Fungal Endophytes: Microfactories of Novel Bioactive Compounds with Therapeutic Interventions; A Comprehensive Review on the Biotechnological Developments in the Field of Fungal Endophytic Biology over the Last Decade
Abstract
1. Introduction
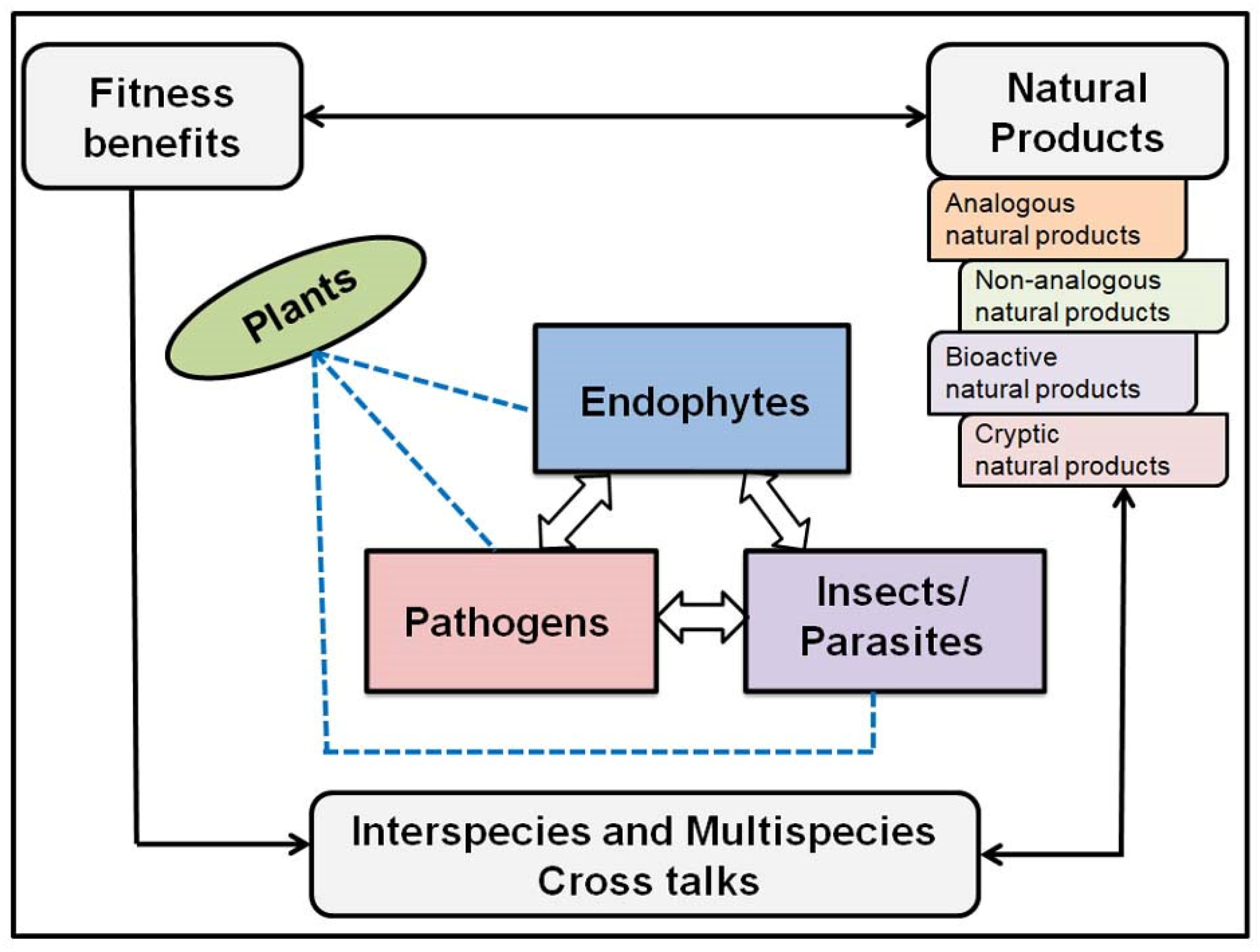
2. What Is an Endophyte?
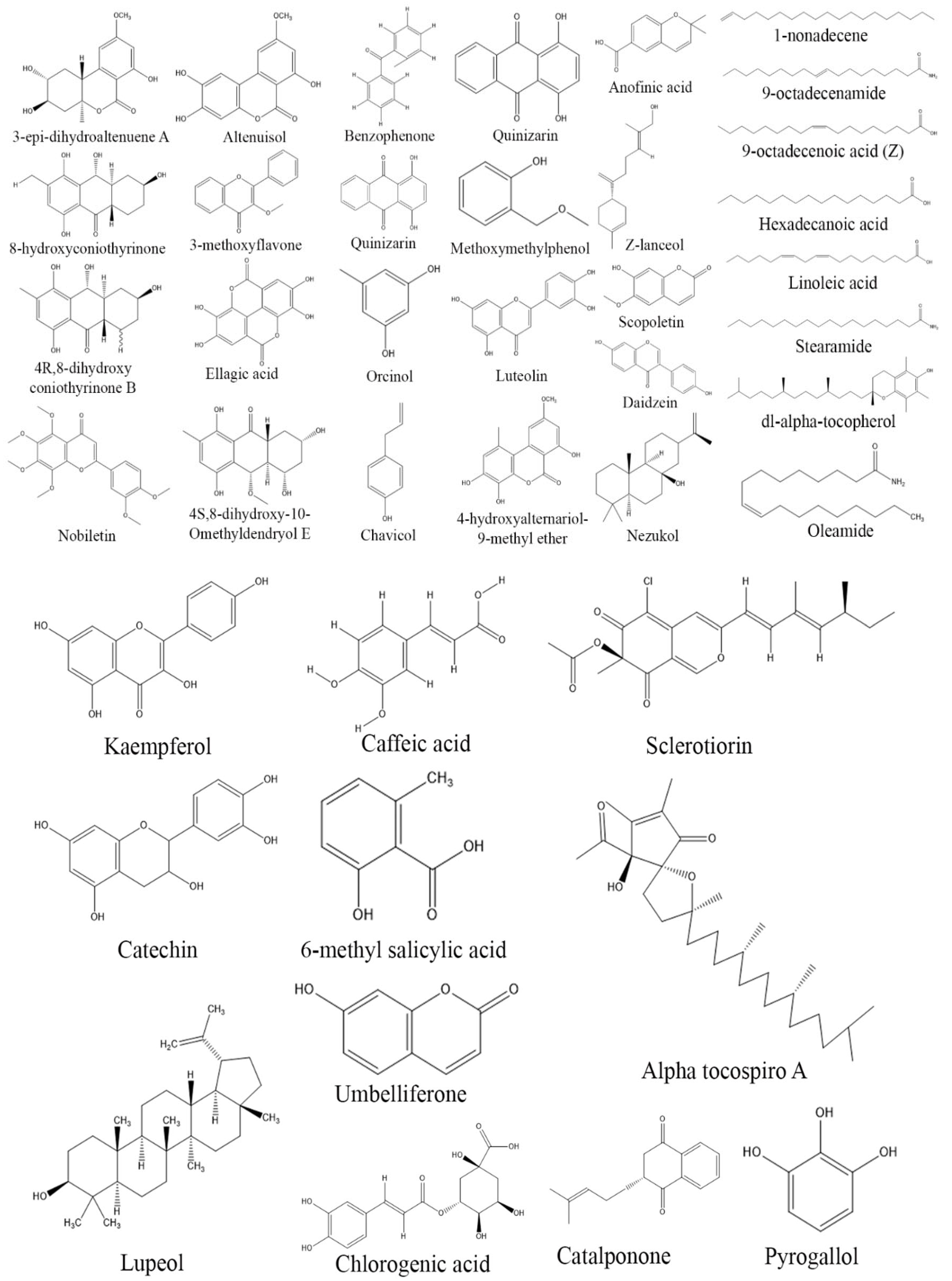
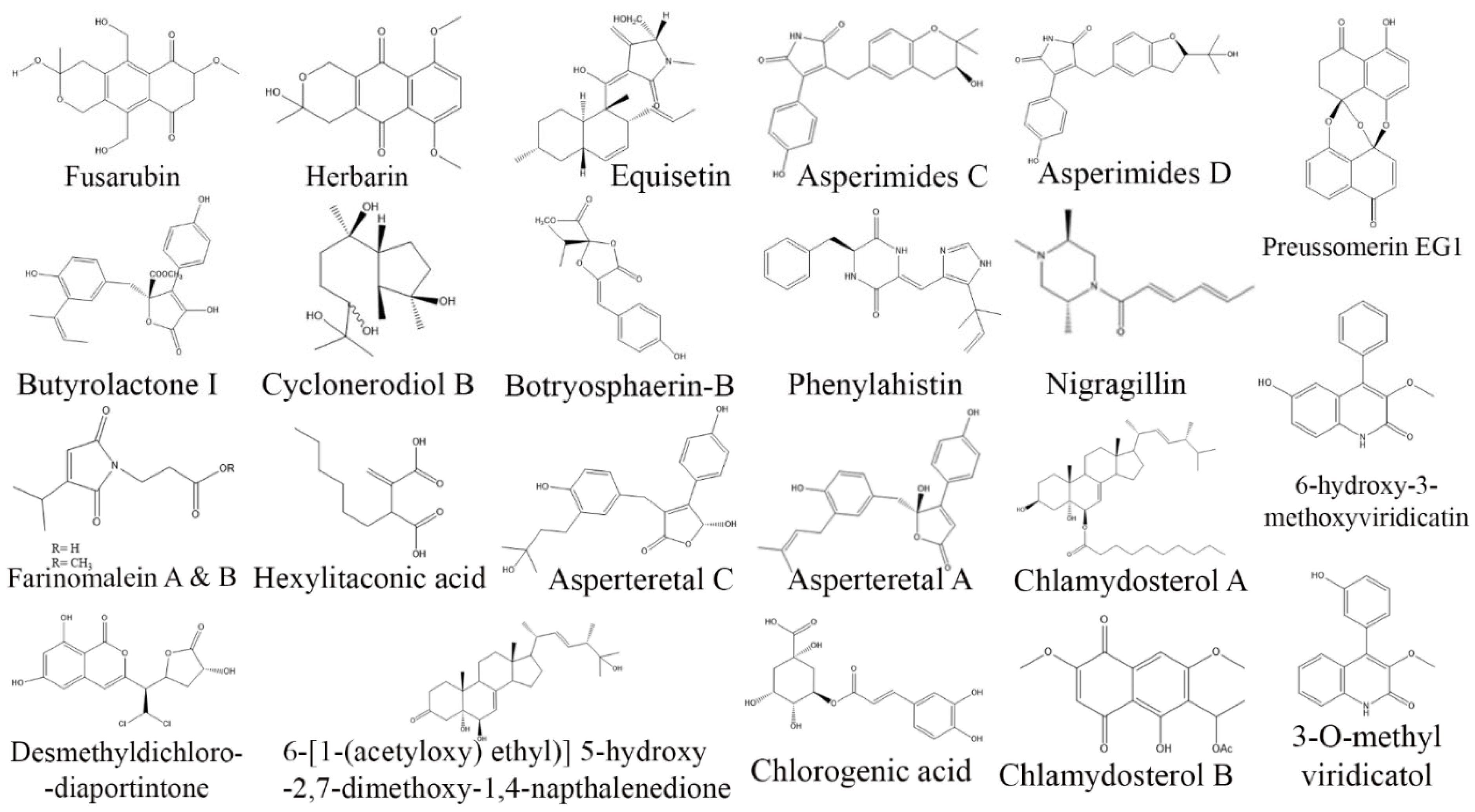
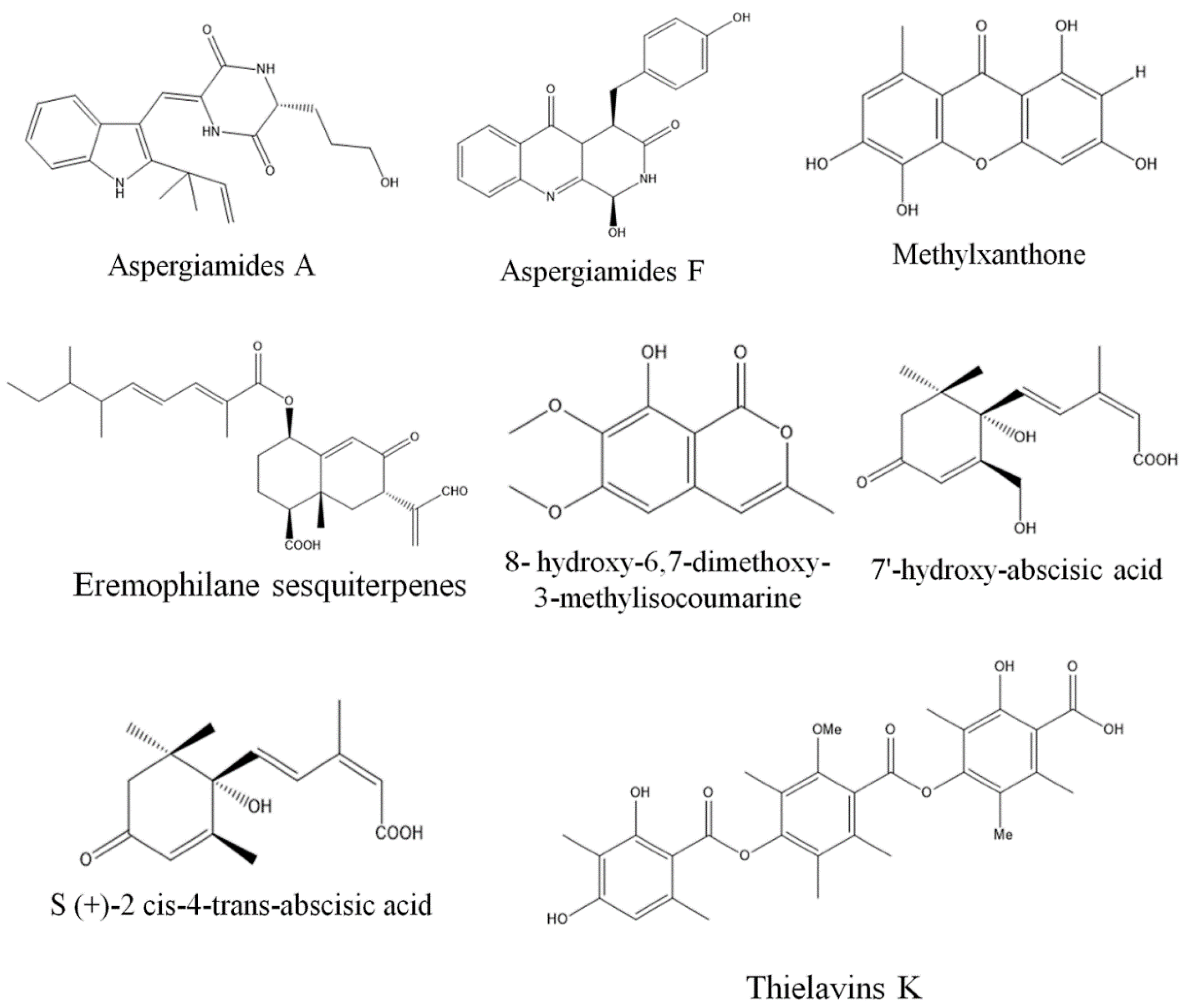
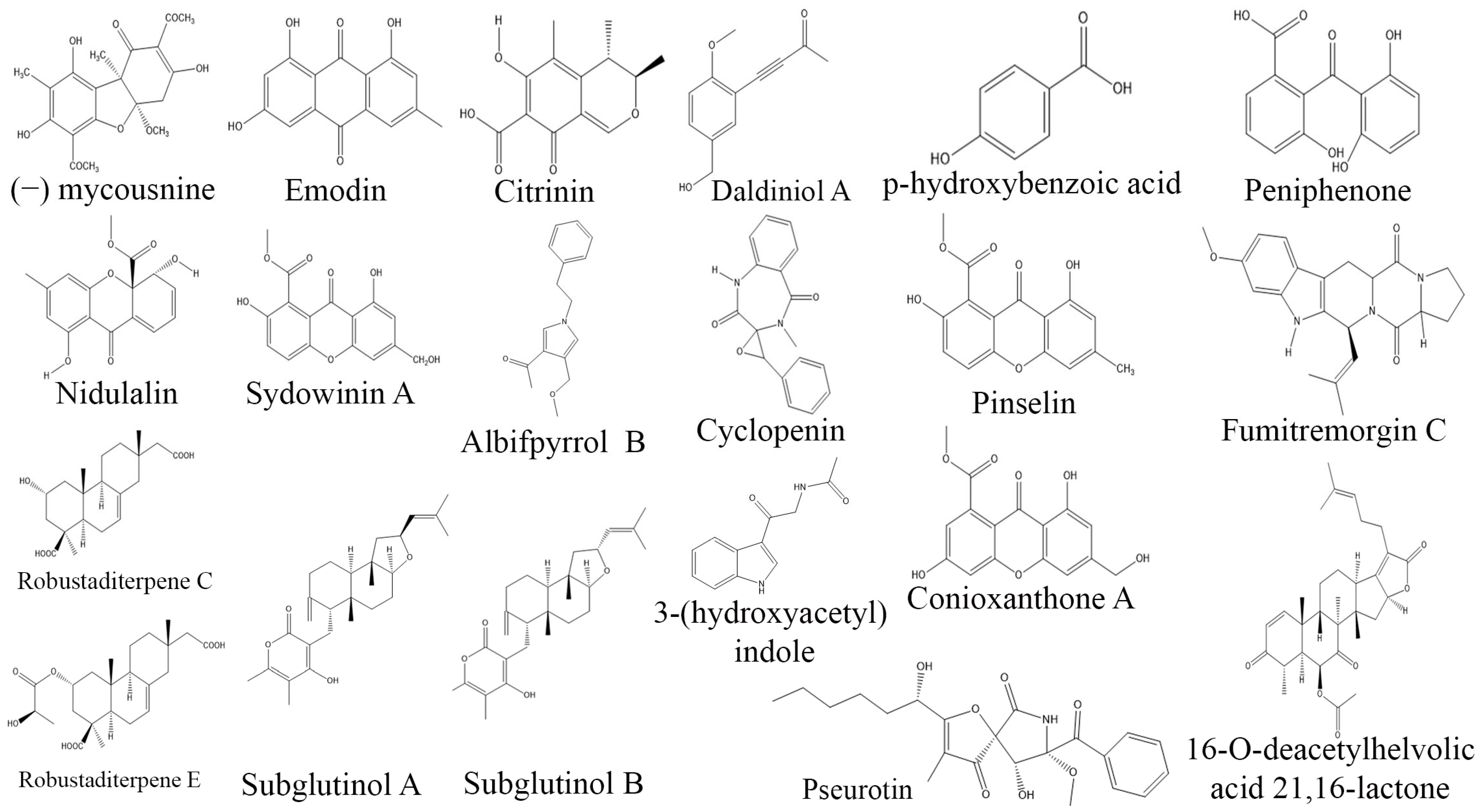
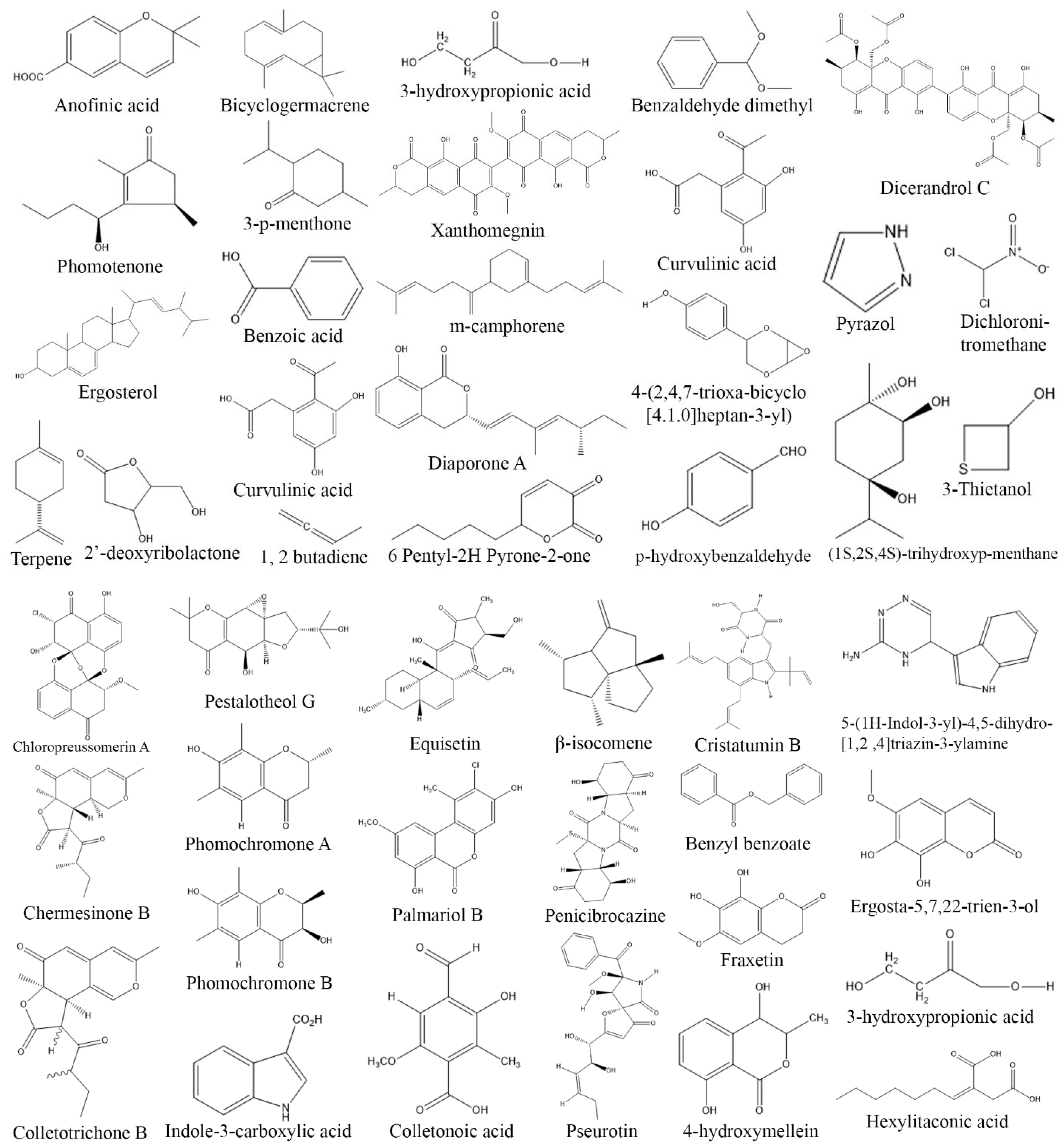
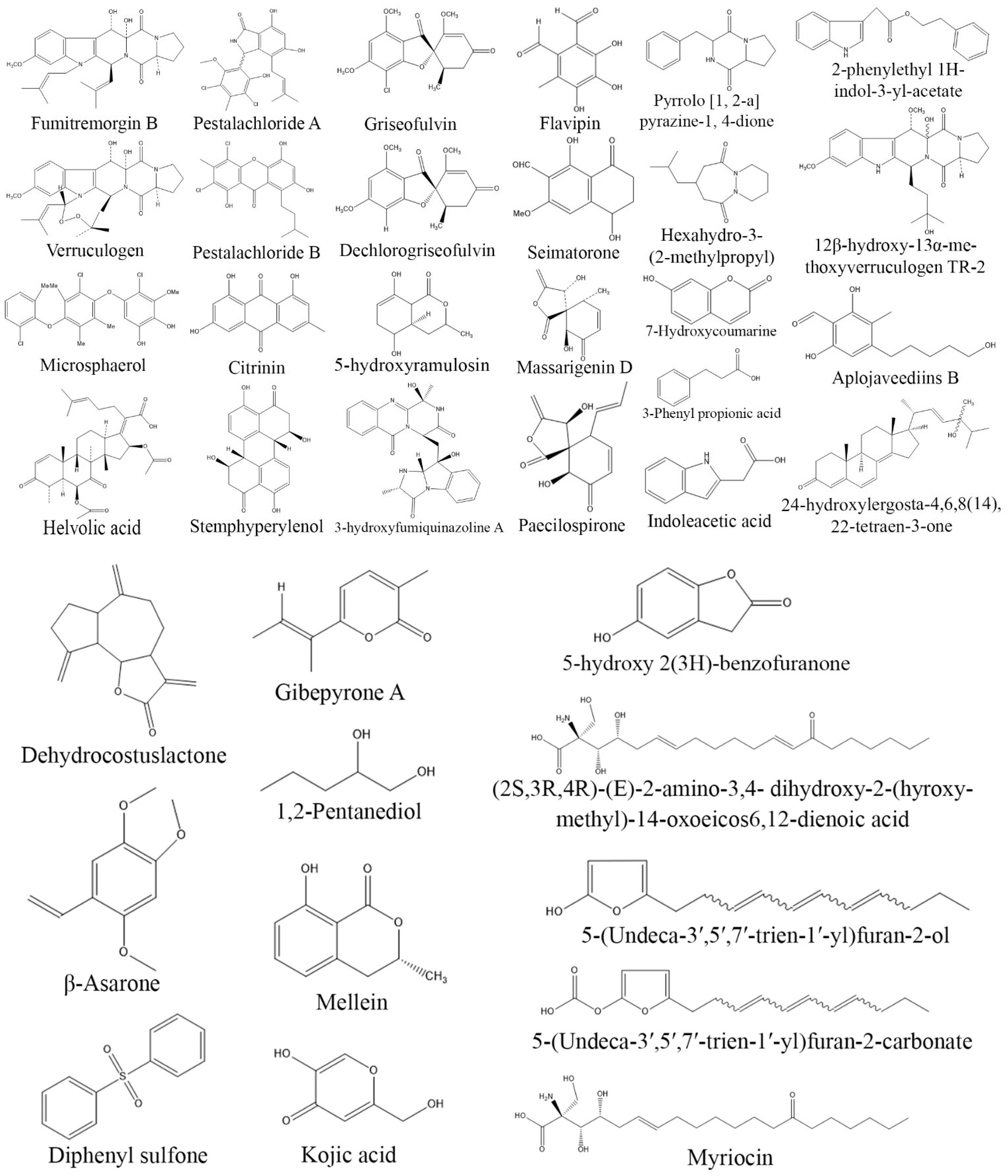
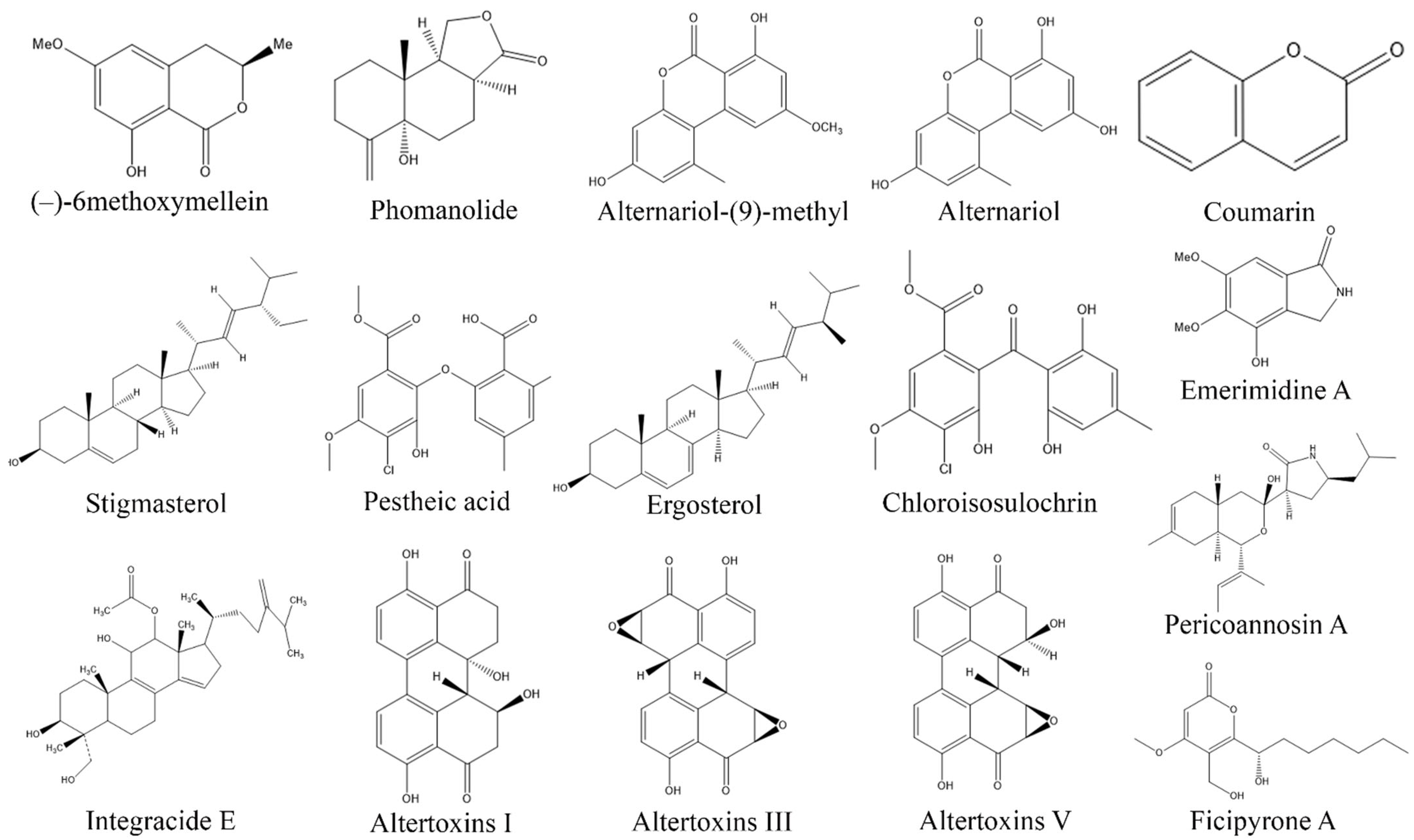
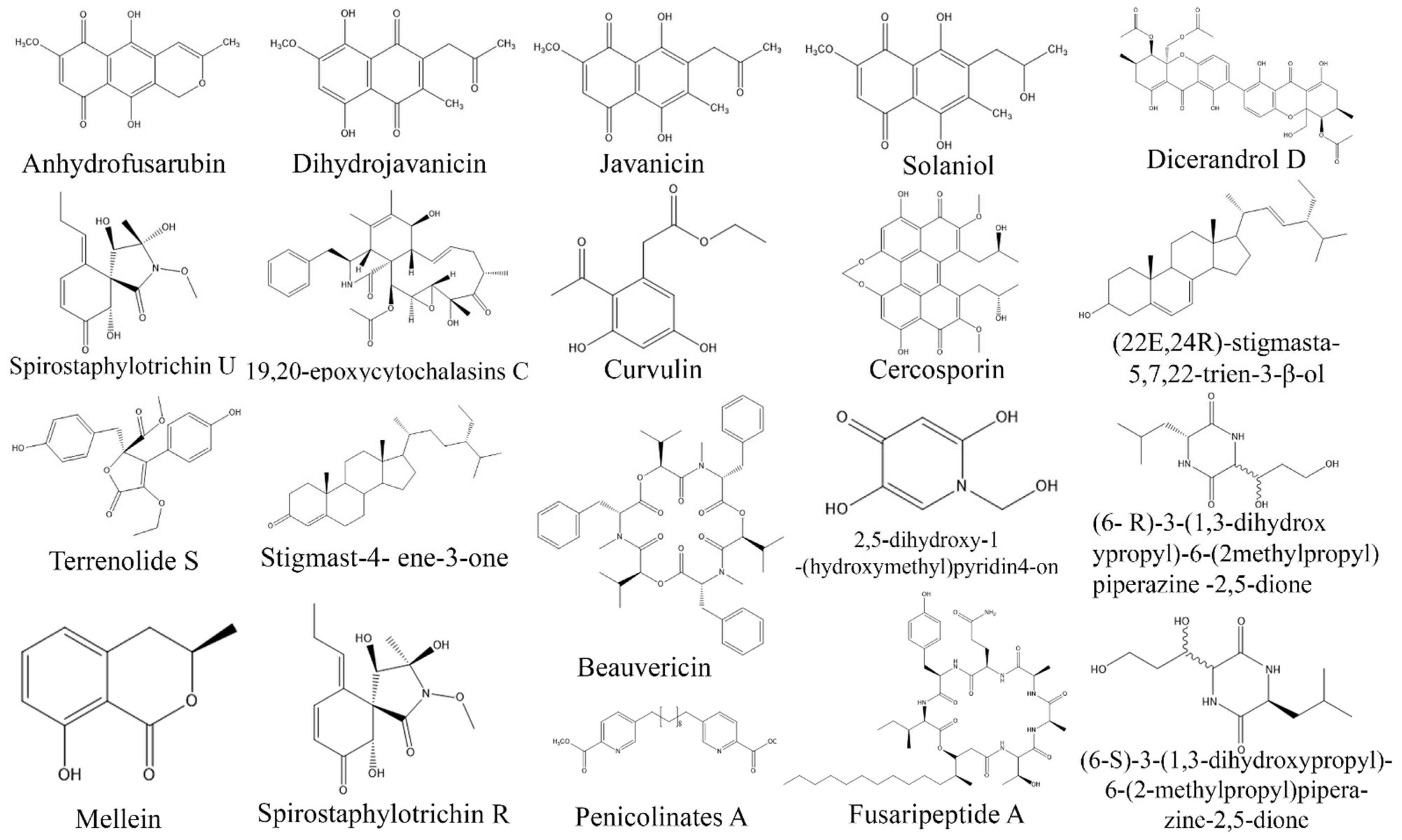
3. Exploring Bioactive Metabolites from Endophytic Fungi: Unveiling Nature’s Treasure Trove
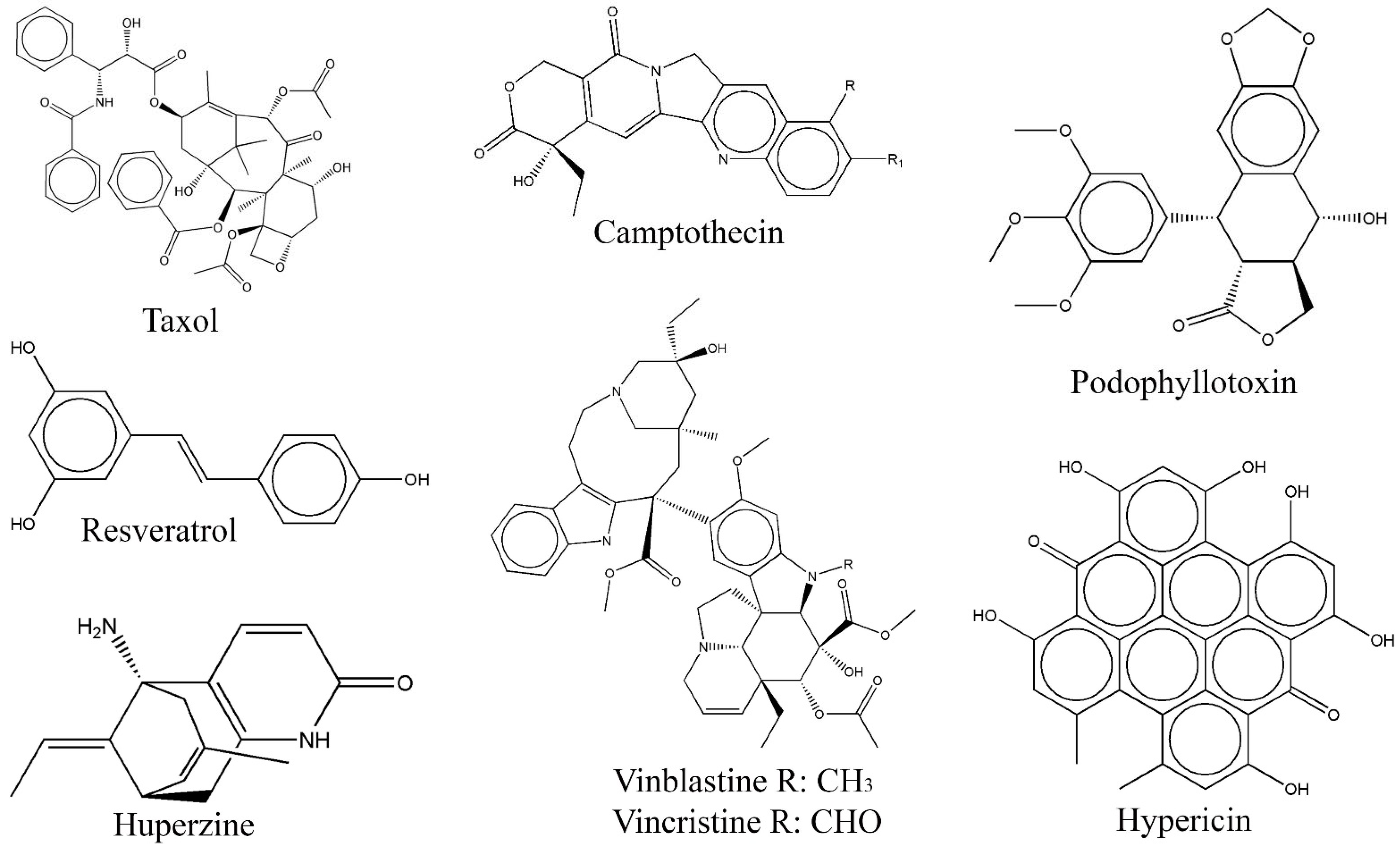
3.1. Anticancer Compounds from Endophytic Fungi
3.2. Antioxidant Compounds from Endophytic Fungi
3.3. Anti-Inflammatory Compounds from Endophytic Fungi
3.4. Antidiabetic Compounds from Endophytic Fungi
3.5. Immunosuppressive Compounds from Endophytic Fungi
3.6. Antimicrobial Compounds from Endophytic Fungi
3.7. Antiprotozoal Compounds from Endophytic Fungi
4. Prospects and Challenges
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caruso, D.J.; Palombo, E.A.; Moulton, S.E.; Zaferanloo, B. Exploring the promise of endophytic fungi: A review of novel antimicrobial compounds. Microorganisms 2022, 10, 1990. [Google Scholar] [CrossRef] [PubMed]
- Pasrija, P.; Girdhar, M.; Kumar, M.; Arora, S.; Katyal, A. Endophytes: An unexplored treasure to combat Multidrug resistance. Phytomed. Plus 2022, 2, 100249. [Google Scholar] [CrossRef]
- Digra, S.; Nonzom, S. An insight into endophytic antimicrobial compounds: An updated analysis. Plant Biotechnol. Rep. 2023, 1–31. [Google Scholar] [CrossRef]
- Deshmukh, S.K.; Verekar, S.A.; Bhave, S.V. Endophytic fungi: A reservoir of antibacterials. Front. Microbiol. 2015, 5, 715. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Babalola, O.O. Pharmacological potential of fungal endophytes associated with medicinal plant: Review. J. Fungi 2021, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Cragg, M.G.; Newman, D.J. Natural products: A continuing source of novel drug leads. BBA Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Cruz, J.S.; Silva, C.A.D.; Hamerski, L. Natural products from endophytic fungi associated with Rubiaceae species. J. Fungi 2020, 6, 128. [Google Scholar] [CrossRef]
- Kaul, S.; Gupta, S.; Ahmed, M.; Dhar, M.K. Endophytic fungi from medicinal plants: A treasure hunt for bioactive metabolites. Phytochem. Rev. 2012, 11, 487–505. [Google Scholar] [CrossRef]
- Abdel-Azeem, A.M.; Azeem, M.A.A.; Hadi, S.Y.A.; Darwish, A.G. Aspergillus: Biodiversity, ecological significances, and industrial applications. In Recent Advancement in White Biotechnology through Fungi Diversity and Enzymes Perspectives; Yadav, A., Mishra, S., Singh, S., Gupta, A., Eds.; Springer: Cham, Switzerland, 2019; Volume 1, pp. 121–179. [Google Scholar]
- Chandra, S. Endophytic fungi: Novel sources of anticancer lead molecules. Appl. Microbiol. Biot. 2012, 95, 47–59. [Google Scholar] [CrossRef]
- Kumar, S.; Aharwal, R.P.; Jain, R.; Sandhu, S.S. Bioactive molecules of endophytic fungi and their potential in anticancer drug development. Curr. Pharmacol. Rep. 2021, 7, 27–41. [Google Scholar] [CrossRef]
- Tavares, D.G.; Barbosa, B.V.L.; Ferreira, R.L.; Duarte, W.F.; Cardoso, P.G. Antioxidant activity and phenolic compounds of the extract from pigment producing fungi isolated from Brazilian caves. BIocatat. Agric. Biotechnol. 2018, 16, 148–154. [Google Scholar] [CrossRef]
- Dudka, M.M.; Jaszek, M.; Blachowicz, A.; Rejczak, T.P.; Matuszewska, A.; Jaroszuk, M.O.; Stefaniuk, D.; Janusz, G.; Sulej, J.; Szerszen, M.K. Fungus Cerrena unicolor as an effective source of new antiviral, immunomodulatory, and anticancer compounds. Int. J. Biol. Macromol. 2015, 79, 459–468. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Rossman, A.Y. Where are all the undescribed fungi? Phytopathology 1997, 87, 888–891. [Google Scholar] [CrossRef]
- UI-Hassan, U.R.; Strobel, G.A.; Booth, E.; Knighton, B.; Floerchinger, C.; Sears, J. Modulation of volatile organic compound formation in the mycodiesel-producing endophyte Hypoxylon sp. CI-4. Microbiology 2012, 158, 465–473. [Google Scholar] [CrossRef]
- Qadri, M.; Johri, S.; Shah, B.A.; Khajuriya, A.; Sidiq, T.; Lattoo, S.K.; Abdin, M.Z.; Riyaz-UI-Hassan, S. Identification and bioactive potential of endophytic fungi isolated from selected medicinal plants of the Western Himalayas. SpringerPlus 2013, 2, 8. [Google Scholar] [CrossRef]
- Deshmukh, S.K.; Gupta, M.K.; Prakash, V.; Saxena, S. Endophytic fungi: A source of potential antifungal compounds. J. Fungi 2018, 4, 77. [Google Scholar] [CrossRef]
- Chowdhary, K.; Kaushik, N.; Coloma, A.G.; Raimundo, C.M. Endophytic fungi and their metabolites isolated from Indian medicinal plant. Phytochem. Rev. 2012, 11, 467–485. [Google Scholar] [CrossRef]
- Meshram, V.; Kapoor, N.; Dwibedi, V.; Srivastava, A.; Saxena, S. Extracellular resveratrol producing endophytic fungus, Quambalaria cyanescens. S. Afr. J. Bot. 2022, 146, 409–416. [Google Scholar] [CrossRef]
- Khare, E.; Mishra, J.; Arora, N.V. Multifaceted interactions between endophytes and plant: Developments and prospects. Front. Microbiol. 2018, 9, 2732. [Google Scholar] [CrossRef]
- Strobel, G. The emergence of endophytic microbes and their biological promise. J. Fungi 2018, 4, 57. [Google Scholar] [CrossRef]
- Meshram, V.; Gupta, M. Endophytic fungi: A quintessential source of potential bioactive compounds. In Sustain: Endophytes for a Growing World; Hodkinson, T., Doohan, F., Saunders, M., Murphy, B., Eds.; Cambridge University Press: Cambridge, UK, 2019; Volume 13, pp. 277–309. [Google Scholar]
- Meshram, V.; Elazar, M.; Maymon, M.; Sharma, G.; Shawahna, R.; Charuvi, D.; Freeman, S. Endophytic Fusarium clavum confers growth and salt tolerance in Cucumis melo. Environ. Exp. Bot. 2023, 206, 105–153. [Google Scholar] [CrossRef]
- Aly, A.H.; Debbab, A.; Proksch, P. Fungal endophytes—Secret producers of bioactive plant metabolites. Int. J. Pharm. Sci. 2013, 68, 499–505. [Google Scholar]
- Saxena, S.; Meshram, V.; Kapoor, N. Musocdor tigerii sp. non-volatile antibiotic producing endophytic fungus from the Northeatern Himalayas. Ann. Microbiol. 2015, 65, 47–57. [Google Scholar] [CrossRef]
- Venugopalan, A.; Srivastava, S. Endophytes as in vitro production platforms of high value plant secondary metabolites. Biotechnol. Adv. 2015, 33, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Kusari, S.; Spiteller, M. The promise of endophytic fungi as sustainable resource of biologically relevant pro-drugs: A focus on Cameroon. In Fungi Applications and Management Strategies; CRC Press: Boca Raton, FL, USA, 2016; pp. 1–13. [Google Scholar]
- Krohn, K.; Ullah, Z.; Hussain, H.; Florke, U.; Schulz, B.; Draeger, S.; Pescitelli, G.; Salvadori, P.; Antus, S.; Kurtan, T. Massarilactones e-g, new metabolites from the endophytic fungus Coniothyrium sp., associated with the plant Artimisia maritime. Chirality 2007, 19, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Kusari, S.; Hertweck, C.; Spiteller, M. Chemical ecology of endophytic fungi: Origins of secondary metabolites. Chem. Biol. 2012, 19, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Howitz, K.T.; Sinclair, D.A. Xenohormesis: Sensing the chemical cues of other spices. Cell 2008, 133, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Kusari, S.; Singh, S.; Jayabaskaran, C. Biotechnological potential of plant-associated endophytic fungi: Hope versus hype. Trends Biotechnol. 2014, 32, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Bedi, A.; Adholeya, A.; Deshmukh, S.K. Novel anticancer compounds from endophytic fungi. Curr. Biotechnol. 2018, 7, 168–184. [Google Scholar] [CrossRef]
- Deshmukh, S.K.; Gupta, M.K.; Prakash, V.; Reddy, M.S. Fungal endophytes: A novel source of cytotoxic compounds. In Endophytes and Secondary Metabolites; Reference Series in Phytochemistry; Jha, S., Ed.; Springer: Cham, Switzerland, 2019; pp. 365–426. [Google Scholar]
- Li, H.L.; Li, X.M.; Li, X.; Wang, C.Y.; Liu, H.; Kassack, M.U.; Meng, L.H.; Wang, B.G. Antioxidant hydroanthraquinones from the marine algal-derived endophytic fungus Talaromyces islandicus EN-501. J. Nat. Prod. 2016, 80, 162–168. [Google Scholar] [CrossRef]
- Kaur, N.; Arora, D.S.; Kalia, N.; Kaur, M. Antibioflm, antiproliferative, antioxidant and antimutagenic activities of an endophytic fungus Aspergillus fumigatus from Moringa oleifera. Mol. Biol. Rep. 2020, 47, 2901–2911. [Google Scholar] [CrossRef]
- Manganyi, M.C.; Ateba, C.N. Untapped potentials of endophytic fungi: A review of novel bioactive compounds with biological applications. Microorganisms 2020, 8, 1934. [Google Scholar] [CrossRef]
- Nischitha, R.; Shivanna, M.B. Screening of secondary metabolites and antioxidant potential of endophytic fungus Penicillium citrinum and host Digitaria bicornis by spectrophotometric and electrochemical methods. Arch. Biol. 2022, 204, 206. [Google Scholar] [CrossRef]
- Pal, P.P.; Shaik, A.B.; Begum, A.S. Prospective leads from endophytic fungi from anti-inflmmatory drug discovery. Planta Med. 2020, 86, 941–959. [Google Scholar] [PubMed]
- Mazumder, K.; Ruma, Y.N.; Akter, R.; Aktar, A.; Hossain, M.M.; Shahina, Z.; Mazumdar, S.; Kerr, P.G. Identification of bioactive metabolites and evaluation of in vitro anti-inflammatory and in vivo antinociceptive and antiarthritic activities of endophyte fungi isolated from Elaeocarpus floribundus blume. J. Ethnopharmacol. 2021, 273, 113975. [Google Scholar] [CrossRef]
- Artanti, N.; Tachibana, S.; Kardono, L.B.; Sukiman, H. Isolation of alpha-glucosidase inhibitors produced by an endophytic fungus, Colletotrichum sp. TSC13 from Taxus sumatrana. Pak. J. Biol. Sci. 2012, 15, 673–679. [Google Scholar] [CrossRef]
- Indrianingsih, A.W.; Tachibana, S. α-Glucosidase inhibitor produced by an endophytic fungus, Xylariaceae sp. QGS 01 from Quercus gilva Blume. Food Sci. Hum. Wellness 2017, 6, 88–95. [Google Scholar] [CrossRef]
- Agrawal, S.; Samanta, S.; Deshmukh, S.K. The antidiabetic potential of endophytic fungi: Future prospects as therapeutic agents. Biotechnol. Appl. Biochem. 2021, 69, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Q.; Zhang, X.; Han, Q.J.; Li, X.B.; Li, G.; Li, R.J.; Jiao, Y.; Zhou, J.C.; Lou, H.X. Xanthone derivatives from Aspergillus sydowii, an endophytic fungus from the liverwort Scapania ciliata S. Lac and their immunosuppressive activities. Phytochem. Lett. 2013, 6, 318–321. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Wei, C.; Deng, X.; Xu, J. Chemical epigenetic modifiers enhance the production of immunosuppressants from the endophytic fungus Aspergillus fumigatus isolated from Cynodon dactylon. Nat. Prod. Res. 2021, 36, 4481–4485. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Chen, X.; Shi, Z.; Xie, S.; Qiao, Y.; Chen, G.; Tan, X.; Lu, Y.; Qi, C.; Zhang, Y. New immunosuppressive secondary metabolites from the endophytic fungus Aspergillus sp. Fitoterapia 2021, 151, 104882. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.P.; Ji, J.C.; Ma, X.J.; Li, Z.H.; Ai, H.L.; Lei, X.X.; Liu, J.K. Three new pyrrole alkaloids from the endophytic fungus Albifmbria viridis. Nat. Prod. Bioprospect. 2022, 12, 5. [Google Scholar] [CrossRef]
- Gupta, M.; Meshram, V. The biological promises of endophytic Muscodor species. In Fungi and Their Role in Sustainable Development: Current Perspectives; Gehlot, P., Singh, J., Eds.; Springer: Cham, Switzerland, 2018; pp. 51–74. [Google Scholar]
- Petrini, O. Fungal endophytes of tree leaves. In Microbial Ecology of Leaves; Andrews, J.H., Hirano, S.S., Eds.; Brock/Springer Series in Contemporary Bioscience; Springer: New York, NY, USA, 1991; pp. 179–197. [Google Scholar]
- Bacon, C.W.; White, J.F. Physiological adaptations in the evolution of endophytism in the clavicipitaceae. In Microbial Endophytes; Bacon, C.W., White, J.F., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 1–26. [Google Scholar]
- Strobel, G.; Daisy, B. Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev. 2003, 67, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Kusari, S.; Pandey, S.P.; Spiteller, M. Untapped mutualistic paradigms linking host plant and endophytic fungal production of similar bioactive secondary metabolites. Phytochem 2013, 91, 81–87. [Google Scholar] [CrossRef]
- Hodkinson, T.R. Evolution and taxonomy of the grasses (poaceae): A model family for the study of species-rich groups. Annu. Plant Rev. 2018, 15, 255–294. [Google Scholar]
- Schulz, B.; Boyle, C. The endophytic continuum. Mycol. Res. 2005, 109, 661–686. [Google Scholar] [CrossRef]
- De-Berry, A. Morphologie und Physiologie der Pilze, Flechten und Myxomyceten; Series: Leipzig: Hofmeister’s Handbook of Physiological Botany; Hansebooks: Norderstedt, Germany, 1866; Volume 2. [Google Scholar]
- Freeman, E.M. The seed-fungus of Lolium temulentum, L., the darnel. Proc. R. Soc. Lond. 1904, 71, 467–476. [Google Scholar]
- Stierle, A.A.; Stierle, D.B. Bioactive secondary metabolites produced by the fungal endophytes of conifers. Nat. Prod. Commun. 2015, 10, 1671–1682. [Google Scholar] [CrossRef]
- Meshram, V.; Saxena, S.; Paul, K. Xylarinase: A novel clot busting enzyme from an endophytic fungus Xylaria curt. J. Enzym. Inhib. Med. Ch. 2016, 31, 1502–1511. [Google Scholar] [CrossRef]
- Promputtha, I.; Lumyong, S.; Dhanasekaran, V.; Mc-Kenzie, E.H.C.; Hyde, K.D.; Jeewon, R. A phylogenetic evolution of whether endophytes become saprotrophs at host senescence. Microb. Ecol. 2007, 53, 579–590. [Google Scholar] [CrossRef]
- Tan, X.; Zhou, Y.; Zhou, X.; Xia, X.; Wei, Y.; He, L.; Tang, H.; Yu, L. Diversity and bioactive potential of culturable fungal endophytes of Dysosma versipellis; a rare medicinal plant endemic to China. Sci. Rep. 2018, 8, 5929. [Google Scholar] [CrossRef]
- Kusari, S.; Zuhlke, S.; Spiteller, M. An endophytic fungus from Camptotheca acuminata that produces camptothecin and analogues. J. Nat. Prod. 2009, 72, 2–7. [Google Scholar] [CrossRef]
- Stierle, A.; Strobel, G.; Stierle, D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of pacific yew. Science 1993, 260, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Stierle, A.; Strobel, G. The search for a taxol-producing microorganism among the endophytic fungi of the pacific yew, Taxus brevifolia. J. Nat. Prod. 1995, 58, 1315–1324. [Google Scholar] [CrossRef]
- Kharwar, R.N.; Mishra, A.; Gond, S.K.; Stierle, A.; Stierle, D. Anticancer compounds derived from fungal endophytes: Their importance and future challenges. Nat. Prod. Rep. 2011, 28, 1208–1288. [Google Scholar] [CrossRef]
- Nicoletti, R.; Fiorentino, A. Plant bioactive metabolites and drugs produced by endophytic fungi of spermatophyta. Agriculture 2015, 5, 918–970. [Google Scholar] [CrossRef]
- Guo, B.; Li, H.; Zhang, L. Isolation of the fungus producing vinblastine. J. Yunnan Univ. (Nat. Sci.) 1998, 20, 214–215. [Google Scholar]
- Puri, S.C.; Verma, V.; Amna, T.; Qazi, G.N.; Spiteller, M. An endophytic fungus from Nothapodytes foetida that produces camptothecin. J. Nat. Prod. 2005, 68, 1717–1719. [Google Scholar] [CrossRef]
- Eyeberger, A.L.; Dondapati, R.; Porter, J.R. Endophytes fungla isolateds from Podophyllum peltatum produce podophyllotoxin. J. Nat. Prod. 2006, 69, 1121–1124. [Google Scholar] [CrossRef]
- Kusari, S.; Lamshoft, M.; Zuhlke, S.; Spiteller, M. An endophytic fungus from Hypericum perforatum that produces hypericin. J. Nat. Prod. 2008, 71, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Porras-Alfaro, A.; Bayman, P. Hidden fungi, emergent properties: Endophytes and microbiomes. Annu. Rev. Phytopathol. 2011, 49, 291–315. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Li, X.Q.; Zhao, D.L.; Zhang, P. Antifungal secondary metabolites produced by the fungal endophytes: Chemical diversity and potential use in the development of biopesticides. Front. Microbiol. 2021, 12, 689527. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.H.; Debbab, A.; Proksch, P. Fungal endophytes: Unique plant inhabitants with great promises. Appl. Microbiol. Biot. 2011, 90, 1829–1845. [Google Scholar] [CrossRef] [PubMed]
- Meshram, V.; Saxena, S.; Paul, K.; Gupta, M.; Kapoor, N. Production, purification and characterization of a potential fibrinolytic protease from endophytic Xylaria curta by solid substrate fermentation. Appl. Biochem. Biotech. 2016, 181, 1496–1512. [Google Scholar] [CrossRef] [PubMed]
- Zilla, M.K.; Qadri, M.; Pathania, A.S.; Strobel, G.A.; Nalli, Y.; Kumar, S.; Guru, S.K.; Bhushan, S.; Singh, S.K.; Vishwakarma, R.A.; et al. Bioactive metabolites from an endophytic Cryptosporiopsis sp. inhabiting Clidemia hirta. Phytochemistry 2013, 95, 291–297. [Google Scholar] [CrossRef]
- Zhang, D.; Tao, X.; Chen, R.; Liu, J.; Li, L.; Fang, X.; Yu, L.; Dai, J. Pericoannosin A, a polyketide synthase−nonribosomal peptide synthetase hybrid metabolite with new carbon skeleton from the endophytic fungus Periconia sp. Org. Lett. 2015, 17, 4304–4307. [Google Scholar] [CrossRef]
- Strobel, G.; Yang, X.; Sears, J.; Kramer, R.; Sidhu, R.S.; Hess, W.M. Taxol from Pestalotiopsis microspora, an endophytic fungus of Taxus wallachiana. Microbiology 1996, 142, 435–440. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Lu, H.; Zheng, Z.; Huang, Y.; Su, W. Taxol from Tubercularia sp. strain TF5, an endophytic fungus of Taxus mairei. FEMS Microbiol. Lett. 2000, 193, 249–253. [Google Scholar] [CrossRef]
- Garyali, S.; Kumar, A.; Reddy, M.S. Taxol production by an endophytic fungus, Fusarium redolens, isolated from Himalayan Yew. J. Microbiol. Biotechnol. 2013, 23, 1372–1380. [Google Scholar] [CrossRef]
- Kumar, A.; Patil, D.; Rajamohanan, P.R.; Ahmad, A. Isolation, purification and characterization of vinblastine and vincristine from endophytic fungus Fusarium oxysporum isolated from Catharanthus roseus. PLoS ONE 2013, 8, e71805. [Google Scholar] [CrossRef]
- Ayob, F.W.; Simarani, K.; Abidin, N.Z.; Mohamed, J. First report on a novel Nigrospora sphaerica isolated from Catharanthus roseus plant with anticarcinogenic properties. Microb. Biotechnol. 2017, 10, 926–932. [Google Scholar] [CrossRef]
- Lingqi, Z.; Bo, G.; Haiyan, L.; Songrong, Z.; Hua, S.; Su, G.; Rongcheng, E. Preliminary study on the isolation of endophytic fungus of Catharanthus roseus and its fermentation to produce products of therapeutic value. Chin. Trad. Herb. Drugs 2000, 31, 805–807. [Google Scholar]
- Palem, P.C.; Kuriakose, G.C.; Jayabaskaran, C. Endophytic fungus, Talaromyces radicus, isolated from Catharanthus roseus, produces vincristine and vinblastine, which induce apoptotic cell death. PLoS ONE 2015, 11, e0144476. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, G.M.; Palem, P.C.; Jayabaskaran, C. Fungal vincristine from Eutypella spp.—CrP14 isolated from Catharanthus roseus induces apoptosis in human squamous carcinoma cell line-A431. BMC Complem. Altern. Med. 2016, 16, 302. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Shawl, A.S.; Kour, A.; Andrabi, R.; Sultan, P.; Verma, V.; Qazi, G.N. An endophytic Neurospora sp. from Nothapodytes foetida producing camptothecin. Appl. Biochem. Microb. 2008, 44, 203–209. [Google Scholar] [CrossRef]
- Rehman, S.; Shawl, A.S.; Kour, A.; Sultan, P.; Ahmad, K.; Khajuria, R.; Qazi, G.N. Comparative studies and identification of camptothecin produced by an endophyte at shake flask and bioreactor. Nat. Prod. Res. 2009, 23, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Qu, X.; Chen, F.; Bao, J.; Zhang, G.; Luo, Y. Camptothecin-producing endophytic fungus Trichoderma atroviride LY357: Isolation, identification, and fermentation conditions optimization for camptothecin production. Appl. Microbiol. Biot. 2013, 97, 9365–9375. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Zhang, G.; Li, S.; Wang, J. Characterization and antitumor activity of camptothecin from endophytic fungus Fusarium solani isolated from Camptotheca acuminate. Afr. Health Sci. 2017, 17, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.C.; Nazir, A.; Chawla, R.; Arora, R.; Riyaz-ul-Hasan, S.; Amna, T.; Ahmed, B.; Verma, V.; Singh, S.; Sagar, R.; et al. The endophytic fungus Trametes hirsuta as a novel alternative source of podophyllotoxin and related aryl tetralin lignans. J. Biotechnol. 2006, 122, 494–510. [Google Scholar] [CrossRef]
- Huang, J.X.; Zhang, J.; Zhang, X.R.; Zhang, K.; Zhang, X.; He, X.R. Mucor fragilis as a novel source of the key pharmaceutical agents podophyllotoxin and kaempferol. Pharm. Biol. 2014, 52, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Wang, J.; Zeng, Q.; Zhang, Z.; Yan, R. A novel endophytic Huperzine A–producing fungus, Shiraia sp. Slf14, isolated from Huperzia serrate. J. Appl. Microbiol. 2010, 109, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.B.; Zeng, Q.G.; Yan, R.W.; Wang, Y.; Zou, Z.R.; Zhu, D. Endophytic fungus Cladosporium cladosporioides LF70 from Huperzia serrata produces Huperzine A. World J. Microbiol. Biotechnol. 2011, 27, 479–486. [Google Scholar] [CrossRef]
- Sun, J.Q.; Yang, M.H. Huperzine A production by Paecilomyces tenuis YS-13, an endophytic fungus isolated from Huperzia serrata. Nat. Prod. Res. 2015, 29, 1035–1041. [Google Scholar]
- Dong, L.H.; Fan, S.W.; Ling, Q.Z.; Huang, B.B.; Wei, Z.J. Indentification of huperzine A–producing endophytic fungi isolated from Huperzia serrate. World J. Microb. Biot. 2014, 30, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zeng, Q.; Liu, Y.; Pan, Z. Alternaria sp. MG1, a resveratrol-producing fungus: Isolation, identification, and optimal cultivation conditions for resveratrol production. Appl. Microbiol. Biotechnol. 2012, 95, 369–379. [Google Scholar] [CrossRef]
- Haq, A.; Sofi, N.Y. Vitamin D and breast cancer: Indian perspective. Clin. Nutr. 2017, 12, 1–10. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Kanwal, S.; Ali, B.; Khalil, A.T. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Med. 2017, 7, 1129–1150. [Google Scholar] [CrossRef]
- Rice, B.A.; Hoeve, E.S.V.; DeLuca, A.N.; Esserman, L.J.; Rugo, H.P.; Melisko, M.E. Registry study to assess hair loss prevention with the Penguin Cold Cap in breast cancer patients receiving chemotherapy. Breast Cancer Res. Treat. 2018, 167, 117–122. [Google Scholar] [CrossRef]
- Zaden, S.Y.B.D.; Qiao, X.; Neefjes, J. New insights into the activities and toxicities of the old anticancer drug doxorubicin. FEBS J. 2020, 288, 6095–6111. [Google Scholar]
- Patil, R.; Patil, M.P.; Maheshwari, V.L. Bioactive secondary metabolites from endophytic fungi: A review of biotechnological production and their potential applications. Stud. Nat. Prod. Chem. 2016, 49, 189–205. [Google Scholar]
- Uzma, F.; Mohan, C.D.; Hashem, A.; Konappa, N.M.; Rangappa, S.; Kamath, P.V.; Singh, B.P.; Mudili, V.; Gupta, V.K.; Siddaiah, C.N.; et al. Endophytic fungi-alternative source of cytotoxic compounds: A review. Front. Pharmacol. 2018, 9, 309. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, L.; Wang, J.; Shan, T.; Zhong, L.; Liu, X.; Gao, X. Endophytic fungi for producing bioactive compounds originally from their host plants. Curr. Res. Technol. Educ. Trop. Appl. Microbial. Microbial. Biotechnol. 2010, 1, 567–576. [Google Scholar]
- Cragg, G.M.; Pezzuto, J.M. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med. Princ. Pract. 2016, 25, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Kusari, S.; Lamshoft, M.; Spiteller, M. Aspergillus fumigatus Fresenius, an endophytic fungus from Juniperus communis L. Horstmann as a novel source of the anticancer pro-drug deoxypodophyllotoxin. J. Appl. Microbiol. 2009, 107, 1019–1030. [Google Scholar] [CrossRef]
- Mohinudeen, I.A.H.K.; Kanumuri, R.; Soujanya, K.N.; Shaanker, R.U.; Rayala, S.K.; Srivastava, S. Sustainable production of camptothecin from an Alternaria sp. isolated from Nothapodytes nimmoniana. Sci. Rep. 2021, 11, 1478. [Google Scholar] [CrossRef]
- Liang, Z.; Xhang, J.; Zhang, X.; Li, J.; Zhanh, X.; Zhao, C. Endophytic fungus from Sinopodophyllum emodi (wall.) ying that produces podophyllotoxin. J. Chromatogr. Sci. 2016, 54, 175–178. [Google Scholar]
- Arora, D.; Sharma, N.; Singamaneni, V.; Sharma, V.; Kushwaha, M.; Abrol, V.; Guru, S.; Sharma, S.; Gupta, A.P.; Bhushan, S.; et al. Isolation and characterization of bioactive metabolites from Xylaria psidii, an endophytic fungus of the medicinal plant Aegle marmelos and their role in mitochondrial dependent apoptosis against pancreatic cancer cells. Phytomedicine 2016, 23, 1312–1320. [Google Scholar] [CrossRef]
- Li, H.; Xiao, J.; Gao, Y.Q.; Tang, J.J.; Zhang, A.N.; Gao, J.M. Chaetoglobosins from Chaetomium globosum, an endophytic fungus in Ginkgo biloba, and their phytotoxic and cytotoxic activities. J. Agric. Food Chem. 2014, 62, 3734–3741. [Google Scholar] [CrossRef]
- Ashoka, G.B.; Shivanna, M.B. Metabolite profiling, in vitro and in silico assessment of antibacterial and anticancer activities of Alternaria alternata endophytic in Jatropha heynei. Arch. Microbiol. 2022, 205, 61. [Google Scholar] [CrossRef]
- Kalimuthu, A.K.; Pavadai, P.; Panneerselvam, T.; Babkiewicz, E.; Pijanowska, J.; Mrowka, P.; Rajagopal, G.; Deepak, V.; Sundar, K.; Maszczyk, P.; et al. Cytotoxic potential of bioactive compounds from Aspergillus flavus, an endophytic fungus isolated from Cynodon dactylon, against breast cancer: Experimental and computational approach. Molecules 2022, 27, 8814. [Google Scholar] [CrossRef] [PubMed]
- Danagoudar, A.; Joshi, C.G.; Ravi, S.K.; Kumar, H.G.R.; Ramesh, B.N. Antioxidant and cytotoxic potential of endophytic fungi isolated from medicinal plant Tragia involucrata L. Pharmacogn. Res. 2021, 10, 188–195. [Google Scholar]
- He, Q.; Zeng, Q.; Shao, Y.; Zhou, H.; Li, T.; Song, F.; Liu, W. Anti-cervical cancer activity of secondary metabolites of endophytic fungi from Ginkgo biloba. Cancer Biomark. 2020, 28, 371–379. [Google Scholar] [CrossRef]
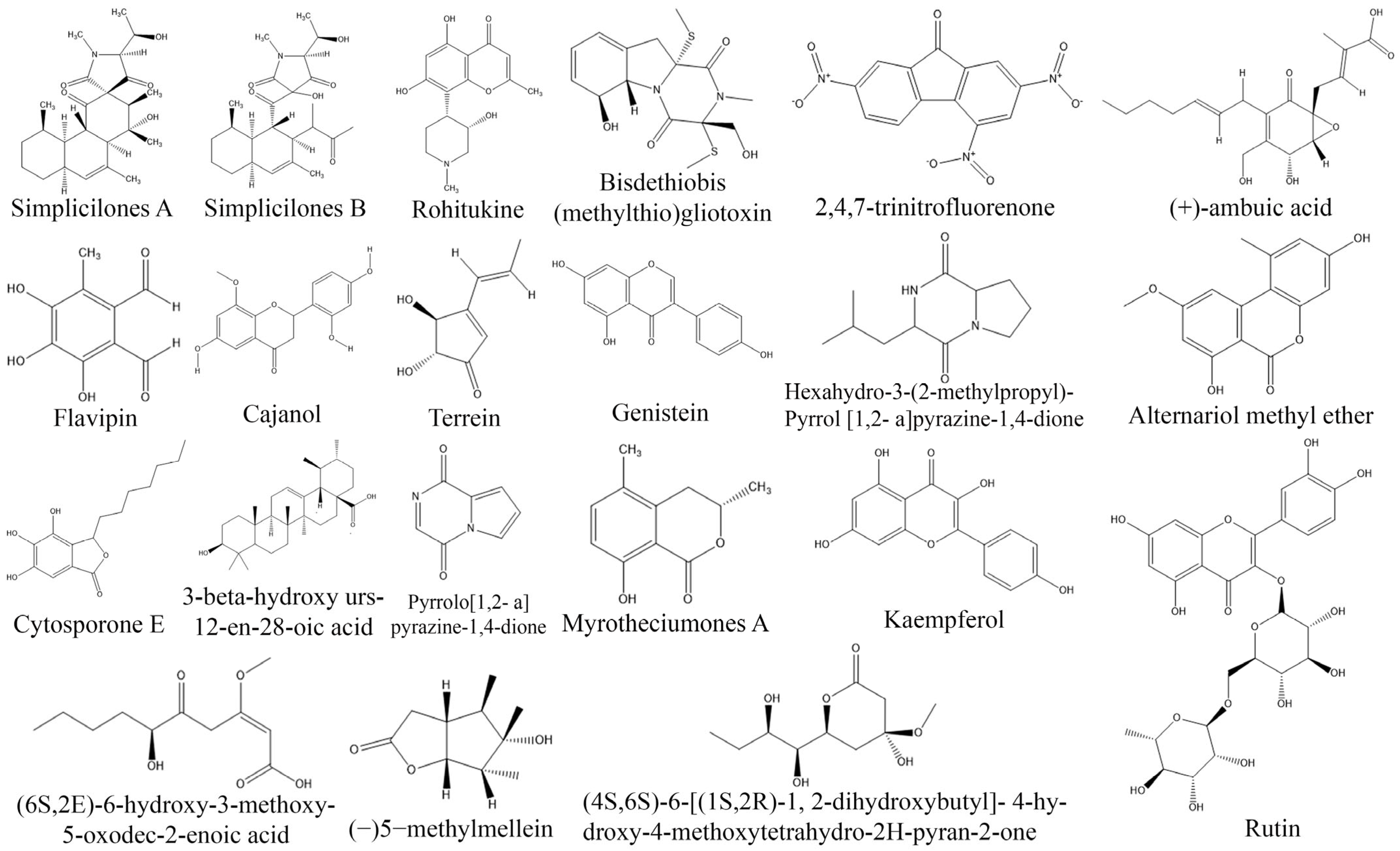
- Anoumedem, E.G.M.; Mountessou, B.Y.G.; Kouam, S.F.; Narmani, A.; Surup, F. Simplicilones A and B isolated from the endophytic fungus Simplicillium subtropicum SPC3. Antibiotics 2020, 9, 753. [Google Scholar] [CrossRef]
- Sheeba, H.; Ali, M.S.; Anuradha, V. In-vitro anti-cancer Activity of endophytic fungi Isolated from Ziziphus mauritiana in cervical cancer cell line. Eur. J. Med. Plants 2020, 31, 38–48. [Google Scholar] [CrossRef]
- Palanichamy, P.; Kannan, S.; Murugan, D.; Alagusundaram, P.; Marudhamuthu, M. Purification, crystallization and anticancer activity evaluation of the compound alternariol methyl ether from endophytic fungi Alternaria alternata. J. Appl. Microbiol. 2019, 127, 1468–1478. [Google Scholar] [CrossRef]
- Ukwatta, K.M.; Lawrence, J.L.; Wijayarathne, C.D. Antimicrobial, anti-cancer, anti-filarial and anti-inflammatory activities of cowabenzophenone a extracted from the endophytic fungus Aspergillus terreus isolated from a mangrove plant Bruguiera gymnorrhyza. Mycology 2020, 11, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.S.; Kumaresan, S.; Tamizh, M.M.; Islam, M.I.H.; Thirugnanasambantham, K. Anticancer potential of NF-κB targeting apoptotic molecule “flavipin” isolated from endophytic Chaetomium globosum. Phytomedicine 2019, 61, 152830. [Google Scholar] [CrossRef]
- Arifni, F.R.; Hasan, A.E.Z.; Julistiono, H.; Bermawie, N.; Riyanti, E.I. Anticancer activities of endophytic fungi isolated from soursop leaves (Annona muricata L.) against WiDr cancer cells. Annu. Res. Rev. Biol. 2017, 18, 1–11. [Google Scholar] [CrossRef]
- Goutam, J.; Sharma, G.; Tiwari, V.K.; Mishra, A.; Kharwar, R.N.; Ramaraj, V.; Koch, B. Isolation and characterization of “Terrin” an antimicrobial and antitumor compound from endophytic fungus Aspergillus terrus (JAS-2) associated from Achyranthus aspera Varanasi, India. Front. Microbiol. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Jinfeng, E.C.; Rafi, M.I.M.; Hoon, K.C.; Lian, H.K.; Kqueen, C.Y. Analysis of chemical constituents, antimicrobial and anticancer activities of dichloromethane extracts of Sordariomycetes sp. endophytic fungi isolated from Strobilanthes crispus. World J. Microb. Biot. 2016, 33, 5. [Google Scholar] [CrossRef]
- Wu, L.S.; Jia, M.; Chen, L.; Zhu, B.; Dong, H.X.; Si, J.P.; Peng, W.; Han, T. Cytotoxic and antifungal constituents isolated from the metabolites of endophytic fungus DO14 from Dendrobium officinale. Molecules 2015, 21, 14. [Google Scholar] [CrossRef]
- Wibowo, M.; Prachyawarakorn, V.; Aree, T.; Wiyakrutta, S.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Tricyclic and spirobicyclic norsesquiterpenes from the endophytic fungus Pseudolagarobasidium acaciicola. Eur. J. Org. Chem. 2014, 19, 3976–3980. [Google Scholar] [CrossRef]
- Suja, M.; Vasuki, S.; Sajitha, N. Anticancer activity of compounds isolated from marine endophytic fungus Aspergillus terrus. World J. Pharm. Pharm. Sci. 2014, 3, 661–672. [Google Scholar]
- Lin, T.; Wang, G.; Shan, W.; Zeng, D.; Jiang, R.D.X.; Zhu, D.; Liu, X.; Yang, S.; Chen, H. Myrotheciumones: Bicyclic cytotoxic lactones isolated from an endophytic fungus of Ajuga decumbens. Bioorg. Med. Chem. Lett. 2014, 24, 2504–2507. [Google Scholar] [CrossRef] [PubMed]
- Verekar, S.A.; Mishra, P.D.; Sreekumar, E.S.; Deshmukh, S.K.; Fiebig, H.H.; Kelter, G.; Maier, A. Anticancer activity of new depsipeptide compound isolated from an endophytic fungus. J. Antibiot. 2014, 67, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, C.; Wang, W.; Zhao, C.; Luo, M.; Mu, F.; Fu, Y.; Zu, Y.; Yao, M. Hypocrea lixii, novel endophytic fungi producing anticancer agent cajanol, isolated from pigeon pea (Cajanus cajan [L.] Millsp). J. Appl. Microbiol. 2013, 115, 102–113. [Google Scholar] [CrossRef]
- Zheng, C.J.; Xu, L.L.; Li, Y.Y.; Han, T.; Zhang, Q.Y.; Ming, Q.L.; Rahman, K.; Qin, L.P. Cytotoxic metabolites from the cultures of endophytic fungi from Panax ginseng. Appl. Microbial. Biot. 2013, 97, 7617–7625. [Google Scholar] [CrossRef] [PubMed]
- Shweta, S.; Gurumurthy, B.R.; Ravikanth, G.; Ramanan, U.S.; Shivanna, M.B. Endophytic fungi from Miquelia dentata Bedd., produce the anti-cancer alkaloid, camptothecine. Phytomedicine 2013, 20, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Kumara, P.M.; Zuehlke, S.; Priti, V.; Ramesha, B.T.; Shweta, S.; Ravikanth, G.; Vasudeva, R.; Santhoshkumar, T.R.; Spiteller, M.; Shaanker, R.M. Fusarium proliferatum, an endophytic fungus from Dysoxylum binectariferum Hook.f, produces rohitukine, a chromane alkaloid possessing anti-cancer activity. Anton Leeuw 2011, 101, 323–329. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Kotwani, A. Implication of oxidative stress in progression of diabetic retinopathy. Surv. Ophthalmol. 2015, 61, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Thiruvengadam, M.; Venkidasamy, B.; Subramanian, U.; Samynathan, R.; Shariati, M.A.; Rebezov, M.; Girish, S.; Thangavel, S.; Dhanapal, A.R.; Fedoseeva, N.; et al. Bioactive compounds in oxidative stress-mediated diseases: Targeting the NRF2/ARE signaling pathway and epigenetic regulation. Antioxidants 2021, 10, 1859. [Google Scholar] [CrossRef]
- Saxena, P.; Selvaraj, K.; Khare, S.K.; Chaudhary, N. Superoxide dismutase as multipotent therapeutic antioxidant enzyme: Role in human diseases. Biotechnol. Lett. 2021, 44, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Gulchin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Mishra, R.C.; Goel, M.; Barrow, C.J.; Deshmukh, S.K. Endophytic fungi- An untapped source of potential antioxidants. Curr. Bioact. Compd. 2020, 16, 944–964. [Google Scholar] [CrossRef]
- Elkhouly, H.I.; Hamed, A.A.; Hosainy, A.M.E.; Ghareeb, M.A.; Sidkey, N.M. Bioactive secondary metabolite from endophytic Aspergillus Tubenginses ASH4 isolated from Hyoscyamus muticus: Antimicrobial, Antibiofilm, Antioxidant and Anticancer Activity. Pharmacogn. J. 2021, 13, 434–442. [Google Scholar] [CrossRef]
- Atiphasaworn, P.; Monggoot, S.; Gentekaki, E.; Brooks, S.; Pripdeevech, P. Antibacterial and antioxidant constituents of extracts of endophytic fungi isolated from Ocimum basilicum var. thyrsiflora leaves. Curr. Microbiol. 2017, 74, 1185–1193. [Google Scholar] [CrossRef]
- Chatterjee, S.; Ghosh, R.; Mandal, N.C. Production of bioactive compounds with bactericidal and antioxidant potential by endophytic fungus Alternaria alternata AE1 isolated from Azadirachta indica A. Juss. PLoS ONE 2019, 14, e0214744. [Google Scholar] [CrossRef]
- Kumaresan, S.; Karthi, V.; Senthilkuma, V.; Balakumar, B.S.; Balakumar, A. Biochemical constituents and antioxidant potential of endophytic fungi isolated from the leaves of Azadirachta indica A. Juss (Neem) from Chennai, India. J. Acad. Ind. Res. 2015, 3, 355–361. [Google Scholar]
- Praptiwi, R.M.; Wulansari, D.; Fathoni, A.; Agusta, A. Antibacterial and antioxidant activities of endophytic fungi extracts of medicinal plants from Central Sulawesi. J. Appl. Pharm. Sci. 2018, 8, 69–74. [Google Scholar]
- Sadrati, N.; Daoud, H.; Zerroug, A.; Dahamna, S.; Bouharati, S. Screening of antimicrobial and antioxidant secondary metabolites from endophytic fungi isolated from wheat (Triticum durum). J. Plant Prot. Res. 2013, 53, 128–136. [Google Scholar] [CrossRef]
- Yadav, M.; Yadav, A.; Yadav, J.P. In vitro antioxidant activity and total phenolic content of endophytic fungi isolated from Eugenia jambolana Lam. Asian Pac. J. Trop. Med. 2014, 7, 256–261. [Google Scholar] [CrossRef]
- Nagda, V.; Gajbhiye, A.; Kumar, D. Isolation and characterization of endophytic fungi from Calotropis Procera for their antioxidant activity. Asian J. Pharm. Clin. Res. 2016, 10, 254–258. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Sathiavelu, M.; Arunachalam, S. In vitro antioxidant and antibacterial activity of endophytic fungi isolated from Mussaenda luteola. J. Pharm. Sci. 2017, 7, 234–238. [Google Scholar]
- Ibrahim, M.; Oyebanji, E.; Fowora, M.; Aiyeolemi, A.; Orabuchi, C.; Akinnawo, B.; Adekunle, A.A. Extracts of endophytic fungi from leaves of selected Nigerian ethnomedicinal plants exhibited antioxidant activity. BMC Complem. Altern. Med. 2021, 21, 98. [Google Scholar] [CrossRef]
- Li, Y.L.; Xin, X.M.; Chang, Z.Y.; Shi, R.J.; Miao, Z.M.; Ding, J.; Hao, G.P. The endophytic fungi of Salvia miltiorrhiza Bge.f. alba are a potential source of natural antioxidants. Bot. Stud. 2015, 56, 5. [Google Scholar] [CrossRef] [PubMed]
- Selim, K.A.; Elkhateeb, W.A.; Tawila, A.M.; Beih, A.A.E.; Rahman, T.M.A.; Diwany, A.I.E.; Ahmed, E.F. Antiviral and antioxidant potential of fungal endophytes of Egyptian medicinal plants. Fermentation 2018, 4, 49. [Google Scholar] [CrossRef]
- Lim, S.M.; Agatonovic-Kustrin, S.A.; Lim, F.T.; Ramasamy, K. High-performance thin layer chromatography-based phytochemical and bioactivity characterisation of anticancer endophytic fungal extracts derived from marine plants. J. Pharmaceut. Biomed. 2021, 193, 113702. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, M.H.; Abdelaziz, A.M.; Kalaba, M.H.; Radwan, A.A.; Hashem, A.H. Antimicrobial, antioxidant, cytotoxic activities and phytochemical analysis of fungal endophytes isolated from Ocimum basilicum. Appl. Biochem. Biotechnol. 2021, 194, 1271–1289. [Google Scholar] [CrossRef]
- Silva, M.H.R.D.; Yesquen, L.G.C.; Junior, S.B.; Garcia, V.L.; Sartoratto, A.; Angelis, D.D.F.D.; Angelis, D.A.D. Endophytic fungi from Passifora incarnata: An antioxidant compound source. Arch. Microbiol. 2020, 202, 2779–2789. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, Y.; Yang, J.; Xiao, Y.; Cai, Y.; Wan, Y.; Chen, H.; Yao, H.; Shan, Z.; Li, C.; et al. Isolation and identification of flavonoid producing endophytic fungi from medicinal plant Conyza blinii H.Lév that exhibit higher antioxidant and antibacterial activities. PeerJ 2020, 8, e8978. [Google Scholar] [CrossRef]
- Tian, J.; Fu, L.; Zhang, Z.; Dong, X.; Xu, D.; Mao, Z.; Liu, Y.; Lai, D.; Zhou, L. Dibenzo-α-pyrones from the endophytic fungus Alternaria sp. Samif01: Isolation, structure elucidation, and their antibacterial and antioxidant activities. Nat. Prod. Res. 2017, 31, 387–396. [Google Scholar] [CrossRef]
- Prihantini, A.I.; Tachibana, S. Antioxidant compounds produced by Pseudocercospora sp. ESL 02, an endophytic fungus isolated from Elaeocarpus sylvestris. Asian Pac. J. Trop. Biomed. 2016, 7, 110–115. [Google Scholar] [CrossRef]
- Dzoyem, J.P.; Melong, R.; Tsamo, A.T.; Maffo, T.; Kapche, D.G.W.F.; Ngadjui, B.T.; Mcgaw, L.J.; Eloff, J.N. Cytotoxicity, antioxidant and antibacterial activity of four compounds produced by an endophytic fungus Epicoccum nigrum associated with Entada abyssinica. Rev. Bras. Farmacogn. 2016, 27, 251–253. [Google Scholar] [CrossRef]
- Zhao, J.T.; Ma, D.H.; Luo, M.; Wang, W.; Zhao, C.; Zu, Y.G.; Fu, Y.; Wink, M. In vitro antioxidant activities and antioxidant enzyme activities in HepG2 cells and main active compounds of endophytic fungus from pigeon pea [Cajanus cajan (L.) Millsp.]. Food Res. Int. 2013, 56, 243–251. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-association diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.R.; Qin, X.; Lin, X.P.; Wang, J.F.; Kaliyaperumal, K.; Tian, Y.Q.; Liu, J.; Liu, F.; Tu, Z.; Xu, S.H.; et al. New phenyl derivatives from endophytic fungus Botryosphaeria sp. SCSIO KcF6 derived of mangrove plant Kandelia candel. Nat. Prod. Res. 2015, 30, 192–198. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, J.L.; Liu, J.M.; Chen, R.D.; Xie, K.B.; Chen, D.W.; Feng, K.P.; Zhang, D.; Dai, J.G. Neural anti-inflammatory sesquiterpenoids from the endophytic fungus Trichoderma sp. Xy24. J. Asian Nat. Prod. Res. 2016, 19, 651–658. [Google Scholar] [CrossRef]
- Harun, A.; Vidyadaran, S.; Lim, S.M.; Cole, A.L.J.; Ramasamy, K. Malaysian endophytic fungal extracts-induced anti-inflammation in Lipopolysaccharide-activated BV-2 microglia is associated with attenuation of NO production and, IL-6 and TNF-α expression. BMC Complem. Altern. Med. 2015, 15, 166. [Google Scholar] [CrossRef]
- Mishra, P.D.; Verekar, S.A.; Almeida, A.K.; Roy, S.K.; Jain, S.; Balakrishnan, A.; Vishwakarma, R.; Deshmukh, S.K. Anti-inflammatory and anti-diabetic napthaquinones from an endophytic fungus Dendryphion nanum (Nees) S. Hughes. Indian J. Chem. 2013, 252B, 565–567. [Google Scholar]
- Govindappa, M.; Farheen, H.; Chandrappa, C.P.; Rai, R.V.; Raghavendra, V.B. Mycosynthesis of silver nanoparticles using extract of endophytic fungi, Penicillium species of Glycosmis mauritiana, and its antioxidant, antimicrobial, anti-inflammatory and tyrokinase inhibitory activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035014. [Google Scholar] [CrossRef]
- Moharram, A.M.; Zohri, A.A.; Omar, H.M.; Ghani, O.A.A.E. In vitro assessment of antimicrobial and anti-inflammatory potential of endophytic fungal metabolites extracts. Eur. J. Biol. Res. 2017, 7, 234–244. [Google Scholar]
- Chen, Y.; Zou, G.; Yang, W.; Zhao, Y.; Tan, Q.; Chen, L.; Wang, J.; Ma, C.; Kang, W.; She, Z. Metabolites with anti-inflammatory activity from the mangrove endophytic fungus Diaporthe sp. QYM12. Mar. Drugs 2021, 19, 56. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yan, C.; Li, C.; You, T.; She, Z. Naphthoquinone derivatives with anti-inflammatory activity from mangrove-derived endophytic fungus Talaromyces sp. SK-S009. Molecules 2020, 25, 576. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Guo, Z.; Zhu, M.; Shi, J.; Li, W.; Jiao, R.; Tan, R.; Ge, H. Anti-inflammatory spirobisnaphthalene natural products from a plant-derived endophytic fungus Edenia gomezpompae. Chin. Chem. Lett. 2020, 31, 1406–1409. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Zou, G.; Li, C.; Yang, W.; Liu, H.; She, Z. Anti-inflammatory activities of alkaloids from the mangrove endophytic fungus Phomopsis sp. SYSUQYP-23. Bioorg. Chem. 2020, 97, 103712. [Google Scholar] [CrossRef]
- Rabia, M.W.A.; Mohamed, G.A.; Ibrahim, S.R.M.; Asfour, H.Z. Anti-inflammatory ergosterol derivatives from the endophytic fungus Fusarium chlamydosporum. Nat. Prod. Res. 2020, 35, 5011–5020. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.N.; Liu, C.Y.; Wang, T.; Li, Y.L.; Xu, K.; Lou, H.X. Two new quinazoline derivatives from the moss endophytic fungus Aspergillus sp. and their antiinfammatory activity. Nat. Prod. Bioprospect. 2021, 11, 105–110. [Google Scholar] [CrossRef]
- Khayat, M.T.; Ibrahim, S.R.M.; Mohamed, G.A.; Abdallah, H.M. Anti-inflammatory metabolites from endophytic fungus Fusarium sp. Phytochem. Lett. 2019, 29, 104–109. [Google Scholar] [CrossRef]
- Liao, G.; Wu, P.; Xue, J.; Liu, L.; Li, H.; Wei, X. Asperimides A–D, anti-inflammatory aromatic butenolides from a tropical endophytic fungus Aspergillus terreus. Fitoterapia 2018, 131, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Z.; Liu, H.; Pan, Y.; Li, J.; Liu, L.; She, Z. Dichloroisocoumarins with potential anti-inflammatory activity from the mangrove endophytic fungus Ascomycota sp. CYSK-4. Mar. Drugs 2018, 16, 54. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, Z.; Liu, H.; Long, Y.; Chen, D.; Lu, Y.; She, Z. Lasiodiplactone A, a novel lactone from the mangrove endophytic fungus Lasiodiplodia theobromae ZJ-HQ1. Org. Biomol. Chem. 2017, 15, 6338–6341. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Li, Z.; Xu, X.; Wang, K.; Shao, M.; Zhao, F.; Wang, H.; Hua, H.; Pei, Y.; Bai, J. Butenolide derivatives from the plant endophytic fungus Aspergillus terreus. Fitoterapia 2016, 113, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ge, H.; Zou, J.H.; Tao, X.; Chen, R.; Dai, J. Periconianone A, a new 6/6/6 carbocyclic sesquiterpenoid from endophytic fungus Periconia sp. with neural anti-inflammatory activity. Org. Lett. 2014, 16, 1410–1413. [Google Scholar] [CrossRef] [PubMed]
- Uzor, P.F.; Osadebe, P.O.; Nwodo, N.J. Antidiabetic activity of extract and compounds from an endophytic fungus Nigrospora oryzae. Drug Res. 2016, 67, 308–311. [Google Scholar] [CrossRef]
- Rivera-Chavez, J.; Gonzalez-Andrade, M.; Gonzalez, M.D.C.; Glenn, A.E.; Mata, R. Thielavins A, J and K: A-Glucosidase inhibitors from MEXU 27095, an endophytic fungus from Hintonia latiflora. Phytochemistry 2013, 94, 198–205. [Google Scholar] [CrossRef]
- Ye, G.; Huang, C.; Li, J.; Chen, T.; Tang, J.; Liu, W.; Long, Y. Isolation, structural characterization and antidiabetic activity of new diketopiperazine alkaloids from mangrove endophytic fungus Aspergillus sp. 16-5c. Mar. Drugs 2021, 19, 402. [Google Scholar] [CrossRef]
- Singh, B.; Kaur, A. Antidiabetic potential of a peptide isolated from an endophytic Aspergillus awamori. J. Appl. Microbiol. 2015, 120, 301–311. [Google Scholar] [CrossRef]
- Ruzieva, D.M.; Abdulmyanova, L.I.; Rasulova, G.A.; Sattarova, R.S.; Gulyamova, T.G. Screening of inhibitory activity against α-amylase of fungal endophytes isolated from medicinal plants in Uzbekistan. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2744–2752. [Google Scholar]
- Ruzieva, D.; Gulyamova, T.; Nasmetova, S.; Mukhammedov, I.; Rasulova, G. Identification of Bioactive Compounds of the Endophytic Fungus Aspergillus egypticus-HT166S Inhibiting the Activity of Pancreatic α-Amylase. Turk. J. Pharm. Sci. 2022, 19, 630–635. [Google Scholar] [CrossRef]
- Pavithra, N.; Sathish, L.; Babu, N.; Venkatarathanamma, V.; Pushpalatha, H.; Reddy, G.B.; Ananda, K. Evaluation of α-Amylase, α-glucosidase and aldose reductase inhibitors in ethyl acetate extracts of endophytic fungi isolated from antidiabetic medicinal plants. Int. J. Pharm. Sci. Res. 2014, 5, 5334–5341. [Google Scholar]
- Ali, S.; Khan, A.L.; Ali, L.; Rizvi, T.S.; Khan, S.A.; Hussain, J.; Hamayun, M.; Al-Harrasi, A. Enzyme inhibitory metabolites from endophytic Penicillium citrinum isolated from Boswellia sacra. Arch. Microbiol. 2017, 199, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, J.; Huang, H.; Ma, L.; Wang, J.; Gu, Y.; Liu, L.; Lin, Y. Four Eremophilane Sesquiterpenes from the Mangrove Endophytic Fungus Xylaria sp. Mar. Drugs 2012, 10, 340–348. [Google Scholar] [CrossRef]
- Xia, X.; Qi, J.; Liu, I.; Jia, A.; Zhang, Y.; Liu, C.; Gao, C.; She, Z. Bioactive isopimarane diterpenes from the fungus, Epicoccum sp. HS-1 associated with Apostichopus japonicas. Mar. Drugs 2015, 13, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Lopéz, D.; Cherigo, L.; Mejia, L.C.; Loza-Mejía, M.A.; Martínez-Luis, S. α-Glucosidase inhibitors from a mangrove associated fungus, Zasmidium sp. strain EM5-10. BMC Chem. 2019, 13, 22. [Google Scholar] [CrossRef]
- Malik, A.; Ardalani, H.; Anam, S.; McNair, L.M.; Kromphardt, K.J.K.; Frandsen, R.J.N.; Franzyk, H.; Staerk, D.; Kongstad, K.T. Antidiabetic xanthones with α-glucosidase inhibitory activities from an endophytic Penicillium canescens. Fitoterapia 2020, 142, 104522. [Google Scholar] [CrossRef]
- Greener, M. Autoimmune diseases: The immunological tightrope. Presecriber 2022, 33, 19–22. [Google Scholar] [CrossRef]
- Mohammedsaleh, Z.M. The use of patient-specific stem cells in different autoimmune diseases. Saudi J. Biol. Sci. 2022, 29, 3338–3346. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.T.; Habbu, P.V.; Kulkarni, V.H.; Jagadish, S.K.; Pandey, A.R.; Sutariya, V.N. Endophytic microbes: A novel source for biologically/pharmacologically active secondary metabolites. Asian J. Pharmacol. Toxicol. 2014, 2, 1–16. [Google Scholar]
- Ujam, N.T.; Ajaghaku, D.L.; Okoye, F.B.C.; Esimone, C.O. Antioxidant and immunosuppressive activities of extracts of endophytic fungi isolated from Psidium guajava and Newbouldia laevis. Phytomed. Plus 2021, 1, 100028. [Google Scholar] [CrossRef]
- Wang, L.W.; Wang, J.L.; Chen, J.; Chen, J.J.; Shen, J.W.; Feng, X.X.; Kubicek, C.P.; Lin, F.C.; Zhang, C.L.; Chen, F.Y. A novel derivative of (-) mycousnine produced by the endophytic fungus Mycosphaerella nawae, exhibits high and selective immunosuppressive activity on T cells. Front. Microbiol. 2017, 8, 1251. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, S.; Liu, W.; Liu, Y.; Huang, X.; She, Z. Polyketides with immunosuppressive activities from mangrove endophytic fungus Penicillium sp. ZJ-SY2. Mar. Drugs 2016, 14, 217. [Google Scholar] [CrossRef]
- Lin, R.; Kim, H.; Hong, J.; Li, Q.J. Biological evaluation of subglutinol a as a novel immunosuppressive agent for inflammation intervention. Med. Chem. Lett. 2014, 5, 485–490. [Google Scholar] [CrossRef]
- Wei, W.; Gao, J.; Shen, Y.; Chu, Y.L.; Xu, Q.; Tan, R.X. Immunosuppressive Diterpenes from Phomopsis sp. S12. Eur. J. Org. Chem. 2014, 26, 5728–5734. [Google Scholar] [CrossRef]
- Katoch, M.; Khajuria, A.; Sharma, P.R.; Saxena, A.K. Immunosuppressive potential of Botryosphaeria dothidea, an endophyte isolated from Kigelia Africana. Pharm. Biol. 2014, 53, 85–91. [Google Scholar] [CrossRef]
- Ye, K.; Lv, X.; Zhang, X.; Wei, P.P.; Li, Z.H.; Ai, H.L.; Zhao, D.K.; Liu, J.K. Immunosuppressive isopimarane diterpenes from cultures of the endophytic fungus Ilyonectria robusta. Front. Pharmacol. 2022, 12, 3847. [Google Scholar] [CrossRef]
- Lin, S.; Yan, S.; Liu, Y.; Zhang, X.; Cao, F.; He, Y.; Li, F.; Liu, J.; Wang, J.; Hu, J.; et al. New secondary metabolites with immunosuppressive and BChE inhibitory activities from an endophytic fungus Daldinia sp. TJ403-LS1. Bioorg. Chem. 2021, 114, 105091. [Google Scholar] [CrossRef]
- Zhang, W.F.; Ma, J.K.; Zhang, X.X.; Qian, Y.N.; Xu, J. Immunosuppressive polyketides from the mangrove endophytic fungus Pestalotiopsis sp. HHL-14. Chem. Nat. Compd. 2021, 57, 1130–1133. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Zhang, X.X.; Ma, J.K.; Yang, Y.; Zhou, J.; Xu, J. Secondary metabolites produced by mangrove endophytic fungus Aspergillus fumigatus HQD24 with immunosuppressive activity. Bochem. Syst. Ecol. 2020, 93, 104166. [Google Scholar] [CrossRef]
- Sandhu, S.S.; Kumar, S.; Aharwal, R.P. Isolation and identification of endophytic fungi from Ricinus communis Linn. and their antibacterial activity. Int. J. Pharm. Pharm. Sci. 2014, 4, 611–618. [Google Scholar]
- Mc-Mullin, D.R.; Green, B.D.; Miller, J.D. Antifungal sesquterpenoids and macrolides from an endophytic Lophodernium species of Pinus strobus. Phytochem. Lett. 2015, 14, 148–152. [Google Scholar] [CrossRef]
- Zhou, M.; Zhou, K.; He, P.; Wang, K.M.; Zhu, R.Z.; Wang, Y.D.; Dong, W.; Li, G.P.; Yang, H.Y.; Ye, Y.Q.; et al. Antiviral and cytotoxic isocoumarin derivatives from an endophytic fungus Aspergillus oryzae. Planta Med. 2015, 82, 414–417. [Google Scholar] [CrossRef]
- Mishra, V.K.; Passari, A.K.; Chandra, P.; Leo, V.V.; Kumar, B.; Uthandi, S.; Thankappan, S.; Gupta, V.K.; Singh, B.P. Determination and production of antimicrobial compounds by Aspergillus clavatonanicus strain MJ31, an endophytic fungus from Mirabilis jalapa L. using UPLC-ESIMS/MS and TD-GC-MS analysis. PLoS ONE 2017, 12, e0186234. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Niu, S.; Chen, S.; Liu, L. Diaporone A, a new antibacterial secondary metabolite from the plant endophytic fungus Diaporthe sp. J. Antibiot. 2019, 73, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Yehia, R.; Shivanna, M.B. Antimicrobial activity and metabolite profling of endophytic fungi in Digitaria bicornis (Lam) Roem. and Schult. and Paspalidium favidum (Retz.) A. Camus. 3 Biotech 2021, 11, 53. [Google Scholar]
- Zihad, S.M.N.K.; Hasan, M.T.; Sultana, M.S.; Nath, S.; Nahar, L.; Rashid, M.A.; Uddin, S.J.; Sarker, S.D.; Shilpi, J.A. Isolation and characterization of antibacterial compounds from Aspergillus fumigatus: An endophytic fungus from a mangrove plant of the Sundarbans. Hindawi Evi. Based Complement. Altern. Med. 2022, 2022, 9600079. [Google Scholar] [CrossRef] [PubMed]
- Elkady, W.M.; Raafat, M.M.; Abdel-Aziz, M.M.; Al-Huqail, A.A.; Ashour, M.L.; Fathallah, N. Endophytic fungus from Opuntina fiscus-indica: A source of potential bioactive antimicrobial compounds against multidrug-resistant bacteria. Plants 2022, 11, 1070. [Google Scholar] [CrossRef]
- Xian, P.J.; Liu, S.Z.; Wang, W.J.; Yang, S.X.; Feng, Z.; Yang, X.L. Undescribed specialised metabolites from the endophytic fungus Emericella sp. XL029 and their antimicrobial activities. Phytochemistry 2022, 202, 113303. [Google Scholar] [CrossRef]
- Astuti, P.; Rollando, R.; Pratoko, D.K.; Wahyuono, S.; Nurrochmad, A. Antimicrobial sand cytotoxic activities of a compound produced by an endophytic fungus isolated from the leaves of Coleus amboinicus Lou. Int. J. Pharm. Res. 2020, 13, 2632–2644. [Google Scholar]
- Khiralla, A.; Spina, R.; Varbanov, M.; Philippot, S.; Lemiere, P.; Deschaumes, S.S.; Andre, P.P.; Mohamed, I.; Yagi, S.M.; Mattar, D.L. Evaluation of antiviral, antibacterial and antiproliferative activities of the endophytic fungus Curvularia papendorfii, and isolation of a new polyhydroxyacid. Microorganisms 2020, 8, 1353. [Google Scholar] [CrossRef]
- Techaoei, S.; Jirayuthcharoenkul, C.; Jarmkom, K.; Dumrongphuttidecha, T.; Khobjai, W. Chemical evaluation and antibacterial activity of novel bioactive compounds from endophytic fungi in Nelumbo nucifera. Saudi J. Biol. Sci. 2020, 27, 2883–2889. [Google Scholar] [CrossRef]
- Hilario, F.; Polinario, G.; Amorim, M.R.D.; Batista, V.D.S.; Junior, N.M.D.N.; Araujo, A.R.; Bauab, T.R.; Santos, L.C.D. Spirocyclic lactams and curvulinic acid derivatives from the endophytic fungus Curvularia lunata and their antibacterial and antifungal activities. Fitoterapia 2019, 141, 104466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yuan, X.L.; Du, Y.M.; Zhang, H.B.; Shen, G.M.; Zhang, Z.F.; Liang, Y.J.; Zhao, D.L.; Xu, K. Angularly prenylated indole alkaloids with antimicrobial and insecticidal activities from an endophytic fungus Fusarium sambucinum TE-6L. J. Agr. Food Chem. 2019, 67, 11994–12001. [Google Scholar] [CrossRef] [PubMed]
- Kaaniche, F.; Hamed, A.; Razek, A.S.A.; Wibberg, D.; Abdissa, N.; Euch, I.Z.E.; Allouche, N.; Mellouli, L.; Shaaban, F.; Sewald, N. Bioactive secondary metabolites from new endophytic fungus Curvularia. sp. isolated from Rauwolfia macrophylla. PLoS ONE 2019, 14, e0217627. [Google Scholar] [CrossRef] [PubMed]
- Pansanit, A.; Pripdeevech, P. Antibacterial secondary metabolites from an endophytic fungus, Arthrinium sp. MFLUCC16-1053 isolated from Zingiber cassumunar. Mycology 2018, 9, 264–272. [Google Scholar] [CrossRef]
- Wang, W.X.; Kusari, S.; Laatsch, H.; Golz, C.; Kusari, P.; Strohmann, C.; Kayser, O.; Spiteller, M. Antibacterial Azaphilones from an endophytic fungus, Colletotrichum sp. BS4. J. Nat. Prod. 2016, 79, 704–710. [Google Scholar] [CrossRef]
- Chen, S.; Chen, D.; Cai, R.; Cui, H.; Long, Y.; Lu, Y.; Li, C.; She, Z. Cytotoxic and antibacterial preussomerins from the mangrove endophytic fungus Lasiodiplodia theobromae ZJ-HQ1. J. Nat. Prod. 2016, 79, 2397–2402. [Google Scholar] [CrossRef]
- Goutam, J.; Kharwar, R.N.; Kharwar, V.K.; Mishra, A.; Singh, S. Isolation and identification of antibacterial compounds isolated from endophytic fungus Emericella qaudrilineata (RS-5). Nat. Prod. Chem. Res. 2016, 4, 2–7. [Google Scholar] [CrossRef]
- Meng, L.H.; Zhang, P.; Li, X.M.; Wang, B.G. Penicibrocazines A–E, five new sulfide diketopiperazines from the marine-derived endophytic Fungus Penicillium brocae. Mar. Drugs 2015, 13, 276–287. [Google Scholar] [CrossRef]
- Ratnaweera, P.B.; Silva, E.D.D.; Williams, D.E.; Andersen, R.J. Antimicrobial activities of endophytic fungi obtained from the arid zone invasive plant Opuntia dillenii and the isolation of equisetin, from endophytic Fusarium sp. BMC Compl. Altern. Med. 2015, 15, 220. [Google Scholar] [CrossRef]
- Hussain, H.; Root, N.; Jabeen, F.; Harrasi, A.A.; Ahmad, M.; Mabood, F.; Hassan, Z.; Shah, A.; Green, I.R.; Schulz, B.; et al. Microsphaerol and seimatorone: Two new compounds isolated from the endophytic fungi, Microsphaeropsis sp. and Seimatosporium sp. Chem. Biodivers. 2014, 12, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Root, N.; Jabeen, F.; Harrasi, A.A.; Rawahi, A.A.; Ahmad, M.; Hassan, Z.; Abbas, G.; Mabood, F.; Shah, A.; et al. Seimatoric acid and colletonoic acid: Two new compounds from the endophytic fungi, Seimatosporium sp. and Colletotrichum sp. Chin. Chem. Lett. 2014, 25, 1577–1579. [Google Scholar] [CrossRef]
- Li, G.; Kusari, S.; Lamshoft, M.; Schuffler, A.; Laatsch, H.; Spiteller, M. Antibacterial secondary metabolites from an endophytic fungus, Eupenicillium sp. LG41. J. Nat. Prod. 2014, 77, 2335–2341. [Google Scholar] [CrossRef]
- Ding, X.; Liu, K.; Deng, B.; Chen, W.; Li, W.; Liu, F. Isolation and characterization of endophytic fungi from Camptotheca acuminata. World J. Microb. Biot. 2013, 29, 1831–1838. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, Y.; Zhao, H.; Yi, Y.; Zhang, C.; Yu, C.; Xu, C. An antimicrobial compound from the endophytic fungus Phoma sp. isolated from the medicinal plant Taraxacum mongolicum. J. Taiwan Inst. Chem. E 2012, 44, 177–181. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, W.; Shan, T.; Mou, Y.; Lou, J.; Li, Y.; Wang, M.; Zhou, L. Antimicrobial metabolites from the endophytic fungus Gliomastix murorum Ppf8 associated with the medicinal plant Paris polyphylla var. yunnanensis. J. Med. Plants Res. 2012, 6, 2100–2104. [Google Scholar]
- Devi, P.; Rodrigues, C.; Naik, C.G.; D’Souza, L. Isolation and characterization of antibacterial compound from a mangrove-endophytic fungus, Penicillium chrysogenum MTCC 5108. Indian J. Microbiol. 2012, 52, 617–623. [Google Scholar] [CrossRef]
- Sebastianes, F.L.S.; Cabedo, N.; Aouad, N.E.; Valente, A.M.M.P.; Lacava, P.T.; Azevedo, J.L.; Kleiner, A.A.P.; Cortes, D. 3-Hydroxypropionic acid as an antibacterial agent from endophytic fungi Diaporthe phaseolorum. Curr. Microbiol. 2012, 65, 622–632. [Google Scholar] [CrossRef]
- Pinheiro, E.A.A.; Carvalho, J.M.; Santos, D.C.P.D.; Feitosa, A.D.O.; Marinho, P.S.B.; Guilhon, G.M.S.P.; Souza, A.D.L.D.; Silva, F.M.A.D.; Marinho, A.M.D.R. Antibacterial activity of alkaloids produced by endophytic fungus Aspergillus sp. EJC08 isolated from medical plant Bauhinia guianensis. Nat. Prod. Res. 2012, 27, 1633–1638. [Google Scholar] [CrossRef]
- Meng, X.; Mao, Z.; Lou, J.; Xu, L.; Zhong, L.; Peng, Y.; Zhou, L.; Wang, M. Benzopyranones from the endophytic fungus Hyalodendriella sp. Ponipodef12 and their bioactivities. Molecules 2012, 17, 11303–11314. [Google Scholar] [CrossRef]
- Erbert, C.; Lopes, A.A.; Yokoya, N.S.; Furtado, N.A.J.C.; Conti, R.; Pupo, M.T.; Lopes, J.L.C.; Debonsi, H.M. Antibacterial compound from the endophytic fungus Phomopsis longicolla isolated from the tropical red seaweed Bostrychia radicans. Bot. Mar. 2012, 55, 435–440. [Google Scholar] [CrossRef]
- Subban, K.; Subramani, R.; Johnpaul, M. A novel antibacterial and antifungal phenolic compound from the endophytic fungus Pestalotiopsis mangiferae. Nat. Prod. Res. 2012, 27, 1445–1449. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Krohn, K.; Hussain, H.; Schulz, B.; Draeger, S. Pestalotheols E–H: Antimicrobial metabolites from an endophytic fungus isolated from the tree Arbutus unedo. Eur. J. Org. Chem. 2011, 2011, 5163–5166. [Google Scholar] [CrossRef]
- Ahmed, I.; Hussain, H.; Schulz, B.; Draeger, S.; Padula, D.; Pescitelli, G.; Ree, T.V.; Krohn, K. Three new antimicrobial metabolites from the endophytic fungus Phomopsis sp. Eur. J. Org. Chem. 2011, 2011, 2867–2873. [Google Scholar] [CrossRef]
- Nithya, K.; Muthumary, J. Bioactive metabolite produced by Phomopsis sp., an endophytic fungus in Allamanda cathartica Linn. Recent Res. Sci. Technol. 2011, 3, 44–48. [Google Scholar]
- Yehia, R.S.; Osman, G.H.; Assaggaf, H.; Salem, R. Isolation of potential antimicrobial metabolites from endophytic fungus Cladosporium cladosporioides from endemic plant Zygophyllum mandavillei. S. Afr. J. Bot. 2020, 15, 313–319. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, L.; Kalscheuer, R.; Liu, Z.; Proksch, P. Antifungal polyketide derivatives from the endophytic fungus Aplosporella javeedii. Bioorg. Med. Chem. 2020, 28, 115456. [Google Scholar] [CrossRef]
- Luo, H.; Qing, Z.; Deng, Y.; Deng, Z.; Tang, X.; Feng, B.; Lin, W. Two polyketides produced by endophytic Penicillium citrinum DBR-9 from medicinal plant Stephania kwangsiensis and their antifungal activity against plant pathogenic fungi. Nat. Prod. Commun. 2019, 14, 1–6. [Google Scholar] [CrossRef]
- Bungtongdee, N.; Sopalun, K.; Laosripaiboon, W.; Lamtham, S. The chemical composition, antifungal, antioxidant and antimutagenicity properties of bioactive compounds from fungal endophytes associated with Thai orchids. J. Phytopathol. 2018, 167, 56–64. [Google Scholar] [CrossRef]
- Venkateshwarulu, N.; Shameer, S.; Bramhachari, P.V.; Basha, S.K.T.; Nagaraju, C.; Vijaya, T. Isolation and characterization of plumbagin (5-hydroxyl-2-methylnaptalene-1,4-dione) producing endophytic fungi Cladosporium delicatulum from endemic medicinal plants. Biotechnol. Rep. 2018, 20, e00282. [Google Scholar] [CrossRef]
- Premjanu, N.; Jaynthy, C.; Diviya, S. Antifungal activity of endophytic fungi isolated from Lannea coromandelicaan in-silico approach. Int. J. Pharm. Pharm. Sci. 2016, 8, 207–210. [Google Scholar]
- Pereira, C.B.; Oliveira, D.M.J.; Hughes, A.F.S.; Kohlhof, M.; Vieira, M.L.A.; Vaz, A.B.M.; Ferreira, M.C.; Carvalho, C.R.; Rosa, L.H.; Rosa, C.A.; et al. Endophytic fungal compounds active against Cryptococcus neoformans and C. gattii. J. Antibiot. 2015, 68, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Nalli, Y.; Mirza, D.N.; WAni, Z.A.; Wadhwa, B.; Mallik, F.A.; Raina, C.; Chaubey, A.; Riyaz-UI-Hassan, S.; Ali, A. Phialomustin A-D, new antimicrobial and cytotoxic metabolites from an endophytic fungus, Phialophora mustea. R. Soc. Chem. 2015, 5, 95307–95312. [Google Scholar] [CrossRef]
- Taware, R.; Abnave, P.; Patil, D.; Rajamohananan, P.R.; Raja, R.; Soundararajan, G.; Kundu, G.C.; Ahmad, A. Isolation, purification and characterization of Trichothecinol-A produced by endophytic fungus Trichothecium sp. and its antifungal, anticancer and antimetastatic activities. Sustain Chem. Process 2014, 2, 8. [Google Scholar] [CrossRef]
- Sumarah, M.W.; Kesting, J.R.; Sorensen, D.; Miller, J.D. Antifungal metabolites from fungal endophytes of Pinus strobus. Phytochemistry 2011, 72, 1833–1837. [Google Scholar] [CrossRef]
- Sun, Z.L.; Zhang, M.; Zhang, J.F.; Feng, F. Antifungal and cytotoxic activities of the secondary metabolites from endophytic fungus Massrison sp. Phytomedicine 2011, 18, 859–862. [Google Scholar] [CrossRef] [PubMed]
- EI-Hawary, S.S.; Mohammed, R.; Bahr, H.S.; Attia, E.Z.; EI-Katatny, M.H.; Abelyan, N.; AI-Sanea, M.M.; Moawad, A.S.; Abdelmohsen, U.R. Soyabean-associated endophytic fungi as potential source of anti-COVID-19 metabolites supported by docking analysis. J. Appl. Microbiol. 2021, 131, 1193–1211. [Google Scholar] [CrossRef]
- Liu, S.S.; Jiang, J.X.; Huang, R.; Wang, Y.T.; Jiang, B.G.; Zheng, K.X.; Wu, S.H. A new antiviral 14-nordrimane sesquiterpenoid from an endophytic fungus Phoma sp. Phytochem. Lett. 2019, 29, 75–78. [Google Scholar] [CrossRef]
- Liang, H.Q.; Zhang, D.W.; Guo, S.X.; Yu, J. Two new tetracyclic triterpenoids from the endophytic fungus Hypoxylon sp. 6269. J. Asian Nat. Prod. Res. 2018, 20, 951–956. [Google Scholar] [CrossRef]
- Pang, X.; Zhao, J.Y.; Fang, X.M.; Zhang, T.; Zhang, D.W.; Liu, H.Y.; Su, J.; Cen, S.; Yu, L.Y. Metabolites from the plant endophytic fungus Aspergillus sp. CPCC 400735 and their anti-HIV activities. J. Nat. Prod. 2017, 80, 2595–2601. [Google Scholar] [CrossRef]
- Zhang, S.P.; Huang, R.; Li, F.F.; Wei, H.X.; Fang, X.W.; Xie, X.S.; Lin, D.G.; Wu, S.H.; He, J. Antiviral anthraquinones and azaphilones produced by an endophytic fungus Nigrospora sp. from Aconitum carmichaeli. Fitoterapia 2016, 112, 85–89. [Google Scholar] [CrossRef]
- Uzor, P.F.; Odimegwu, D.C.; Ebrahim, W.; Osadebe, P.O.; Nwodo, N.J.; Okoye, F.B.; Liu, Z.; Proksch, P. Anti-Respiratory Syncytial Virus Compounds from Two Endophytic Fungi Isolated from Nigerian Medicinal Plants. Drug Res. (Stuttg) 2016, 66, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Govindappa, M.; Hemmanur, K.C.; Nithin, S.; Poojari, C.C.; Kakunje, G.B.; Channabasava, K. In vitro anti-HIV activity of partially purified coumarin(s) isolated from fungal endophyte, Alternaria species of Calophyllum inophyllum. Pharm. Pharmacol. 2015, 6, 321–328. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, H.; Wang, J.; Yang, J.; Li, X.N.; Wang, J.; Chen, K.; Wang, Y.; Luo, Z.; Yao, G.; et al. Armochaetoglobins K–R, anti-HIV pyrrole-based cytochalasans from Chaetomium globosum TW1-1. Eur. J. Org. Chem. 2015, 13, 3086–3094. [Google Scholar] [CrossRef]
- Liu, X.; Guo, W.J.; Fu, W.J.; Fan, M. Steroids from an anti-HIV active endophyte. Chem. Nat. Compd. 2014, 50, 387–388. [Google Scholar] [CrossRef]
- Bashyal, B.P.; Wellensiek, B.P.; Ramakrishnan, R.; Faeth, S.H.; Ahmad, N.; Gunatilaka, A.A.L. Altertoxins with potent anti-HIV activity from Alternaria tenuissima QUE1Se, a fungal endophyte of Quercus emoryi. Bioorg. Med. Chem. 2014, 22, 6112–6116. [Google Scholar] [CrossRef]
- Wellensiek, B.P.; Ramakrishnan, R.; Bashyal, B.P.; Eason, Y.; Gunatilaka, A.A.L.; Ahmad, N. Inhibition of HIV-1 replication by secondary metabolites from endophytic fungi of desert plants. Virol. J. 2013, 7, 72–80. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, S.; Zhu, T.; Gu, Z.L.J.; Li, D.; Gu, Q. Antiviral isoindolone derivatives from an endophytic fungus Emericella sp. associated with Aegiceras corniculatum. Phytochemistry 2011, 72, 1436–1442. [Google Scholar] [CrossRef]
- Gendy, M.M.A.E.; Bondkly, A.M.A.E.; Yahya, S.M.M. Production and evaluation of antimycotic and antihepatitis C virus potential of Fusant MERV6270 derived from mangrove endophytic fungi using novel substrates of agroindustrial wastes. Appl. Biochem. Biotech. 2014, 174, 2674–2701. [Google Scholar] [CrossRef]
- Cholewinski, M.; Derda, M.; Hadas, E. Parasitic diseases in humans transmitted by vectors. Ann. Parasitol. 2015, 61, 137–157. [Google Scholar]
- Capela, R.; Moreira, R.; Lopes, F. An overview of drug resistancein protozoal diseases. Int. J. Mol. Sci. 2019, 20, 5748. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Pastor, I.; Gonzalez-Menéndez, V.; Annang, F.; Toro, C.; Mackenzie, T.A.; Bosch-Navarrete, C.; Genilloud, O.; Reyes, F. Pipecolisporin, a novel cyclic peptide with antimalarial and antitrypanosome activities from a wheat endophytic Nigrospora oryzae. Pharmaceuticals 2021, 14, 268. [Google Scholar] [CrossRef]
- Alhadrami, H.A.; Sayed, A.M.; El Gendy, A.O.; Shamikh, Y.I.; Gaber, Y.; Bakeer, W.; Sheirf, N.H.; Attia, E.Z.; Shaban, G.M.; Khalifa, B.A.; et al. A metabolomic approach to target antimalarial metabolites in the Artemisia annua fungal endophytes. Sci. Rep. 2021, 11, 2770. [Google Scholar] [CrossRef]
- Purwantini, I.; Wahyono, M.; Susidarti, R.A.; Sholikhah, E.N.; Hestiyani, R.A.N. Antiplasmodial activity of endophytic fungi isolated from Artemisia Annua, L. Int. J. Pharm. Clin. Res. 2016, 8, 341–344. [Google Scholar]
- Kumarihamy, M.; Ferreira, D.; Croom, E.C.M., Jr.; Sahu, R.; Tekwani, B.L.; Duke, S.O.; Khan, S.; Techen, N.; Nanayakkara, N.P.D. Antiplasmodial and cytotoxic cytochalasins from an endophytic fungus, Nemania sp. UM10M, isolated from a diseased Torreya taxifolia leaf. Molecules 2019, 24, 777. [Google Scholar] [CrossRef]
- Ateba, J.E.T.; Toghueo, R.M.K.; Awantu, A.F.; Ning, B.M.M.; Gohlke, S.; Sahal, D.; Filho, E.R.; Tsamo, E.; Boyom, F.F.; Sewald, N.; et al. Antiplasmodial Properties and Cytotoxicity of Endophytic Fungi from Symphonia globulifera (Clusiaceae). J. Fungi 2018, 4, 70. [Google Scholar] [CrossRef]
- Tawfike, A.F.; Romli, M.; Clements, C.; Abbott, G.; Young, L.; Schumacher, M.; Diederich, M.; Farag, M.; Ebel, R.A.E. Isolation of anticancer and anti-trypanosome secondary metabolites from the endophytic fungus Aspergillus flocculus via bioactivity guided isolation and MS based metabolomics. J. Chromatogr. B 2019, 1106, 71–83. [Google Scholar] [CrossRef]
- Santiago, I.M.; Alves, T.M.A.; Rabello, A.; Junior, P.A.S.; Romanha, A.J.; Zani, C.L.; Rosa, C.A.; Rosa, L.H. Leishmanicidal and antitumoral activities of endophytic fungi associated with the Antarctic angiosperms Deschampsia antarctica Desv. and Colobanthus quitensis (Kunth) Bartl. Extremophiles 2012, 16, 95–103. [Google Scholar] [CrossRef]
- Fatima, N.; Muhammad, S.A.; Mumtaz, A.; Tariq, H.; Shahzadi, I.; Said, M.S.; Dawood, M. Fungal metabolites and leishmaniasis: A review. Br. J. Pharm. Res. 2016, 12, 101631759. [Google Scholar] [CrossRef]
- Fatima, N.; Mumtaz, A.; Shamim, R.; Qadir, M.I.; Muhammad, S.A. In silico analyses of epicoccamides on selected leishmania trypanothione reductase enzyme-based target. Indian J. Pharm. Sci. 2016, 78, 259–266. [Google Scholar] [CrossRef]
- Mazlan, N.W.; Tate, R.; Yusoff, Y.M.; Clements, C.; Ebel, R.A.E. Metabolomics-guided isolation of anti-trypanosomal com-pounds from endophytic fungi of the mangrove plant Avicennia lanata. Curr. Med. Chem. 2018, 27, 1815–1835. [Google Scholar] [CrossRef] [PubMed]
- Almeida, T.T.D.; Ribeiro, M.A.D.S.; Polonio, J.C.; Garcia, F.P.; Nakamura, C.V.; Meurer, E.C.; Sarragiotto, M.H.; Baldoqui, D.C.; Azevedo, J.L.; Pamphile, J.A. Curvulin and spirostaphylotrichins R and U from extracts produced by two endophytic Bipolaris sp. associated to aquatic macrophytes with antileishmanial activity. Nat. Prod. Res. 2017, 32, 2783–2790. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Abdallah, H.M.; Elkhayat, E.S.; Musayeib, N.M.A.; Asfour, H.Z.; Zayed, M.F.; Mohamed, M.A. Fusaripeptide A: New antifungal and anti-malarial cyclodepsipeptide from the endophytic fungus Fusarium sp. J. Asian Nat. Prod. Res. 2017, 20, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.C.; Cantrell, C.L.; Wedge, D.E.; Goncalves, C.N.; Jacob, M.R.; Khan, S.; Rosa, C.A.; Rosa, L.H. Antimycobacterial and antimalarial activities of endophytic fungi associated with the ancient and narrowly endemic neotropical plant Vellozia gigantea from Brazil. Mem. Inst. Oswaldo Cruz. 2017, 112, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Metwaly, A.M.; Ghoneim, M.M.; Musa, A. Two new antileishmanial diketopiperazine alkaloids from the endophytic fungus Trichosporum sp. Pharma Chem. 2015, 7, 322–327. [Google Scholar]
- Campos, F.F.; Junior, P.A.S.; Romanha, A.J.; Araujo, M.S.S.; Siqueira, E.P.; Resende, J.M.; Alves, T.M.A.; Filho, O.A.M.; Santos, V.L.D.; Rosa, C.A.; et al. Bioactive endophytic fungi isolated from Caesalpinia echinata Lam. (Brazilwood) and identification of beauvericin as a trypanocidal metabolite from Fusarium sp. Mem. Inst. Oswaldo Cruz. 2015, 110, 65–74. [Google Scholar] [CrossRef]
- Elkhayat, E.S.; Ibrahim, S.R.M.; Mohamed, G.A.; Ross, S.A. Terrenolide S, a new antileishmanial butenolide from the endophytic fungus Aspergillus terreus. Nat. Prod. Res. 2015, 30, 814–820. [Google Scholar] [CrossRef]
- Calcul, L.; Waterman, C.; Ma, W.S.; Lebar, M.D.; Harter, C.; Mutka, T.; Morton, L.; Maignan, P.; Olphen, A.V.; Kyle, D.E.; et al. Screening mangrove endophytic fungi for antimalarial natural products. Mar. Drugs 2013, 11, 5036–5050. [Google Scholar] [CrossRef]
- Intaraudom, C.; Boonyuen, N.; Suvannakad, R.; Rachtawee, P.; Pittayakhajonwut, P. Penicolinates A–E from endophytic Penicillium sp. BCC16054. Tetrahedron Lett. 2012, 54, 744–748. [Google Scholar] [CrossRef]
- Elfita, E.; Muharni, M.; Munawar, M.; Legasari, L.; Darwati, D. Antimalarial compounds from endophytic fungi of brotowali. (Tinaspora crispa L.). Indones. J. Chem. 2011, 11, 53–58. [Google Scholar] [CrossRef]
- Moreno, E.; Varughese, T.; Spadafora, C.; Arnold, A.E.; Coley, P.D.; Kursar, T.A.; Gerwick, W.H.; Rios, L.C. Chemical constituents of the new endophytic fungus Mycosphaerella sp. nov. and their anti-parasitic activity. Nat. Prod. Commun. 2011, 6, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lin, P.; Wang, K.; Gu, C.C.; Kusari, S. Artificial intelligence-guided discovery of anticancer lead compounds from plants and associated microorganisms. Trends Cancer 2022, 8, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Y.; Byrne, R.; Schneider, G.; Yang, S. Concepts of artificial intelligence for computer-assisted drug discovery. Chem. Rev. 2019, 119, 10520–10594. [Google Scholar] [CrossRef] [PubMed]
- Barre, P.; Stover, B.C.; Muller, K.F.; Steinhage, V. LeafNet: A computer vision system for automatic plant species identification. Ecol. Inform. 2017, 40, 50–56. [Google Scholar] [CrossRef]
- Kumar, N.; Belhumeur, P.N.; Biswas, A.; Jacobs, D.W.; Kress, W.H.; Lopez, I.C.; Soares, J.V.B. Leafsnap: A computer vision system for automatic plant species identification. In Computer Vision—ECCV 2012; Springer: Berlin/Heidelberg, Germany, 2012; pp. 502–516. [Google Scholar]
- Sun, Y.; Liu, Y.; Wang, G.; Zhang, H. Deep learning for plant identification in natural environment. Comput. Intell. Neurosci. 2017, 2017, 7361042. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.M.; Costa, M.S.; Sanchez, L.M.; Murphy, B.T. Coupling MALDI-TOF mass spectrometry protein and specialized metabolite analyses to rapidly discriminate bacterial function. Proc. Natl. Acad. Sci. USA 2018, 115, 4981–4986. [Google Scholar] [CrossRef]
- Dumolin, C.; Aerts, M.; Verheyde, B.; Schellaert, S.; Vandamme, T.; Jeugt, F.V.D.; Canck, E.D.; Cnockaert, M.; Wieme, A.D.; Cleenwerck, I.; et al. Introducing SPeDE: High-throughput dereplication and accurate determination of microbial diversity from matrix-assisted laser desorption-ionization time of flight mass spectrometry data. mSystems 2019, 4, 1359. [Google Scholar] [CrossRef]
- Kautsar, S.A.; Blin, K.; Shaw, S.; Navarro-Munoz, J.C.; Terlouw, B.R.; Hooft, J.J.J.V.D.; Santen, J.A.V.; Tracanna, V.; Duran, H.G.S.; Andreu, V.P.; et al. MIBiG 2.0: A repository for biosynthetic gene clusters of known function. Nucleic Acids Res. 2019, 48, 454–458. [Google Scholar] [CrossRef]
- Palaniappan, K.; Chen, I.M.A.; Chu, K.; Ratner, A.; Seshadri, R.; Kyrpides, N.C.; Ivanova, N.N.; Mouncey, N.J. IMG-ABC v.5.0: An update to the IMG/atlas of biosynthetic gene clusters knowledgebase. Nucleic Acids Res. 2019, 48, 422–430. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Steinke, K.; Villebro, R.; Ziemert, N.; Lee, S.Y.; Medema, M.H.; Weber, T. antiSMASH 5.0: Updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019, 47, 81–87. [Google Scholar] [CrossRef]
- Ricart, E.; Pupin, M.; Muller, M.; Lisacek, F. Automatic annotation and dereplication of tandem mass spectra of peptidic natural products. Anal. Chem. 2020, 92, 15862–15871. [Google Scholar] [CrossRef] [PubMed]
- Kurita, K.L.; Glassey, E.; Linington, R.G. Integration of high-content screening and untargeted metabolomics for comprehensive functional annotation of natural product libraries. Proc. Natl. Acad. Sci. USA 2015, 112, 11999–12004. [Google Scholar] [CrossRef]
- Grienke, U.; Foster, P.A.; Zwirchmayr, J.; Tahir, A.; Rollinger, J.M.; Mikros, E. 1H NMR-MS-based heterocovariance as a drug discovery tool for fishing bioactive compounds out of a complex mixture of structural analogues. Sci. Rep. 2019, 9, 11113. [Google Scholar] [CrossRef] [PubMed]
- Zani, C.L.; Carroll, A.R. Database for rapid dereplication of known natural products using data from MS and fast NMR experiments. J. Nat. Prod. 2017, 80, 1758–1766. [Google Scholar] [CrossRef] [PubMed]
- Phosrithong, N.; Ungwitayatorn, J. Molecular docking study on anticancer activity of plant-derived natural products. Med. Chem. Res. 2010, 19, 817–835. [Google Scholar] [CrossRef]
- Sharma, V.; Sharma, P.C.; Kumar, V. In silico molecular docking analysis of natural pyridoacridines as anticancer agents. Adv. Chem. 2016, 2016, 409387. [Google Scholar] [CrossRef]
- Reker, D.; Rodrigues, T.; Schneider, P.; Schneider, G. Identifying the macromolecular targets of de novo-designed chemical entities through self-organizing map consensus. Proc. Natl. Acad. Sci. USA 2014, 111, 4067–4072. [Google Scholar] [CrossRef]
- Madhukar, N.S.; Khade, P.K.; Huang, L.; Gayvert, K.; Galletti, G.; Stogniew, M.; Allen, J.E.; Giannakakou, P.; Elemento, O. A Bayesian machine learning approach for drug target identification using diverse data types. Nat. Commun. 2019, 10, 5221. [Google Scholar] [CrossRef]
| Compound | Fungal Endophyte | Host Plant | Bioactivity | References |
|---|---|---|---|---|
| Taxol | Taxomyces andreanae | Taxus brevifolia | Cytotoxic | [62] |
| Pestalotiopsis microspora | Taxus wallachiana | Cytotoxic | [76] | |
| Tubercularia sp. TF5 | Taxus mairei | Cytotoxic | [77] | |
| Fusarium redolens | Taxus baccata | Antimitotic | [78] | |
| Vinblastine | Alternaria sp. | Catharanthus roseus | - | [66] |
| Fusarium oxysporum | Catharanthus roseus | - | [79] | |
| Nigrospora sphaerica | Catharanthus roseus | Cytotoxic | [80] | |
| Vincristine | Fusarium oxysporum | Catharanthus roseus | - | [81] |
| Fusarium oxysporum AA-CRL-6 | Catharanthus roseus | - | [79] | |
| Talaromyces radicus CrP20 | Catharanthus roseus | Cytotoxic | [82] | |
| Eutypella sp. CrP14 | Catharanthus roseus | Cytotoxic | [83] | |
| Camptothecin | Entrophospora infrequens | Nothapodytes foetida | Cytotoxic | [67] |
| Neurospora crassa | Nothapodytes foetida | Cytotoxic | [84] | |
| Nodulisporium sp. | Nothapodytes foetida | - | [85] | |
| Fusarium solani | Camptotheca acuminata | - | [61] | |
| Trichoderma atroviride LY357 | Camptotheca acuminata | - | [86] | |
| Fusarium solani S-019 | Camptotheca acuminata | Cytotoxic | [87] | |
| Podophyllotoxin | Phialocephala fortinii (PPE5 and PPE7) | Podophyllum peltatum | Cytotoxicity | [68] |
| Trametes hirsute | Sinopodophyllum hexandrum | Cytotoxic | [88] | |
| Mucor fragilis TW5 | Sinopodophyllum hexandrum | - | [89] | |
| Huperzine | Shiraia sp. Slf14 | Huperzia serrata | Acetylcholinesterase inhibition | [90] |
| Cladosporium cladosporioides LF70 | Huperzia serrata | Acetylcholinesterase inhibition | [91] | |
| Paecilomyces tenuis YS-13 | Huperzia serrata | Acetylcholinesterase inhibition | [92] | |
| Trichoderma sp. L44 | Huperzia serrata | Acetylcholinesterase inhibition | [93] | |
| Hypericin | Thielavia subthermophila | Hypericum perforatum | Antimicrobial, cytotoxic | [69] |
| Resveratrol | Alternaria sp. MG1 | Vitis vinifera | - | [94] |
| Quambalaria cyanescens | Vitis vinifera | Antibacterial, antioxidant, cytotoxic | [20] |
| Fungal Endophyte | Host Plant | Bioactive Compounds | Tested Cell Lines | IC50 or Inhibition (%) | References |
|---|---|---|---|---|---|
| Alternaria alternata | Jatropha heynei | Kaempferol | Lung carcinoma cancer cell line (A549) | 393.52 µg/mL | [108] |
| Aspergillus flavus | Cynodon dactylon | 2,4,7-trinitrofluorenone and 22t-triene-6beta-ol | MCF-7 breast cancer cell line | 16.25 μg/mL | [109] |
| Quambalaria cyanescens | Vitis vinifera | Resveratrol | A549 cell line | 82% | [20] |
| Penicillium citrinum CGJ-C2 | Tragia involucrata | Quercetin | MCF-7 cell line | 1 µg/mL | [110] |
| J-1, J-2, and J-3 | Ginkgo biloba | Podophyllotoxin | HeLa cell lines | 75% | [111] |
| Simplicillium subtropicum SPC3 | Duguetia staudtii | Simplicilones A and B | Cervix carcinoma cell line KB3.1 | 25–29 μg/mL | [112] |
| Trichoderma viride | Ziziphus mauritiana | 3-beta-hydroxyurs-12-en-28-oic acid | HeLa cell lines | 23 μg/mL | [113] |
| Xylaria sp. ZJWCF255 | Ficus carica | Cytochalasin Q | SMMC-772, MCF-7, MGc 80-3 cell lines | 7–17 μg/mL | [34] |
| Phomopsis sp. BCC 45011 | Xylocarpus granatum | Phomoxydiene C and Cytosporone E | KB, MCF-7, NCI-H187, Vero cells | 1.49–40.17 μg/mL | [34] |
| Pestalotiopsis uvicola | Artemisia japonica | Kaempferol, Quercetin, Rutin, Genistein | Adriamycin-resistant (ADR) MCF-7, ADR, and ovarian paclitaxel-resistant cell A2780 cells | - | [34] |
| Pestalotiopsis sp. FT172 | Myrsine sandwicensis | (+)-ambuic acid | Cisplatin-resistant A2780 cell lines | 3–17 μM | [34] |
| A. alternata MGTMMP031 | Vitex negundo | Alternariol methyl ether | Hepatocellular carcinoma HepG2 | - | [114] |
| Aspergillus terreus | Bruguiera gymnorrhyza | Cowabenzophenone A | Colon cancer cell line | 10 μM | [115] |
| Chaetomium globosum | Couroupita guianensis | Flavipin | A549, colorectal adenocarcinoma cells (HT-29), MCF-7 cancer cell lines | 9–54 µg/mL | [116] |
| Fusarium solani | Camptotheca acuminata | Camptothecin | Vero, prostatic adenocarcinoma cells (PC-3) cells | - | [87] |
| Sir-SM2 | Annona muricata | Hexahydro-3-(2-methylpropyl)-Pyrrolo[1,2-a]pyrazine-1,4-dione | WiDr cell lines | 20 µg/mL | [117] |
| Aspergillus terreus | Achyranthus aspera | Terrein (4,5-Dihydroxy-3-(1-propenyl)-2-cyclopenten-1-one) | A-549 | 121 µg/mL | [118] |
| Xylaria psidii | Aegle marmelos | (−) 5-methylmellein | MCF-7, MIA-Pa-Ca-2, NCI-H226, HepG2, and DU145 | 16–37 μM | [104] |
| Sordariomycetes sp. (PDA)BL5 | Strobilanthes crispus | Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl) | PC-3, HepG2, A-549, HT-29, MCF-7 | 27–161 µg/mL | [119] |
| Pestalotiopsis sp. | Dendrobium officinale | (4S,6S)-6-[(1S,2R)-1,2-dihydroxybutyl]- 4-hydroxy-4-methoxytetrahydro-2H-pyran-2-one, (6S,2E)-6-hydroxy-3-methoxy-5-oxodec-2-enoic acid | HL-60 cell lines | 183 μM | [120] |
| Pseudolagarobasidium acaciicola | Bruguiera gymnorrhiz | Merulin B and C, steperoxide A | HL-60, HepG2 | 0.08–49.08 µg/mL | [121] |
| A. terreus | Codium decorticatum | F8 | HepG2 | 7 µg/mL | [122] |
| C. globosum | Ginkgo biloba | Chaetoglobosin A | Colon cancer cell lines (HCT116) | 3–8 µM | [107] |
| Myrothecium roridum | Ajuga decumbens | Myrotheciumones A | HepG2 | 5 µM | [123] |
| Phomopsis glabrae | Pongamia pinnata | Depsipeptide (PM181110) | BXFT24, CXF 269L (colon), LXFA 629L (lung), PAXF 546L cell lines | 0.04–0.055 µM | [124] |
| Hypocrea lixii | Cajanus cajan | Cajanol | A549 | 20 µg/mL | [125] |
| Penicillium janthinellum Yuan-27 | Panax ginseng | Brefeldin A | MKN45, LOVO, A549, MDA-MB-435, HepG2, HL-60 cell lines | 0.49–7.46 μg/mL | [126] |
| Fomitopsis sp. (MTCC 10177) | Miquelia dentata | Camptothecin | HCT-116, SW-480 | 5–23 μg/mL | [127] |
| Fusarium proliferatum (MTCC 9690) | Dysoxylum binectariferum | Rohitukine | HCT-116, MCF-7 cancer cell lines | 10 μg/mL | [128] |
| Fungal Endophyte | Host Plant | Bioactive Compounds | IC50 or Inhibition (%) | References |
|---|---|---|---|---|
| Penicilliumcitrinum | Digitaria bicornis | DL-carnitine, α-Eleostearic acid, Benzophenone, Sclerotiorin, Cafeic acid, Oleamide, Stearamide | 0.76–55 µg/mL | [38] |
| Penicillium decumbens | - | Sulforhodamine B | - | [146] |
| Aspergillus tubenginses ASH4 | Hyoscyamus muticus | Anofinic acid | - | [134] |
| ZA 163, MO 211, LO 261, FE 082, and FE 084 | Albizia zygia, Millettia thonningii, Alchornea cordifolia, Ficus exasperat | Pyrogallol, Di-alpha-tocopherol, Alpha tocospiro, Linoleic acid, 9-octadecenamide, Lupeol, and 9-octadecenoic acid (Z) | - | [14] |
| Aspergillus nidulans, Aspergillus fumigatus, Aspergillus favus | Ocimum basilicum | 9-Octadecenoic acid (Z)-, Hexadecanoic acid, 1-nonadecene | 68–347 µg/mL | [147] |
| A. fumigatus | Moringa oleifera | Cafeic acid, Rutin, Ellagic acid, Quercetin, Kaempferol | 40 µg/mL | [36] |
| Chaetomium globosum, Aspergillus nidulans | Passifora incarnata | Methoxymethylphenol, Orcinol, Sorbicillin | 0.21–0.324 mg/mL | [148] |
| C. globosum | Moringa oleifera | Catechin, Chlorogenic acid, Cafeic acid, Umbelliferone, Coumaric acid, Kaempferol | 45–50 µg/mL | [36] |
| C. globosum | Conyza blinii | 3-methoxyflavone, Nobiletin, Scopoletin, and Daidzein | 0.01–0.11 mg/mL | [149] |
| Alternaria sp. Samif01 | Salvia miltiorrhiza | 3-epi-dihydroaltenuene A, Altenuisol, 4-hydroxyalternariol-9-methyl ether | 474 μM | [150] |
| Nigrospora sp. | O. basilicum | Nezukol, Z-lanceol, Chavicol, Catalponone | 15 μg/mL | [135] |
| Talaromyces islandicus EN-501 | Laurencia okamurai | 8-hydroxyconiothyrinone B, 8,11-dihydroxyconiothyrinone B | 61 μM | [35] |
| Pseudocercospora sp. ESL 02 | Elaeocarpus sylvestris | Terreic acid and 6-methylsalicylic acid | 30 mg/mL | [151] |
| Epicoccum nigrum | Entada abyssinica | Quinizarin | 11 μg/mL | [152] |
| A. fumigates | Cajanus cajan | Luteolin | 22 μg/mL | [153] |
| Fungal Endophyte | Host Plant | Bioactive Compounds | Inhibition | IC50 or Inhibition (%) | References |
|---|---|---|---|---|---|
| Aspergillus niger | Elaeocarpus floribundus | Asnipyrone B, Hexylitaconic acid, Chlorogenic acid, Nigragillin, Fusarubin | NO, COX-II, IL-1, IL-6, and TNF-α | - | [40] |
| Diaporthe sp. QYM12 | Kandelia candel | Diaporpenoid A, Diaporpyrones A | NO | 12–21 µM | [161] |
| Talaromyces sp. SK-S009 | Kandelia obovata | 6-[1-(acetyloxy) ethyl)] 5-hydroxy-2,7-dimethoxy-1,4-napthalenedione | NO | 1.7 µM | [162] |
| Edenia gomezpompae | Unidentified plant | Preussomerin EG1 | NO | - | [163] |
| Phomopsis sp. SYSUQYP-23 | Kandelia candel | Farinomalein A, B, and H, Phenylahistin | NO | 15–25 μM | [164] |
| Fusarium chlamydosporum | Anvillea garcinii | Chlamydosterol A and B | 5-lipoxygenase | 3.57 μM | [165] |
| Aspergillus sp. | Trichocoleaceae sp. | 6-hydroxy-3-methoxyviridicatin, notoamide B, 3-O-methylviridicatol | NO | 22–50 μM | [166] |
| Fusarium sp. | Mentha longifolia | Fusaristerols B | 5-lipoxygenase | 2–4 μM | [167] |
| A. terreus | Strigamia maritima | Asperimides C and D | NO | 1.26 μM | [168] |
| Ascomycota sp. CYSK-4 | Pluchea indica | Desmethyldichlorodiaportintone | NO | 15.8 µM | [169] |
| Lasiodiplodia theobromae ZJ-HQ1 | Acanthus ilicifolius | Lasiodiplactone A | NO | 23.5 μM | [170] |
| Trichoderma sp. Xy24 | Xylocarpus granatum | Cyclonerodiol B | NO | 75% | [155] |
| A. terreus PR-P-2 | Camellia sinensis | Asperteretal C, butyrolactone I | NO | 16–27 μM | [171] |
| Botryosphaeria sp. SCSIO KcF6 | Kandelia candel | Botryosphaerin-B | COX-2 | 1.12 μM | [155] |
| Periconia sp. | Annonsa muricata | Periconianone A | NO | 0.15–0.38 μM | [172] |
| Dendryphion nanum | Ficus religiosa | Herbarin | Cytokines TNF-α and IL-6 | 0.60 µM | [158] |
| Fungal Endophyte | Host Plant | Bioactive Compounds | Inhibition | IC50 or Inhibition (%) | References |
|---|---|---|---|---|---|
| Aspergillus sp. | Sonneratia apetala | Aspergiamides A, F | α-glucosidase | 40–83 µM | [173] |
| Penicillium canescens | Juniperus polycarpos | Methylxanthone | α-glucosidase | 32 μM | [184] |
| Xylariaceae sp. QGS01 | Querus gilva | 8-Hydroxy-6,7-dimethoxy-3-methylisocoumarine | α-glucosidase | 41.75 μg/mL | [42] |
| Nigrospora oryzae | Combretum dolichopetalum | S (+)-2 cis-4-trans-abscisic acid, 7-hydroxy-abscisic acid, 4-des-hydroxy altersolanol A | Diabetic- induced mice | 30–46% | [173] |
| Epicoccum sp. HS-1 | Apostichopus japonicus | Isopimarane diterpene | α-glucosidase | 4.6–11.9 μM | [183] |
| Aspergillus awamori | Acacia nilotica | Peptides | α-amylase, α-glucosidase | 3–6 μg/mL | [176] |
| Chaetomiaceae sp. MEXU 27095 | Hintonia latiflora | Thielavins A, J, and K | α-glucosidase | 15–23 μM | [174] |
| Xylaria sp. | - | Eremophilane sesquiterpenes | α-glucosidase | 6.54 μM | [181] |
| Fungal Endophyte | Host Plant | Bioactive Compounds | Inhibition | Percentage Inhibition or IC50 | References |
|---|---|---|---|---|---|
| Albifmbria viridis | Coptis chinensis | Albifpyrrol B | LPS (B cells) | 16.16 μM | [47] |
| Ilyonectria robusta | Bletilla striata | Robustaditerpene C and E | Concanavalin (Con) A (T cells) and LPS (B cells) | 17–75 μM | [194] |
| Aspergillus sp. | Tripterygium wilfordii | Pseurotin | anti-CD3/anti-CD28 mAbs | 8–9 μM | [46] |
| Daldinia sp. TJ403-LS1 | Anoectochilus roxburghii | Daldiniol A | LPS and antiCD3/anti-CD28 mAbs | 0.06 μM | [195] |
| Aspergillus fumigatus | Cynodon dactylon | Bisdethiobis (methylthio) Gliotoxin, Fumitremorgin C, 3-(hydroxyacetyl) indole | Con A (T cells) and LPS (B cells) | 1.08–97 μM | [45] |
| Fusarium sp. and Cladosporium sp. | Psidium guajava and Newbouldia laevis | Citrinin, Nidulalin, p-hydroxybenzoic acid, Cyclopenin | - | - | [188] |
| Pestalotiopsis sp. HHL-14 | Rhizophora stylosa | Phomoxydiene C, Z-isomer, mycoepoxydiene | Con A (T cells) and LPS (B cells) | 33–97 μM | [196] |
| A. fumigatus HQD24 | Rhizophora mucronata | 16-O-deacetylhelvolic acid 21,16-lactone | Con A (T cells) and LPS (B cells) | 12–62 μM | [197] |
| Mycospaerella nawae ZJLQ129 | Smilax china | (−) mycousnine | Antigens CD25 and CD69 | - | [189] |
| Penicillium sp. ZJ-SY2 | Sonneratia apetala | Peniphenone, Conioxanthone A, Pinselin, Sydowinin A | Con A (T cells) and LPS (B cells) | 6–9 µg/mL | [190] |
| Fusarium subglutinans | Tripterygium wilfordii | Subglutinol A and B | Proinflammatory IFNγ and IL-17 | - | [191] |
| Phomopsis sp. S12 | Illigera rhodantha | Libertellenone J | LPS | 3–15 μM | [192] |
| Aspergillus sydowii | Scapania ciliata | Emodin | Con A- and LPS | 8–10 µg/ml | [44] |
| Fungal Endophyte | Host Plant | Bioactive Compounds | Tested Pathogen(s) | IC50 or Inhibition (%) | References |
|---|---|---|---|---|---|
| Antibacterial compounds | |||||
| Aspergillus fumigatus | Ceriops decandra | Fumigaclavine C, Azaspirofuran B, Fraxetin | Staphylococcus aureus, Micrococcus luteus, Escherichia coli, Pseudomonas aeruginosa | 0.078–5 mg/mL | [204] |
| Emericella sp. | Panax notoginseng | Quiannulatic acid | Multidrug-resistant (MDR) Enterococcus faecium | 12.5 µg/mL | [206] |
| Aspergillus niger | Opuntia ficus-indica | Cristatumin B, Dihydroauroglaucin | MDR S. aureus, E. faecalis, Klebsiella. pneumonia, P. aeruginosa | 2–125 µg/mL | [205] |
| Penicillium citrinum | Digitaria bicornis | Ciprofloxacin | S. aureus, Salmonella typhi, E. faecalis, E. coli | 9–20% | [203] |
| Curvularia papendorfii | Vernonia amygdalina | Polyhydroxyacid, Kheiric acid | Methicillin resistant S. aureus (MRSA) | 62.5 µg/mL | [208] |
| Aspergillus cejpii | Nelumbo nucifera | 5-(1H-Indol-3-yl)-4,5-dihydro-[1,2,4]triazin-3-ylamine | MRSA | - | [209] |
| Diaporthe sp. | Pteroceltis tatarinowii | Diaporone A | Bacillus subtilis | 66.7 μM | [202] |
| Curvularia lunata | Paepalanthus chiquitensis | Curvulinic acid | E. coli | 62.5 μg/mL | [210] |
| Alternaria alternata AE1 | Azadirachta indica | Phenanthrene, 7-isopropyl-1-methyl (Retene), Dichloronitromethane | B. subtilis, Listeria monocytogenes, S. aureus, E. coli, Salmonella typhimurium | - | [136] |
| Fusarium sambucinum TE-6L | Nicotiana tabacum | Amoenamide C, Sclerotiamide B | E. coli, M. luteus, P. aeruginosa | 4–8 μg/mL | [211] |
| Curvularia sp. T12 | Rauwolfia macrophylla | 2′-deoxyribolactone, Hexylitaconic acid, Ergosterol | Pseudomonas agarici, E. coli, Staphylococcus warneri, M. luteus | - | [212] |
| Arthrinium sp. MFLUCC16-1053 | Zingiber cassumunar | 3-p-menthone, Bornyl acetate, γ-curcumene, Bicyclogermacrene, and β-isocomene | S. aureus, E. coli | 7–31 µg/mL | [213] |
| Aspergillus clavatonanicus MJ31 | Mirabilis jalapa | 6-PP (6 Pentyl-2H Pyrone-2-one), 1, 2 butadiene, m-camphorene, 3-Thietanol, Thiopivalic acid, Pthalic acid, Heneicosane, Pyrazol, and benzene derivatives | E. coli, S. aureus, M. luteus, B. subtilis | 0.078–0.62 mg/mL | [201] |
| Colletotrichum sp. BS4 | Buxus sinica | Colletotrichone A, B, C and Chermesinone B | S. aureus, E. coli, B. subtilis, P. aeruginosa | >10 µg/mL | [214] |
| Lasiodiplodia theobromae ZJ-HQ1 | Acanthus ilicifolius | Chloropreussomerin A and B, Preussomerin M | S. aureus, B. subtilis, E. coli, P. aeruginosa, Salmonella enteritidis | 1–13 μg/mL | [215] |
| Epicoccum nigrum | Entada abyssinica | Quinizarin, indole-3-carboxylic acid, and Parahydroxybenzaldehyde | B. cereus, S. typhimurium | 3–6.25 μg/mL | [152] |
| Emericella qaudrilineata (RS-5) | Pteris pellucid | Benzyl benzoate, Benzaldehyde dimethyl acetal, and Benzoic acid | S. aureus, Aeromonas hydrophilla | - | [216] |
| Penicillium brocae MA-231 | Avicennia marina | Penicibrocazine A–E | S. aureus, M. luteus, Gaeumannomyces graminis | 0.25–64 μg/mL | [217] |
| Fusarium sp. | Opuntia dillenii | Equisetin | B. subtilis, MRSA | 8–16 μg/mL | [218] |
| Microsphaeropsis sp., Seimatosporium sp. | Salsola oppositifolia | Microsphaerol, Seimatorone | E. coli, Bacillus megaterium | 8–9 mg/mL | [219] |
| Colletotrichum sp. | Umbelliferae | Colletonoic acid | B. megaterium | 8 mg/mL | [220] |
| Eupenicillium sp. LG41 | Xanthium sibiricum | Eupenicinicols A and B, (2S)-butylitaconic acid, (2S)-hexylitaconic acid | B. subtilis, S. aureus, E. coli, Acinetobacter sp. | 1- > 10.0 μg/mL | [221] |
| Botryosphaeria dothidea X-4 | Camptotheca acuminata | 9-methoxycamptothecin | B. subtilis, E. coli, | 47.6% | [222] |
| Phoma sp. PG23 | Taraxacum mongolicum | 2-hydroxy-6-methylbenzoic acid | E. coli, S. aureus, A. hydrophila, Edwardsiella tarda, Pasteurella multocida | - | [223] |
| Gliomastix murorum Ppf8 | Paris polyphylla | Ergosta-5,7,22-trien-3-ol, 2,3-dihydro-5-hydroxy-α,α-dimethyl-2-benzofuranmethanol | E. coli, Pseudomonas lachrymans, B. subtilis, Staphylococcus haemolyticus | 65–146 µg/mL | [224] |
| Penicillium chrysogenum | Porteresia coarctata | Dipodazine D | Vibrio cholerae | - | [225] |
| Diaporthe phaseolorum | Laguncularia racemosa | 3-hydroxypropionic acid | S. aureus, S. typhi | 64 µg/mL | [226] |
| Aspergillus sp. EJC08 | Bauhinia guianensis | Funigaclavine C, Pseurotin A | B. subtilis, E. coli, P. aeruginosa, S. aureus | 7–31 µg/mL | [227] |
| Hyalodendriella sp. Ponipodef12 | Populus deltoides | Palmariol B, 4-hydroxymellein, Alternariol 9-methyl ether | B. subtilis, P. lachrymans | 16–19 µg/mL | [228] |
| Phomopsis longicolla | Bostrychia radicans | Dicerandrol C | S. aureus | 1.33 µg/mL | [229] |
| Pestalotiopsis mangiferae | Mangifera indica | 4-(2,4,7-trioxa-bicyclo[4.1.0]heptan-3-yl) | B. subtilis, K. pneumoniae, E. coli, M. luteus, P. aeruginosa | - | [230] |
| Pestalotiopsis sp. | Arbutus unedo | Pestalotheol G, Anofinic acid | E. coli, B. megaterium | 7–12 µg/mL | [231] |
| Phomopsis sp. | Cistus monspeliensis | Phomochromone A-B, Phomotenone, (1S,2S,4S)-trihydroxy-p-menthane | E. coli, B. megaterium | 6–8 µg/mL | [232] |
| Phomopsis sp. | Allamanda cathartica | Terpene | S. aureus, B. subtilis, S. typhi | - | [233] |
| Antifungal compounds | |||||
| Curvularia protuberate | Paspalidium favidum | Diphenyl sulfone, 7-Hydroxycoumarine, Griseofulvin, β-Asarone | Alternaria alternata, Fusarium oxysporum | 31–62 µg/mL | [203] |
| Cladosporium cladosporioides | Zygophyllum mandavillei | 3-phenylpropionic acid | Aspergillus flavus and Fusaroum solani | 3.90–15.62 mg/mL | [234] |
| Aplosporella javeedii, | Orychophragmus violaceus | Aplojaveediins A–F | Candida albicans ATCC 24433 | - | [235] |
| Alternaria tenuissima OE7 | Ocimum tenuiflorum | 1,2-Pentanediol | C. albican | 100–500 µg/mL | [136] |
| P. citrinum | Stephania kwangsiensis | Citrinin, Emodin | Alternaria citri | 3 μg/mL | [236] |
| F. oxysporum KU527806 | Dendrobim lindley | Gibepy- rone A, Pyrrolo[1,2-a] pyrazine-1, 4-dione, hexahydro-3-(2-methylpropyl), and in- doleacetic acid | C. albicans, C. tropicalis, Curvularia sp., f. sp. | - | [237] |
| Cladosporium delicatulum | Terminalia pallida, Rhychosia beddomei, Pterocarpu santalinus | Plumbagin (5-hydroxyl- 2- methylnaptalene-1,4-dione) | C. albicans, C. tropicalis, F. moniliforme | 6–12 mg/mL | [238] |
| Aspergillus flavus | Lannea coromandelica | Kojic acid, Octadecanoic acid, Diethyl phylate, 3-Phenyl propionic acid | C. ablicans, Malassezia pachydermis | - | [239] |
| Mycosphaerella sp. UFMGCB 2032 | Eugenia bimarginata | (2S,3R,4R)-(E)-2-amino-3,4-Antifungal dihydroxy-2-(hydroxymethyl)-14-oxoeicos6,12-dienoic acid, and Myriocin | Cryptococcus neoformans, Cryptococcus gattii | 7–31 µg/mL | [240] |
| Lophodermium nitens DAOM 250027 | Pinus strobus | (7R)-(-)-methoxysydonol and its derivatives, (7R,7′R)-(-)-pyrenophorin | Saccharomyces cerevisiae | 5 µM | [199] |
| Phialophora mustea | Crocus sativus | Phialomustin | C. albicans | 73.6 µM | [241] |
| Trichothecium sp. | Phyllanthus amarus | Trichothecinol-A | Cryptococcus albidus | 25 µg/mL | [242] |
| Lophodermium sp. | Pinus strobus | Methyl (2Z,4E)-6(acetyloxy)-5-formyl-7-oxoocta-2,4-dienoate, 5-(hydroxymethyl)-2-(20, 60, 60-trimethyltetrahydro-2H-pyran-2- yl)phenol, pyrenophorol | S. cerevisae | 2 μM | [243] |
| Massrison sp. | Rehmannia glutinosa | Massarigenin D, Spiromassaritone, Paecilospirone | C. albicans, C. neoformans, T. rubrum, A. fumigatus | 1–4 μg/mL | [244] |
| Antiviral compounds | |||||
| A. terreus | Glycine max | Aspulvinone E | HIV | - | [245] |
| Phoma sp.YE3135 | Aconitum vilmorinianum | Phomanolide | H1N1 | 2–20 μg/mL | [246] |
| Pleospora tarda | Ephedra aphylla | Alternariol and Alternariol-(9)-methyl ether | HSV | 15–40% | [145] |
| Hypoxylon sp. 6269 | Artemisia annua | Integracide E and Isointegracide E | HIV | 31–100 μM | [247] |
| Aspergillus sp. CPCC 400735 | Kadsura longipedunculata | Asperphenalenone A and D | HIV | 2–9 μM | [248] |
| Nigrospora sp. YE3033 | Aconitum carmichaeli | 6-O-demethyl-4-dehydroxyaltersolanol A, 4-dehydroxyaltersolanol A, Altersolanol B, Cermesinone B | H1N1 | 0.80–8 µg/mL | [249] |
| Pestalotiopsis thea | Fagara zanthoxyloides | Chloroisosulochrin ficipyrone A, and Pestheic acid | RSV | 0.57–2 µg/mL | [250] |
| Periconia sp. F-31 | Annona muricata | Pericoannosin A, Periconiasins F | HIV | 67 μM | [75] |
| Alternaria sp. | Calophyllum inophyllum | Coumarin | HIV | - | [251] |
| Chaetomium globosum TW1-1 | Armadillidium vulgare | Armochaetoglobins K–R | HIV | 0 0.11–0.55 μM | [252] |
| Cercosporella sp. | Schisandra chinensis | Ergosterol Peroxide, Ergosterol, β-Sitosterol, Stigmasterol | HIV | - | [253] |
| A. tenuissima QUE1Se | Quercus emoryi | Altertoxins V, Altertoxins I-III | HIV | 0.5–2 μg/mL | [254] |
| A. tenuissima | Quercus emoryi | DK, DL, DM, and DP | HIV | 1.5 μg/mL | [255] |
| Emericella sp. (HK-ZJ) | Aegiceras corniculatum | Emerimidine A–B and Emeriphenolicins A–D | H1N1 | 50% | [256] |
| Fungal Endophyte | Host Plant | Bioactive Compounds | Inhibition | IC50 or Inhibition (%) | References |
|---|---|---|---|---|---|
| Nigrospora oryzae CF-298113 | Triticum sp. | Pipecolisporin | Plasmodium falciparum, Trypanosoma cruzi | 3.21 µM | [260] |
| Nemania sp. UM10M | Torreya taxifolia | 19,20-epoxycytochalasins C | P. falciparum, P. berghei | 0.05 µM | [263] |
| Fusarium sp., Lasiodiplodia theobromae | Avicennia lanata | Anhydrofusarubin, Javanicin, Dihydrojavanicin, Solaniol, (-)-mellein | Trypanosoma brucei brucei | 0.047–0.276 µg/mL | [269] |
| Aspergillus flocculus | Markhamia platycalyx | Ergosterol, Ergosterol peroxide | T. brucei brucei | 7.3–31.6 μM | [265] |
| Bipolaris sp. C36, Bipolaris sp. AZ26 | Deschampsia antarctica, Colobanthus quitensis | Curvulin, Spirostaphylotrichin R, U | Leishmania amazonensis | 70–84 µg/mL | [270] |
| Fusarium sp. | Mentha longifolia | Fusaripeptide A | P. falciparum | 0.34 μM | [271] |
| Diaporthe miriciae | Vellozia gigantea | Epoxicitocalasin H | P. falciparum chloroquine-sensitive and resistant strains | 39–51 µg/mL | [272] |
| E. nigrum | - | Epicoccamide | Leishmania sp. | - | [268] |
| Trichosporum sp. | Trigonella foenum-graecum | (6-S)-3-(1,3-dihydroxypropyl)-6-(2-methylpropyl)piperazine-2,5-dione (6-R)-3-(1,3-dihydroxypropyl)-6-(2methylpropyl)piperazine-2,5-dione | Leishmania donovani | 82–96 µg/mL | [273] |
| Fusarium sp. WC 9 | Caesalpinia echinata | Beauvericin | T. cruzi | 1.9 μg/mL | [274] |
| A. terreus | Carthamus lanatus | (22E,24R)-stigmasta-5,7,22-trien-3-β-ol, stigmast-4-ene-3-one, terrenolide S | L. donovani | 15–27 µM | [275] |
| Diaporthe sp. CY-5188 | Kandelia obovate, Avicennia marina | Dicerandrol D | P. falciparum | - | [276] |
| Penicillium sp. BCC16054 | Grass | Penicolinates A–C | P. falciparum | 3.07–3.25 µg/mL | [277] |
| BB1-BB5, BD4-BD6 | Tinaspora crispa | 2,5-dihydroxy-1-(hydroxymethyl)pyridin4-on | P. falciparum | 0.129 µM | [278] |
| Mycosphaerella sp. F2140, | Psychotria horizontalis | Cercosporin | P. falciparum, L. donovani, T. cruzi | 0.46–1.08 µM | [279] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, A.; Meshram, V.; Gupta, M.; Goyal, S.; Qureshi, K.A.; Jaremko, M.; Shukla, K.K. Fungal Endophytes: Microfactories of Novel Bioactive Compounds with Therapeutic Interventions; A Comprehensive Review on the Biotechnological Developments in the Field of Fungal Endophytic Biology over the Last Decade. Biomolecules 2023, 13, 1038. https://doi.org/10.3390/biom13071038
Gupta A, Meshram V, Gupta M, Goyal S, Qureshi KA, Jaremko M, Shukla KK. Fungal Endophytes: Microfactories of Novel Bioactive Compounds with Therapeutic Interventions; A Comprehensive Review on the Biotechnological Developments in the Field of Fungal Endophytic Biology over the Last Decade. Biomolecules. 2023; 13(7):1038. https://doi.org/10.3390/biom13071038
Chicago/Turabian StyleGupta, Aditi, Vineet Meshram, Mahiti Gupta, Soniya Goyal, Kamal Ahmad Qureshi, Mariusz Jaremko, and Kamlesh Kumar Shukla. 2023. "Fungal Endophytes: Microfactories of Novel Bioactive Compounds with Therapeutic Interventions; A Comprehensive Review on the Biotechnological Developments in the Field of Fungal Endophytic Biology over the Last Decade" Biomolecules 13, no. 7: 1038. https://doi.org/10.3390/biom13071038
APA StyleGupta, A., Meshram, V., Gupta, M., Goyal, S., Qureshi, K. A., Jaremko, M., & Shukla, K. K. (2023). Fungal Endophytes: Microfactories of Novel Bioactive Compounds with Therapeutic Interventions; A Comprehensive Review on the Biotechnological Developments in the Field of Fungal Endophytic Biology over the Last Decade. Biomolecules, 13(7), 1038. https://doi.org/10.3390/biom13071038








