Diagnostic and Prognostic Role of CD93 in Cardiovascular Disease: A Systematic Review
Abstract
1. Introduction
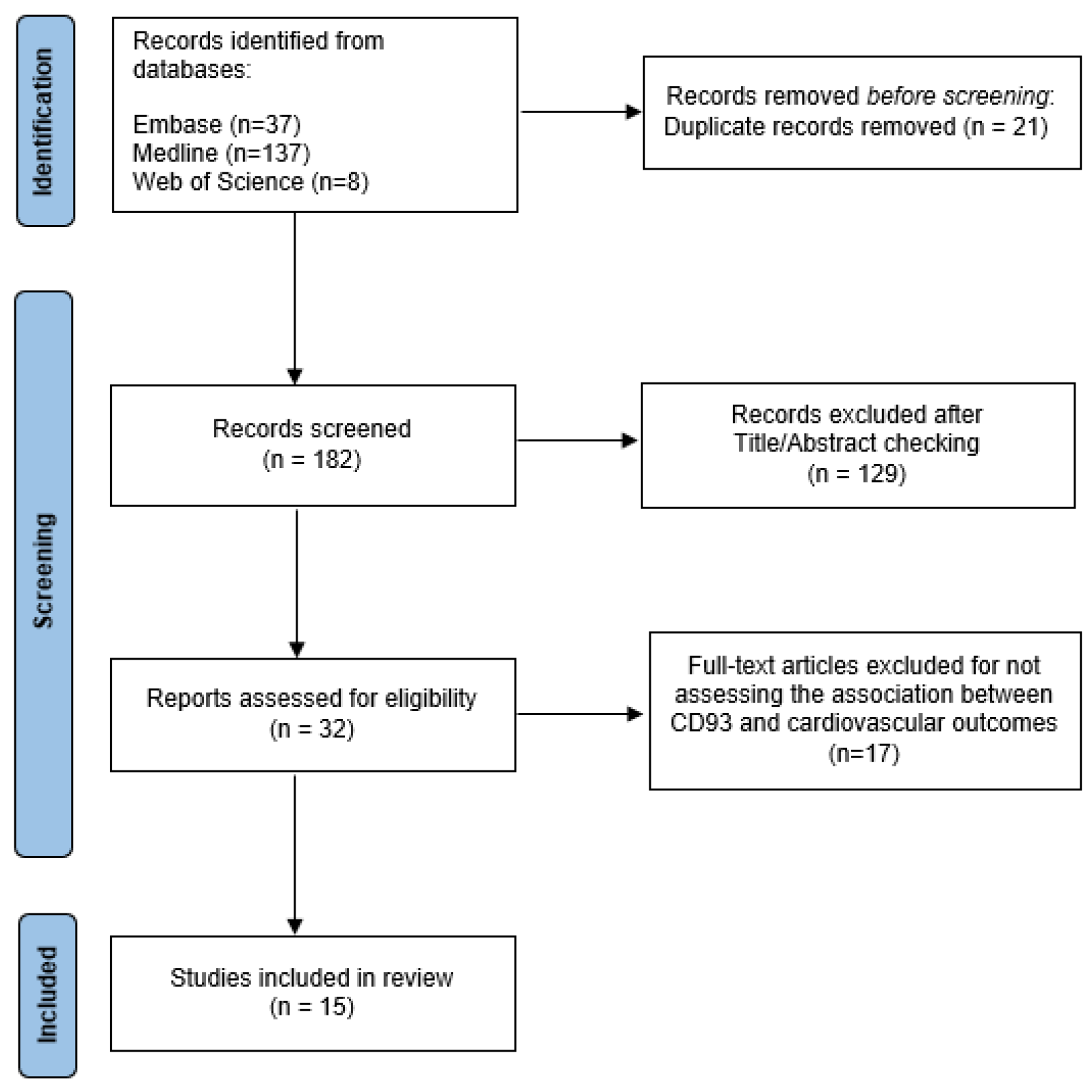
2. Materials and Methods
2.1. Protocol
2.2. Eligibility Criteria
2.3. Information Sources and Search Terms
2.4. Study Selection, Data Extraction, and Quality Assessment
3. Results
4. Discussion
4.1. CD93 and Lipid Metabolism
4.2. CD93 and Diabetes
4.3. CD93 and Arterial Hypertension
4.4. CD93 and Obesity
4.5. CD93 and Hyperuricemia
4.6. CD93 and Coronary Artery Disease
4.7. CD93 and Heart Failure
4.8. CD93 and Stroke
4.9. CD93 and Peripheral Artery Disease
4.10. CD93 and CV Mortality
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Benincasa, G.; Coscioni, E.; Napoli, C. Cardiovascular risk factors and molecular routes underlying endothelial dysfunction: Novel opportunities for primary prevention. Biochem. Pharmacol. 2022, 202, 115108. [Google Scholar] [CrossRef]
- Fonseca, M.I.; Carpenter, P.M.; Park, M.; Palmarini, G.; Nelson, E.L.; Tenner, A.J. C1qR(P), a myeloid cell receptor in blood, is predominantly expressed on endothelial cells in human tissue. J. Leukoc. Biol. 2001, 70, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Nepomuceno, R.R.; Tenner, A.J. C1qRP, the C1q receptor that enhances phagocytosis, is detected specifically in human cells of myeloid lineage, endothelial cells, and platelets. J. Immunol. 1998, 160, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Lovik, G.; Larsen Sand, K.; Iversen, J.G.; Rolstad, B. C1qRp elicits a Ca++ response in rat NK cells but does not influence NK-mediated cytotoxicity. Scand. J. Immunol. 2001, 53, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Fantone, S.; Tossetta, G.; Di Simone, N.; Tersigni, C.; Scambia, G.; Marcheggiani, F.; Giannubilo, S.R.; Marzioni, D. CD93 a potential player in cytotrophoblast and endothelial cell migration. Cell Tissue Res. 2022, 387, 123–130. [Google Scholar] [CrossRef]
- Ikewaki, N.; Yamao, H.; Kulski, J.K.; Inoko, H. Flow cytometric identification of CD93 expression on naive T lymphocytes (CD4(+)CD45RA (+) cells) in human neonatal umbilical cord blood. J. Clin. Immunol. 2010, 30, 723–733. [Google Scholar] [CrossRef]
- Chevrier, S.; Genton, C.; Kallies, A.; Karnowski, A.; Otten, L.A.; Malissen, B.; Malissen, M.; Botto, M.; Corcoran, L.M.; Nutt, S.L.; et al. CD93 is required for maintenance of antibody secretion and persistence of plasma cells in the bone marrow niche. Proc. Natl. Acad. Sci. USA 2009, 106, 3895–3900. [Google Scholar] [CrossRef]
- Greenlee-Wacker, M.C.; Briseno, C.; Galvan, M.; Moriel, G.; Velazquez, P.; Bohlson, S.S. Membrane-associated CD93 regulates leukocyte migration and C1q-hemolytic activity during murine peritonitis. J. Immunol. 2011, 187, 3353–3361. [Google Scholar] [CrossRef]
- Rossi, G.; Giger, S.; Hübscher, T.; Lutolf, M.P. Gastruloids as in vitro models of embryonic blood development with spatial and temporal resolution. Sci. Rep. 2022, 12, 13380. [Google Scholar] [CrossRef]
- Khan, K.A.; McMurray, J.L.; Mohammed, F.; Bicknell, R. C-type lectin domain group 14 proteins in vascular biology, cancer and inflammation. FEBS J. 2019, 286, 3299–3332. [Google Scholar] [CrossRef]
- Patel, V.K.; Williams, H.; Li, S.C.H.; Fletcher, J.P.; Medbury, H.J. Monocyte inflammatory profile is specific for individuals and associated with altered blood lipid levels. Atherosclerosis 2017, 263, 15–23. [Google Scholar] [CrossRef]
- Torzewski, M.; Bhakdi, S. Complement and atherosclerosis-united to the point of no return? Clin. Biochem. 2013, 46, 20–25. [Google Scholar] [CrossRef]
- Linton, M.F.; Babaev, V.R.; Huang, J.; Linton, E.F.; Tao, H.; Yancey, P.G. Macrophage Apoptosis and Efferocytosis in the Pathogenesis of Atherosclerosis. Circ. J. 2016, 80, 2259–2268. [Google Scholar] [CrossRef]
- Badimon, L.; Suades, R.; Arderiu, G.; Peña, E.; Chiva-Blanch, G.; Padró, T. Microvesicles in Atherosclerosis and Angiogenesis: From Bench to Bedside and Reverse. Front. Cardiovasc. Med. 2017, 4, 77. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- van der Net, J.B.; Oosterveer, D.M.; Versmissen, J.; Defesche, J.C.; Yazdanpanah, M.; Aouizerat, B.E.; Steyerberg, E.W.; Malloy, M.J.; Pullinger, C.R.; Kastelein, J.J.; et al. Replication study of 10 genetic polymorphisms associated with coronary heart disease in a specific high-risk population with familial hypercholesterolemia. Eur. Heart J. 2008, 29, 2195–2201. [Google Scholar] [CrossRef]
- Malarstig, A.; Silveira, A.; Wagsater, D.; Ohrvik, J.; Backlund, A.; Samnegard, A.; Khademi, M.; Hellenius, M.L.; Leander, K.; Olsson, T.; et al. Plasma CD93 concentration is a potential novel biomarker for coronary artery disease. J. Intern. Med. 2011, 270, 229–236. [Google Scholar] [CrossRef]
- Adamski, M.G.; Li, Y.; Wagner, E.; Yu, H.; Seales-Bailey, C.; Soper, S.A.; Murphy, M.; Baird, A.E. Expression profile based gene clusters for ischemic stroke detection. Genomics 2014, 104, 163–169. [Google Scholar] [CrossRef]
- Chan, K.H.; Huang, Y.T.; Meng, Q.; Wu, C.; Reiner, A.; Sobel, E.M.; Tinker, L.; Lusis, A.J.; Yang, X.; Liu, S. Shared molecular pathways and gene networks for cardiovascular disease and type 2 diabetes mellitus in women across diverse ethnicities. Circ. Cardiovasc. Genet. 2014, 7, 911–919. [Google Scholar] [CrossRef]
- Youn, J.C.; Yu, H.T.; Jeon, J.W.; Lee, H.S.; Jang, Y.; Park, Y.W.; Park, Y.B.; Shin, E.C.; Ha, J.W. Soluble CD93 levels in patients with acute myocardial infarction and its implication on clinical outcome. PLoS ONE 2014, 9, e96538. [Google Scholar] [CrossRef] [PubMed]
- Strawbridge, R.J.; Hilding, A.; Silveira, A.; Osterholm, C.; Sennblad, B.; McLeod, O.; Tsikrika, P.; Foroogh, F.; Tremoli, E.; Baldassarre, D.; et al. Soluble CD93 Is Involved in Metabolic Dysregulation but Does Not Influence Carotid Intima-Media Thickness. Diabetes 2016, 65, 2888–2899. [Google Scholar] [CrossRef] [PubMed]
- Bouwens, E.; van den Berg, V.J.; Akkerhuis, K.M.; Baart, S.J.; Caliskan, K.; Brugts, J.J.; Mouthaan, H.; Ramshorst, J.V.; Germans, T.; Umans, V.; et al. Circulating Biomarkers of Cell Adhesion Predict Clinical Outcome in Patients with Chronic Heart Failure. J. Clin. Med. 2020, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Alehagen, U.; Shamoun, L.; Wagsater, D. Genetic variance and plasma concentration of CD93 is associated with cardiovascular mortality: Results from a 6.7—Year follow—Up of a healthy community—Living elderly population. Mol. Med. Rep. 2020, 22, 4629–4636. [Google Scholar] [CrossRef]
- Lee, M.; Park, H.S.; Choi, M.Y.; Kim, H.Z.; Moon, S.J.; Ha, J.Y.; Choi, A.; Park, Y.W.; Park, J.S.; Shin, E.C.; et al. Significance of Soluble CD93 in Type 2 Diabetes as a Biomarker for Diabetic Nephropathy: Integrated Results from Human and Rodent Studies. J. Clin. Med. 2020, 9, 1394. [Google Scholar] [CrossRef]
- Sharma, K.; Chanana, N.; Mohammad, G.; Thinlas, T.; Gupta, M.; Syed, M.A.; Das, R.S.; Pasha, Q.; Mishra, A. Hypertensive Patients Exhibit Enhanced Thrombospondin-1 Levels at High-Altitude. Life 2021, 11, 893. [Google Scholar] [CrossRef]
- Bicvic, A.; Scherrer, N.; Schweizer, J.; Fluri, F.; Christ-Crain, M.; De Marchis, G.M.; Luft, A.R.; Katan, M. A novel biomarker panel index improves risk stratification after ischemic stroke. Eur. Stroke J. 2022, 7, 158–165. [Google Scholar] [CrossRef]
- Chandramouli, C.; Ting, T.W.; Tromp, J.; Agarwal, A.; Svedlund, S.; Saraste, A.; Hage, C.; Tan, R.S.; Beussink-Nelson, L.; Lagerström Fermer, M.; et al. Sex differences in proteomic correlates of coronary microvascular dysfunction among patients with heart failure and preserved ejection fraction. Eur. J. Heart Fail. 2022, 24, 681–684. [Google Scholar] [CrossRef]
- Helleberg, S.; Engel, A.; Ahmed, S.; Ahmed, A.; Rådegran, G. Higher plasma IL-6 and PTX3 are associated with worse survival in left heart failure with pulmonary hypertension. Am. Heart J. Plus: Cardiol. Res. Pract. 2022, 20, 100190. [Google Scholar] [CrossRef]
- Snelder, S.M.; Pouw, N.; Aga, Y.; Castro Cabezas, M.; Biter, L.U.; Zijlstra, F.; Kardys, I.; van Dalen, B.M. Cardiovascular Biomarker Profiles in Obesity and Relation to Normalization of Subclinical Cardiac Dysfunction after Bariatric Surgery. Cells 2022, 11, 422. [Google Scholar] [CrossRef]
- Su, C.; Han, Y.; Qu, B.; Zhang, C.; Liang, T.; Gao, F.; Hou, G. CD93 in macrophages: A novel target for atherosclerotic plaque imaging? J. Cell Mol. Med. 2022, 26, 2152–2162. [Google Scholar] [CrossRef]
- Kuznetsova, T.; Prange, K.H.M.; Glass, C.K.; de Winther, M.P.J. Transcriptional and epigenetic regulation of macrophages in atherosclerosis. Nat. Rev. Cardiol. 2020, 17, 216–228. [Google Scholar] [CrossRef]
- Remmerie, A.; Scott, C.L. Macrophages and lipid metabolism. Cell Immunol. 2018, 330, 27–42. [Google Scholar] [CrossRef]
- Engelen, S.E.; Robinson, A.J.B.; Zurke, Y.X.; Monaco, C. Therapeutic strategies targeting inflammation and immunity in atherosclerosis: How to proceed? Nat. Rev. Cardiol. 2022, 19, 522–542. [Google Scholar] [CrossRef]
- Blackburn, J.W.D.; Lau, D.H.C.; Liu, E.Y.; Ellins, J.; Vrieze, A.M.; Pawlak, E.N.; Dikeakos, J.D.; Heit, B. Soluble CD93 is an apoptotic cell opsonin recognized by α(x) β(2). Eur. J. Immunol. 2019, 49, 600–610. [Google Scholar] [CrossRef]
- Feingold, K.R.; Grunfeld, C. The Effect of Inflammation and Infection on Lipids and Lipoproteins. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Guo, B.; Rao, J.; Wang, Q.; He, R.; Jiang, X.; Xie, N. The Diagnostic Value Of Soluble CD93 In Chinese Patients With Type 2 Diabetic Nephropathy. Res. Sq. 2022, 2022. [Google Scholar] [CrossRef]
- Shalaeva, E.V.; Messerli, F.H. What is resistant arterial hypertension? Blood Press. 2023, 32, 2185457. [Google Scholar] [CrossRef]
- Narvaez-Guerra, O.; Herrera-Enriquez, K.; Medina-Lezama, J.; Chirinos, J.A. Systemic Hypertension at High Altitude. Hypertension 2018, 72, 567–578. [Google Scholar] [CrossRef]
- Lastra, G.; Sowers, J.R. Obesity and cardiovascular disease: Role of adipose tissue, inflammation, and the renin-angiotensin-aldosterone system. Horm. Mol. Biol. Clin. Investig. 2013, 15, 49–57. [Google Scholar] [CrossRef]
- Rubio-Almanza, M.; Cámara-Gómez, R.; Merino-Torres, J.F. Obesity and type 2 diabetes: Also linked in therapeutic options. Endocrinol. Diabetes Nutr. (Engl. Ed.) 2019, 66, 140–149. [Google Scholar] [CrossRef]
- Borghi, C.; Piani, F. Uric Acid and Risk of Cardiovascular Disease: A Question of Start and Finish. Hypertension 2021, 78, 1219–1221. [Google Scholar] [CrossRef] [PubMed]
- Borghi, C.; Fogacci, F.; Piani, F. Not all the eggs and the chickens are the same: The case of uric acid and metabolic syndrome. Eur. J. Intern. Med. 2022, 103, 36–37. [Google Scholar] [CrossRef] [PubMed]
- Piani, F.; Cicero, A.F.G.; Borghi, C. Uric Acid and Hypertension: Prognostic Role and Guide for Treatment. J. Clin. Med. 2021, 10, 448. [Google Scholar] [CrossRef] [PubMed]
- Shaya, G.E.; Leucker, T.M.; Jones, S.R.; Martin, S.S.; Toth, P.P. Coronary heart disease risk: Low-density lipoprotein and beyond. Trends Cardiovasc. Med. 2022, 32, 181–194. [Google Scholar] [CrossRef]
- Lugano, R.; Vemuri, K.; Barbera, S.; Orlandini, M.; Dejana, E.; Claesson-Welsh, L.; Dimberg, A. CD93 maintains endothelial barrier function by limiting the phosphorylation and turnover of VE-cadherin. FASEB J. 2023, 37, e22894. [Google Scholar] [CrossRef]
- Barbera, S.; Lugano, R.; Pedalina, A.; Mongiat, M.; Santucci, A.; Tosi, G.M.; Dimberg, A.; Galvagni, F.; Orlandini, M. The C-type lectin CD93 controls endothelial cell migration via activation of the Rho family of small GTPases. Matrix Biol. 2021, 99, 1–17. [Google Scholar] [CrossRef]
- Newell, S.; Gorman, J.C.; Bell, E.; Atkinson, J.P. Hemolytic and antigenic measurements of complement. A comparison of serum and plasma samples in normal individuals and patients. J. Lab. Clin. Med. 1982, 100, 437–444. [Google Scholar]
- Roger, V.L. Epidemiology of Heart Failure: A Contemporary Perspective. Circ. Res. 2021, 128, 1421–1434. [Google Scholar] [CrossRef]
- Saini, V.; Guada, L.; Yavagal, D.R. Global Epidemiology of Stroke and Access to Acute Ischemic Stroke Interventions. Neurology 2021, 97, S6–S16. [Google Scholar] [CrossRef]

| Population | CD93 Measurement | Outcome | Results | |
|---|---|---|---|---|
| Van der Net J.B. et al., 2008 [17] | Cohort study of 2145 patients with heterozygous familial hypercholesterolemia | Genotyping of CD93 rs3746731 polymorphism | Longitudinal study with a mean follow-up of 5 years and an outcome of the development of coronary heart disease. | The rs3746731 polymorphism was associated with an increased risk of coronary heart disease (HR 1.26, 95% CI 1.06–1.49, p = 0.01). Additional adjustments for CV risk factors yielded similar results. Patients homozygous for the T-allele of the CD93 polymorphism had a further increase in the risk of coronary artery disease (p = 0.01). |
| Mälarstig A. et al., 2011 [18] | Population study of 764 individuals of both sexes (predominantly men) with median age 54 years | ELISA assay for plasma sCD93 | Cross-sectional differences in the whole population plus the risk of premature MI in 316 individuals (159 had premature MI and 157 did not). | In the cross-sectional analysis, elevated levels of sCD93 were associated with increased plasma fibrinogen (p = 0.03) and cystatin-C (p < 0.001). No significant associations with the risk of MI were observed among different sCD93 tertiles after adjustment. In the unadjusted analysis, the middle tertile was associated with a decreased risk of MI. |
| Adamski M.G. et al., 2014 [19] | Case-control study (18 ischemic stroke patients vs. 15 matched controls) | High-throughput next-generation qPCR for CD93 gene transcript | Cross-sectional comparison of 40 transcripts between patients with ischemic stroke and controls. | CD93 transcript was 2-times higher in patients who suffered from ischemic stroke compared with control subjects (adjusted p value = 0.03). |
| Chan K.H. et al., 2014 [20] | Longitudinal genome-wide study on 15,346 women of different ethnicities | Genome-wide genotyping (Affymetrix and Illumina), standard SNP analysis | CVD development (MI, stroke, deep vein thrombosis, and pulmonary embolism) and/or type 2 diabetes development. | CD93 ranked among the top 10 key driver genes, which, when perturbed, can potentially affect a large number of genes involved in cardiovascular disease and type 2 diabetes pathways. |
| Youn J.-C. et al., 2014 [21] | 120 patients with acute MI and 120 matched controls | ELISA assay for serum sCD93 | Cross-sectional differences plus all-cause and CV deaths in a mean follow-up of 7 months. | sCD93 was significantly higher in patients with acute MI (552.1 ± 293.7 vs. 429.8 ± 114.2 ng/mL, p < 0.0001). Initial sCD93 level was an independent predictor of all-cause (p = 0.002) and CV mortality (p = 0.033) when controlled for age and EF. |
| Strawbridge R.J. et al., 2016 [22] | Different cohorts of subjects with type 2 diabetes (n = 901) and subjects without diabetes (n = 2470) | Quantification of blood sCD93 by Meso Scale platform and SECTOR Imager 2400 | Cross-sectional differences of diabetic vs. non-diabetic subjects and associations with IMT modifications (follow-up at 15 and 30 months) or development of T2D or glucose intolerance (follow-up of 8–10 years). | Levels of sCD93 were significantly lower in subjects with T2D (157 ± 40 vs. 164 ± 45 ng/mL, p < 0.0001) and higher in subjects who remained normal glucose tolerant at follow-up (166 ± 44 vs. 158 ± 45 ng/mL, respectively; p = 0.016). In lipid-lowering-naive subjects, sCD93 levels were positively associated with HDL, adiponectin, and vitamin D and negatively with TG, BMI, insulin, and HOMA. sCD93 levels at baseline were not associated with any baseline or progression measures of IMT. |
| Patel V.K. et al., 2017 [11] | Healthy individuals with heterogenous lipid profiles (n = 30) | Whole blood flow cytometry for monocyte expression of CD93 | Cross-sectional study to assess the associations between monocyte phenotype and lipid profile. | In all monocyte types, CD93 expression was positively associated with ApoB levels. CD93 in non-classical monocytes is negatively associated with ApoA1/ApoB ratio (R = −0.445, p = 0.049); in intermediate monocytes, it is positively associated with TC/HDL-C ratio (R = 0.459, p = 0.042) and negatively correlated with HDL-C (R = −0.457, p = 0.043). |
| Bouwens E. et al., 2019 [23] | 263 patients with HF | Serum sCD93 by high-throughput proximity extension assays (Olink Proteomics Cardiovascular III multiplex assay) | Composite outcome: CV mortality, HF-hospitalisation, heart transplantation, or LVAD implantation. Median follow-up of 2.2 years. | In patients who met the primary outcome, sCD93 was higher both at baseline and at follow-up. Adjusted HR for the primary outcome per 0.1 standard deviation of the annual slope of CD93 at any point in time during follow-up (estimated by joint modelling analysis) was 1.43 (CI 1.13–1.92, p = 0.002). |
| Alehagen U. et al., 2020 [24] | Population study of 457 individuals of both sexes aged between 70 and 85 years | PCR quantification of CD93 SNP rs2749812 in plasma | Cross-sectional differences plus all-cause and cardiovascular deaths in a mean follow-up of 6.7 years. | CD93 rs2749812 was associated with higher concentration of plasma NT-proBNP (p = 0.03). People in the highest quartile of CD93 rs2749812 showed a significant increase in all-cause mortality (p = 0.037) but not in CV mortality (p = 0.43). |
| Lee M. et al., 2020 [25] | 97 subjects with T2D (mean age 56 years) | ELISA assay for serum sCD93 | Cross-sectional analysis of the associations between sCD93 levels, participant characteristics, and markers of diabetic micro- and macrovascular complications. | Higher sCD93 levels were associated with higher proteinuria and ACR values and lower eGFR values. After adjustment for multiple confounders, sCD92 was still associated with a decreased eGFR (β = −14.734, standard error (SE) = 5.564, p = 0.010) and an increased ACR (β = 387.943, SE = 191.129, p = 0.046). |
| Sharma K. et al., 2021 [26] | Case–control study of 349 subjects (hypertensive vs. controls) living at high-altitude (≥3500 mt) | Genotype analysis of SNPs by SNPs Fluidigm 48.48 SNPtype assay | Cross-sectional study to investigate the associations between genetic variants of THBS-CD Family Genes and hypertension at high altitude. | Intergenic SNPs rs1998081 and rs2424515 of thrombomodulin and CD93, and CD93 SNPs rs7492 and rs2749812 were associated with the presence of hypertension at high-altitude. |
| Bicvic A. et al., 2022 [27] | 320 patients who had an ischemic stroke within 72 h | Serum sCD93 by high-throughput proximity extension assays (Olink Proteomics multiplex assay) | Longitudinal study on the associations between 92 biomarkers and mortality from any cause within 90 days after stroke. | sCD93 was significantly associated with increased mortality at 90 days after suffering an ischemic stroke (OR 4.30, CI 1.99–9.30; p value 0.0002). |
| Chandramouli C. et al., 2022 [28] | 182 patients of both sexes with HFpEF (mean age 74.2 years) | Serum sCD93 by high-throughput proximity extension assays (Olink Proseek Multiplexcardiovascular II and III) | Cross-sectional study on sex-related differences in biomarkers associated with coronary microvascular dysfunction. | In lasso-penalized regression analyses, sCD93 was associated with coronary microvascular dysfunction in men but not in women. |
| Helleberg S. et al., 2022 [29] | Case-control study (20 healthy controls, 67 patients with left HF and pulmonary hypertension, and 19 who underwent heart transplantation) | Serum sCD93 by high-throughput proximity extension assays (Olink Proteomics, Proseek Multiplex immunoassay reagent kits) | Cross-sectional and longitudinal study on differences in 67 inflammatory proteins in controls vs. left HF and pulmonary hypertension and in a subgroup before and after heart transplantation. | Patients with left HF and pulmonary hypertension showed higher levels of sCD93 compared with healthy controls (p < 0.0001). Patients who underwent heart transplantation had lower levels of sCD93 compared with the pre-transplantation timepoint (p < 0.0001). sCD93 levels after heart transplant were similar to those of healthy controls. |
| Snelder S.M. et al., 2022 [30] | 72 obese patients who underwent bariatric surgery | Serum sCD93 by high-throughput proximity extension assays (Olink Proteomics Cardiovascular III multiplex assay) | Longitudinal study to investigate cardiovascular biomarkers pre- and after bariatric surgery and their predictive ability for cardiac dysfunction reversal after surgery. | sCD93 levels significantly diminished from before to one year after bariatric surgery. Although not significant, a trend towards decreased levels of sCD93 in patients who had a reversal of pre-surgery cardiac dysfunction was observed (2200 vs. 2572, p = 0.22). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piani, F.; Tossetta, G.; Cara-Fuentes, G.; Agnoletti, D.; Marzioni, D.; Borghi, C. Diagnostic and Prognostic Role of CD93 in Cardiovascular Disease: A Systematic Review. Biomolecules 2023, 13, 910. https://doi.org/10.3390/biom13060910
Piani F, Tossetta G, Cara-Fuentes G, Agnoletti D, Marzioni D, Borghi C. Diagnostic and Prognostic Role of CD93 in Cardiovascular Disease: A Systematic Review. Biomolecules. 2023; 13(6):910. https://doi.org/10.3390/biom13060910
Chicago/Turabian StylePiani, Federica, Giovanni Tossetta, Gabriel Cara-Fuentes, Davide Agnoletti, Daniela Marzioni, and Claudio Borghi. 2023. "Diagnostic and Prognostic Role of CD93 in Cardiovascular Disease: A Systematic Review" Biomolecules 13, no. 6: 910. https://doi.org/10.3390/biom13060910
APA StylePiani, F., Tossetta, G., Cara-Fuentes, G., Agnoletti, D., Marzioni, D., & Borghi, C. (2023). Diagnostic and Prognostic Role of CD93 in Cardiovascular Disease: A Systematic Review. Biomolecules, 13(6), 910. https://doi.org/10.3390/biom13060910










