A Truncated 14-Amino-Acid Myelin Protein-Zero-Targeting Peptide for Fluorescence-Guided Nerve-Preserving Surgery
Abstract
1. Introduction
2. Materials and Methods
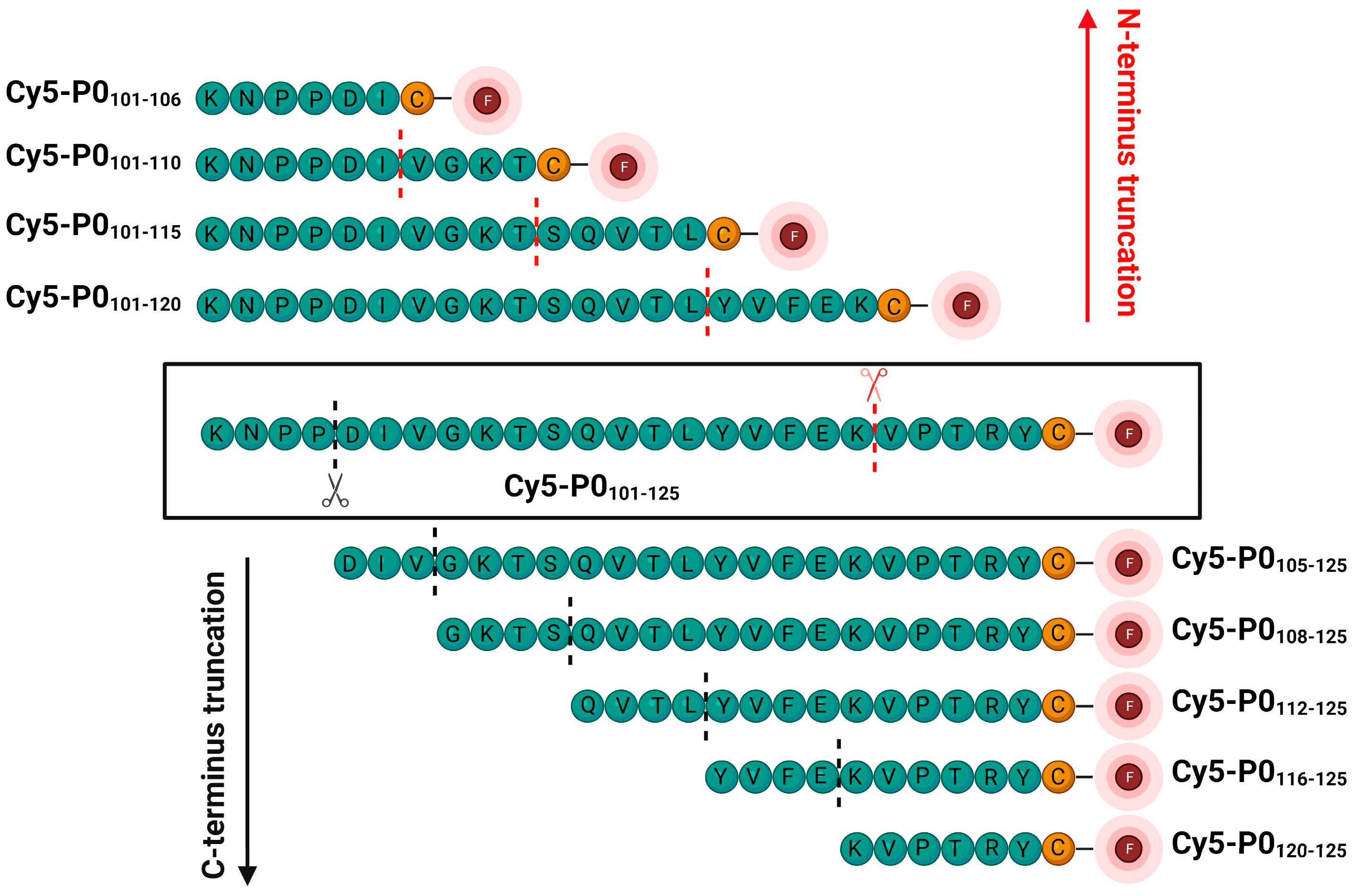
2.1. Synthetic Procedures
2.1.1. Dye Synthesis
2.1.2. General Synthetic Procedure for the Fluorescent C-Terminal Labelling of Truncation Peptides
2.2. Experimental Procedures
2.2.1. Assessment of P0 Staining via Fluorescence Confocal Microscopy
2.2.2. Affinity Assessment via Flow Cytometry
2.2.3. Chemical and Photophysical Properties
2.2.4. Correlation Assessment and P0-Related Interactions
2.2.5. In Vivo Imaging of the Optimized Peptide
2.3. Statistical Evaluation
3. Results
3.1. Synthesis and Compound Properties
3.2. P0 Staining by Fluorescence Confocal Microscopy
3.2.1. C-Terminal Matrix
3.2.2. N-Terminal Matrix
3.3. P0 Affinity Determined by Flow Cytometry
3.4. Relationships between Chemical and Biological Features
3.5. In Vivo Imaging of Nerves in the Head and Neck Region
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berger, D.; van den Berg, N.; van der Noort, V.; van der Hiel, B.; Valdés Olmos, R.A.; Buckle, T.A.; KleinJan, G.; Brouwer, O.R.; Vermeeren, L.; Karakullukçu, B.; et al. Technological (R)Evolution Leads to Detection of More Sentinel Nodes in Patients with Melanoma in the Head and Neck Region. J. Nucl. Med. 2021, 62, 1357–1362. [Google Scholar] [CrossRef]
- KleinJan, G.H.; Bunschoten, A.; van den Berg, N.S.; Olmos, R.A.; Klop, W.M.; Horenblas, S.; van der Poel, H.G.; Wester, H.J.; van Leeuwen, F.W. Fluorescence guided surgery and tracer-dose, fact or fiction? Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Krishnan, G.; Nishio, N.; van den Berg, N.S.; Lu, G.; Martin, B.A.; van Keulen, S.; Colevas, A.D.; Kapoor, S.; Liu, J.T.C.; et al. Intraoperative Fluorescence-Guided Surgery in Head and Neck Squamous Cell Carcinoma. Laryngoscope 2021, 131, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Vonk, J.; de Wit, J.G.; Voskuil, F.J.; Witjes, M.J.H. Improving oral cavity cancer diagnosis and treatment with fluorescence molecular imaging. Oral. Dis. 2021, 27, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.; Juhl, K.; Kiss, K.; Lelkaitis, G.; Charabi, B.W.; Mortensen, J.; Kjær, A.; von Buchwald, C. Near-infrared fluorescence imaging improves the nodal yield in neck dissection in oral cavity cancer—A randomized study. Eur. J. Surg. Oncol. 2019, 45, 2151–2158. [Google Scholar] [CrossRef]
- Zanoni, D.K.; Stambuk, H.E.; Madajewski, B.; Montero, P.H.; Matsuura, D.; Busam, K.J.; Ma, K.; Turker, M.Z.; Sequeira, S.; Gonen, M.; et al. Use of Ultrasmall Core-Shell Fluorescent Silica Nanoparticles for Image-Guided Sentinel Lymph Node Biopsy in Head and Neck Melanoma: A Nonrandomized Clinical Trial. JAMA Netw. Open 2021, 4, e211936. [Google Scholar] [CrossRef]
- KleinJan, G.H.; van Werkhoven, E.; van den Berg, N.S.; Karakullukcu, M.B.; Zijlmans, H.; van der Hage, J.A.; van de Wiel, B.A.; Buckle, T.; Klop, W.M.C.; Horenblas, S.; et al. The best of both worlds: A hybrid approach for optimal pre- and intraoperative identification of sentinel lymph nodes. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1915–1925. [Google Scholar] [CrossRef]
- Maurer, T.; Robu, S.; Schottelius, M.; Schwamborn, K.; Rauscher, I.; van den Berg, N.S.; van Leeuwen, F.W.B.; Haller, B.; Horn, T.; Heck, M.M.; et al. (99m)Technetium-based Prostate-specific Membrane Antigen-radioguided Surgery in Recurrent Prostate Cancer. Eur. Urol. 2019, 75, 659–666. [Google Scholar] [CrossRef]
- Maurer, T.; Weirich, G.; Schottelius, M.; Weineisen, M.; Frisch, B.; Okur, A.; Kübler, H.; Thalgott, M.; Navab, N.; Schwaiger, M.; et al. Prostate-specific membrane antigen-radioguided surgery for metastatic lymph nodes in prostate cancer. Eur. Urol. 2015, 68, 530–534. [Google Scholar] [CrossRef]
- Nishio, N.; van den Berg, N.S.; van Keulen, S.; Martin, B.A.; Fakurnejad, S.; Teraphongphom, N.; Chirita, S.U.; Oberhelman, N.J.; Lu, G.; Horton, C.E.; et al. Optical molecular imaging can differentiate metastatic from benign lymph nodes in head and neck cancer. Nat. Commun. 2019, 10, 5044. [Google Scholar] [CrossRef]
- Voskuil, F.J.; de Jongh, S.J.; Hooghiemstra, W.T.R.; Linssen, M.D.; Steinkamp, P.J.; de Visscher, S.; Schepman, K.P.; Elias, S.G.; Meersma, G.J.; Jonker, P.K.C.; et al. Fluorescence-guided imaging for resection margin evaluation in head and neck cancer patients using cetuximab-800CW: A quantitative dose-escalation study. Theranostics 2020, 10, 3994–4005. [Google Scholar] [CrossRef] [PubMed]
- Buckle, T.; KleinJan, G.H.; Engelen, T.; van den Berg, N.S.; DeRuiter, M.C.; van der Heide, U.; Valdes Olmos, R.A.; Webb, A.; van Buchem, M.A.; Balm, A.J.; et al. Diffusion-weighted-preparation (D-prep) MRI as a future extension of SPECT/CT based surgical planning for sentinel node procedures in the head and neck area? Oral. Oncol. 2016, 60, 48–54. [Google Scholar] [CrossRef]
- Kolokythas, A. Long-term surgical complications in the oral cancer patient: A comprehensive review. Part I. J. Oral. Maxillofac. Res. 2010, 1, e1. [Google Scholar] [CrossRef] [PubMed]
- Godballe, C.; Madsen, A.R.; Sørensen, C.H.; Schytte, S.; Trolle, W.; Helweg-Larsen, J.; Barfoed, L.; Kristiansen, L.; Sørensen, V.Z.; Samuelsen, G.; et al. Risk factors for recurrent nerve palsy after thyroid surgery: A national study of patients treated at Danish departments of ENT Head and Neck Surgery. Eur. Arch. Otorhinolaryngol. 2014, 271, 2267–2276. [Google Scholar] [CrossRef] [PubMed]
- Møller, M.N.; Sørensen, C.H. Risk of marginal mandibular nerve injury in neck dissection. Eur. Arch. Otorhinolaryngol. 2012, 269, 601–605. [Google Scholar] [CrossRef]
- van Beurden, F.; van Willigen, D.M.; Vojnovic, B.; van Oosterom, M.N.; Brouwer, O.R.; der Poel, H.G.V.; Kobayashi, H.; van Leeuwen, F.W.B.; Buckle, T. Multi-Wavelength Fluorescence in Image-Guided Surgery, Clinical Feasibility and Future Perspectives. Mol. Imaging 2020, 19, 1536012120962333. [Google Scholar] [CrossRef]
- van Leeuwen, F.W.; Hardwick, J.C.; van Erkel, A.R. Luminescence-based Imaging Approaches in the Field of Interventional Molecular Imaging. Radiology 2015, 276, 12–29. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Lei, Z.; Zhang, F. Recent Advances in Intraoperative Nerve Bioimaging: Fluorescence-Guided Surgery for Nerve Preservation. Small Struct. 2020, 1, 2000036. [Google Scholar] [CrossRef]
- Barth, C.W.; Gibbs, S.L. Direct Administration of Nerve-Specific Contrast to Improve Nerve Sparing Radical Prostatectomy. Theranostics 2017, 7, 573–593. [Google Scholar] [CrossRef]
- Gibbs-Strauss, S.L.; Nasr, K.A.; Fish, K.M.; Khullar, O.; Ashitate, Y.; Siclovan, T.M.; Johnson, B.F.; Barnhardt, N.E.; Tan Hehir, C.A.; Frangioni, J.V. Nerve-highlighting fluorescent contrast agents for image-guided surgery. Mol. Imaging 2011, 10, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, T.P.; Yan, Y.; Newton, P.; Hunter, D.A.; Achilefu, S.; Akers, W.J.; Mackinnon, S.E.; Johnson, P.J.; Berezin, M.Y. A NIR Dye for Development of Peripheral Nerve Targeted Probes. Medchemcomm 2012, 3, 685–690. [Google Scholar] [CrossRef]
- Park, M.H.; Hyun, H.; Ashitate, Y.; Wada, H.; Park, G.; Lee, J.H.; Njiojob, C.; Henary, M.; Frangioni, J.V.; Choi, H.S. Prototype nerve-specific near-infrared fluorophores. Theranostics 2014, 4, 823–833. [Google Scholar] [CrossRef]
- Hingorani, D.V.; Whitney, M.A.; Friedman, B.; Kwon, J.K.; Crisp, J.L.; Xiong, Q.; Gross, L.; Kane, C.J.; Tsien, R.Y.; Nguyen, Q.T. Nerve-targeted probes for fluorescence-guided intraoperative imaging. Theranostics 2018, 8, 4226–4237. [Google Scholar] [CrossRef]
- Hussain, T.; Mastrodimos, M.B.; Raju, S.C.; Glasgow, H.L.; Whitney, M.; Friedman, B.; Moore, J.D.; Kleinfeld, D.; Steinbach, P.; Messer, K.; et al. Fluorescently labeled peptide increases identification of degenerated facial nerve branches during surgery and improves functional outcome. PLoS ONE 2015, 10, e0119600. [Google Scholar] [CrossRef]
- Whitney, M.A.; Crisp, J.L.; Nguyen, L.T.; Friedman, B.; Gross, L.A.; Steinbach, P.; Tsien, R.Y.; Nguyen, Q.T. Fluorescent peptides highlight peripheral nerves during surgery in mice. Nat. Biotechnol. 2011, 29, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Berehova, N.; Buckle, T.; van Meerbeek, M.P.; Bunschoten, A.; Velders, A.H.; van Leeuwen, F.W.B. Nerve Targeting via Myelin Protein Zero and the Impact of Dimerization on Binding Affinity. Molecules 2022, 27, 9015. [Google Scholar] [CrossRef] [PubMed]
- Buckle, T.; Hensbergen, A.W.; van Willigen, D.M.; Bosse, F.; Bauwens, K.; Pelger, R.C.M.; van Leeuwen, F.W.B. Intraoperative visualization of nerves using a myelin protein-zero specific fluorescent tracer. EJNMMI Res. 2021, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Raasakka, A.; Kursula, P. How Does Protein Zero Assemble Compact Myelin? Cells 2020, 9, 1832. [Google Scholar] [CrossRef]
- Buckle, T.; van Willigen, D.M.; Welling, M.M.; van Leeuwen, F.W.B. Pre-clinical development of fluorescent tracers and translation towards clinical application. In Nuclear Medicine and Molecular Imaging; Signore, A., Ed.; Elsevier: Oxford, UK, 2022; pp. 644–661. [Google Scholar]
- D’Urso, D.; Brophy, P.J.; Staugaitis, S.M.; Gillespie, C.S.; Frey, A.B.; Stempak, J.G.; Colman, D.R. Protein zero of peripheral nerve myelin: Biosynthesis, membrane insertion, and evidence for homotypic interaction. Neuron 1990, 4, 449–460. [Google Scholar] [CrossRef]
- Bunschoten, A.; van Willigen, D.M.; Buckle, T.; van den Berg, N.S.; Welling, M.M.; Spa, S.J.; Wester, H.J.; van Leeuwen, F.W. Tailoring Fluorescent Dyes To Optimize a Hybrid RGD-Tracer. Bioconjug Chem. 2016, 27, 1253–1258. [Google Scholar] [CrossRef]
- Lopalco, M.; Koini, E.N.; Cho, J.K.; Bradley, M. Catch and release microwave mediated synthesis of cyanine dyes. Org. Biomol. Chem. 2009, 7, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Shershov, V.E.; Spitsyn, M.A.; Kuznetsova, V.E.; Timofeev, E.N.; Ivashkina, O.A.; Abramov, I.S.; Nasedkina, T.V.; Zasedatelev, A.S.; Chudinov, A.V. Near-infrared heptamethine cyanine dyes. Synthesis, spectroscopic characterization, thermal properties and photostability. Dye Pigment. 2013, 97, 353–360. [Google Scholar] [CrossRef]
- Kuil, J.; Buckle, T.; Yuan, H.; van den Berg, N.S.; Oishi, S.; Fujii, N.; Josephson, L.; van Leeuwen, F.W. Synthesis and evaluation of a bimodal CXCR4 antagonistic peptide. Bioconjug. Chem. 2011, 22, 859–864. [Google Scholar] [CrossRef]
- Berrens, A.C.; van Oosterom, M.N.; Slof, L.J.; van Leeuwen, F.W.B.; van der Poel, H.G.; Buckle, T. Three-way multiplexing in prostate cancer patients—Combining a bimodal sentinel node tracer with multicolor fluorescence imaging. Eur. J. Nucl. Med. Mol. Imaging 2022, 50, 1262–1263. [Google Scholar] [CrossRef]
- Spa, S.J.; Hensbergen, A.W.; van der Wal, S.; Kuil, J.; van Leeuwen, F.W.B. The influence of systematic structure alterations on the photophysical properties and conjugation characteristics of asymmetric cyanine 5 dyes. Dye Pigment. 2018, 152, 19–28. [Google Scholar] [CrossRef]
- Azargoshasb, S.; Boekestijn, I.; Roestenberg, M.; KleinJan, G.H.; van der Hage, J.A.; van der Poel, H.G.; Rietbergen, D.D.D.; van Oosterom, M.N.; van Leeuwen, F.W.B. Quantifying the Impact of Signal-to-background Ratios on Surgical Discrimination of Fluorescent Lesions. Mol. Imaging Biol. 2023, 25, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.; Doyle, J.P.; Hensley, P.; Colman, D.R.; Hendrickson, W.A. Structure of Myelin Membrane Adhesion Molecule P0. Available online: https://www.rcsb.org/structure/1NEU (accessed on 29 March 2023).
- van den Berg, N.S.; Miwa, M.; KleinJan, G.H.; Sato, T.; Maeda, Y.; van Akkooi, A.C.; Horenblas, S.; Karakullukcu, B.; van Leeuwen, F.W. (Near-Infrared) Fluorescence-Guided Surgery Under Ambient Light Conditions: A Next Step to Embedment of the Technology in Clinical Routine. Ann. Surg. Oncol. 2016, 23, 2586–2595. [Google Scholar] [CrossRef]
- Buckle, T.; van Alphen, M.; van Oosterom, M.N.; van Beurden, F.; Heimburger, N.; van der Wal, J.E.; van den Brekel, M.; van Leeuwen, F.W.B.; Karakullukcu, B. Translation of c-Met Targeted Image-Guided Surgery Solutions in Oral Cavity Cancer-Initial Proof of Concept Data. Cancers 2021, 13, 2674. [Google Scholar] [CrossRef]
- Shapiro, L.; Doyle, J.P.; Hensley, P.; Colman, D.R.; Hendrickson, W.A. Crystal structure of the extracellular domain from P0, the major structural protein of peripheral nerve myelin. Neuron 1996, 17, 435–449. [Google Scholar] [CrossRef]
- Morris, J.C.; Roe, C.M.; Grant, E.A.; Head, D.; Storandt, M.; Goate, A.M.; Fagan, A.M.; Holtzman, D.M.; Mintun, M.A. Pittsburgh Compound B Imaging and Prediction of Progression from Cognitive Normality to Symptomatic Alzheimer Disease. Arch. Neurol. 2009, 66, 1469–1475. [Google Scholar] [CrossRef]
- Puchtler, H.; Sweat, F.; Levine, M. On The Binding of Congo Red by Amyloid. J. Histochem. Cytochem. 1962, 10, 355–364. [Google Scholar] [CrossRef]
- KleinJan, G.H.; Buckle, T.; van Willigen, D.M.; van Oosterom, M.N.; Spa, S.J.; Kloosterboer, H.E.; van Leeuwen, F.W. Fluorescent lectins for local in vivo visualization of peripheral nerves. Molecules 2014, 19, 9876–9892. [Google Scholar] [CrossRef] [PubMed]
- De Vries, H.M.; Bekers, E.; van Oosterom, M.N.; Karakullukcu, M.B.; van der Poel, H.G.; van Leeuwen, F.W.B.; Buckle, T.; Brouwer, O.R. c-MET Receptor-Targeted Fluorescence on the Road to Image-Guided Surgery in Penile Squamous Cell Carcinoma Patients. J. Nucl. Med. 2022, 63, 51–56. [Google Scholar] [CrossRef] [PubMed]





| Solubility (µM) | LogP | Serum Binding | Brightness (in HSA) | Net Charge | |
| Cy5-P0101–125 | 172 | −1.39 + 0.09 | 89 + 2% | 67,760 | −1 |
| Cy5-P0101–120 | 15 | −0.99 ± 0.1 | 66 ± 12% | 137,940 | −2 |
| Cy5-P0101–115 | >400 | −0.80 ± 0.16 | 60 ± 9% | 217,800 | −2 |
| Cy5-P0101–110 | >400 | −0.73 ± 0.12 | 55 ± 8% | 111,320 | −2 |
| Cy5-P0101–106 | >400 | −0.95 ± 0.05 | 47 ± 7% | 118,580 | −3 |
| Cy5-P0105–125 | 9 | −0.60 ± 0.04 | 56 ± 8% | 157,300 | −2 |
| Cy5-P0108–125 | 25 | −1.34 + 0.03 | 69 ± 7% | 58,080 | −1 |
| Cy5-P0112–125 | 71 | −1.08 + 0.03 | 50 + 3% | 121,000 | −2 |
| Cy5-P0116–125 | 150 | −1.58 + 0.03 | 81 + 5% | 60,500 | −2 |
| Cy5-P0120–125 | 150 | −1.59 + 0.01 | 71 ± 11% | 70,180 | −1 |
| No. of Acceptors | No. of Donors | No. of Intramolecular Interactions | No. of Intermolecular Interactions | |
|---|---|---|---|---|
| Cy5-P0101–125 | 40 | 39 | 26 | 3 |
| Cy5-P0101–120 | 29 | 29 | 26 | 2 |
| Cy5-P0101–115 | 24 | 22 | 18 | 2 |
| Cy5-P0101–110 | 16 | 14 | 8 | 1 |
| Cy5-P0101–106 | 10 | 9 | 2 | 0 |
| Cy5-P0105–125 | 36 | 33 | 24 | 3 |
| Cy5-P0108–125 | 32 | 28 | 23 | 2 |
| Cy5-P0112–125 | 26 | 21 | 16 | 2 |
| Cy5-P0116–125 | 20 | 15 | 8 | 1 |
| Cy5-P0120–125 | 13 | 10 | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berehova, N.; van Meerbeek, M.P.; Azargoshasb, S.; van Willigen, D.M.; Slof, L.J.; Navaei Lavasani, S.; van Oosterom, M.N.; van Leeuwen, F.W.B.; Buckle, T. A Truncated 14-Amino-Acid Myelin Protein-Zero-Targeting Peptide for Fluorescence-Guided Nerve-Preserving Surgery. Biomolecules 2023, 13, 942. https://doi.org/10.3390/biom13060942
Berehova N, van Meerbeek MP, Azargoshasb S, van Willigen DM, Slof LJ, Navaei Lavasani S, van Oosterom MN, van Leeuwen FWB, Buckle T. A Truncated 14-Amino-Acid Myelin Protein-Zero-Targeting Peptide for Fluorescence-Guided Nerve-Preserving Surgery. Biomolecules. 2023; 13(6):942. https://doi.org/10.3390/biom13060942
Chicago/Turabian StyleBerehova, Nataliia, Maarten P. van Meerbeek, Samaneh Azargoshasb, Danny M. van Willigen, Leon J. Slof, Saaedeh Navaei Lavasani, Matthias N. van Oosterom, Fijs W. B. van Leeuwen, and Tessa Buckle. 2023. "A Truncated 14-Amino-Acid Myelin Protein-Zero-Targeting Peptide for Fluorescence-Guided Nerve-Preserving Surgery" Biomolecules 13, no. 6: 942. https://doi.org/10.3390/biom13060942
APA StyleBerehova, N., van Meerbeek, M. P., Azargoshasb, S., van Willigen, D. M., Slof, L. J., Navaei Lavasani, S., van Oosterom, M. N., van Leeuwen, F. W. B., & Buckle, T. (2023). A Truncated 14-Amino-Acid Myelin Protein-Zero-Targeting Peptide for Fluorescence-Guided Nerve-Preserving Surgery. Biomolecules, 13(6), 942. https://doi.org/10.3390/biom13060942







