 | 0.09 ± 0.03 | 0.04 ± 0.02 | 0.05 ± 0.01 | 0.06 ± 0.02 | 0.09 ± 0.03 | 0.07 ± 0.04 | 0.04 ± 0.01 | |
| Oxygenated monoterpenes | |||||||||
| 1,8-Cineole | 1037 | 0.40 ± 0.09 | 0.54 ± 0.12 | 0.54 ± 0.21 | 0.11 ± 0.02 | 0.43 ± 0.22 | 0.35 ± 0.10 | 0.39 ± 0.12 | 0.07 ± 0.03 |
| trans-Linalool oxide | 1078 | 0.20 ± 0.08 | 0.40 ± 0.15 | 0.17 ± 0.08 | 0.21 ± 0.05 | ||||
| Linalool | 1103 | 2.74 ± 0.45 | 3.21 ± 0.22 | 4.39 ± 0.78 | 3.84 ± 1.75 | 3.86 ± 1.31 | 3.34 ± 0.20 | 2.79 ± 0.41 | 2.76 ± 0.87 |
| Camphor | 1150 | 1.20 ± 0.31 | 1.01 ± 0.06 | 1.44 ± 0.39 | 1.26 ± 0.12 | 1.30 ± 0.88 | 1.23 ± 0.23 | 0.98 ± 0.61 | 1.14 ± 0.56 |
| Menthone | 1160 | 0.34 ± 0.12 | 0.11 ± 0.04 | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.41 ± 0.18 | 0.34 ± 0.04 | 0.29 ± 0.12 | 0.60 ± 0.23 |
| Borneol | 1170 | 4.63 ± 1.23 | 3.59 ± 0.83 | 6.07 ± 1.05 | 4.93 ± 1.54 | 5.49 ± 0.71 | 5.90 ± 0.71 | 4.94 ± 0.15 | 2.80 ± 0.48 |
| Menthol | 1171 | 0.22 ± 0.15 | 0.23 ± 0.06 | 1.32 ± 0.44 | 0.48 ± 0.23 | 0.78 ± 0.12 | 0.68 ± 0.14 | 0.78 ± 0.15 | |
| Terpinene-4-ol | 1182 | 6.79 ± 2.60 | 6.28 ± 1.36 | 8.29 ± 1.28 | 7.29 ± 2.04 | 7.32 ± 0.47 | 8.41 ± 2.47 | 6.44 ± 2.51 | 5.95 ± 0.56 |
| p-Cymen-8-ol | 1190 | 0.26 ± 0.14 | 0.12 ± 0.05 | 0.28 ± 0.12 | 0.42 ± 0.04 | 0.19 ± 0.04 | 0.32 ± 0.05 | 0.29 ± 0.14 | |
| α-Terpineol | 1194 | 0.84 ± 0.06 | 0.52 ± 0.15 | 0.54 ± 0.04 | 0.22 ± 0.04 | 0.65 ± 0.23 | 0.22 ± 0.10 | 0.12 ± 0.10 | 0.33 ± 0.10 |
| cis-Dihydrocarvone | 1199 | 0.01 ± 0.01 | 0.21 ± 0.09 | 0.09 ± 0.01 | 0.09 ± 0.06 | 0.10 ± 0.02 | |||
| Verbenone | 1224 | 0.01 | 0.03 ± 0.01 | 0.12 ± 0.01 | 0.03 ± 0.02 | 0.01 ± 0.00 | 0.02 ± 0.01 | ||
| trans-Carveol | 1230 | 0.01 ± 0.01 | 0.05 ± 0.02 | 0.10 ± 0.01 | 0.01 ± 0.01 | 0.07 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 | |
| Thymol methyl ether | 1241 | 0.24 ± 0.14 | 0.09 ± 0.03 | 0.10 ± 0.02 | 0.11 ± 0.03 | 0.19 ± 0.11 | 0.08 ± 0.02 | 0.14 ± 0.04 | 0.03 ± 0.01 |
| Cuminal | 1246 | 0.14 ± 0.08 | 0.15 ± 0.05 | 0.18 ± 0.12 | 0.12 ± 0.03 | 0.29 ± 0.14 | |||
| Carvacrol methyl ether | 1250 | 3.75 ± 1.54 | 4.95 ± 0.87 | 8.96 ± 1.41 | 7.03 ± 2.87 | 3.98 ± 0.96 | 2.98 ± 0.41 | 3.18 ± 0.58 | 9.44 ± 1.58 |
| Thymoquinone | 1258 | 12.51 ± 2.08 | 15.39 ± 2.05 | 8.96 ± 1.69 | 12.58 ± 3.16 | 13.17 ± 1.58 | 7.26 ± 1.41 | 12.46 ± 2.27 | 16.64 ± 2.66 |
| Bornyl acetate | 1297 | 0.39 ± 0.07 | 0.13 ± 0.04 | 0.37 ± 0.10 | 0.35 ± 0.10 | 0.35 ± 0.12 | 0.77 ± 0.14 | ||
| Thymol | 1297 | 20.38 ± 3.15 | 20.67 ± 0.47 | 18.36 ± 2.12 | 16.67 ± 1.89 | 19.12 ± 1.06 | 22.94 ± 1.89 | 22.17 ± 1.36 | 8.71 ± 0.57 |
| Carvacrol | 1307 | 28.95 ± 2.08 | 27.34 ± 1.51 | 25.31 ± 0.25 | 22.19 ± 0.87 | 25.50 ± 2.71 | 31.44 ± 3.21 | 29.72 ± 2.14 | 11.61 ± 0.66 |
| Dihydroactinidiolide | 1532 | 0.04 ± 0.01 | 0.08 ± 0.04 | 0.10 ± 0.06 | 0.09 ± 0.02 | 0.39 ± 0.16 | |||
| α-Copaene | 1378 | 0.13 ± 0.06 | 0.16 ± 0.09 | 0.03 ± 0.00 | 0.10 ± 0.08 | 0.35 ± 0.12 | |||
| β-Bourbonene | 1386 | 0.37 ± 0.09 | 0.23 ± 0.05 | 0.10 ± 0.04 | 0.11 ± 0.01 | 0.41 ± 0.17 | 0.29 ± 0.09 | 0.28 ± 0.11 | 1.00 ± 0.33 |
| trans-Caryophyllene | 1419 | 2.58 ± 0.21 | 0.93 ± 0.41 | 4.23 ± 0.09 | 2.83 ± 0.81 | 1.93 ± 0.47 | 2.51 ± 0.88 | 2.00 ± 0.36 | 2.93 ± 0.74 |
| α-Humulene | 1456 | 0.18 ± 0.04 | 0.15 ± 0.09 | 0.13 ± 0.04 | 0.10 ± 0.01 | 0.13 ± 0.07 | 0.39 ± 0.14 | ||
| γ-Muurolene | 1477 | 0.27 ± 0.18 | 0.16 ± 0.04 | 0.07 ± 0.06 | 0.11 ± 0.03 | 0.25 ± 0.15 | 0.19 ± 0.08 | 0.76 ± 0.26 | |
| Germacrene D | 1482 | 0.12 ± 0.01 | 0.12 ± 0.04 | 0.23 ± 0.03 | 0.06 ± 0.02 | ||||
| β-Selinene | 1486 | 0.17 ± 0.05 | 0.30 ± 0.10 | ||||||
| Ledene | 1493 | 0.12 ± 0.03 | 0.13 ± 0.06 | ||||||
| α-Muurolene | 1499 | 0.04 ± 0.01 | 0.13 ± 0.01 | 0.07 ± 0.02 | 0.05 ± 0.01 | 0.01 ± 0.00 | |||
| β-Bisabolene | 1500 | 1.41 ± 0.31 | 1.19 ± 0.62 | 1.48 ± 0.27 | 2.08 ± 0.19 | 1.13 ± 0.14 | 0.79 ± 0.14 | 1.14 ± 0.54 | 1.91 ± 0.87 |
| γ-Cadinene | 1505 | 0.29 ± 0.06 | 0.22 ± 0.02 | 0.28 ± 0.13 | 0.17 ± 0.02 | 0.06 ± 0.02 | 0.21 ± 0.12 | 0.65 ± 0.14 | |
| Δ-Cadinene | 1525 | 0.46 ± 0.08 | 0.45 ± 0.58 | 0.15 ± 0.07 | 0.62 ± 0.14 | 0.35 ± 0.17 | 0.36 ± 0.12 | 0.43 ± 0.18 | 0.89 ± 0.33 |
| α-Calacorene | 1546 | 0.11 ± 0.10 | 0.08 ± 0.01 | 0.18 ± 0.12 | |||||
| Oxygenated sesquiterpenes | |||||||||
| Spathulenol | 1581 | 0.92 ± 0.14 | 0.16 ± 0.02 | 1.00 ± 0.15 | 0.23 ± 0.03 | 1.01 ± 0.18 | 0.92 ± 0.17 | 1.23 ± 0.48 | |
| Caryophyllene oxide | 1581 | 3.54 ± 0.95 | 6.56 ± 1.86 | 3.45 ± 0.74 | 4.91 ± 2.66 | 5.37 ± 0.66 | 4.84 ± 1.24 | 4.09 ± 0.74 | 12.04 ± 1.97 |
| Others | |||||||||
| Acetic acid | <900 | 1.29 ± 0.28 | |||||||
| Oct-1-en-3-ol | 986 | 1.02 ± 0.40 | 0.86 ± 0.15 | 1.30 ± 0.31 | 1.10 ± 0.14 | 1.29 ± 0.41 | 1.13 ± 0.18 | 1.03 ± 0.38 | 0.65 ± 0.39 |
| Octan-3-one | 989 | 0.07 ± 0.02 | 0.06 ± 0.01 | 0.04 ± 0.02 | 0.02 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.01 | ||
| 2-Phenylethanol | 1112 | 0.07 ± 0.01 | |||||||
| Compound | RI | Sample | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Glyc/Glu | Bet/Suc/Pro/W | Bet/Gly/Suc/W | Bet/Sor/W | Pro/Gly/Sor/W | Gly/Glu/Sor/W | Bet/EG | ||
| Monoterpene hydrocarbons | |||||||||
| α-Pinene | 940 | 0.05 ± 0.02 | 0.05 ± 0.01 | 0.03 ± 0.02 | 0.16 ± 0.08 | ||||
| Camphene | 955 | 0.25 ± 0.04 | 0.09 ± 0.03 | 0.09 ± 0.03 | 0.08 ± 0.04 | 0.04 ± 0.03 | 0.09 ± 0.02 | 0.28 ± 0.14 | |
| α-Phellandrene | 1011 | 0.06 ± 0.01 | 0.09 ± 0.02 | 0.08 ± 0.01 | 0.10 ± 0.04 | 0.08 ± 0.05 | 0.06 ± 0.01 | ||
| α-Terpinene | 1023 | 0.16 ± 0.08 | 0.11 ± 0.04 | 0.13 ± 0.08 | 0.11 ± 0.04 | 0.09 ± 0.03 | 0.10 ± 0.02 | 0.14 ± 0.04 | 0.18 ± 0.05 |
| p-Cymene | 1030 | 2.58 ± 0.18 | 1.18 ± 0.35 | 1.84 ± 0.26 | 3.06 ± 1.32 | 1.61 ± 0.19 | 1.46 ± 0.74 | 2.48 ± 1.14 | 6.38 ± 0.63 |
| Limonene | 1035 | 0.13 ± 0.03 | 0.12 ± 0.04 | 0.12 ± 0.01 | 0.16 ± 0.04 | 0.10 ± 0.07 | |||
| γ-Terpinene | 1065 | 0.03 ± 0.01 | 0.05 ± 0.01 | 0.11 ± 0.04 | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.08 ± 0.04 | 0.04 ± 0.01 | 0.11 ± 0.07 |
| Oxygenated monoterpenes | |||||||||
| 1,8-Cineole | 1037 | 0.59 ± 0.25 | 0.11 ± 0.03 | 0.31 ± 0.26 | 0.32 ± 0.14 | 0.28 ± 0.04 | 0.37 ± 0.14 | 0.39 ± 0.25 | 0.70 ± 0.28 |
| cis-Linalool oxide | 1087 | ||||||||
| trans-Linalool oxide | 1078 | 0.29 ± 0.10 | 0.14 ± 0.05 | 0.20 ± 0.15 | 0.18 ± 0.05 | 0.22 ± 0.05 | 0.22 ± 0.07 | 0.21 ± 0.17 | 0.10 ± 0.04 |
| Linalool | 1103 | 3.14 ± 1.03 | 3.40 ± 1.04 | 4.57 ± 1.39 | 3.49 ± 1.99 | 3.75 ± 0.94 | 3.78 ± 0.51 | 3.19 ± 0.96 | 3.13 ± 0.68 |
| α-Thujone | 1104 | 0.13 ± 0.04 | 0.08 ± 0.02 | 0.07 ± 0.02 | 0.10 ± 0.02 | 0.10 ± 0.02 | 0.10 ± 0.01 | 0.12 ± 0.04 | 0.17 ± 0.12 |
| cis-p-Mentha-2-en-1-ol | 1126 | 0.25 ± 0.06 | 0.09 ± 0.04 | 0.28 ± 0.12 | 0.26 ± 0.04 | 0.28 ± 0.10 | 0.27 ± 0.08 | 0.14 ± 0.08 | 0.13 ± 0.09 |
| β-Thujone | 1114 | 0.06 ± 0.01 | |||||||
| Camphor | 1150 | 1.25 ± 0.15 | 0.91 ± 0.42 | 1.35 ± 0.24 | 1.12 ± 0.45 | 1.15 ± 0.09 | 1.24 ± 0.61 | 1.14 ± 0.31 | 1.32 ± 0.54 |
| Menthone | 1160 | 0.48 ± 0.13 | 0.31 ± 0.15 | 0.49 ± 0.12 | 0.47 ± 0.18 | 0.41 ± 0.19 | 0.46 ± 0.14 | 0.42 ± 0.12 | 0.70 ± 0.24 |
| Borneol | 1170 | 4.85 ± 1.39 | 3.73 ± 0.65 | 5.61 ± 0.54 | 4.56 ± 2.04 | 4.94 ± 1.04 | 5.43 ± 0.98 | 4.84 ± 1.58 | 3.64 ± 1.87 |
| Menthol | 1171 | 1.47 ± 0.58 | 1.58 ± 0.41 | 2.11 ± 0.21 | 1.66 ± 0.85 | 1.75 ± 0.66 | 1.90 ± 0.41 | 1.59 ± 0.69 | 1.32 ± 0.65 |
| Terpinene-4-ol | 1182 | 5.73 ± 1.52 | 5.49 ± 1.58 | 6.72 ± 1.08 | 5.41 ± 1.24 | 5.67 ± 0.71 | 6.55 ± 0.24 | 5.35 ± 0.45 | 5.98 ± 1.00 |
| p-Cymen-8-ol | 1190 | 1.16 ± 0.26 | 0.37 ± 0.12 | 1.10 ± 0.05 | 0.86 ± 0.04 | 0.99 ± 0.21 | 1.04 ± 0.12 | 0.93 ± 0.31 | 0.43 ± 0.05 |
| Terpenediol I | 1192 | 0.05 ± 0.01 | |||||||
| α-Terpineol | 1194 | 0.97 ± 0.22 | 0.81 ± 0.28 | 1.41 ± 0.78 | 1.00 ± 0.21 | 1.20 ± 0.14 | 1.14 ± 0.27 | 0.96 ± 0.41 | 0.67 ± 0.24 |
| cis-Dihydrocarvone | 1199 | 0.20 ± 0.06 | 0.07 ± 0.02 | 0.27 ± 0.02 | 0.47 ± 0.12 | 0.23 ± 0.02 | 0.21 ± 0.08 | 0.18 ± 0.08 | 0.20 ± 0.05 |
| trans-Dihydrocarvone | 1207 | 0.04 ± 0.01 | |||||||
| Verbenone | 1224 | 0.31 ± 0.15 | 0.05 ± 0.01 | 0.15 ± 0.04 | 0.21 ± 0.10 | 0.15 ± 0.04 | 0.04 ± 0.01 | 0.08 ± 0.02 | 0.07 ± 0.04 |
| trans-Carveol | 1230 | 0.21 ± 0.08 | 0.15 ± 0.05 | 0.20 ± 0.02 | 0.22 ± 0.07 | 0.22 ± 0.09 | 0.01 ± 0.00 | ||
| Thymol methyl ether | 1241 | 0.36 ± 0.14 | 0.30 ± 0.14 | 0.44 ± 0.17 | 0.56 ± 0.19 | 0.34 ± 0.11 | 0.33 ± 0.05 | 0.31 ± 0.17 | 0.77 ± 0.18 |
| Cuminal | 1246 | 0.31 ± 0.06 | 0.11 ± 0.08 | 0.26 ± 0.10 | 0.32 ± 0.11 | 0.31 ± 0.12 | 0.26 ± 0.05 | 0.25 ± 0.05 | 0.21 ± 0.10 |
| Carvacrol methyl ether | 1250 | 3.58 ± 1.58 | 3.99 ± 0.05 | 4.75 ± 0.88 | 5.57 ± 1.05 | 4.03 ± 0.31 | 3.45 ± 1.25 | 3.52 ± 0.28 | 7.91 ± 1.84 |
| Thymoquinone | 1258 | 13.50 ± 2.56 | 14.97 ± 1.04 | 6.62 ± 2.00 | 13.07 ± 3.58 | 14.52 ± 1.88 | 5.88 ± 0.48 | 12.85 ± 1.28 | 15.19 ± 0.84 |
| Geraniol | 1262 | 0.40 ± 0.16 | 0.74 ± 0.18 | 0.75 ± 0.18 | 0.82 ± 0.22 | 0.97 ± 0.31 | 1.04 ± 0.22 | 0.83 ± 0.14 | 0.70 ± 0.25 |
| Piperitone | 1263 | 0.13 ± 0.04 | 0.18 ± 0.04 | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.13 ± 0.04 | 0.10 ± 0.02 | 0.09 ± 0.01 | 0.16 ± 0.07 |
| Bornyl acetate | 1297 | 0.52 ± 0.32 | 0.66 ± 0.32 | 0.71 ± 0.22 | 0.69 ± 0.14 | 0.67 ± 0.18 | 0.54 ± 0.14 | 0.55 ± 0.00 | 1.01 ± 0.15 |
| Thymol | 1297 | 15.41 ± 1.56 | 16.36 ± 2.58 | 15.84 ± 2.84 | 14.02 ± 3.14 | 15.13 ± 2.08 | 18.40 ± 1.44 | 17.62 ± 1.05 | 8.69 ± 1.65 |
| Carvacrol | 1307 | 18.68 ± 3.15 | 20.66 ± 1.88 | 19.45 ± 0.41 | 16.70 ± 2.50 | 18.66 ± 0.39 | 23.05 ± 1.84 | 21.48 ± 0.41 | 10.53 ± 2.84 |
| Limonen-1,2-diol | 1342 | 0.17 ± 0.05 | 0.17 ± 0.04 | 0.11 ± 0.08 | 0.07 ± 0.01 | ||||
| Dihydroactinidiolide | 1532 | 0.20 ± 0.10 | 0.13 ± 0.00 | 0.27 ± 0.11 | 0.24 ± 0.15 | 0.31 ± 0.07 | 0.29 ± 0.15 | 0.24 ± 0.04 | 0.11 ± 0.05 |
| Sesquiterpene hydrocarbons | |||||||||
| α-Copaene | 1378 | 0.17 ± 0.06 | 0.32 ± 0.12 | 0.31 ± 0.04 | 0.34 ± 0.12 | 0.20 ± 0.04 | 0.16 ± 0.05 | 0.14 ± 0.04 | 0.33 ± 0.14 |
| β-Bourbonene | 1386 | 0.48 ± 0.24 | 0.79 ± 0.24 | 0.92 ± 0.10 | 1.07 ± 0.17 | 0.66 ± 0.28 | 0.56 ± 0.14 | 0.45 ± 0.12 | 1.14 ± 0.35 |
| trans-Caryophyllene | 1419 | 2.23 ± 1.26 | 0.73 ± 0.42 | 4.55 ± 1.85 | 2.03 ± 1.54 | 1.39 ± 0.77 | 2.89 ± 0.88 | 1.80 ± 0.58 | 2.87 ± 0.12 |
| α-Humulene | 1456 | 0.11 ± 0.06 | 0.44 ± 0.14 | 0.26 ± 0.14 | 0.22 ± 0.11 | 0.34 ± 0.14 | 0.21 ± 0.14 | 0.45 ± 0.08 | |
| γ-Muurolene | 1477 | 0.58 ± 0.21 | 0.11 ± 0.02 | 0.11 ± 0.01 | 0.12 ± 0.02 | 0.32 ± 0.05 | 0.28 ± 0.16 | 0.18 ± 0.02 | |
| Germacrene D | 1482 | 0.13 ± 0.02 | 0.05 ± 0.01 | 0.37 ± 0.11 | 0.11 ± 0.03 | 0.16 ± 0.05 | 0.24 ± 0.07 | 0.08 ± 0.01 | 0.21 ± 0.05 |
| ar-Curcumene | 1483 | 0.05 ± 0.02 | 0.07 ± 0.02 | ||||||
| β-Selinene | 1486 | 0.22 ± 0.07 | 0.10 ± 0.01 | 0.25 ± 0.12 | 0.29 ± 0.07 | 0.40 ± 0.09 | 0.69 ± 0.11 | 0.24 ± 0.09 | |
| Ledene | 1493 | 0.10 ± 0.05 | |||||||
| α-Muurolene | 1499 | 0.88 ± 0.35 | 0.11 ± 0.04 | 0.11 ± 0.07 | 0.17 ± 0.11 | 0.97 ± 0.27 | 0.11 ± 0.08 | 0.28 ± 0.08 | 0.57 ± 0.14 |
| β-Bisabolene | 1500 | 0.87 ± 0.28 | 1.62 ± 0.41 | 1.86 ± 0.05 | 2.14 ± 0.41 | 1.07 ± 0.64 | 0.82 ± 0.22 | 0.61 ± 0.14 | 1.62 ± 0.36 |
| γ-Cadinene | 1505 | 0.41 ± 0.11 | 0.77 ± 0.31 | 0.75 ± 0.06 | 0.78 ± 0.13 | 0.57 ± 0.29 | 0.63 ± 0.14 | 0.45 ± 0.25 | |
| Δ-Cadinene | 1525 | 0.69 ± 0.24 | 1.13 ± 0.78 | 1.08 ± 0.21 | 1.02 ± 0.22 | 0.69 ± 0.24 | 0.63 ± 0.42 | 0.56 ± 0.25 | 1.12 ± 0.44 |
| α-Calacorene | 1546 | 0.12 ± 0.04 | 0.22 ± 0.10 | 0.15 ± 0.04 | 0.11 ± 0.02 | 0.05 ± 0.01 | 0.14 ± 0.04 | 0.12 ± 0.01 | 0.21 ± 0.05 |
| Oxygenated sesquiterpenes | |||||||||
| Spathulenol | 1581 | 1.41 ± 0.66 | 2.06 ± 0.65 | 2.05 ± 0.11 | 1.97 ± 0.23 | 2.14 ± 0.41 | 2.31 ± 0.75 | 1.69 ± 0.14 | 2.32 ± 0.41 |
| Caryophyllene oxide | 1581 | 2.78 ± 1.15 | 5.07 ± 1.59 | 3.96 ± 0.54 | 5.38 ± 1.52 | 4.69 ± 0.67 | 3.58 ± 0.22 | 3.40 ± 0.64 | 7.76 ± 0.87 |
| Veridiflorol | 1592 | 0.27 ± 0.06 | 0.40 ± 0.13 | 0.34 ± 0.12 | 0.36 ± 0.14 | 0.40 ± 0.14 | 0.38 ± 0.04 | 0.29 ± 0.04 | 0.48 ± 0.01 |
| Others | |||||||||
| Acetic acid | <900 | 1.45 ± 0.60 | |||||||
| γ-Butyrolactone | 921 | 0.07 ± 0.02 | |||||||
| Oct-1-en-3-ol | 986 | 1.45 ± 0.12 | 1.54 ± 0.62 | 1.77 ± 0.41 | 2.39 ± 0.76 | 1.43 ± 0.76 | 1.56 ± 0.81 | 2.42 ± 1.03 | 1.01 ± 0.02 |
| (E,E)-Hepta-2,4-dienal | 1016 | 0.13 ± 0.05 | |||||||
| 5-Methyl-5-vinyldihydrofuran-2(3H)-one | 1047 | 0.10 ± 0.03 | 0.04 ± 0.00 | ||||||
| 4-Methyl-benzaldehyde | 1086 | 0.18 ± 0.07 | 0.19 ± 0.09 | 0.15 ± 0.02 | 0.13 ± 0.09 | 0.07 ± 0.02 | 0.08 ± 0.05 | ||
| Octan-3-one | 989 | 0.10 ± 0.01 | 0.15 ± 0.09 | 0.09 ± 0.04 | 0.11 ± 0.01 | ||||
| 2-Phenylethanol | 1112 | 0.26 ± 0.06 | 0.08 ± 0.03 | 0.16 ± 0.09 | 0.11 ± 0.06 | 0.12 ± 0.04 | 0.14 ± 0.02 | 0.15 ± 0.07 | 0.14 ± 0.06 |
| Vanillin | 1398 | 0.10 ± 0.01 | |||||||
| Eugenol | 1359 | 0.04 ± 0.02 | 0.03 ± 0.00 | ||||||
| 1,3,5-Trimethoxy-benzene | 1410 | 0.13 ± 0.03 | 0.14 ± 0.05 | 0.18 ± 0.04 | 0.18 ± 0.07 | 0.14 ± 0.04 | 0.12 ± 0.05 | 0.19 ± 0.11 | 0.07 ± 0.03 |
| Start of the Monitoring | Monitoring after 3 Months | Monitoring after 6 Months | ||
|---|---|---|---|---|
| Cont0 | Cont3 | Cont6 | ||
| N valid = 54 T = 476.5 Z = 2.290 p = 0.022 | N valid = 62 T = 500.5 Z = 3.337 p = 0.001 | |||
| Gly/Glu0 | Gly/Glu3 | Gly/Glu6 | ||
| N valid = 48 T = 472.5 Z = 1.185 p = 0.236 | N valid = 51 T = 423.5 Z = 2.245 p = 0.025 | |||
| Bet/Suc/Pro/W0 | Bet/Suc/Pro/W3 | Bet/Suc/Pro/W6 | ||
| N valid = 52 T = 474.5 Z = 1.953 p = 0.05 | N valid = 55 T = 412.0 Z = 3000 p = 0.003 | |||
| Bet/Gly/Suc/W0 | Bet/Gly/Suc/W3 | Bet/Gly/Suc/W6 | ||
| N valid = 49 T = 444.5 Z = 1.671 p = 0.095 | N valid = 50 T = 356.0 Z = 2.717 p = 0.007 | |||
| Bet/Sor/W0 | Beth/Sor/W3 | Beth/Sor/W6 | ||
| N valid = 50 T = 556.0 Z = 0.787 p = 0.431 | N valid = 52 T = 458.0 Z = 2.104 p = 0.035 | |||
| Pro/Gly/Sor/W0 | Pro/Gly/Sor/W3 | Pro/Gly/Sor/W6 | ||
| N valid = 49 T = 420.5 Z = 1.910 p = 0.06 | N valid = 51 T = 292.0 Z = 3.478 p = 0.0005 | |||
| Gly/Glu/Sor/W0 | Gly/Glu/Sor/W3 | Gly/Glu/Sor/W6 | ||
| N valid = 50 T = 554.0 Z = 0.806 p = 0.420 | N valid = 49 T = 315.0 Z = 2.959 p = 0.003 | |||
| Bet/EG0 | Bet/EG3 | Bet/EG6 | ||
| N valid = 48 T = 493.5 Z = 0.969 p = 0.332 | N valid = 49 T = 398.5 Z = 2.129 p = 0.033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vladić, J.; Kovačević, S.; Aladić, K.; Jokić, S.; Radman, S.; Podunavac-Kuzmanović, S.; Duarte, A.R.C.; Jerković, I. Innovative Strategy for Aroma Stabilization Using Green Solvents: Supercritical CO2 Extracts of Satureja montana Dispersed in Deep Eutectic Solvents. Biomolecules 2023, 13, 1126. https://doi.org/10.3390/biom13071126
Vladić J, Kovačević S, Aladić K, Jokić S, Radman S, Podunavac-Kuzmanović S, Duarte ARC, Jerković I. Innovative Strategy for Aroma Stabilization Using Green Solvents: Supercritical CO2 Extracts of Satureja montana Dispersed in Deep Eutectic Solvents. Biomolecules. 2023; 13(7):1126. https://doi.org/10.3390/biom13071126
Chicago/Turabian StyleVladić, Jelena, Strahinja Kovačević, Krunoslav Aladić, Stela Jokić, Sanja Radman, Sanja Podunavac-Kuzmanović, Ana Rita C. Duarte, and Igor Jerković. 2023. "Innovative Strategy for Aroma Stabilization Using Green Solvents: Supercritical CO2 Extracts of Satureja montana Dispersed in Deep Eutectic Solvents" Biomolecules 13, no. 7: 1126. https://doi.org/10.3390/biom13071126
APA StyleVladić, J., Kovačević, S., Aladić, K., Jokić, S., Radman, S., Podunavac-Kuzmanović, S., Duarte, A. R. C., & Jerković, I. (2023). Innovative Strategy for Aroma Stabilization Using Green Solvents: Supercritical CO2 Extracts of Satureja montana Dispersed in Deep Eutectic Solvents. Biomolecules, 13(7), 1126. https://doi.org/10.3390/biom13071126














