Targeted Disruption of the MORG1 Gene in Mice Causes Embryonic Resorption in Early Phase of Development
Abstract
1. Introduction
2. Materials and Methods
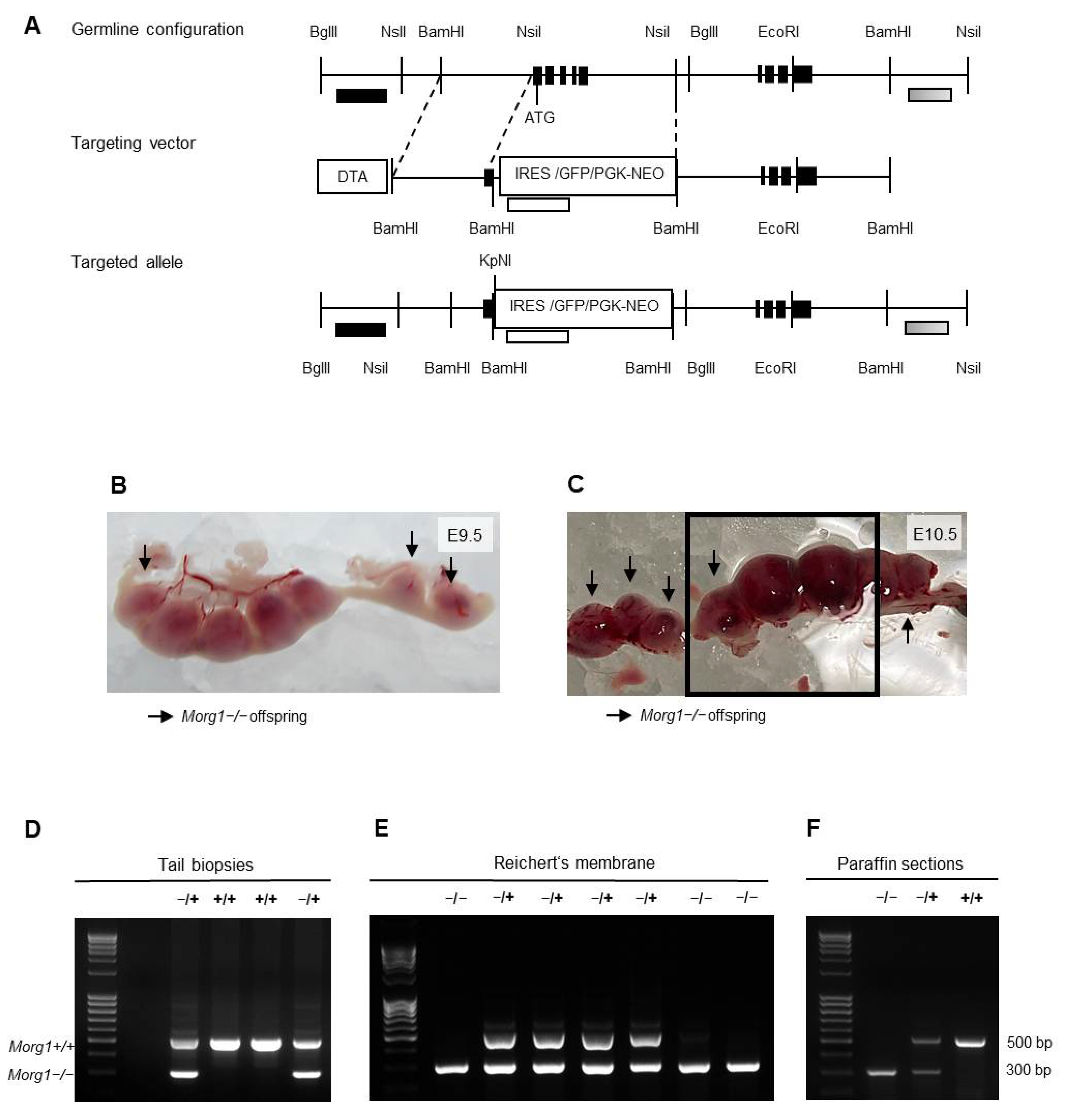
2.1. Construction of the Targeting Vector
2.2. MRI
2.3. Genotyping
2.4. Preparation, Fixation, Embedding, Sectioning
2.5. Immunohistochemistry, Immunofluorescence
2.6. Hematoxylin and Eosin Staining (H&E)
2.7. Microscopy
2.8. Multiplex Immunofluorescence Labeling (mIF) and Multispectral Imaging
2.9. Whole Mount Staining
2.10. Statement about Sample Size Estimate, Blinding and Statistics
3. Results and Discussion
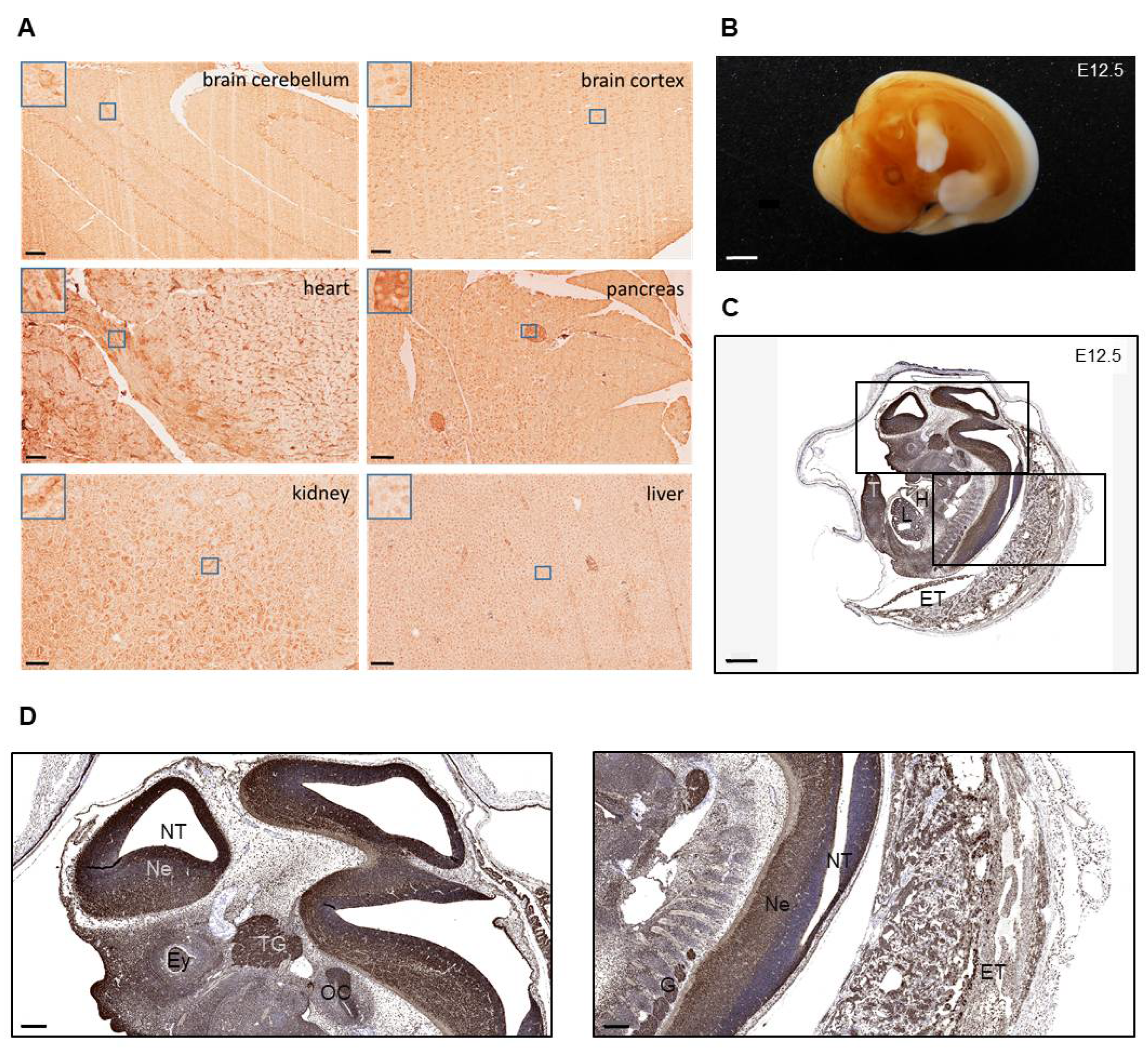
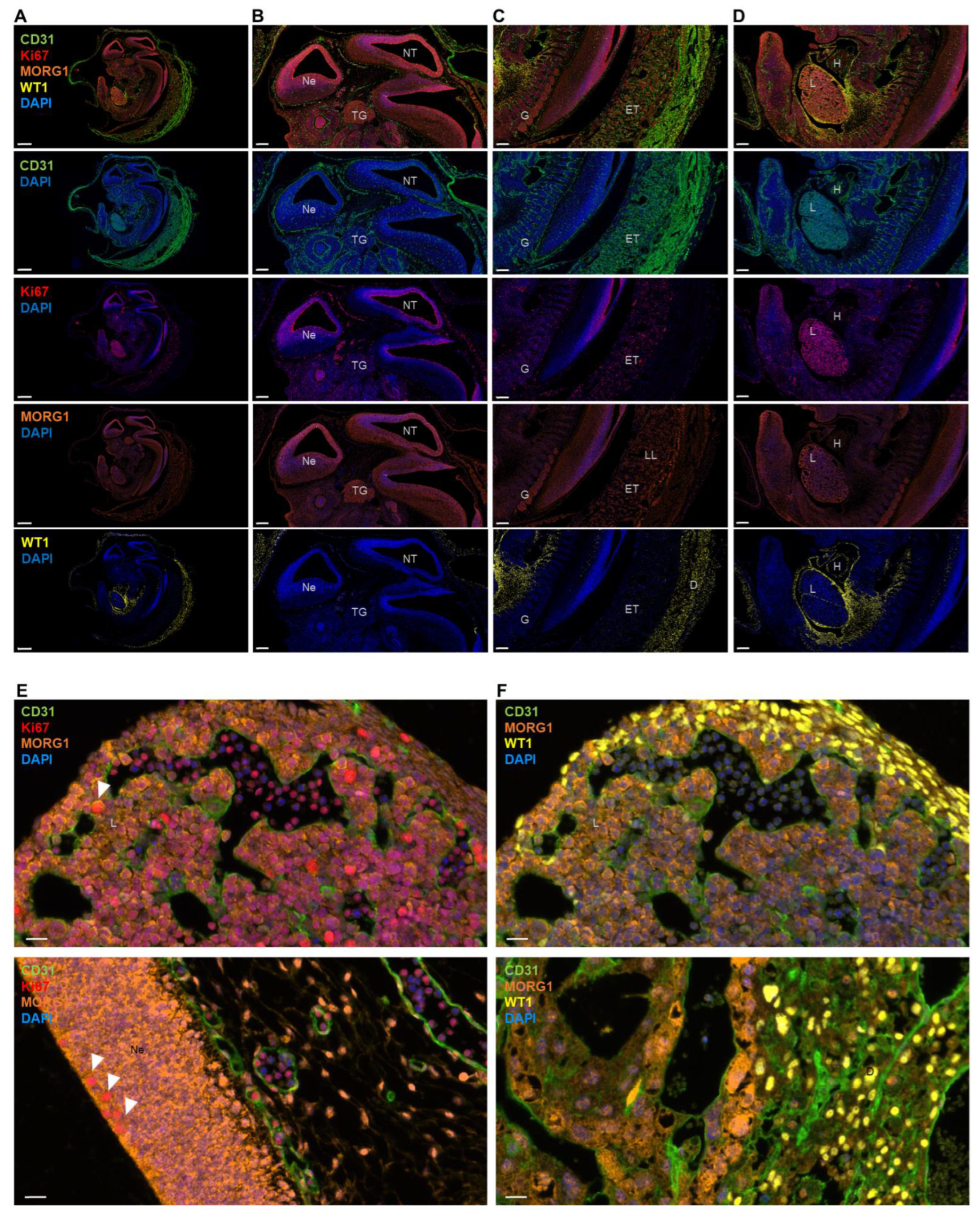
3.1. Spatial Expression Pattern of MORG1 Protein in Organs of Adult and Embryonic Mice
3.2. Generation of Morg1 Knockout Mice and Characterization of Offspring
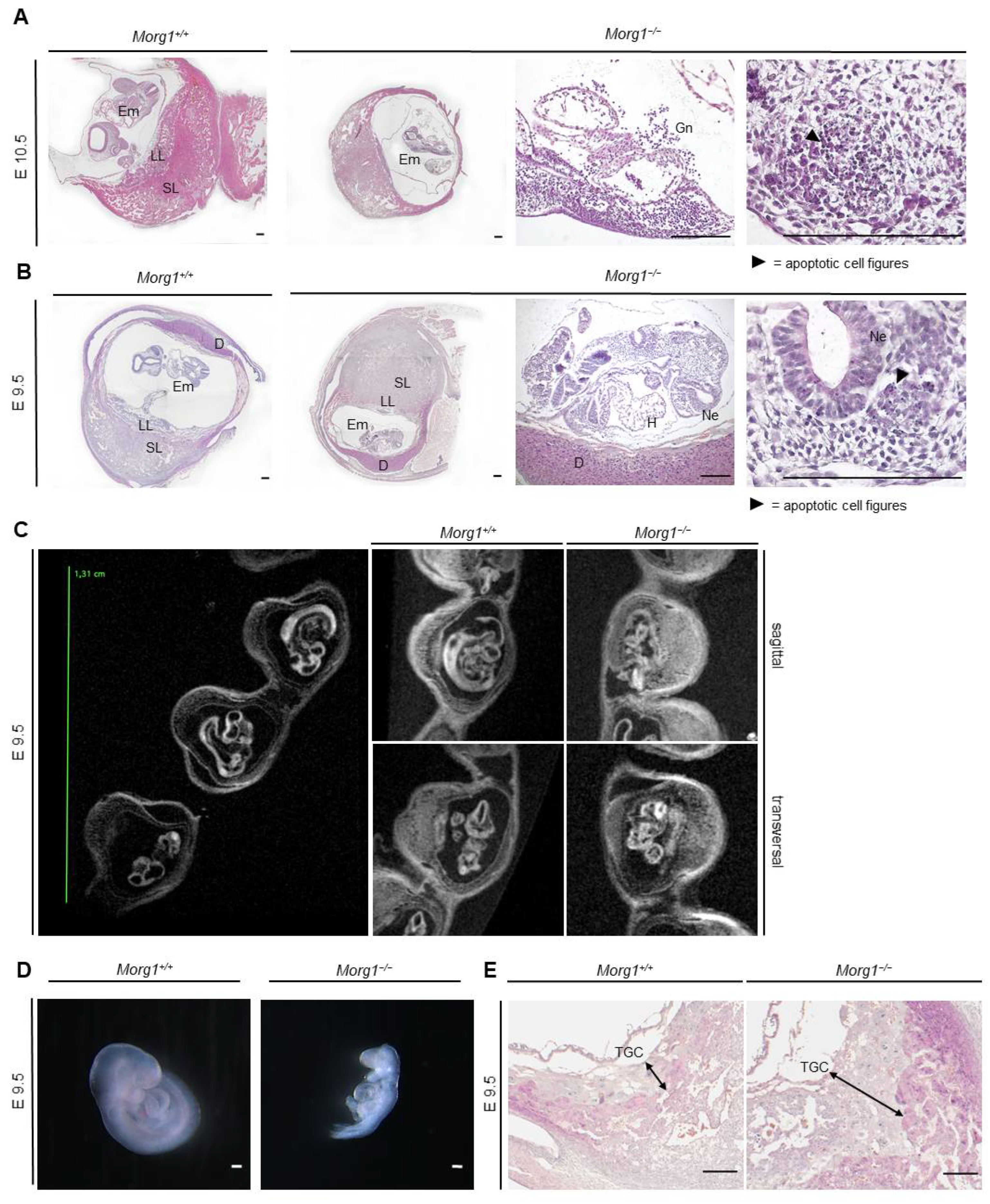
3.3. Homozygous Morg1 Knockout in Mice Leads to Embryonic Lethality
3.4. Resorption of Embryonic Tissue on E12.5 and E11.5 as Well as Apoptosis and Decomposition of Embryos on E10.5 and E9.5 in the Morg1−/− Genotype
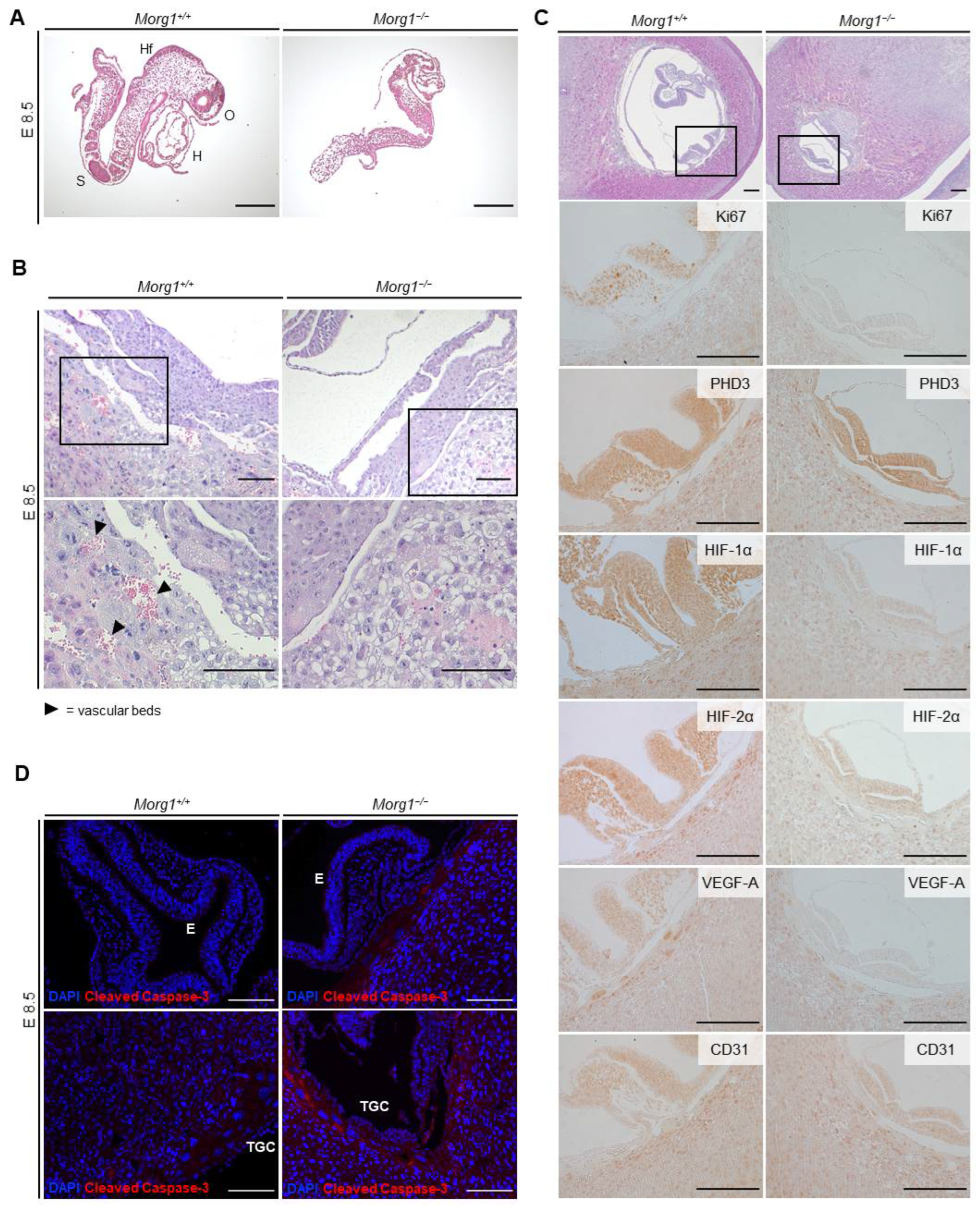
3.5. Growth Retardation and Maldevelopment of Morg1−/− Embryos on E9.5 and E8.5
3.6. Severe Placental Abnormalities and Delayed Neural Tube Closure in Morg1−/− Embryos
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, C.; Min, J. Structure and function of WD40 domain proteins. Protein Cell 2011, 2, 202–214. [Google Scholar] [CrossRef]
- Vomastek, T.; Schaeffer, H.J.; Tarcsafalvi, A.; Smolkin, M.E.; Bissonette, E.A.; Weber, M.J. Modular construction of a signaling scaffold: MORG1 interacts with components of the ERK cascade and links ERK signaling to specific agonists. Proc. Natl. Acad. Sci. USA 2004, 101, 6981–6986. [Google Scholar] [CrossRef]
- Teis, D.; Taub, N.; Kurzbauer, R.; Hilber, D.; de Araujo, M.E.; Erlacher, M.; Offterdinger, M.; Villunger, A.; Geley, S.; Bohn, G.; et al. p14-MP1-MEK1 signaling regulates endosomal traffic and cellular proliferation during tissue homeostasis. J. Cell Biol. 2006, 175, 861–868. [Google Scholar] [CrossRef]
- Albadari, N.; Deng, S.; Li, W. The transcriptional factors HIF-1 and HIF-2 and their novel inhibitors in cancer therapy. Expert Opin. Drug Discov. 2019, 14, 667–682. [Google Scholar] [CrossRef]
- Lee, J.W.; Ko, J.; Ju, C.; Eltzschig, H.K. Hypoxia signaling in human diseases and therapeutic targets. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Loeffler, I.; Wolf, G. Morg1 heterozygous deficiency ameliorates hypoxia-induced acute renal injury. Am. J. Physiol. Renal. Physiol. 2015, 308, F511–F521. [Google Scholar] [CrossRef]
- Hopfer, U.; Hopfer, H.; Jablonski, K.; Stahl, R.A.; Wolf, G. The novel WD-repeat protein Morg1 acts as a molecular scaffold for hypoxia-inducible factor prolyl hydroxylase 3 (PHD3). J. Biol. Chem. 2006, 281, 8645–8655. [Google Scholar] [CrossRef]
- Hayase, J.; Kamakura, S.; Iwakiri, Y.; Yamaguchi, Y.; Izaki, T.; Ito, T.; Sumimoto, H. The WD40 protein Morg1 facilitates Par6-aPKC binding to Crb3 for apical identity in epithelial cells. J. Cell Biol. 2013, 200, 635–650. [Google Scholar] [CrossRef]
- Rappel, W.J.; Edelstein-Keshet, L. Mechanisms of Cell Polarization. Curr. Opin. Syst. Biol. 2017, 3, 43–53. [Google Scholar] [CrossRef]
- Loeffler, I.; Wolf, G. MORG1 (Mitogen-Activated Protein Kinase Organizer 1). In Encyclopedia of Signaling Molecules; Choi, S., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; p. 6060. [Google Scholar]
- Hammerschmidt, E.; Loeffler, I.; Wolf, G. Morg1 heterozygous mice are protected from acute renal ischemia-reperfusion injury. Am. J. Physiol. Renal. Physiol. 2009, 297, F1273–F1287. [Google Scholar] [CrossRef]
- Loeffler, I.; Liebisch, M.; Daniel, C.; Amann, K.; Wolf, G. Heterozygosity of mitogen-activated protein kinase organizer 1 ameliorates diabetic nephropathy and suppresses epithelial-to-mesenchymal transition-like changes in db/db mice. Nephrol. Dial. Transplant. 2017, 32, 2017–2034. [Google Scholar] [CrossRef]
- Jankowski, E.; Wulf, S.; Ziller, N.; Wolf, G.; Loeffler, I. MORG1-A Negative Modulator of Renal Lipid Metabolism in Murine Diabetes. Biomedicines 2021, 10, 30. [Google Scholar] [CrossRef]
- Herrmann, K.H.; Hung, L.Y.; Reichenbach, J.R. Fast 3D Isotropic High-Resolution MRI of Mouse Brain Using a Variable Flip Angle RARE Sequence with T2 Compensation @9. 4T. In Proceedings of the 30th Annual Meeting ISMRM, London, UK, 7–12 May 2022. [Google Scholar]
- Sun, X.; Kaufman, P.D. Ki-67: More than a proliferation marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef]
- Wilm, B.; Muñoz-Chapuli, R. The Role of WT1 in Embryonic Development and Normal Organ Homeostasis. Methods Mol. Biol. 2016, 1467, 23–39. [Google Scholar] [CrossRef]
- Capecchi, M.R. Altering the genome by homologous recombination. Science 1989, 244, 1288–1292. [Google Scholar] [CrossRef]
- Friedel, R.H.; Wurst, W.; Wefers, B.; Kuhn, R. Generating conditional knockout mice. Methods Mol. Biol. 2011, 693, 205–231. [Google Scholar] [CrossRef]
- Perez-Garcia, V.; Fineberg, E.; Wilson, R.; Murray, A.; Mazzeo, C.I.; Tudor, C.; Sienerth, A.; White, J.K.; Tuck, E.; Ryder, E.J.; et al. Placentation defects are highly prevalent in embryonic lethal mouse mutants. Nature 2018, 555, 463–468. [Google Scholar] [CrossRef]
- Papaioannou, V.E.; Behringer, R.R. Early embryonic lethality in genetically engineered mice: Diagnosis and phenotypic analysis. Vet. Pathol. 2012, 49, 64–70. [Google Scholar] [CrossRef]
- Stumpo, D.J.; Byrd, N.A.; Phillips, R.S.; Ghosh, S.; Maronpot, R.R.; Castranio, T.; Meyers, E.N.; Mishina, Y.; Blackshear, P.J. Chorioallantoic fusion defects and embryonic lethality resulting from disruption of Zfp36L1, a gene encoding a CCCH tandem zinc finger protein of the Tristetraprolin family. Mol. Cell Biol. 2004, 24, 6445–6455. [Google Scholar] [CrossRef]
- Morin-Kensicki, E.M.; Boone, B.N.; Howell, M.; Stonebraker, J.R.; Teed, J.; Alb, J.G.; Magnuson, T.R.; O’Neal, W.; Milgram, S.L. Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Mol. Cell Biol. 2006, 26, 77–87. [Google Scholar] [CrossRef]
- Rada, C.C.; Murray, G.; England, S.K. The SK3 channel promotes placental vascularization by enhancing secretion of angiogenic factors. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E935–E943. [Google Scholar] [CrossRef]
- Hemberger, M.; Hanna, C.W.; Dean, W. Mechanisms of early placental development in mouse and humans. Nat. Rev. Genet. 2020, 21, 27–43. [Google Scholar] [CrossRef]
- Eom, D.S.; Amarnath, S.; Agarwala, S. Apicobasal polarity and neural tube closure. Dev. Growth Differ. 2013, 55, 164–172. [Google Scholar] [CrossRef]
- Hohenstein, P.; Kielman, M.F.; Breukel, C.; Bennett, L.M.; Wiseman, R.; Krimpenfort, P.; Cornelisse, C.; van Ommen, G.J.; Devilee, P.; Fodde, R. A targeted mouse Brca1 mutation removing the last BRCT repeat results in apoptosis and embryonic lethality at the headfold stage. Oncogene 2001, 20, 2544–2550. [Google Scholar] [CrossRef]
- Chu, C.T.; Levinthal, D.J.; Kulich, S.M.; Chalovich, E.M.; DeFranco, D.B. Oxidative neuronal injury. The dark side of ERK1/2. Eur. J. Biochem. 2004, 271, 2060–2066. [Google Scholar] [CrossRef]
- Hofer, T.; Desbaillets, I.; Hopfl, G.; Gassmann, M.; Wenger, R.H. Dissecting hypoxia-dependent and hypoxia-independent steps in the HIF-1alpha activation cascade: Implications for HIF-1alpha gene therapy. FASEB J. 2001, 15, 2715–2717. [Google Scholar] [CrossRef]
- Ayala, R.; Shu, T.; Tsai, L.H. Trekking across the brain: The journey of neuronal migration. Cell 2007, 128, 29–43. [Google Scholar] [CrossRef]
- Scholzke, M.N.; Schwaninger, M. Transcriptional regulation of neurogenesis: Potential mechanisms in cerebral ischemia. J. Mol. Med. 2007, 85, 577–588. [Google Scholar] [CrossRef]
- Saba-El-Leil, M.K.; Vella, F.D.; Vernay, B.; Voisin, L.; Chen, L.; Labrecque, N.; Ang, S.L.; Meloche, S. An essential function of the mitogen-activated protein kinase Erk2 in mouse trophoblast development. EMBO Rep. 2003, 4, 964–968. [Google Scholar] [CrossRef]
- Giroux, S.; Tremblay, M.; Bernard, D.; Cardin-Girard, J.F.; Aubry, S.; Larouche, L.; Rousseau, S.; Huot, J.; Landry, J.; Jeannotte, L.; et al. Embryonic death of Mek1-deficient mice reveals a role for this kinase in angiogenesis in the labyrinthine region of the placenta. Curr. Biol. 1999, 9, 369–372. [Google Scholar] [CrossRef]
- Adelman, D.M.; Gertsenstein, M.; Nagy, A.; Simon, M.C.; Maltepe, E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 2000, 14, 3191–3203. [Google Scholar] [CrossRef]
- Iyer, N.V.; Kotch, L.E.; Agani, F.; Leung, S.W.; Laughner, E.; Wenger, R.H.; Gassmann, M.; Gearhart, J.D.; Lawler, A.M.; Yu, A.Y.; et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998, 12, 149–162. [Google Scholar] [CrossRef]
- Rojas, D.A.; Perez-Munizaga, D.A.; Centanin, L.; Antonelli, M.; Wappner, P.; Allende, M.L.; Reyes, A.E. Cloning of hif-1alpha and hif-2alpha and mRNA expression pattern during development in zebrafish. Gene Expr. Patterns 2007, 7, 339–345. [Google Scholar] [CrossRef]
- Cowden Dahl, K.D.; Fryer, B.H.; Mack, F.A.; Compernolle, V.; Maltepe, E.; Adelman, D.M.; Carmeliet, P.; Simon, M.C. Hypoxia-inducible factors 1alpha and 2alpha regulate trophoblast differentiation. Mol. Cell Biol. 2005, 25, 10479–10491. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef]
- Holmes, D.I.; Zachary, I. The vascular endothelial growth factor (VEGF) family: Angiogenic factors in health and disease. Genome Biol. 2005, 6, 209. [Google Scholar] [CrossRef]
- Jiang, L.; Lin, L.; Li, R.; Yuan, C.; Xu, M.; Huang, J.H.; Huang, M. Dimer conformation of soluble PECAM-1, an endothelial marker. Int. J. Biochem. Cell Biol. 2016, 77, 102–108. [Google Scholar] [CrossRef]
- Ali-Khan, S.E.; Hales, B.F. Caspase-3 mediates retinoid-induced apoptosis in the organogenesis-stage mouse limb. Birth Defects Res. A Clin. Mol. Teratol. 2003, 67, 848–860. [Google Scholar] [CrossRef]
- Crowley, L.C.; Waterhouse, N.J. Detecting Cleaved Caspase-3 in Apoptotic Cells by Flow Cytometry. Cold Spring Harb. Protoc. 2016, 2016, pdb-prot087312. [Google Scholar] [CrossRef]
- Theiler, K. The House Mouse: Atlas of Embryonic Development; Springer: Berlin/Heidelberg, Germany, 1989. [Google Scholar]
- Haase, D.; Keiner, S.; Mawrin, C.; Wolf, G. Reduced Morg1 expression in ischemic human brain. Neurosci. Lett. 2009, 455, 46–50. [Google Scholar] [CrossRef]
- Stahr, A.; Frahm, C.; Kretz, A.; Bondeva, T.; Witte, O.W.; Wolf, G. Morg1(+/-) heterozygous mice are protected from experimentally induced focal cerebral ischemia. Brain Res. 2012, 1482, 22–31. [Google Scholar] [CrossRef]
- Peng, G.; Suo, S.; Cui, G.; Yu, F.; Wang, R.; Chen, J.; Chen, S.; Liu, Z.; Chen, G.; Qian, Y.; et al. Molecular architecture of lineage allocation and tissue organization in early mouse embryo. Nature 2019, 572, 528–532. [Google Scholar] [CrossRef]
- Peng, G.; Suo, S.; Chen, J.; Chen, W.; Liu, C.; Yu, F.; Wang, R.; Chen, S.; Sun, N.; Cui, G.; et al. Spatial Transcriptome for the Molecular Annotation of Lineage Fates and Cell Identity in Mid-gastrula Mouse Embryo. Dev. Cell 2020, 55, 802–804. [Google Scholar] [CrossRef]
- Chen, J.; Suo, S.; Tam, P.P.; Han, J.J.; Peng, G.; Jing, N. Spatial transcriptomic analysis of cryosectioned tissue samples with Geo-seq. Nat. Protoc. 2017, 12, 566–580. [Google Scholar] [CrossRef]
| Embryonic Day | Total | Morg1 Genotype | |||
|---|---|---|---|---|---|
| +/+ | +/− | −/− | Unknown | ||
| E8.5 | 29 | 27.6% (25%) | 44.9% (50%) | 24.1% (25%) | 3.4% * |
| E9.5 | 67 | 20.9% (25%) | 46.3% (50%) | 31.3% (25%) | 1.5% * |
| E10.5 | 155 | 31.6% (25%) | 46.5% (50%) | 21.9% (25%) | 0% |
| E11.5 | 80 | 26.3% (25%) | 53.7% (50%) | 0% (25%) | 20% * |
| E12.5 | 38 | 23.7% (25%) | 55.3% (50%) | 0% (25%) | 21% * |
| postnatal | 75 | 34.7% (25%) | 65.3% (50%) | 0% (25%) | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wulf, S.; Mizko, L.; Herrmann, K.-H.; Sánchez-Carbonell, M.; Urbach, A.; Lemke, C.; Berndt, A.; Loeffler, I.; Wolf, G. Targeted Disruption of the MORG1 Gene in Mice Causes Embryonic Resorption in Early Phase of Development. Biomolecules 2023, 13, 1037. https://doi.org/10.3390/biom13071037
Wulf S, Mizko L, Herrmann K-H, Sánchez-Carbonell M, Urbach A, Lemke C, Berndt A, Loeffler I, Wolf G. Targeted Disruption of the MORG1 Gene in Mice Causes Embryonic Resorption in Early Phase of Development. Biomolecules. 2023; 13(7):1037. https://doi.org/10.3390/biom13071037
Chicago/Turabian StyleWulf, Sophie, Luisa Mizko, Karl-Heinz Herrmann, Marta Sánchez-Carbonell, Anja Urbach, Cornelius Lemke, Alexander Berndt, Ivonne Loeffler, and Gunter Wolf. 2023. "Targeted Disruption of the MORG1 Gene in Mice Causes Embryonic Resorption in Early Phase of Development" Biomolecules 13, no. 7: 1037. https://doi.org/10.3390/biom13071037
APA StyleWulf, S., Mizko, L., Herrmann, K.-H., Sánchez-Carbonell, M., Urbach, A., Lemke, C., Berndt, A., Loeffler, I., & Wolf, G. (2023). Targeted Disruption of the MORG1 Gene in Mice Causes Embryonic Resorption in Early Phase of Development. Biomolecules, 13(7), 1037. https://doi.org/10.3390/biom13071037







