New Targets and Strategies for Rheumatoid Arthritis: From Signal Transduction to Epigenetic Aspect
Abstract
1. Introduction
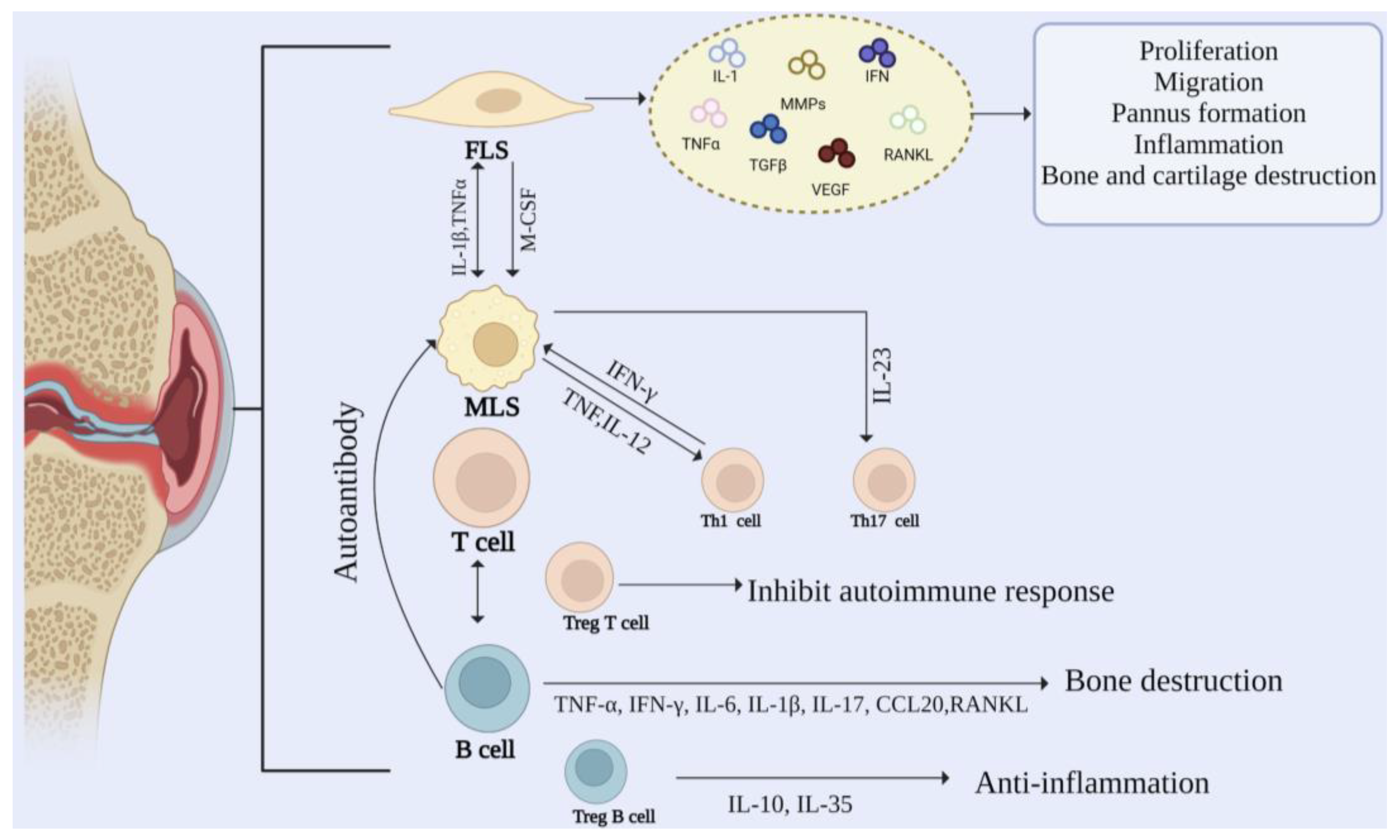
2. Cellular Mechanisms in RA
2.1. Fibroblast-Like Synovial (FLSs)
2.2. Macrophage-Like Synovial (MLSs)
2.3. T Cells
2.4. B Cells
3. Signaling Pathways in RA
4. Epigenetic Regulation in RA
4.1. Histone Modification
4.2. DNA Methylation
4.3. Micro RNA
| Targets | Effects | References |
|---|---|---|
| JMJD3 | Regulating the proliferation and migration ability of FLS through the AKT signaling pathway Regulating symptoms of CIA mice | [107,108] |
| GATA4 | Mediating the proliferation and migration ability of FLS through MAPK signaling pathway Promoting angiogenesis | [109] |
| SIRT3 | Regulating of ROS levels in miceInducing osteoarthritis in mice Inducing osteoarthritis in mice | [110,111] |
| SIRT4 | Regulating the expression of SOD1, SOD2, and catalase Targeting osteoarthritis with inflammatory factors | [112] |
| HDAC1 | Knockdown relieves joint swelling and synovial inflammation in arthritic mice | [115] |
| HDAC6 | Increase in the synovium tissues of adjuvant-induced arthritic rats | [116] |
| miR-137 | Decreasing expression is due to ROS activation of MAPK in AIA rats | [133] |
| miR-155 | Upregulating FOXO3 and reducing inflammation and proliferation of FLS | [134] |
| miR-146a | Targeting miR-146a reduces the invasion and migration of FLSs via the miR-146a/GATA6 axis | [135] |
| NAV2 | Mediating the proliferation and migration of MH7A via Wnt/β-catenin signaling pathway | [140,141] |
| miR-203 | Promoting the production of MMP1 and IL-6 induces RA | [142] |
| miR-19 | Inducing inflammation through regulation of TLR2, IL-6, and MMP3 in FLS stimulated by TNFα Having an anti-inflammatory effect in FLS stimulated by LPS by regulating IL-6 and IL-1β | [143,144,145] |
| miR-10a | Mediating the production of inflammatory factors through NF-κB in RA-FLS | [146] |
| miR-124a | Reducing the proliferation of synovial cells Relieving cartilage or bone destruction and the symptoms in AIA rats | [147] |
| miR-152 | Upregulating the expression of SFRP4 (negative regulator of Wnt signaling pathway) by targeting DNMT1, and reducing the proliferation of FLSs by inhibiting the activation of Wnt pathway | [148] |
| miR-375 | Decreasing the expression of FZD8 (a member of Wnt pathway), inhibiting the expression of IL-6 and IL-8, and relieving the symptoms of AIA rats | [149] |
5. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vesperini, V.; Lukas, C.; Fautrel, B.; Le Loet, X.; Rincheval, N.; Combe, B. Association of Tobacco Exposure and Reduction of Radiographic Progression in Early Rheumatoid Arthritis: Results from a French Multicenter Cohort. Arthritis Care Res. 2013, 65, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef]
- Qin, B.; Yang, M.; Fu, H.; Ma, N.; Wei, T.; Tang, Q.; Hu, Z.; Liang, Y.; Yang, Z.; Zhong, R. Body mass index and the risk of rheumatoid arthritis: A systematic review and dose-response meta-analysis. Arthritis Res. Ther. 2015, 17, 86. [Google Scholar] [CrossRef]
- Gioia, C.; Lucchino, B.; Tarsitano, M.G.; Iannuccelli, C.; Di Franco, M. Dietary Habits and Nutrition in Rheumatoid Arthritis: Can Diet Influence Disease Development and Clinical Manifestations? Nutrients 2020, 12, 1456. [Google Scholar] [CrossRef]
- Smith, M.D. The normal synovium. Open Rheumatol. J. 2011, 5, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.E.; Souza, P.R.; Colas, R.A.; Dalli, J. 13-Series resolvins mediate the leukocyte-platelet actions of atorvastatin and pravastatin in inflammatory arthritis. FASEB J. 2017, 31, 3636–3648. [Google Scholar] [CrossRef]
- Misharin, A.V.; Cuda, C.M.; Saber, R.; Turner, J.D.; Gierut, A.K.; Haines, G.K., 3rd; Berdnikovs, S.; Filer, A.; Clark, A.R.; Buckley, C.D.; et al. Nonclassical Ly6C(-) monocytes drive the development of inflammatory arthritis in mice. Cell Rep. 2014, 9, 591–604. [Google Scholar] [CrossRef]
- Thieblemont, N.; Wright, H.L.; Edwards, S.W.; Witko-Sarsat, V. Human neutrophils in auto-immunity. Semin. Immunol. 2016, 28, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Filer, A.; Pitzalis, C.; Buckley, C.D. Targeting the stromal microenvironment in chronic inflammation. Curr. Opin. Pharmacol. 2006, 6, 393–400. [Google Scholar] [CrossRef]
- Wojcik, P.; Gegotek, A.; Zarkovic, N.; Skrzydlewska, E. Oxidative Stress and Lipid Mediators Modulate Immune Cell Functions in Autoimmune Diseases. Int. J. Mol. Sci. 2021, 22, 723. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, W.; Wei, S.; Wei, D.; Li, R.; Liu, J.; Peng, L.; Yang, S.; Gao, Y.; Wu, C.; et al. Guizhi-Shaoyao-Zhimu decoction possesses anti-arthritic effects on type II collagen-induced arthritis in rats via suppression of inflammatory reactions, inhibition of invasion & migration and induction of apoptosis in synovial fibroblasts. Biomed. Pharmacother. 2019, 118, 109367. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Zhang, M.; Wei, S.; Li, R.; Gao, Y.; Peng, W.; Wu, C. Apoptosis Induction of Fibroblast-Like Synoviocytes Is an Important Molecular-Mechanism for Herbal Medicine along with its Active Components in Treating Rheumatoid Arthritis. Biomolecules 2019, 9, 795. [Google Scholar] [CrossRef] [PubMed]
- Bolam, A.; Manandhar, D.S.; Shrestha, P.; Ellis, M.; Costello, A.M. The effects of postnatal health education for mothers on infant care and family planning practices in Nepal: A randomised controlled trial. BMJ 1998, 316, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Mousavi, M.J.; Jamalzehi, S.; Alimohammadi, R.; Bezvan, M.H.; Mohammadi, H.; Aslani, S. Strategies toward rheumatoid arthritis therapy; the old and the new. J. Cell Physiol. 2019, 234, 10018–10031. [Google Scholar] [CrossRef]
- Ding, Q.; Hu, W.; Wang, R.; Yang, Q.; Zhu, M.; Li, M.; Cai, J.; Rose, P.; Mao, J.; Zhu, Y.Z. Signaling pathways in rheumatoid arthritis: Implications for targeted therapy. Signal Transduct. Target. Ther. 2023, 8, 68. [Google Scholar] [CrossRef]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; McShane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S.; et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988, 31, 315–324. [Google Scholar] [CrossRef]
- Filer, A. The fibroblast as a therapeutic target in rheumatoid arthritis. Curr. Opin. Pharmacol. 2013, 13, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, G.; Firestein, G.S. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat. Rev. Rheumatol. 2020, 16, 316–333. [Google Scholar] [CrossRef]
- Shin, Y.J.; Han, S.H.; Kim, D.S.; Lee, G.H.; Yoo, W.H.; Kang, Y.M.; Choi, J.Y.; Lee, Y.C.; Park, S.J.; Jeong, S.K.; et al. Autophagy induction and CHOP under-expression promotes survival of fibroblasts from rheumatoid arthritis patients under endoplasmic reticulum stress. Arthritis Res. Ther. 2010, 12, R19. [Google Scholar] [CrossRef]
- Hua, S.; Dias, T.H. Hypoxia-Inducible Factor (HIF) as a Target for Novel Therapies in Rheumatoid Arthritis. Front. Pharmacol. 2016, 7, 184. [Google Scholar] [CrossRef]
- Tsaltskan, V.; Firestein, G.S. Targeting fibroblast-like synoviocytes in rheumatoid arthritis. Curr. Opin. Pharmacol. 2022, 67, 102304. [Google Scholar] [CrossRef]
- Wu, Z.; Ma, D.; Yang, H.; Gao, J.; Zhang, G.; Xu, K.; Zhang, L. Fibroblast-like synoviocytes in rheumatoid arthritis: Surface markers and phenotypes. Int. Immunopharmacol. 2021, 93, 107392. [Google Scholar] [CrossRef]
- Bartok, B.; Firestein, G.S. Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunol. Rev. 2010, 233, 233–255. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Hong, W.; Zhang, P.; Wang, X.; Korner, H.; Wei, W. Ontology and Function of Fibroblast-Like and Macrophage-Like Synoviocytes: How Do They Talk to Each Other and Can They Be Targeted for Rheumatoid Arthritis Therapy? Front. Immunol. 2018, 9, 1467. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007, 7, 429–442. [Google Scholar] [CrossRef]
- Burger, D.; Dayer, J.M. The role of human T-lymphocyte-monocyte contact in inflammation and tissue destruction. Arthritis Res. 2002, 4 (Suppl. 3), S169–S176. [Google Scholar] [CrossRef]
- Mellado, M.; Martinez-Munoz, L.; Cascio, G.; Lucas, P.; Pablos, J.L.; Rodriguez-Frade, J.M. T Cell Migration in Rheumatoid Arthritis. Front. Immunol. 2015, 6, 384. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Kuroda, T.; Kobayashi, D. Cytokine Networks in the Pathogenesis of Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 10922. [Google Scholar] [CrossRef]
- Jang, S.; Kwon, E.J.; Lee, J.J. Rheumatoid Arthritis: Pathogenic Roles of Diverse Immune Cells. Int. J. Mol. Sci. 2022, 23, 905. [Google Scholar] [CrossRef]
- Onishi, R.M.; Gaffen, S.L. Interleukin-17 and its target genes: Mechanisms of interleukin-17 function in disease. Immunology 2010, 129, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Yang, G.; Liu, Q.; Wang, S.; Cui, D. Function and Role of Regulatory T Cells in Rheumatoid Arthritis. Front. Immunol. 2021, 12, 626193. [Google Scholar] [CrossRef]
- Li, X.; Zheng, Y. Regulatory T cell identity: Formation and maintenance. Trends Immunol. 2015, 36, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Floudas, A.; Canavan, M.; McGarry, T.; Mullan, R.; Nagpal, S.; Veale, D.J.; Fearon, U. ACPA Status Correlates with Differential Immune Profile in Patients with Rheumatoid Arthritis. Cells 2021, 10, 647. [Google Scholar] [CrossRef] [PubMed]
- Meednu, N.; Zhang, H.; Owen, T.; Sun, W.; Wang, V.; Cistrone, C.; Rangel-Moreno, J.; Xing, L.; Anolik, J.H. Production of RANKL by Memory B Cells: A Link Between B Cells and Bone Erosion in Rheumatoid Arthritis. Arthritis Rheumatol. 2016, 68, 805–816. [Google Scholar] [CrossRef]
- Filipowicz, W.; Bhattacharyya, S.N.; Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat. Rev. Genet. 2008, 9, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Thorarinsdottir, K.; Camponeschi, A.; Jonsson, C.; Granhagen Onnheim, K.; Nilsson, J.; Forslind, K.; Visentini, M.; Jacobsson, L.; Martensson, I.L.; Gjertsson, I. CD21(-/low) B cells associate with joint damage in rheumatoid arthritis patients. Scand. J. Immunol. 2019, 90, e12792. [Google Scholar] [CrossRef] [PubMed]
- Yanaba, K.; Bouaziz, J.D.; Haas, K.M.; Poe, J.C.; Fujimoto, M.; Tedder, T.F. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 2008, 28, 639–650. [Google Scholar] [CrossRef]
- Gomez-Puerta, J.A.; Celis, R.; Hernandez, M.V.; Ruiz-Esquide, V.; Ramirez, J.; Haro, I.; Canete, J.D.; Sanmarti, R. Differences in synovial fluid cytokine levels but not in synovial tissue cell infiltrate between anti-citrullinated peptide/protein antibody-positive and -negative rheumatoid arthritis patients. Arthritis Res. Ther. 2013, 15, R182. [Google Scholar] [CrossRef]
- Sun, W.; Meednu, N.; Rosenberg, A.; Rangel-Moreno, J.; Wang, V.; Glanzman, J.; Owen, T.; Zhou, X.; Zhang, H.; Boyce, B.F.; et al. B cells inhibit bone formation in rheumatoid arthritis by suppressing osteoblast differentiation. Nat. Commun. 2018, 9, 5127. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Gao, J.; Kang, J.; Wang, X.; Niu, Q.; Liu, J.; Zhang, L. B Cells in Rheumatoid Arthritis: Pathogenic Mechanisms and Treatment Prospects. Front. Immunol. 2021, 12, 750753. [Google Scholar] [CrossRef]
- Nishimura, R.; Hata, K.; Takahata, Y.; Murakami, T.; Nakamura, E.; Ohkawa, M.; Ruengsinpinya, L. Role of Signal Transduction Pathways and Transcription Factors in Cartilage and Joint Diseases. Int. J. Mol. Sci. 2020, 21, 1340. [Google Scholar] [CrossRef] [PubMed]
- Asahara, H.; Fujisawa, K.; Kobata, T.; Hasunuma, T.; Maeda, T.; Asanuma, M.; Ogawa, N.; Inoue, H.; Sumida, T.; Nishioka, K. Direct evidence of high DNA binding activity of transcription factor AP-1 in rheumatoid arthritis synovium. Arthritis Rheum. 1997, 40, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Chi, P.L.; Chen, Y.W.; Hsiao, L.D.; Chen, Y.L.; Yang, C.M. Heme oxygenase 1 attenuates interleukin-1beta-induced cytosolic phospholipase A2 expression via a decrease in NADPH oxidase/reactive oxygen species/activator protein 1 activation in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2012, 64, 2114–2125. [Google Scholar] [CrossRef]
- Thalhamer, T.; McGrath, M.A.; Harnett, M.M. MAPKs and their relevance to arthritis and inflammation. Rheumatology 2008, 47, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Yu, P.; Jiang, H.; Yuan, T.; Liu, N.C.; Tong, J.; Chen, H.Y.; Bao, N.R.; Zhao, J.N. Molecular hydrogen decelerates rheumatoid arthritis progression through inhibition of oxidative stress. Am. J. Transl. Res. 2016, 8, 4472–4477. [Google Scholar]
- Wang, M.; Zhu, S.; Peng, W.; Li, Q.; Li, Z.; Luo, M.; Feng, X.; Lin, Z.; Huang, J. Sonic hedgehog signaling drives proliferation of synoviocytes in rheumatoid arthritis: A possible novel therapeutic target. J. Immunol. Res. 2014, 2014, 401903. [Google Scholar] [CrossRef]
- Skoda, A.M.; Simovic, D.; Karin, V.; Kardum, V.; Vranic, S.; Serman, L. The role of the Hedgehog signaling pathway in cancer: A comprehensive review. Bosn. J. Basic Med. Sci. 2018, 18, 8–20. [Google Scholar] [CrossRef]
- Liu, F.; Feng, X.X.; Zhu, S.L.; Huang, H.Y.; Chen, Y.D.; Pan, Y.F.; June, R.R.; Zheng, S.G.; Huang, J.L. Sonic Hedgehog Signaling Pathway Mediates Proliferation and Migration of Fibroblast-Like Synoviocytes in Rheumatoid Arthritis via MAPK/ERK Signaling Pathway. Front. Immunol. 2018, 9, 2847. [Google Scholar] [CrossRef]
- Cici, D.; Corrado, A.; Rotondo, C.; Cantatore, F.P. Wnt Signaling and Biological Therapy in Rheumatoid Arthritis and Spondyloarthritis. Int. J. Mol. Sci. 2019, 20, 5552. [Google Scholar] [CrossRef] [PubMed]
- Maruotti, N.; Corrado, A.; Neve, A.; Cantatore, F.P. Systemic effects of Wnt signaling. J. Cell. Physiol. 2013, 228, 1428–1432. [Google Scholar] [CrossRef]
- Sen, M. Wnt signalling in rheumatoid arthritis. Rheumatology 2005, 44, 708–713. [Google Scholar] [CrossRef]
- Sen, M.; Reifert, J.; Lauterbach, K.; Wolf, V.; Rubin, J.S.; Corr, M.; Carson, D.A. Regulation of fibronectin and metalloproteinase expression by Wnt signaling in rheumatoid arthritis synoviocytes. Arthritis Rheum. 2002, 46, 2867–2877. [Google Scholar] [CrossRef]
- Tao, S.S.; Cao, F.; Sam, N.B.; Li, H.M.; Feng, Y.T.; Ni, J.; Wang, P.; Li, X.M.; Pan, H.F. Dickkopf-1 as a promising therapeutic target for autoimmune diseases. Clin. Immunol. 2022, 245, 109156. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Cai, J.; Chen, K.; Zhu, M.; Li, Z.; Liu, H.; Liu, T.; Mao, J.; Ding, Q.; Zhu, Y.Z. STAT3-NAV2 axis as a new therapeutic target for rheumatoid arthritis via activating SSH1L/Cofilin-1 signaling pathway. Signal Transduct. Target. Ther. 2022, 7, 209. [Google Scholar] [CrossRef] [PubMed]
- Kiu, H.; Nicholson, S.E. Biology and significance of the JAK/STAT signalling pathways. Growth Factors 2012, 30, 88–106. [Google Scholar] [CrossRef] [PubMed]
- Baldini, C.; Moriconi, F.R.; Galimberti, S.; Libby, P.; De Caterina, R. The JAK-STAT pathway: An emerging target for cardiovascular disease in rheumatoid arthritis and myeloproliferative neoplasms. Eur. Heart J. 2021, 42, 4389–4400. [Google Scholar] [CrossRef]
- Villarino, A.V.; Kanno, Y.; O’Shea, J.J. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat. Immunol. 2017, 18, 374–384. [Google Scholar] [CrossRef]
- Delgoffe, G.M.; Vignali, D.A. STAT heterodimers in immunity: A mixed message or a unique signal? JAKSTAT 2013, 2, e23060. [Google Scholar] [CrossRef]
- Simon, L.S.; Taylor, P.C.; Choy, E.H.; Sebba, A.; Quebe, A.; Knopp, K.L.; Porreca, F. The Jak/STAT pathway: A focus on pain in rheumatoid arthritis. Semin. Arthritis Rheum. 2021, 51, 278–284. [Google Scholar] [CrossRef]
- Sun, K.; Luo, J.; Guo, J.; Yao, X.; Jing, X.; Guo, F. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: A narrative review. Osteoarthr. Cartil. 2020, 28, 400–409. [Google Scholar] [CrossRef]
- Ito, Y.; Hart, J.R.; Vogt, P.K. Isoform-specific activities of the regulatory subunits of phosphatidylinositol 3-kinases—Potentially novel therapeutic targets. Expert Opin. Ther. Targets 2018, 22, 869–877. [Google Scholar] [CrossRef]
- Ersahin, T.; Tuncbag, N.; Cetin-Atalay, R. The PI3K/AKT/mTOR interactive pathway. Mol. Biosyst. 2015, 11, 1946–1954. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Q.; Zhang, Y.; Liu, D.; Qian, Y.Y.; Zhang, H.; Guo, S.Y.; Sunagawa, M.; Hisamitsu, T.; Liu, Y.Q. PI3 kinase/Akt/HIF-1alpha pathway is associated with hypoxia-induced epithelial-mesenchymal transition in fibroblast-like synoviocytes of rheumatoid arthritis. Mol. Cell. Biochem. 2013, 372, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lee, S.W.; Kim, H.Y.; Lee, W.S.; Hong, K.W.; Kim, C.D. HMGB1 induces angiogenesis in rheumatoid arthritis via HIF-1alpha activation. Eur. J. Immunol. 2015, 45, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Mysler, E.; Moots, R.J. Etanercept for the treatment of rheumatoid arthritis. Immunotherapy 2018, 10, 433–445. [Google Scholar] [CrossRef]
- Feldman, M.; Taylor, P.; Paleolog, E.; Brennan, F.M.; Maini, R.N. Anti-TNF alpha therapy is useful in rheumatoid arthritis and Crohn’s disease: Analysis of the mechanism of action predicts utility in other diseases. Transplant. Proc. 1998, 30, 4126–4127. [Google Scholar] [CrossRef]
- Gholami, A.; Azizpoor, J.; Aflaki, E.; Rezaee, M.; Keshavarz, K. Cost-Effectiveness Analysis of Biopharmaceuticals for Treating Rheumatoid Arthritis: Infliximab, Adalimumab, and Etanercept. BioMed Res. Int. 2021, 2021, 4450162. [Google Scholar] [CrossRef]
- Cvetkovic, R.S.; Scott, L.J. Adalimumab: A review of its use in adult patients with rheumatoid arthritis. BioDrugs 2006, 20, 293–311. [Google Scholar] [CrossRef]
- Zhao, S.; Chadwick, L.; Mysler, E.; Moots, R.J. Review of Biosimilar Trials and Data on Adalimumab in Rheumatoid Arthritis. Curr. Rheumatol. Rep. 2018, 20, 57. [Google Scholar] [CrossRef]
- Cohen, M.D.; Keystone, E.C. Intravenous golimumab in rheumatoid arthritis. Expert Rev. Clin. Immunol. 2014, 10, 823–830. [Google Scholar] [CrossRef]
- Markatseli, T.E.; Papagoras, C.; Nikoli, A.; Voulgari, P.V.; Drosos, A.A. Drosos. Certolizumab for rheumatoid arthritis. Clin. Exp. Rheumatol. 2014, 32, 415–423. [Google Scholar] [PubMed]
- Mok, C.C. Rituximab for the treatment of rheumatoid arthritis: An update. Drug Des. Dev. Ther. 2013, 8, 87–100. [Google Scholar] [CrossRef]
- Blair, H.A.; Deeks, E.D. Abatacept: A Review in Rheumatoid Arthritis. Drugs 2017, 77, 1221–1233. [Google Scholar] [CrossRef]
- Scott, L.J. Tocilizumab: A Review in Rheumatoid Arthritis. Drugs 2017, 77, 1865–1879. [Google Scholar] [CrossRef]
- Pelechas, E.; Voulgari, P.V.; Drosos, A.A. Sirukumab: A promising therapy for rheumatoid arthritis. Expert Opin. Biol. Ther. 2017, 17, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.; Bourne, T.; Meier, C.; Carrington, B.; Gelinas, R.; Henry, A.; Popplewell, A.; Adams, R.; Baker, T.; Rapecki, S.; et al. Discovery and characterization of olokizumab: A humanized antibody targeting interleukin-6 and neutralizing gp130-signaling. MAbs 2014, 6, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Serio, I.; Tovoli, F. Rheumatoid arthritis: New monoclonal antibodies. Drugs Today 2018, 54, 219–230. [Google Scholar] [CrossRef]
- Bauer, E.; Lucier, J.; Furst, D.E. Brodalumab -an IL-17RA monoclonal antibody for psoriasis and psoriatic arthritis. Expert Opin. Biol. Ther. 2015, 15, 883–893. [Google Scholar] [CrossRef]
- Dhillon, S. Tofacitinib: A Review in Rheumatoid Arthritis. Drugs 2017, 77, 1987–2001. [Google Scholar] [CrossRef]
- Baricitinib for rheumatoid arthritis. Aust. Prescr. 2019, 42, 34–35. [CrossRef]
- Serhal, L.; Edwards, C.J. Upadacitinib for the treatment of rheumatoid arthritis. Expert Rev. Clin. Immunol. 2019, 15, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kavanaugh, A.; Wicklund, J.; McInnes, I.B. Filgotinib, a novel JAK1-preferential inhibitor for the treatment of rheumatoid arthritis: An overview from clinical trials. Mod. Rheumatol. 2022, 32, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Feng, Q.; Tan, X.; Guo, M. JAK3-selective inhibitor peficitinib for the treatment of rheumatoid arthritis. Expert Rev. Clin. Pharmacol. 2019, 12, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, L.; Ding, Y.; Tao, X.; Ji, C.; Dong, X.; Lu, J.; Wu, L.; Wang, R.; Lu, Q.; et al. Efficacy and Safety of SHR0302, a Highly Selective Janus Kinase 1 Inhibitor, in Patients with Moderate to Severe Atopic Dermatitis: A Phase II Randomized Clinical Trial. Am. J. Clin. Dermatol. 2021, 22, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Genovese, M.C.; van Vollenhoven, R.F.; Pacheco-Tena, C.; Zhang, Y.; Kinnman, N. VX-509 (Decernotinib), an Oral Selective JAK-3 Inhibitor, in Combination with Methotrexate in Patients With Rheumatoid Arthritis. Arthritis Rheumatol. 2016, 68, 46–55. [Google Scholar] [CrossRef]
- Peng, S.; Hu, C.; Liu, X.; Lei, L.; He, G.; Xiong, C.; Wu, W. Rhoifolin regulates oxidative stress and proinflammatory cytokine levels in Freund’s adjuvant-induced rheumatoid arthritis via inhibition of NF-kappaB. Braz. J. Med. Biol. Res. 2020, 53, e9489. [Google Scholar] [CrossRef]
- Puppala, E.R.; Jain, S.; Saha, P.; Rachamalla, M.; Np, S.; Yalamarthi, S.S.; Abubakar, M.; Chaudhary, A.; Chamundeswari, D.; Usn, M.; et al. Perillyl alcohol attenuates rheumatoid arthritis via regulating TLR4/NF-kappaB and Keap1/Nrf2 signaling pathways: A comprehensive study onin-vitro and in-vivo experimental models. Phytomedicine 2022, 97, 153926. [Google Scholar] [CrossRef]
- Wang, G.; Xie, X.; Yuan, L.; Qiu, J.; Duan, W.; Xu, B.; Chen, X. Resveratrol ameliorates rheumatoid arthritis via activation of SIRT1-Nrf2 signaling pathway. Biofactors 2020, 46, 441–453. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, G.; Wang, T.; Cao, W.; Zhang, L.; Chen, X. Nrf2-Keap1 pathway-mediated effects of resveratrol on oxidative stress and apoptosis in hydrogen peroxide-treated rheumatoid arthritis fibroblast-like synoviocytes. Ann. N. Y. Acad. Sci. 2019, 1457, 166–178. [Google Scholar] [CrossRef]
- Xia, Z.B.; Yuan, Y.J.; Zhang, Q.H.; Li, H.; Dai, J.L.; Min, J.K. Salvianolic Acid B Suppresses Inflammatory Mediator Levels by Downregulating NF-kappaB in a Rat Model of Rheumatoid Arthritis. Med. Sci. Monit. 2018, 24, 2524–2532. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, L.; Chitti, R.; Sreeharsha, N.; Mishra, A.; Gubbiyappa, S.K.; Singh, Y. Wogonin ameliorate complete Freund’s adjuvant induced rheumatoid arthritis via targeting NF-kappaB/MAPK signaling pathway. Biofactors 2020, 46, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Fan, X.; Qu, Y.; Tang, M.; Huang, Y.; Peng, Y.; Fu, Q. Magnoflorine attenuates inflammatory responses in RA by regulating the PI3K/Akt/NF-kappaB and Keap1-Nrf2/HO-1 signalling pathways in vivo and in vitro. Phytomedicine 2022, 104, 154339. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhang, L.C.; Zhao, R.J.; Pu, L.M.; Chen, K.Y.; Nasim, A.A.; Leung, E.L.; Fan, X.X. Chelerythrine ameliorates rheumatoid arthritis by modulating the AMPK/mTOR/ULK-1 signaling pathway. Phytomedicine 2022, 104, 154140. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Xie, N.; Hou, Y.; Chen, X.; Hu, Y.; Zhang, Y.; Meng, X.; Wang, X.; Tang, C. The enhanced mitochondrial dysfunction by cantleyoside confines inflammatory response and promotes apoptosis of human HFLS-RA cell line via AMPK/Sirt 1/NF-kappaB pathway activation. Biomed. Pharmacother. 2022, 149, 112847. [Google Scholar] [CrossRef]
- Wu, Z.M.; Xiang, Y.R.; Zhu, X.B.; Shi, X.D.; Chen, S.; Wan, X.; Guo, J. Icariin represses the inflammatory responses and survival of rheumatoid arthritis fibroblast-like synoviocytes by regulating the TRIB1/TLR2/NF-kB pathway. Int. Immunopharmacol. 2022, 110, 108991. [Google Scholar] [CrossRef]
- Wang, X.H.; Dai, C.; Wang, J.; Liu, R.; Li, L.; Yin, Z.S. Therapeutic effect of neohesperidin on TNF-alpha-stimulated human rheumatoid arthritis fibroblast-like synoviocytes. Chin. J. Nat. Med. 2021, 19, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Mittal, S.; Gulia, R.; Alam, K.; Saha, T.K.; Arif, Z.; Nafees, K.A.; Al-Shaghdali, K.; Ahmad, S. Therapeutic role of hesperidin in collagen-induced rheumatoid arthritis through antiglycation and antioxidant activities. Cell Biochem. Funct. 2022, 40, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.; Yang, J.; Zhang, L.; Liu, J.; Xu, S.; Wang, M.; Zhang, L.; Sun, Y.; Yan, W.; Hou, G.; et al. Celastrol inhibits rheumatoid arthritis through the ROS-NF-kappaB-NLRP3 inflammasome axis. Int. Immunopharmacol. 2021, 98, 107879. [Google Scholar] [CrossRef]
- Yang, J.; Liu, J.; Li, J.; Jing, M.; Zhang, L.; Sun, M.; Wang, Q.; Sun, H.; Hou, G.; Wang, C.; et al. Celastrol inhibits rheumatoid arthritis by inducing autophagy via inhibition of the PI3K/AKT/mTOR signaling pathway. Int. Immunopharmacol. 2022, 112, 109241. [Google Scholar] [CrossRef]
- Villa, T.; Kim, M.; Oh, S. Fangchinoline Has an Anti-Arthritic Effect in Two Animal Models and in IL-1beta-Stimulated Human FLS Cells. Biomol. Ther. 2020, 28, 414–422. [Google Scholar] [CrossRef]
- Liao, K.; Su, X.; Lei, K.; Liu, Z.; Lu, L.; Wu, Q.; Pan, H.; Huang, Q.; Zhao, Y.; Wang, M.; et al. Sinomenine protects bone from destruction to ameliorate arthritis via activating p62(Thr269/Ser272)-Keap1-Nrf2 feedback loop. Biomed. Pharmacother. 2021, 135, 111195. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhang, Q.; Zeng, F.; Cai, M.; Ding, D. The protective effect of gentisic acid on rheumatoid arthritis via the RAF/ERK signaling pathway. J. Orthop. Surg. Res. 2022, 17, 109. [Google Scholar] [CrossRef]
- Xu, Z.; Shang, W.; Zhao, Z.; Zhang, B.; Liu, C.; Cai, H. Curcumin alleviates rheumatoid arthritis progression through the phosphatidylinositol 3-kinase/protein kinase B pathway: An in vitro and in vivo study. Bioengineered 2022, 13, 12899–12911. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.; Gay, S. Epigenetics in rheumatoid arthritis. Curr. Opin. Rheumatol. 2015, 27, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Nemtsova, M.V.; Zaletaev, D.V.; Bure, I.V.; Mikhaylenko, D.S.; Kuznetsova, E.B.; Alekseeva, E.A.; Beloukhova, M.I.; Deviatkin, A.A.; Lukashev, A.N.; Zamyatnin, A.A., Jr. Epigenetic Changes in the Pathogenesis of Rheumatoid Arthritis. Front. Genet. 2019, 10, 570. [Google Scholar] [CrossRef]
- Jia, W.; Wu, W.; Yang, D.; Xiao, C.; Su, Z.; Huang, Z.; Li, Z.; Qin, M.; Huang, M.; Liu, S.; et al. Histone demethylase JMJD3 regulates fibroblast-like synoviocyte-mediated proliferation and joint destruction in rheumatoid arthritis. FASEB J. 2018, 32, 4031–4042. [Google Scholar] [CrossRef]
- Wu, W.; Qin, M.; Jia, W.; Huang, Z.; Li, Z.; Yang, D.; Huang, M.; Xiao, C.; Long, F.; Mao, J.; et al. Cystathionine-γ-lyase ameliorates the histone demethylase JMJD3-mediated autoimmune response in rheumatoid arthritis. Cell. Mol. Immunol. 2019, 16, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Wu, W.; Yang, D.; Xiao, C.; Huang, M.; Long, F.; Su, Z.; Qin, M.; Liu, X.; Zhu, Y.Z. GATA4 regulates angiogenesis and persistence of inflammation in rheumatoid arthritis. Cell Death Dis. 2018, 9, 503. [Google Scholar] [CrossRef]
- Jing, E.; Emanuelli, B.; Hirschey, M.D.; Boucher, J.; Lee, K.Y.; Lombard, D.; Verdin, E.M.; Kahn, C.R. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc. Natl. Acad. Sci. USA 2011, 108, 14608–14613. [Google Scholar] [CrossRef]
- Tseng, A.H.; Shieh, S.S.; Wang, D.L. SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free. Radic. Biol. Med. 2013, 63, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.; Grether-Beck, S.; Singh, M.; Kuck, F.; Jakob, S.; Kefalas, A.; Altinoluk-Hambuchen, S.; Graffmann, N.; Schneider, M.; Lindecke, A.; et al. MicroRNA-15b regulates mitochondrial ROS production and the senescence-associated secretory phenotype through sirtuin 4/SIRT4. Aging 2016, 8, 484–505. [Google Scholar] [CrossRef] [PubMed]
- Angiolilli, C.; Grabiec, A.M.; Ferguson, B.S.; Ospelt, C.; Malvar Fernandez, B.; van Es, I.E.; van Baarsen, L.G.; Gay, S.; McKinsey, T.A.; Tak, P.P.; et al. Inflammatory cytokines epigenetically regulate rheumatoid arthritis fibroblast-like synoviocyte activation by suppressing HDAC5 expression. Ann. Rheum. Dis. 2016, 75, 430–438. [Google Scholar] [CrossRef]
- Park, J.K.; Shon, S.; Yoo, H.J.; Suh, D.H.; Bae, D.; Shin, J.; Jun, J.H.; Ha, N.; Song, H.; Choi, Y.I.; et al. Inhibition of histone deacetylase 6 suppresses inflammatory responses and invasiveness of fibroblast-like-synoviocytes in inflammatory arthritis. Arthritis Res. Ther. 2021, 23, 177. [Google Scholar] [CrossRef] [PubMed]
- Goschl, L.; Preglej, T.; Boucheron, N.; Saferding, V.; Muller, L.; Platzer, A.; Hirahara, K.; Shih, H.Y.; Backlund, J.; Matthias, P.; et al. Histone deacetylase 1 (HDAC1): A key player of T cell-mediated arthritis. J. Autoimmun. 2020, 108, 102379. [Google Scholar] [CrossRef]
- Li, M.; Hu, W.; Wang, R.; Li, Z.; Yu, Y.; Zhuo, Y.; Zhang, Y.; Wang, Z.; Qiu, Y.; Chen, K.; et al. Sp1 S-Sulfhydration Induced by Hydrogen Sulfide Inhibits Inflammation via HDAC6/MyD88/NF-κB Signaling Pathway in Adjuvant-Induced Arthritis. Antioxidants 2022, 11, 732. [Google Scholar] [CrossRef]
- Chung, Y.L.; Lee, M.Y.; Wang, A.J.; Yao, L.F. A therapeutic strategy uses histone deacetylase inhibitors to modulate the expression of genes involved in the pathogenesis of rheumatoid arthritis. Mol. Ther. 2003, 8, 707–717. [Google Scholar] [CrossRef]
- Nishida, K.; Komiyama, T.; Miyazawa, S.; Shen, Z.N.; Furumatsu, T.; Doi, H.; Yoshida, A.; Yamana, J.; Yamamura, M.; Ninomiya, Y.; et al. Histone deacetylase inhibitor suppression of autoantibody-mediated arthritis in mice via regulation of p16INK4a and p21(WAF1/Cip1) expression. Arthritis Rheum. 2004, 50, 3365–3376. [Google Scholar] [CrossRef]
- Park, J.K.; Jang, Y.J.; Oh, B.R.; Shin, J.; Bae, D.; Ha, N.; Choi, Y.I.; Youn, G.S.; Park, J.; Lee, E.Y.; et al. Therapeutic potential of CKD-506, a novel selective histone deacetylase 6 inhibitor, in a murine model of rheumatoid arthritis. Arthritis Res. Ther. 2020, 22, 176. [Google Scholar] [CrossRef]
- Oh, B.R.; Suh, D.H.; Bae, D.; Ha, N.; Choi, Y.I.; Yoo, H.J.; Park, J.K.; Lee, E.Y.; Lee, E.B.; Song, Y.W. Therapeutic effect of a novel histone deacetylase 6 inhibitor, CKD-L, on collagen-induced arthritis in vivo and regulatory T cells in rheumatoid arthritis in vitro. Arthritis Res. Ther. 2017, 19, 154. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.; Sun, X.; Wang, Z.; Guo, W.; Hu, F.; Yao, H.; Cao, X.; Jin, J.; Wang, P.G.; et al. Therapeutic effects of NK-HDAC-1, a novel histone deacetylase inhibitor, on collagen-induced arthritis through the induction of apoptosis of fibroblast-like synoviocytes. Inflammation 2013, 36, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Ciechomska, M.; Roszkowski, L.; Maslinski, W. DNA Methylation as a Future Therapeutic and Diagnostic Target in Rheumatoid Arthritis. Cells 2019, 8, 953. [Google Scholar] [CrossRef]
- Karouzakis, E.; Gay, R.E.; Michel, B.A.; Gay, S.; Neidhart, M. DNA hypomethylation in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2009, 60, 3613–3622. [Google Scholar] [CrossRef] [PubMed]
- Richardson, B. Effect of an inhibitor of DNA methylation on T cells. II. 5-Azacytidine induces self-reactivity in antigen-specific T4+ cells. Hum. Immunol. 1986, 17, 456–470. [Google Scholar] [CrossRef] [PubMed]
- Nile, C.J.; Read, R.C.; Akil, M.; Duff, G.W.; Wilson, A.G. Methylation status of a single CpG site in the IL6 promoter is related to IL6 messenger RNA levels and rheumatoid arthritis. Arthritis Rheum. 2008, 58, 2686–2693. [Google Scholar] [CrossRef]
- Richardson, B.; Scheinbart, L.; Strahler, J.; Gross, L.; Hanash, S.; Johnson, M. Evidence for impaired T cell DNA methylation in systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 1990, 33, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Pieper, J.; Johansson, S.; Snir, O.; Linton, L.; Rieck, M.; Buckner, J.H.; Winqvist, O.; van Vollenhoven, R.; Malmstrom, V. Peripheral and site-specific CD4+ CD28null T cells from rheumatoid arthritis patients show distinct characteristics. Scand. J. Immunol. 2014, 79, 149–155. [Google Scholar] [CrossRef]
- Ai, R.; Boyle, D.L.; Wang, W.; Firestein, G.S. Distinct DNA Methylation Patterns of Rheumatoid Arthritis Peripheral Blood and Synovial Tissue T Cells. ACR Open Rheumatol. 2021, 3, 127–132. [Google Scholar] [CrossRef]
- Kolarz, B.; Ciesla, M.; Dryglewska, M.; Majdan, M. Peptidyl Arginine Deiminase Type 4 Gene Promoter Hypo-Methylation in Rheumatoid Arthritis. J. Clin. Med. 2020, 9, 2049. [Google Scholar] [CrossRef]
- Fu, L.H.; Ma, C.L.; Cong, B.; Li, S.J.; Chen, H.Y.; Zhang, J.G. Hypomethylation of proximal CpG motif of interleukin-10 promoter regulates its expression in human rheumatoid arthritis. Acta Pharmacol. Sin. 2011, 32, 1373–1380. [Google Scholar] [CrossRef]
- Evangelatos, G.; Fragoulis, G.E.; Koulouri, V.; Lambrou, G.I. MicroRNAs in rheumatoid arthritis: From pathogenesis to clinical impact. Autoimmun. Rev. 2019, 18, 102391. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, C.; Jia, X.; Yu, J. Circulating Exosomal miR-17 Inhibits the Induction of Regulatory T Cells via Suppressing TGFBR II Expression in Rheumatoid Arthritis. Cell. Physiol. Biochem. 2018, 50, 1754–1763. [Google Scholar] [CrossRef]
- Tsai, M.H.; Chi, M.C.; Hsu, J.F.; Lee, I.T.; Lin, K.M.; Fang, M.L.; Lee, M.H.; Lee, C.W.; Liu, J.F. Urban Particulate Matter Enhances ROS/IL-6/COX-II Production by Inhibiting MicroRNA-137 in Synovial Fibroblast of Rheumatoid Arthritis. Cells 2020, 9, 1378. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Feng, X.; Wang, W.; Zhao, X.; Li, X. Paeonol protects against TNF-α-induced proliferation and cytokine release of rheumatoid arthritis fibroblast-like synoviocytes by upregulating FOXO3 through inhibition of miR-155 expression. Inflamm. Res. 2017, 66, 603–610. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, B.; Peng, X.; Wang, C.; Wang, K.; Han, F.; Xu, J. Quercetin suppresses migration and invasion by targeting miR-146a/GATA6 axis in fibroblast-like synoviocytes of rheumatoid arthritis. Immunopharmacol. Immunotoxicol. 2020, 42, 221–227. [Google Scholar] [CrossRef]
- Pauley, K.M.; Cha, S. miRNA-146a in rheumatoid arthritis: A new therapeutic strategy. Immunotherapy 2011, 3, 829–831. [Google Scholar] [CrossRef] [PubMed]
- Mucientes, A.; Lisbona, J.M.; Mena-Vázquez, N.; Ruiz-Limón, P.; Manrique-Arija, S.; Fernández-Nebro, A. miRNA-Mediated Epigenetic Regulation of Treatment Response in RA Patients-A Systematic Review. Int. J. Mol. Sci. 2022, 23, 12989. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Pillman, K.A.; Hayball, J.; Su, Y.W.; Xian, C.J. Differentially expressed miRNAs in bone after methotrexate treatment. J. Cell. Physiol. 2022, 237, 965–982. [Google Scholar] [CrossRef]
- Bure, I.V.; Mikhaylenko, D.S.; Kuznetsova, E.B.; Alekseeva, E.A.; Bondareva, K.I.; Kalinkin, A.I.; Lukashev, A.N.; Tarasov, V.V.; Zamyatnin, A.A., Jr.; Nemtsova, M.V. Analysis of miRNA Expression in Patients with Rheumatoid Arthritis during Olokizumab Treatment. J. Pers. Med. 2020, 10, 205. [Google Scholar] [CrossRef]
- Wang, R.; Li, M.; Ding, Q.; Cai, J.; Yu, Y.; Liu, X.; Mao, J.; Zhu, Y.Z. Neuron navigator 2 is a novel mediator of rheumatoid arthritis. Cell. Mol. Immunol. 2021, 18, 2288–2289. [Google Scholar] [CrossRef]
- Wang, R.; Li, M.; Wu, W.; Qiu, Y.; Hu, W.; Li, Z.; Wang, Z.; Yu, Y.; Liao, J.; Sun, W.; et al. NAV2 positively modulates inflammatory response of fibroblast-like synoviocytes through activating Wnt/β-catenin signaling pathway in rheumatoid arthritis. Clin. Transl. Med. 2021, 11, e376. [Google Scholar] [CrossRef] [PubMed]
- Stanczyk, J.; Ospelt, C.; Karouzakis, E.; Filer, A.; Raza, K.; Kolling, C.; Gay, R.; Buckley, C.D.; Tak, P.P.; Gay, S.; et al. Altered expression of microRNA-203 in rheumatoid arthritis synovial fibroblasts and its role in fibroblast activation. Arthritis Rheum. 2011, 63, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Philippe, L.; Alsaleh, G.; Suffert, G.; Meyer, A.; Georgel, P.; Sibilia, J.; Wachsmann, D.; Pfeffer, S. TLR2 expression is regulated by microRNA miR-19 in rheumatoid fibroblast-like synoviocytes. J. Immunol. 2012, 188, 454–461. [Google Scholar] [CrossRef]
- Gantier, M.P.; Stunden, H.J.; McCoy, C.E.; Behlke, M.A.; Wang, D.; Kaparakis-Liaskos, M.; Sarvestani, S.T.; Yang, Y.H.; Xu, D.; Corr, S.C.; et al. A miR-19 regulon that controls NF-κB signaling. Nucleic Acids Res. 2012, 40, 8048–8058. [Google Scholar] [CrossRef] [PubMed]
- Philippe, L.; Alsaleh, G.; Pichot, A.; Ostermann, E.; Zuber, G.; Frisch, B.; Sibilia, J.; Pfeffer, S.; Bahram, S.; Wachsmann, D.; et al. MiR-20a regulates ASK1 expression and TLR4-dependent cytokine release in rheumatoid fibroblast-like synoviocytes. Ann. Rheum. Dis. 2013, 72, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Mu, N.; Gu, J.; Huang, T.; Zhang, C.; Shu, Z.; Li, M.; Hao, Q.; Li, W.; Zhang, W.; Zhao, J.; et al. A novel NF-κB/YY1/microRNA-10a regulatory circuit in fibroblast-like synoviocytes regulates inflammation in rheumatoid arthritis. Sci. Rep. 2016, 6, 20059. [Google Scholar] [CrossRef]
- Nakamachi, Y.; Ohnuma, K.; Uto, K.; Noguchi, Y.; Saegusa, J.; Kawano, S. MicroRNA-124 inhibits the progression of adjuvant-induced arthritis in rats. Ann. Rheum. Dis. 2016, 75, 601–608. [Google Scholar] [CrossRef]
- Miao, C.G.; Yang, Y.Y.; He, X.; Huang, C.; Huang, Y.; Qin, D.; Du, C.L.; Li, J. MicroRNA-152 modulates the canonical Wnt pathway activation by targeting DNA methyltransferase 1 in arthritic rat model. Biochimie 2014, 106, 149–156. [Google Scholar] [CrossRef]
- Miao, C.G.; Shi, W.J.; Xiong, Y.Y.; Yu, H.; Zhang, X.L.; Qin, M.S.; Du, C.L.; Song, T.W.; Li, J. miR-375 regulates the canonical Wnt pathway through FZD8 silencing in arthritis synovial fibroblasts. Immunol. Lett. 2015, 164, 1–10. [Google Scholar] [CrossRef]
- McNeill, E.M.; Roos, K.P.; Moechars, D.; Clagett-Dame, M. Nav2 is necessary for cranial nerve development and blood pressure regulation. Neural Dev. 2010, 5, 6. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Hore, Z.; Pattison, L.A.; Lalnunhlimi, S.; Bhebhe, C.N.; Callejo, G.; Bulmer, D.C.; Taams, L.S.; Denk, F.; Smith, E.S.J. Sensitization of knee-innervating sensory neurons by tumor necrosis factor-α-activated fibroblast-like synoviocytes: An in vitro, coculture model of inflammatory pain. Pain 2020, 161, 2129–2141. [Google Scholar] [CrossRef]
- Wu, W.J.; Jia, W.W.; Liu, X.H.; Pan, L.L.; Zhang, Q.Y.; Yang, D.; Shen, X.Y.; Liu, L.; Zhu, Y.Z. S-propargyl-cysteine attenuates inflammatory response in rheumatoid arthritis by modulating the Nrf2-ARE signaling pathway. Redox Biol. 2016, 10, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, Z.; Yang, Q.; Ding, Q.; Wang, R.; Li, Z.; Fang, Y.; Liao, J.; Qi, W.; Chen, K.; et al. A novel dendritic mesoporous silica based sustained hydrogen sulfide donor for the alleviation of adjuvant-induced inflammation in rats. Drug Deliv. 2021, 28, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, Z.; Ding, Q.; Yu, X.; Yang, Q.; Wang, R.; Fang, Y.; Qi, W.; Liao, J.; Hu, W.; et al. The Preparation of a Novel Poly(Lactic Acid)-Based Sustained H(2)S Releasing Microsphere for Rheumatoid Arthritis Alleviation. Pharmaceutics 2021, 13, 742. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Du, J.; Pu, X.; Zheng, L.; Chen, S.; Wang, N.; Li, J.; Chen, S.; Pan, S.; Shen, B. The Gut Microbiome and Metabolites Are Altered and Interrelated in Patients with Rheumatoid Arthritis. Front. Cell. Infect. Microbiol. 2021, 11, 763507. [Google Scholar] [CrossRef]
- Bergot, A.S.; Giri, R.; Thomas, R. The microbiome and rheumatoid arthritis. Best Pr. Res. Clin. Rheumatol. 2019, 33, 101497. [Google Scholar] [CrossRef]
- Shaw, A.M.; Qasem, A.; Naser, S.A. Modulation of PTPN2/22 Function by Spermidine in CRISPR-Cas9-Edited T-Cells Associated with Crohn’s Disease and Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 8883. [Google Scholar] [CrossRef]
- Ding, J.; Orozco, G. Identification of rheumatoid arthritis causal genes using functional genomics. Scand. J. Immunol. 2019, 89, e12753. [Google Scholar] [CrossRef]
- Lee, M.H.; Shin, J.I.; Yang, J.W.; Lee, K.H.; Cha, D.H.; Hong, J.B.; Park, Y.; Choi, E.; Tizaoui, K.; Koyanagi, A.; et al. Genome Editing Using CRISPR-Cas9 and Autoimmune Diseases: A Comprehensive Review. Int. J. Mol. Sci. 2022, 23, 1337. [Google Scholar] [CrossRef]
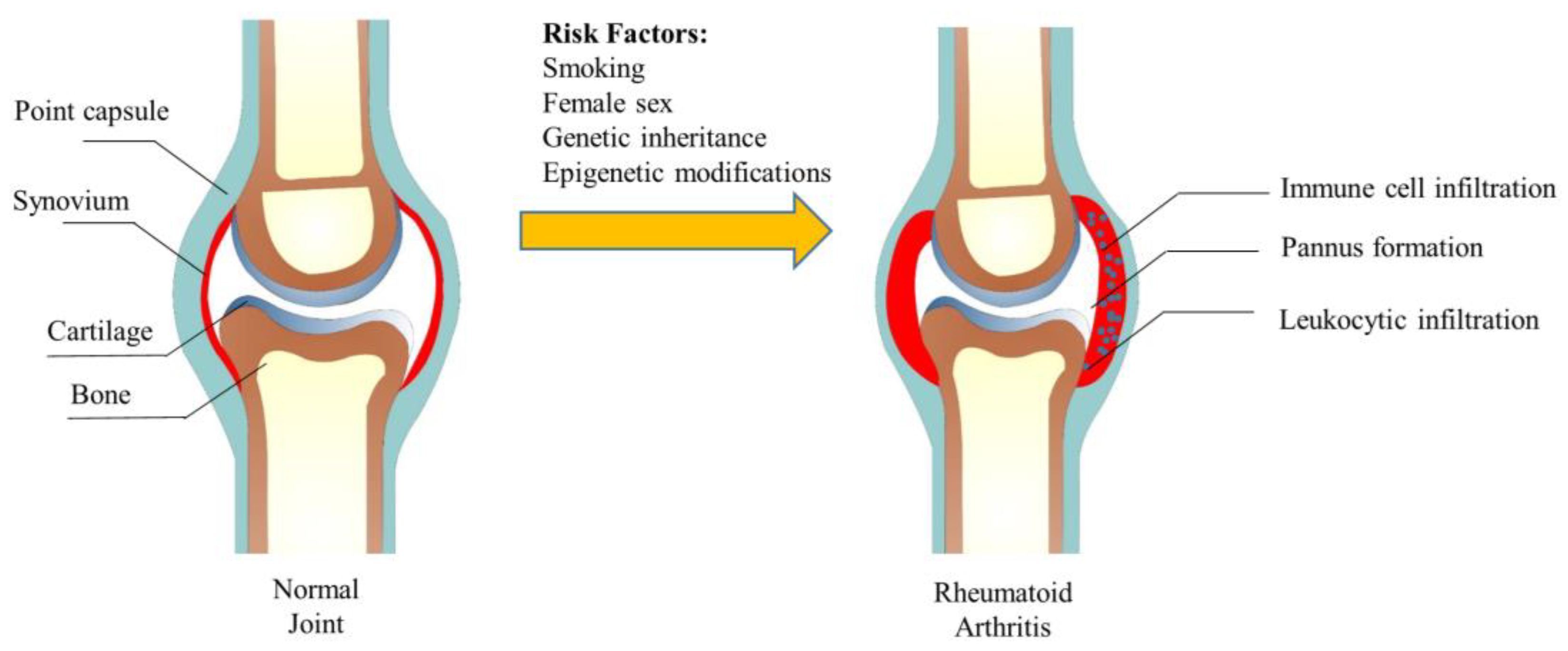


| Biological Agents | Target Cell | Target Factor | Mechanisms | Status | References |
|---|---|---|---|---|---|
| Etanercept (ENT) | TNF-α | In binding to TNFα and TNFβ, it has a more remarkable ability than endogenous soluble TNFRs to prevent the proinflammatory reaction | Approved | [66] | |
| Infliximab (IFX) | TNF-α | It binds to the target receptor of TNFα and inhibits the production of inflammatory factors | Approved | [67,68] | |
| Adalimumab (ADA) | TNF-α | It specifically binds to TNFα and blocks its interaction with TNF receptors on p55 and p75 cells | Approved | [69,70] | |
| Golimumab | TNF-α | Like Infliximab, it has a strong binding force on soluble and transmembrane TNFα, resulting in TNFα neutralization | Approved | [71] | |
| Certolizumab pegol | TNF-α | It is a human antibody consisting of an antigen-binding fragment (Fab ‘) of an anti-TNF-α monoclonal antibody | Approved | [72] | |
| Rituximab | B cells | It removes all B cells except the pre-B cells and plasma cells | Approved | [73] | |
| Abatacept (CTLA4-Ig) | T cells | By binding to CD80 and CD86 on the surface of B cells, it inhibits co-stimulation and activation of T cells, leading to the downregulation of inflammatory mediators | Approved | [74] | |
| Tocilizumab | IL-6 | It inhibits IL-6-mediated inflammatory responses, induces/expanses Breg cells, and down-regulates the expression of pro-inflammatory factors. | Approved | [75] | |
| Sirukumab | IL-6 | It binds IL-6R and blocks its binding with IL-6 molecules to inhibit the IL-6 signaling pathway to slow the disease | Approved | [76] | |
| Olokizumab | IL-6 | It recognizes the “site 3” site of IL-6, thus preventing the binding of IL-6 to gp130 and achieving the purpose of inhibiting the IL-6 pathway | Approved | [77] | |
| Clazakizumab | IL-6 | It targets soluble and membrane-bound IL-6 receptors to prevent IL-6 receptor complex formation and inhibit IL-6 signaling | Approved | [78] | |
| Brodalumab | IL-17 | It leads to the disruption of the IL-17 pathway by blocking the activities of 17A, 17E, and 17F | Approved | [79] | |
| Tofacitinib | JAK | It inhibits STAT phosphorylation and activation through JAK1/JAK3 dimer-mediated signaling and inhibits B lymphocyte activation and differentiation | Approved | [80] | |
| Baricitinib | JAK | It selectively inhibits JAK1 and JAK2 | Approved | [81] | |
| Upadacitinib | JAK | It selectively inhibits JAK1 and effectively inhibits STAT3 phosphorylation induced by IL-6 and IFNγ | Approved | [82] | |
| Filgotinib | JAK | It causes the selective inhibition of JAK1 and reduces the pro-inflammatory response | Approved | [83] | |
| Peficitinib | JAK | It is a JAK3 inhibitor, restraining the activation of pro-inflammatory cytokine signaling | Approved | [84] | |
| SHR0302 | JAK | Its highly selective inhibition of JAK1 suppresses the inflammatory response | Clinical trial phase 3 (NCT04333771) | [85] | |
| VX-509 | JAK | It causes selective JAK3 inhibition and mitigates the inflammatory response | Clinical trial phase 2/3 (NCT01830985) | [86] |
| Extract | Source | In Vivo | In Vitro | Potential Pathway | References |
|---|---|---|---|---|---|
| Rhoifolin | A flavanone extracted from Rhus succedanea | Alleviating the symptoms of CIA mice, and improving cytokines and oxidation indexes | NF-κB | [87] | |
| Perillyl alcohol | A monoterpene separated and collected from the essential oils of citronella, lavandin, peppermint, spearmint, celery seeds, and a few different plants | Improving the levels of MDA, GSH, NO, IL-1β, IL-10, IL-6, TNFα, and PGE2 in CIA mice | Protecting LPS-induced RAW 264.7 activation and improving inflammatory and oxidative markers | TLR4/NF-κB and Keap1/Nrf2 | [88] |
| Resveratrol | A natural polyphenol found in grapes and Polygonum cuspidatum | Attenuating paw swelling and decreasing serum antioxidant enzyme levels in AA rats | Activating antioxidant pathways and inhibiting the proliferation of FLS | Nrf2-ARE | [89] |
| Reducing H2O2-induced mitochondrial ROS production | Nrf2–Keap1 | [90] | |||
| Salvianolic acid | A major phytoconstituent of the plant Radix Salvia miltiorrhiza. | Alleviating oxidative stress state in mice and antagonizes free radicals | [91] | ||
| wogonin | A flavonoid contained in the Scutellaria Baicalensis Georgi root | Decreasing the content of lipid peroxides in CFA rats | [92] | ||
| Magnoflorine | A main component purified from Clematis manshurica Rupr. | Decreasing inflammatory responses in AIA rats | Decreasing the proliferation, migration, invasion, and reactive oxygen species levels in IL-1β-treated MH7A | PI3K/Akt/NF-κBKeap1-Nrf2/HO-1 | [93] |
| Chelerythrine | A benzo phenanthridine alkaloid isolated from the root of Papaveraceae. | Protecting AIA rats from inflammation and bone damage. | Inhibiting the migration and colony-formation of the HFLS-RA, increasing the intracellular level of ROS, promoting apoptosis | AMPK/mTOR/ULK-1 | [94] |
| Cantleyoside | A iridoid glycosides in P. hookeri | Leading to mitochondrial damage by Ca2+ overload and ROS release, promotes apoptosis and inhibits proliferation of HFLS-RA | AMPK/Sirt 1/NF-κB | [95] | |
| Icariin | A prenylated flavonol glycoside isolated from Epimedium | Interfering with cell cycle progression, increasing intracellular ROS level, inducing cell apoptosis, and inhibiting the proliferation of FLS | [96] | ||
| Neohesperidin | A flavone compound isolated from various dietary sources | Anti-migration, anti-invasion, anti-oxidative, and apoptosis-induced in RA-FLS | MAPK | [97] | |
| Hesperidin | A flavanone present in large quantities in citrus fruits | Reducing inflammation in CIA rats, regulating adenosine nucleotide and nucleoside hydrolases, reducing intracellular ROS, attenuating the apoptotic process of bone marrow cells | [98] | ||
| Celastrol | A quinone-methylated triterpenoid extracted from Tripterygium wilfordii | Reducing the degree of joint swelling, arthritis index score, inflammatory cell infiltration and synovial hyperplasia | Inhibiting the activation of NLRP3 inflammasome and the production of ROS induced by LPS and ATP in THP-1 cells. | [99] | |
| Reducing the expression of inflammatory factors in the serum of CIA-induced RA mice, alleviate cartilage tissue damage and inflammatory invasion in mice | Inhibiting FLSs proliferation and enhancing autophagy | PI3K/AKT/mTOR | [100] | ||
| Fangchinoline | An alkaloid can be extracted from the roots of Stephaniae tetrandra | Improving behavioral parameters and inflammatory signs in C/K rats and CIA mouse | Decreasing the production of inflammatory cytokines and ROS in HFLS | MAPK NF-κB | [101] |
| Sinomenine | Alleviating symptoms in CAIA mice by protecting their joints from destruction | Inhibiting the secretion of IL-6 and IL-33 and ROS production in RASFs, thereby mediating the protective effect against bone destruction | Keap1-Nrf2 | [102] | |
| Gentiolic acid | An active component in the whole plant or root of Gaultheria leucocarpa Bl.va. crenulata (Kurz) t.z.Hsu | Reducing the expression of inflammatory factors in serum of CIA rats and relieving the symptoms of RA | Inhibiting proliferation and invasion of MH7A, and the apoptosis was promoted | RAF/ERK | [103] |
| Curcumin | A tetraterpenoid obtained from Curcuma longa | Inhibiting the expression of pro-inflammatory cytokines TNF-α, IL-6, and IL-17 in CIA-stimulated mice, relieving hind paw edema in RA mice | Reducing TNFα-induced MH7A proliferation and migration ability and promoting cell apoptosis. | PI3K/AKT | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.; Ding, Q.; Lin, Z.; Fu, R.; Zhang, F.; Li, Z.; Zhang, M.; Zhu, Y. New Targets and Strategies for Rheumatoid Arthritis: From Signal Transduction to Epigenetic Aspect. Biomolecules 2023, 13, 766. https://doi.org/10.3390/biom13050766
Zhu M, Ding Q, Lin Z, Fu R, Zhang F, Li Z, Zhang M, Zhu Y. New Targets and Strategies for Rheumatoid Arthritis: From Signal Transduction to Epigenetic Aspect. Biomolecules. 2023; 13(5):766. https://doi.org/10.3390/biom13050766
Chicago/Turabian StyleZhu, Menglin, Qian Ding, Zhongxiao Lin, Rong Fu, Fuyuan Zhang, Zhaoyi Li, Mei Zhang, and Yizhun Zhu. 2023. "New Targets and Strategies for Rheumatoid Arthritis: From Signal Transduction to Epigenetic Aspect" Biomolecules 13, no. 5: 766. https://doi.org/10.3390/biom13050766
APA StyleZhu, M., Ding, Q., Lin, Z., Fu, R., Zhang, F., Li, Z., Zhang, M., & Zhu, Y. (2023). New Targets and Strategies for Rheumatoid Arthritis: From Signal Transduction to Epigenetic Aspect. Biomolecules, 13(5), 766. https://doi.org/10.3390/biom13050766









