Trametinib-Induced Epidermal Thinning Accelerates a Mouse Model of Junctional Epidermolysis Bullosa
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Mice Implantation of Trametinib
2.3. Mice Water Treatment with Losartan
2.4. Severity Scoring
2.5. Immunohistochemistry (IHC)
2.6. Aperio Staining Analysis
2.7. Hematoxylin and Eosin (H&E) Staining
2.8. Statistics
3. Results
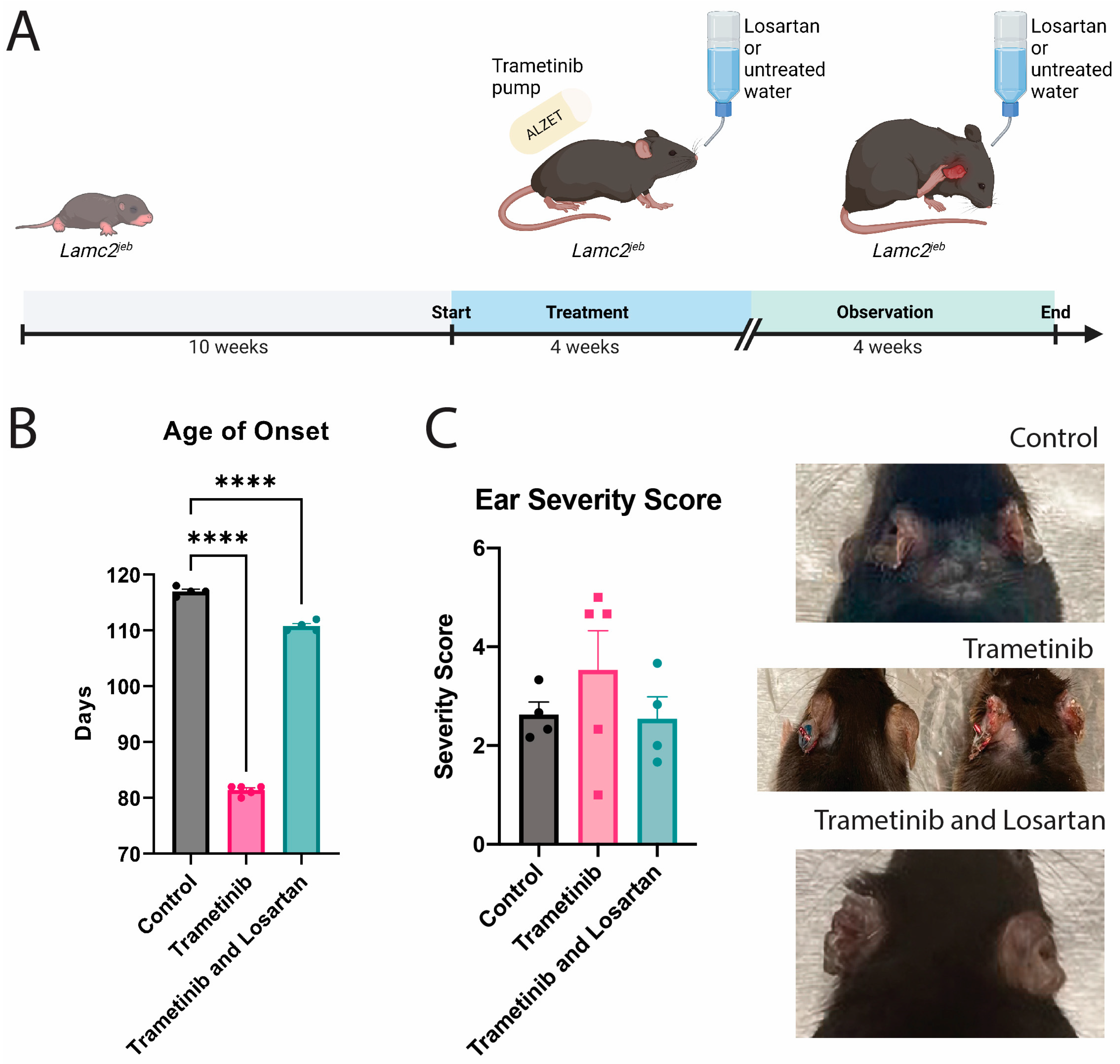
3.1. Trametinib Had a Wide Range of Effect on Lamc2jeb Mice Ear Damage
3.2. Severe Reactions to Trametinib Contribute to a Thinner Epidermis
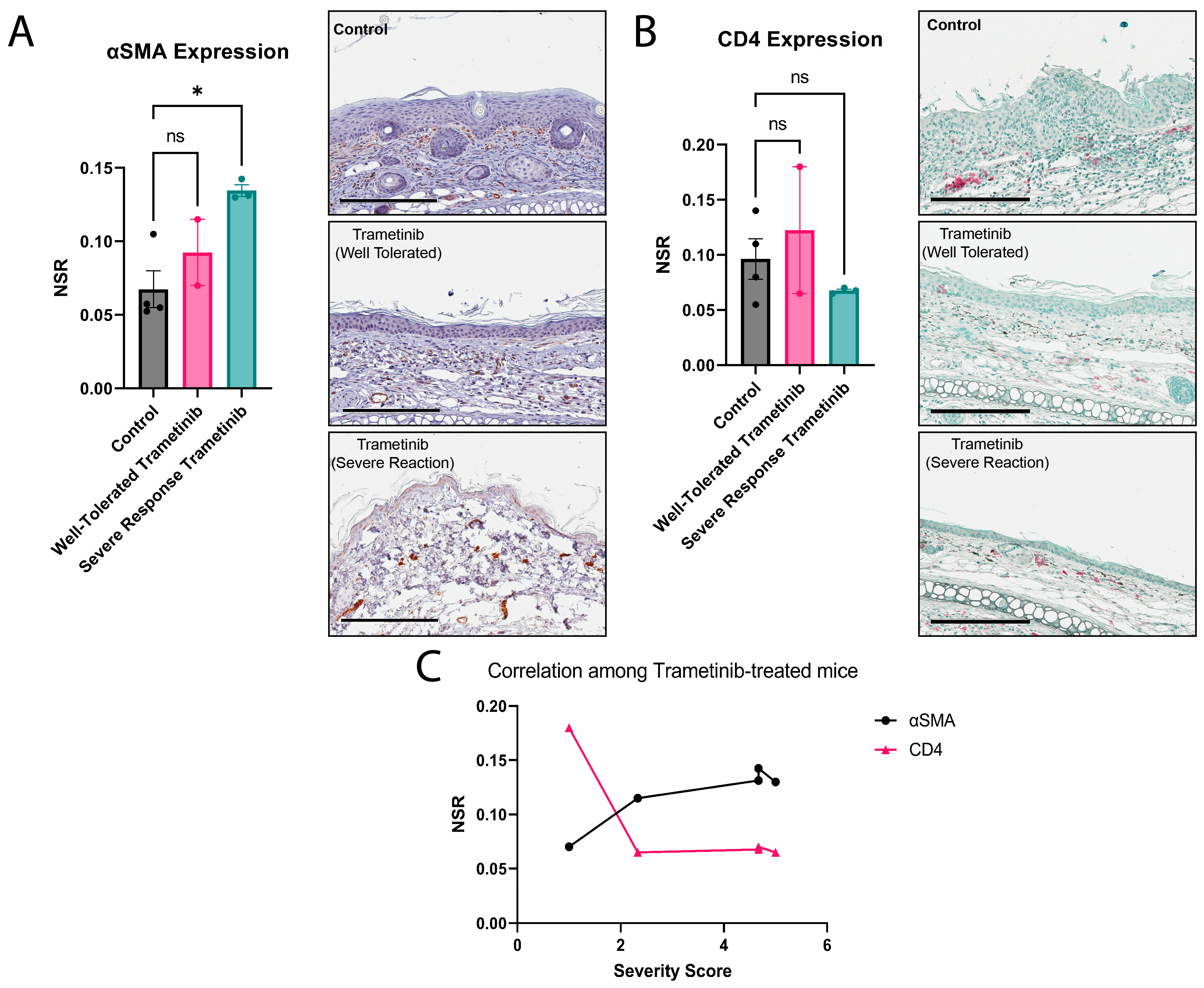
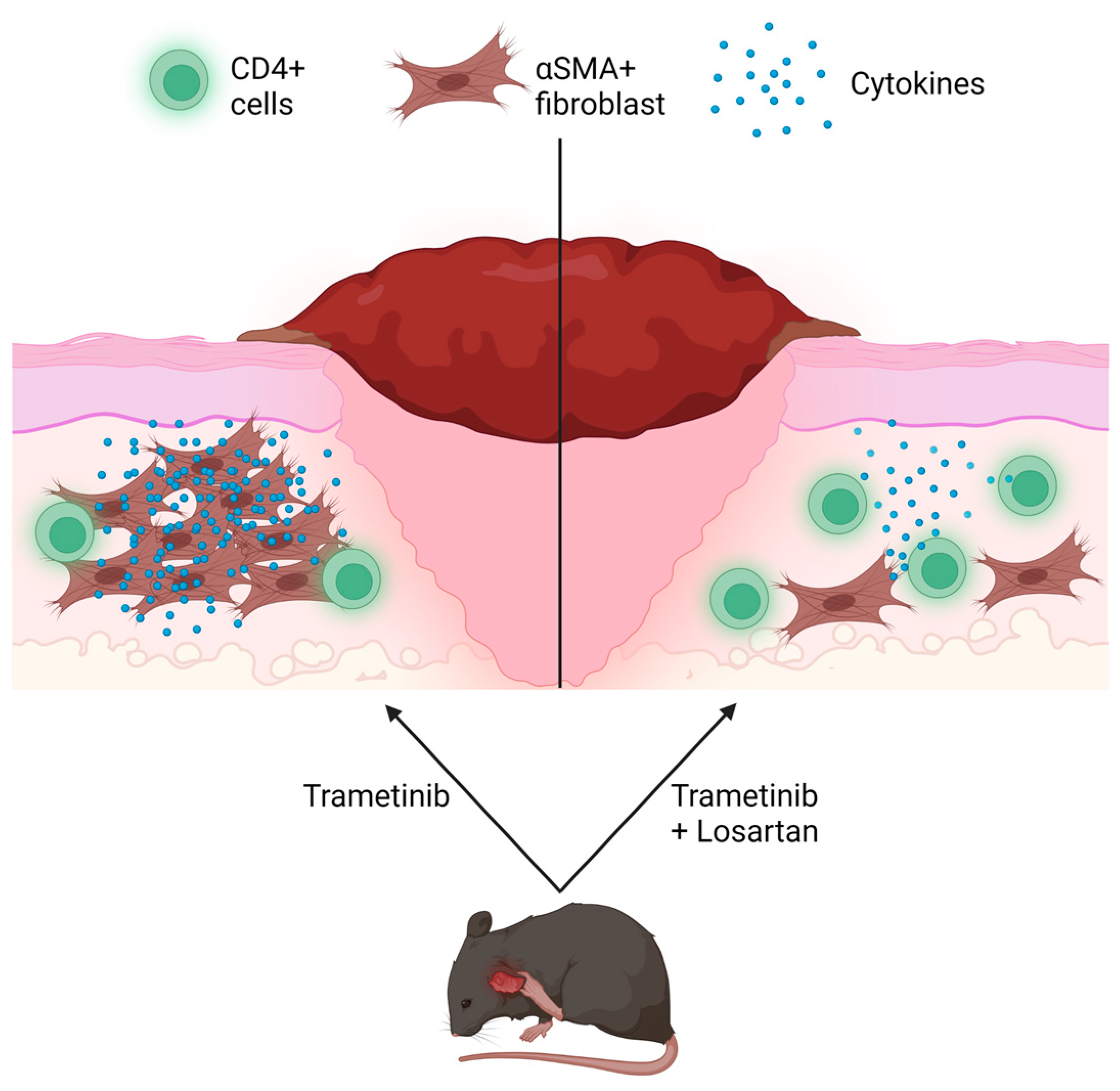
3.3. Severe Reactions to Trametinib Experience More Fibrosis with a Non-Significant Reduction in CD4 Expression
3.4. Losartan Ameliorates Trametinib’s Fibrotic Effect
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Antibodies | Company | Catalog Number | Dilution |
|---|---|---|---|
| CD3 | abcam | ab5690 | 1:1000 |
| CD4 | abcam | ab183685 | 1:1000 |
| CD8 | Cell signaling | 98941 | 1:100 |
| CD45 | BD Biosciences | 550539 | 1:200 |
| αSMA | abcam | ab5694 | 1:1000 |
References
- Yuen, W.Y.; Lemmink, H.H.; van Dijk-Bos, K.K.; Sinke, R.J.; Jonkman, M.F. Herlitz junctional epidermolysis bullosa: Diagnostic features, mutational profile, incidence and population carrier frequency in the Netherlands. Br. J. Dermatol. 2011, 165, 1314–1322. [Google Scholar] [CrossRef]
- Fine, J.D.; Mellerio, J.E. Extracutaneous manifestations and complications of inherited epidermolysis bullosa: Part I. Epithelial associated tissues. J. Am. Acad. Dermatol. 2009, 61, 367–384. [Google Scholar] [CrossRef]
- Aumailley, M.; Bruckner-Tuderman, L.; Carter, W.G.; Deutzmann, R.; Edgar, D.; Ekblom, P.; Engel, J.; Engvall, E.; Hohenester, E.; Jones, J.C.; et al. A simplified laminin nomenclature. Matrix Biol. 2005, 24, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Yancey, K.B.; Hintner, H. Non-herlitz junctional epidermolysis bullosa. Dermatol. Clin. 2010, 28, 67–77. [Google Scholar] [CrossRef]
- Has, C.; Bauer, J.W.; Bodemer, C.; Bolling, M.C.; Bruckner-Tuderman, L.; Diem, A.; Fine, J.D.; Heagerty, A.; Hovnanian, A.; Marinkovich, M.P.; et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br. J. Dermatol. 2020, 183, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Bubier, J.A.; Sproule, T.J.; Alley, L.M.; Webb, C.M.; Fine, J.D.; Roopenian, D.C.; Sundberg, J.P. A mouse model of generalized non-Herlitz junctional epidermolysis bullosa. J. Investig. Dermatol. 2010, 130, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Sproule, T.J.; Bubier, J.A.; Grandi, F.C.; Sun, V.Z.; Philip, V.M.; McPhee, C.G.; Adkins, E.B.; Sundberg, J.P.; Roopenian, D.C. Molecular identification of collagen 17a1 as a major genetic modifier of laminin gamma 2 mutation-induced junctional epidermolysis bullosa in mice. PLoS Genet. 2014, 10, e1004068. [Google Scholar] [CrossRef]
- Hinz, B. Formation and function of the myofibroblast during tissue repair. J. Investig. Dermatol. 2007, 127, 526–537. [Google Scholar] [CrossRef]
- Gabbiani, G. The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 2003, 200, 500–503. [Google Scholar] [CrossRef]
- Shinde, A.V.; Humeres, C.; Frangogiannis, N.G. The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 298–309. [Google Scholar] [CrossRef]
- Pfisterer, K.; Shaw, L.E.; Symmank, D.; Weninger, W. The Extracellular Matrix in Skin Inflammation and Infection. Front. Cell Dev. Biol. 2021, 9, 682414. [Google Scholar] [CrossRef]
- Diller, R.B.; Tabor, A.J. The Role of the Extracellular Matrix (ECM) in Wound Healing: A Review. Biomimetics 2022, 7, 87. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.; Peng, N.; Xiao, F.; Hu, D.; Wang, X.; Lu, L. The Roles of Immune Cells in the Pathogenesis of Fibrosis. Int. J. Mol. Sci. 2020, 21, 5203. [Google Scholar] [CrossRef]
- García Nores, G.D.; Ly, C.L.; Cuzzone, D.A.; Kataru, R.P.; Hespe, G.E.; Torrisi, J.S.; Huang, J.J.; Gardenier, J.C.; Savetsky, I.L.; Nitti, M.D.; et al. CD4(+) T cells are activated in regional lymph nodes and migrate to skin to initiate lymphedema. Nat. Commun. 2018, 9, 1970. [Google Scholar] [CrossRef]
- Zampell, J.C.; Yan, A.; Elhadad, S.; Avraham, T.; Weitman, E.; Mehrara, B.J. CD4(+) cells regulate fibrosis and lymphangiogenesis in response to lymphatic fluid stasis. PLoS ONE 2012, 7, e49940. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.; Munoz, E.; Johnston, R.; Korn, J.H.; Jimenez, S.A. Regulation of human lung fibroblast alpha 1(I) procollagen gene expression by tumor necrosis factor alpha, interleukin-1 beta, and prostaglandin E2. J. Biol. Chem. 1993, 268, 10364–10371. [Google Scholar] [CrossRef] [PubMed]
- Barbul, A.; Breslin, R.J.; Woodyard, J.P.; Wasserkrug, H.L.; Efron, G. The effect of in vivo T helper and T suppressor lymphocyte depletion on wound healing. Ann. Surg. 1989, 209, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Pasare, C. Location, location, location: Tissue-specific regulation of immune responses. J. Leukoc. Biol. 2013, 94, 409–421. [Google Scholar] [CrossRef]
- Pao, W.; Ooi, C.H.; Birzele, F.; Ruefli-Brasse, A.; Cannarile, M.A.; Reis, B.; Scharf, S.H.; Schubert, D.A.; Hatje, K.; Pelletier, N.; et al. Tissue-Specific Immunoregulation: A Call for Better Understanding of the “Immunostat” in the Context of Cancer. Cancer Discov. 2018, 8, 395–402. [Google Scholar] [CrossRef]
- Poholek, A.C. Tissue-Specific Contributions to Control of T Cell Immunity. Immunohorizons 2021, 5, 410–423. [Google Scholar] [CrossRef]
- Varanasi, S.K.; Kumar, S.V.; Rouse, B.T. Determinants of Tissue-Specific Metabolic Adaptation of T Cells. Cell Metab. 2020, 32, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Garlapati, V.; Molitor, M.; Michna, T.; Harms, G.S.; Finger, S.; Jung, R.; Lagrange, J.; Efentakis, P.; Wild, J.; Knorr, M.; et al. Targeting myeloid cell coagulation signaling blocks MAP kinase/TGF-β1-driven fibrotic remodeling in ischemic heart failure. J. Clin. Investig. 2023, 133, e156436. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, Q.; Tian, H.; Yao, X.; Bakina, O.; Zhang, H.; Lei, T.; Hu, F. MEK inhibitor trametinib attenuates neuroinflammation and cognitive deficits following traumatic brain injury in mice. Am. J. Transl. Res. 2020, 12, 6351–6365. [Google Scholar] [PubMed]
- Pourani, M.R.; Vahidnezhad, H.; Mansouri, P.; Youssefian, L.; Rakhshan, A.; Hajimoradi, B.; Abdollahimajd, F.; Uitto, J. Losartan treatment improves recessive dystrophic epidermolysis bullosa: A case series. Dermatol. Ther. 2022, 35, e15515. [Google Scholar] [CrossRef]
- Losartan Showing Promise in Pediatric Epidermolysis Bullosa Trial. Available online: https://www.mdedge.com/dermatology/article/216653/wounds/losartan-showing-promise-pediatric-epidermolysis-bullosa-trial (accessed on 24 April 2023).
- Nyström, A.; Thriene, K.; Mittapalli, V.; Kern, J.S.; Kiritsi, D.; Dengjel, J.; Bruckner-Tuderman, L. Losartan ameliorates dystrophic epidermolysis bullosa and uncovers new disease mechanisms. EMBO Mol. Med. 2015, 7, 1211–1228. [Google Scholar] [CrossRef] [PubMed]
- Chai, C.K. Response of inbred and F1 hybrid mice to hormone. Nature 1960, 185, 514–518. [Google Scholar] [CrossRef]
- Huff, S.D. A Genetically Controlled Response to the Drug Chlorpromazine; The Jackson Laboratory: Bar Harbor, ME, USA, 1962; p. 50. [Google Scholar]
- Plotnikoff, N. Drug Resistance due to Inbreeding. Science 1961, 134, 1881–1882. [Google Scholar] [CrossRef]
- Graham, J.B.; Swarts, J.L.; Mooney, M.; Choonoo, G.; Jeng, S.; Miller, D.R.; Ferris, M.T.; McWeeney, S.; Lund, J.M. Extensive Homeostatic T Cell Phenotypic Variation within the Collaborative Cross. Cell Rep. 2017, 21, 2313–2325. [Google Scholar] [CrossRef]
- Thota, R.; Johnson, D.B.; Sosman, J.A. Trametinib in the treatment of melanoma. Expert Opin. Biol. Ther. 2015, 15, 735–747. [Google Scholar] [CrossRef]
- Infante, J.R.; Fecher, L.A.; Falchook, G.S.; Nallapareddy, S.; Gordon, M.S.; Becerra, C.; DeMarini, D.J.; Cox, D.S.; Xu, Y.; Morris, S.R.; et al. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: A phase 1 dose-escalation trial. Lancet Oncol. 2012, 13, 773–781. [Google Scholar] [CrossRef]
- Falchook, G.S.; Lewis, K.D.; Infante, J.R.; Gordon, M.S.; Vogelzang, N.J.; DeMarini, D.J.; Sun, P.; Moy, C.; Szabo, S.A.; Roadcap, L.T.; et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: A phase 1 dose-escalation trial. Lancet Oncol. 2012, 13, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Gandara, D.R.; Leighl, N.; Delord, J.P.; Barlesi, F.; Bennouna, J.; Zalcman, G.; Infante, J.R.; Reckamp, K.L.; Kelly, K.; Shepherd, F.A.; et al. A Phase 1/1b Study Evaluating Trametinib Plus Docetaxel or Pemetrexed in Patients With Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, 556–566. [Google Scholar] [CrossRef]
- De Rosa, L.; Secone Seconetti, A.; De Santis, G.; Pellacani, G.; Hirsch, T.; Rothoeft, T.; Teig, N.; Pellegrini, G.; Bauer, J.W.; De Luca, M. Laminin 332-Dependent YAP Dysregulation Depletes Epidermal Stem Cells in Junctional Epidermolysis Bullosa. Cell Rep. 2019, 27, 2036–2049.e6. [Google Scholar] [CrossRef] [PubMed]
- Mavilio, F.; Pellegrini, G.; Ferrari, S.; Di Nunzio, F.; Di Iorio, E.; Recchia, A.; Maruggi, G.; Ferrari, G.; Provasi, E.; Bonini, C.; et al. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat. Med. 2006, 12, 1397–1402. [Google Scholar] [CrossRef]
- Naska, S.; Yuzwa, S.A.; Johnston, A.P.; Paul, S.; Smith, K.M.; Paris, M.; Sefton, M.V.; Datti, A.; Miller, F.D.; Kaplan, D.R. Identification of Drugs that Regulate Dermal Stem Cells and Enhance Skin Repair. Stem Cell Rep. 2016, 6, 74–84. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ota, S.; Terada, S.; Kawakami, Y.; Otsuka, T.; Fu, F.H.; Huard, J. The Combined Use of Losartan and Muscle-Derived Stem Cells Significantly Improves the Functional Recovery of Muscle in a Young Mouse Model of Contusion Injuries. Am. J. Sports Med. 2016, 44, 3252–3261. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Ki, M.R.; Lee, E.M.; Kim, A.Y.; You, S.Y.; Han, S.Y.; Lee, E.J.; Hong, I.H.; Kwon, S.H.; Kim, S.J.; et al. Losartan improves adipose tissue-derived stem cell niche by inhibiting transforming growth factor-β and fibrosis in skeletal muscle injury. Cell Transplant. 2012, 21, 2407–2424. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, S. T Cells in Fibrosis and Fibrotic Diseases. Front. Immunol. 2020, 11, 1142. [Google Scholar] [CrossRef]
- Jacobs, H.; Vandeputte, D.; Tolkamp, L.; de Vries, E.; Borst, J.; Berns, A. CD3 components at the surface of pro-T cells can mediate pre-T cell development in vivo. Eur. J. Immunol. 1994, 24, 934–939. [Google Scholar] [CrossRef]
- Kamphorst, A.O.; Ahmed, R. CD4 T-cell immunotherapy for chronic viral infections and cancer. Immunotherapy 2013, 5, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Irie-Sasaki, J.; Sasaki, T.; Matsumoto, W.; Opavsky, A.; Cheng, M.; Welstead, G.; Griffiths, E.; Krawczyk, C.; Richardson, C.D.; Aitken, K.; et al. CD45 is a JAK phosphatase and negatively regulates cytokine receptor signalling. Nature 2001, 409, 349–354. [Google Scholar] [CrossRef]
- Ledbetter, J.A.; Deans, J.P.; Aruffo, A.; Grosmaire, L.S.; Kanner, S.B.; Bolen, J.B.; Schieven, G.L. CD4, CD8 and the role of CD45 in T-cell activation. Curr. Opin. Immunol. 1993, 5, 334–340. [Google Scholar] [CrossRef]
- Allegrezza, M.J.; Rutkowski, M.R.; Stephen, T.L.; Svoronos, N.; Tesone, A.J.; Perales-Puchalt, A.; Nguyen, J.M.; Sarmin, F.; Sheen, M.R.; Jeng, E.K.; et al. IL15 Agonists Overcome the Immunosuppressive Effects of MEK Inhibitors. Cancer Res. 2016, 76, 2561–2572. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Mayes, P.A.; Eastman, S.; Shi, H.; Yadavilli, S.; Zhang, T.; Yang, J.; Seestaller-Wehr, L.; Zhang, S.Y.; Hopson, C.; et al. The BRAF and MEK Inhibitors Dabrafenib and Trametinib: Effects on Immune Function and in Combination with Immunomodulatory Antibodies Targeting PD-1, PD-L1, and CTLA-4. Clin. Cancer Res. 2015, 21, 1639–1651. [Google Scholar] [CrossRef]
- Accogli, T.; Bruchard, M.; Végran, F. Modulation of CD4 T Cell Response According to Tumor Cytokine Microenvironment. Cancers 2021, 13, 373. [Google Scholar] [CrossRef] [PubMed]
- Knosp, C.A.; Johnston, J.A. Regulation of CD4+ T-cell polarization by suppressor of cytokine signalling proteins. Immunology 2012, 135, 101–111. [Google Scholar] [CrossRef]
- Yoshimura, A.; Ito, M.; Chikuma, S.; Akanuma, T.; Nakatsukasa, H. Negative Regulation of Cytokine Signaling in Immunity. Cold Spring Harb. Perspect. Biol. 2018, 10, a028571. [Google Scholar] [CrossRef]
- Vignali, D.A.; Collison, L.W.; Workman, C.J. How regulatory T cells work. Nat. Rev. Immunol. 2008, 8, 523–532. [Google Scholar] [CrossRef]
- Baker, J.V.; Wolfson, J.; Collins, G.; Morse, C.; Rhame, F.; Liappis, A.P.; Rizza, S.; Temesgen, Z.; Mystakelis, H.; Deeks, S.; et al. Losartan to reduce inflammation and fibrosis endpoints in HIV disease. Aids 2021, 35, 575–583. [Google Scholar] [CrossRef]
- Torres, B.; Guardo, A.C.; Squarcia, M.; Diaz, A.; Fabra, A.; Caballero, M.; Ugarte, A.; Leal, L.; Gatell, J.M.; Plana, M.; et al. Impact of switching to raltegravir and/or adding losartan in lymphoid tissue fibrosis and inflammation in people living with HIV. A randomized clinical trial. HIV Med. 2021, 22, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, C.; Paradis, P.; Schiffrin, E.L. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol. Sci. 2008, 29, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Campistol, J.M.; Iñigo, P.; Jimenez, W.; Lario, S.; Clesca, P.H.; Oppenheimer, F.; Rivera, F. Losartan decreases plasma levels of TGF-beta1 in transplant patients with chronic allograft nephropathy. Kidney Int. 1999, 56, 714–719. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tartaglia, G.; Park, P.H.; Alexander, M.H.; Nyström, A.; Rosenbloom, J.; South, A.P. Trametinib-Induced Epidermal Thinning Accelerates a Mouse Model of Junctional Epidermolysis Bullosa. Biomolecules 2023, 13, 740. https://doi.org/10.3390/biom13050740
Tartaglia G, Park PH, Alexander MH, Nyström A, Rosenbloom J, South AP. Trametinib-Induced Epidermal Thinning Accelerates a Mouse Model of Junctional Epidermolysis Bullosa. Biomolecules. 2023; 13(5):740. https://doi.org/10.3390/biom13050740
Chicago/Turabian StyleTartaglia, Grace, Pyung Hun Park, Michael H. Alexander, Alexander Nyström, Joel Rosenbloom, and Andrew P. South. 2023. "Trametinib-Induced Epidermal Thinning Accelerates a Mouse Model of Junctional Epidermolysis Bullosa" Biomolecules 13, no. 5: 740. https://doi.org/10.3390/biom13050740
APA StyleTartaglia, G., Park, P. H., Alexander, M. H., Nyström, A., Rosenbloom, J., & South, A. P. (2023). Trametinib-Induced Epidermal Thinning Accelerates a Mouse Model of Junctional Epidermolysis Bullosa. Biomolecules, 13(5), 740. https://doi.org/10.3390/biom13050740






