Plasma Cytokeratin-18 Fragment Level Reflects the Metabolic Phenotype in Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Anthropometric Measures and Blood Collection
2.3. Blood Analyses
2.4. Data Analysis
3. Results
3.1. Participant Characteristics
3.2. Liver and Inflammatory Markers
3.3. Associations of CK-18 with Anthropometric and Metabolic Markers of Obesity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Targher, G.; Tilg, H.; Byrne, C.D. Non-alcoholic fatty liver disease: A multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol. Hepatol. 2021, 6, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Armandi, A.; Rosso, C.; Younes, R.; Leeming, D.J.; Karsdal, M.A.; Caviglia, G.P.; Pérez-Diaz-Del-Campo, N.; D’Amato, D.; Abdulle, A.; Nicolosi, A.; et al. Cross-Sectional and Longitudinal Performance of Non-Invasive Tests of Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. J. Clin. Med. 2023, 12, 650. [Google Scholar] [CrossRef]
- Jiang, Z.G.; Tapper, E.B.; Connelly, M.A.; Pimentel, C.F.; Feldbrügge, L.; Kim, M.; Krawczyk, S.; Afdhal, N.; Robson, S.C.; Herman, M.A.; et al. Steatohepatitis and liver fibrosis are predicted by the characteristics of very low density lipoprotein in nonalcoholic fatty liver disease. Liver Int. 2016, 36, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Ku, N.O. Revealing the roles of keratin 8/18-associated signaling proteins involved in the development of hepatocellular carcinoma. Int. J. Mol. Sci. 2021, 22, 6401. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Pessoa, J.; Teixeira, J. Cytoskeleton alterations in non-alcoholic fatty liver disease. Metabolism 2022, 128, 155115. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The fatty liver index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Petta, S.; Vanni, E.; Bugianesi, E.; Di Marco, V.; Cammà, C.; Cabibi, D.; Mezzabotta, L.; Craxì, A. The combination of liver stiffness measurement and NAFLD fibrosis score improves the noninvasive diagnostic accuracy for severe liver fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2015, 35, 1566–1573. [Google Scholar] [CrossRef]
- European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J. Hepatol. 2015, 63, 237–264. [Google Scholar] [CrossRef]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive Assessment of Liver Disease in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264–1281.e4. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.H.; She, Z.G.; Li, H.L.; Cai, J.J. Noninvasive evaluation of nonalcoholic fatty liver disease: Current evidence and practice. World J. Gastroenterol. 2019, 25, 1307–1326. [Google Scholar] [CrossRef] [PubMed]
- Grigorescu, M.; Crisan, D.; Radu, C.; Grigorescu, M.D.; Sparchez, Z.; Serban, A. A novel pathophysiologicalbased panel of biomarkers for the diagnosis of nonalcoholic steatohepatitis. J. Physiol. Pharmacol. 2012, 63, 347–353. [Google Scholar] [PubMed]
- Zhou, Y.; Orešič, M.; Leivonen, M.; Gopalacharyulu, P.; Hyysalo, J.; Arola, J.; Verrijken, A.; Francque, S.; Van Gaal, L.; Hyötyläinen, T. Noninvasive Detection of Nonalcoholic Steatohepatitis Using Clinical Markers and Circulating Levels of Lipids and Metabolites. Clin. Gastroenterol. Hepatol. 2016, 14, 1463–1472.e6. [Google Scholar] [CrossRef]
- Lang, A.L.; Beier, J.I. Interaction of volatile organic compounds and underlying liver disease: A new paradigm for risk. Biol. Chem. 2018, 399, 1237. [Google Scholar] [CrossRef]
- Becker, P.P.; Rau, M.; Schmitt, J.; Malsch, C.; Hammer, C.; Bantel, H.; Müllhaupt, B.; Geier, A. Performance of serum microRNAs-122,-192 and-21 as biomarkers in patients with non-Alcoholic steatohepatitis. PLoS ONE 2015, 10, e0142661. [Google Scholar] [CrossRef]
- You, H.; Wang, L.; Bu, F.; Meng, H.; Pan, X.; Li, J.; Zhang, Y.; Wang, A.; Yin, N.; Huang, C.; et al. The miR-455-3p/HDAC2 axis plays a pivotal role in the progression and reversal of liver fibrosis and is regulated by epigenetics. FASEB J. 2021, 35, e21700. [Google Scholar] [CrossRef]
- Paolini, E.; Longo, M.; Corsini, A.; Dongiovanni, P. The Non-Invasive Assessment of Circulating D-Loop and mt-ccf Levels Opens an Intriguing Spyhole into Novel Approaches for the Tricky Diagnosis of NASH. Int. J. Mol Sci. 2023, 24, 2331. [Google Scholar] [CrossRef]
- Strnad, P.; Zatloukal, K.; Stumptner, C.; Kulaksiz, H.; Denk, H. Mallory-Denk-bodies: Lessons from keratin-containing hepatic inclusion bodies. Biochim. Biophys. Acta Mol. Basis Dis. 2008, 1782, 764–774. [Google Scholar] [CrossRef]
- Strnad, P.; Stumptner, C.; Zatloukal, K.; Denk, H. Intermediate filament cytoskeleton of the liver in health and disease. Histochem. Cell Biol. 2008, 129, 735–749. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhong, X.; Shen, H.; Sheng, L.; Liangpunsakul, S.; Lok, A.S.; Omary, M.B.; Wang, S.; Rui, L. Biliary NIK promotes ductular reaction and liver injury and fibrosis in mice. Nat. Commun. 2022, 13, 5111. [Google Scholar] [CrossRef] [PubMed]
- Ku, N.O.; Strnad, P.; Bantel, H.; Omary, M.B. Keratins: Biomarkers and modulators of apoptotic and necrotic cell death in the liver. Hepatology 2016, 64, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; Cassader, M.; Pagano, G. Meta-analysis: Natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann. Med. 2011, 43, 617–649. [Google Scholar] [CrossRef] [PubMed]
- Strnad, P.; Paschke, S.; Jang, K.H.; Ku, N.O. Keratins: Markers and modulators of liver disease. Curr. Opin. Gastroenterol. 2012, 28, 209–216. [Google Scholar] [CrossRef]
- Wong, V.W.S.; Adams, L.A.; de Lédinghen, V.; Wong, G.L.H.; Sookoian, S. Noninvasive biomarkers in NAFLD and NASH—current progress and future promise. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 461–478. [Google Scholar] [CrossRef]
- Razny, U.; Goralska, J.; Zdzienicka, A.; Gruca, A.; Zapala, B.; Micek, A.; Dembinska-Kiec, A.; Solnica, B.; Malczewska-Malec, M. High fat mixed meal tolerance test leads to suppression of osteocalcin decrease in obese insulin resistant subjects compared to healthy adults. Nutrients 2018, 10, 1611. [Google Scholar] [CrossRef]
- Feldstein, A.E.; Wieckowska, A.; Lopez, A.R.; Liu, Y.C.; Zein, N.N.; McCullough, A.J. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: A multicenter validation study. Hepatology 2009, 50, 1072–1078. [Google Scholar] [CrossRef]
- Safarian, M.; Mohammadpour, S.; Shafiee, M.; Ganji, A.; Soleimani, A.; Nematy, M.; Bahari, A. Effect of diet-induced weight loss on cytokeratin-18 levels in overweight and obese patients with liver fibrosis. Diabetes Metab. Syndr. 2019, 13, 989–994. [Google Scholar] [CrossRef]
- Takahashi, A.; Abe, K.; Fujita, M.; Hayashi, M.; Okai, K.; Ohira, H. Simple resistance exercise decreases cytokeratin 18 and fibroblast growth factor 21 levels in patients with nonalcoholic fatty liver disease: A retrospective clinical study. Medicine 2020, 99, e20399. [Google Scholar] [CrossRef]
- Hempel, F.; Roderfeld, M.; Müntnich, L.J.; Albrecht, J.; Oruc, Z.; Arneth, B.; Karrasch, T.; Pons-Kühnemann, J.; Padberg, W.; Renz, H.; et al. Caspase-cleaved keratin 18 measurements identified ongoing liver injury after bariatric surgery. J. Clin. Med. 2021, 10, 1233. [Google Scholar] [CrossRef]
- Wu, G.; Li, H.; Fang, Q.; Zhang, J.; Zhang, M.; Zhang, L.; Wu, L.; Hou, X.; Lu, J.; Bao, Y.; et al. Complementary Role of Fibroblast Growth Factor 21 and Cytokeratin 18 in Monitoring the Different Stages of Nonalcoholic Fatty Liver Disease. Sci. Rep. 2017, 7, 5095. [Google Scholar] [CrossRef] [PubMed]
- Werder, E.J.; Beier, J.I.; Sandler, D.P.; Falkner, K.C.; Gripshover, T.; Wahlang, B.; Engel, L.S.; Cave, M.C. Blood BTEXS and heavy metal levels are associated with liver injury and systemic inflammation in Gulf states residents. Food Chem. Toxicol. 2020, 139, 111242. [Google Scholar] [CrossRef] [PubMed]
- Qadri, S.; Ahlholm, N.; Lønsmann, I.; Pellegrini, P.; Poikola, A.; Luukkonen, P.K.; Porthan, K.; Juuti, A.; Sammalkorpi, H.; Penttilä, A.K.; et al. Obesity Modifies the Performance of Fibrosis Biomarkers in Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2022, 107, E2008–E2020. [Google Scholar] [CrossRef]
- Qian, L.; Zhang, L.; Wu, L.; Zhang, J.; Fang, Q.; Hou, X.; Gao, Q.; Li, H.; Jia, W. Elevated Serum Level of Cytokeratin 18 M65ED Is an Independent Indicator of Cardiometabolic Disorders. J. Diabetes Res. 2020, 2020, 5198359. [Google Scholar] [CrossRef]
- Vuppalanch, R.; Jain, A.K.; Deppe, R.; Yates, K.; Comerford, M.; Masuoka, H.C.; Neuschwander-Tetri, B.A.; Loomba, R.; Brunt, E.M.; Kleiner, D.E.; et al. Relationship Between Changes in Serum Levels of Keratin 18 and Changes in Liver Histology in Children and Adults with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2014, 12, 2121–2130.e2. [Google Scholar] [CrossRef] [PubMed]
- Thulin, P.; Nordahl, G.; Gry, M.; Yimer, G.; Aklillu, E.; Makonnen, E.; Aderaye, G.; Lindquist, L.; Mattsson, C.M.; Ekblom, B.; et al. Keratin-18 and microRNA-122 complement alanine aminotransferase as novel safety biomarkers for drug-induced liver injury in two human cohorts. Liver Int. 2014, 34, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Korver, S.; Bowen, J.; Pearson, K.; Gonzalez, R.J.; French, N.; Park, K.; Jenkins, R.; Goldring, C. The application of cytokeratin-18 as a biomarker for drug-induced liver injury. Arch. Toxicol. 2021, 95, 3435–3448. [Google Scholar] [CrossRef]
- Chuah, K.H.; Wan Yusoff, W.N.I.; Sthaneshwar, P.; Nik Mustapha, N.R.; Mahadeva, S.; Chan, W.K. MACK-3 (combination of hoMa, Ast and CK18): A promising novel biomarker for fibrotic non-alcoholic steatohepatitis. Liver Int. 2019, 39, 1315–1324. [Google Scholar] [CrossRef]
- Góralska, J.; Raźny, U.; Polus, A.; Dziewońska, A.; Gruca, A.; Zdzienicka, A.; Dembińska-Kieć, A.; Solnica, B.; Micek, A.; Kapusta, M.; et al. Enhanced GIP secretion in obesity is associated with biochemical alteration and miRNA contribution to the development of liver steatosis. Nutrients 2020, 12, 476. [Google Scholar] [CrossRef]
- López-Sánchez, G.N.; Montalvo-Javé, E.; Domínguez-Perez, M.; Antuna-Puente, B.; Beltrán-Anaya, F.O.; Hidalgo-Miranda, A.; Chávez-Tapia, N.C.; Uribe, M.; Nuño-Lámbarri, N. Hepatic mir-122-3p, mir-140-5p and mir-148b-5p expressions are correlated with cytokeratin-18 serum levels in MAFLD. Ann. Hepatol. 2022, 27, 100756. [Google Scholar] [CrossRef]
- He, L.; Deng, L.; Zhang, Q.; Guo, J.; Zhou, J.; Song, W.; Yuan, F. Diagnostic value of CK-18, FGF-21, and related biomarker panel in nonalcoholic fatty liver disease: A systematic review and meta-analysis. Biomed Res. Int. 2017, 2017, 9729107. [Google Scholar] [CrossRef] [PubMed]
- Vatsalya, V.; Kong, M.; Gobejishvili, L.; Chen, W.Y.; Srivastava, S.; Barve, S.; McClain, C.; Joshi-Barve, S. Urinary acrolein metabolite levels in severe acute alcoholic hepatitis patients. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G115–G122. [Google Scholar] [CrossRef] [PubMed]
- Darweesh, S.K.; Abdelaziz, R.A.; Abd-Elfatah, D.S.; Abdelazim, N.A.; Fathi, S.A.; Attia, D.; AbdAllah, M. Serum cytokeratin-18 and its relation to liver fibrosis and steatosis diagnosed by FibroScan and controlled attenuation parameter in nonalcoholic fatty liver disease and hepatitis C virus patients. Eur. J. Gastroenterol. Hepatol. 2019, 31, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Wu, D.; Jiang, W.; Li, J.; Long, J.; Jia, C.; Zhou, T. Molecular biomarkers in drug-induced liver injury: Challenges and future perspectives. Front. Pharmacol. 2020, 10, 1667. [Google Scholar] [CrossRef]
- Possamai, L.A.; McPhail, M.J.W.; Quaglia, A.; Zingarelli, V.; Abeles, R.D.; Tidswell, R.; Puthucheary, Z.; Rawal, J.; Karvellas, C.J.; Leslie, E.M.; et al. Character and temporal evolution of apoptosis in acetaminophen-induced acute liver failure. Crit. Care Med. 2013, 41, 2543–2550. [Google Scholar] [CrossRef]
- Nikam, A.; Patankar, J.V.; Somlapura, M.; Lahiri, P.; Sachdev, V.; Kratky, D.; Helmut, D.; Zatloukal, K.; Abuja, P.M. The PPARα Agonist Fenofibrate Prevents Formation of Protein Aggregates (Mallory-Denk bodies) in a Murine Model of Steatohepatitis-like Hepatotoxicity. Sci. Rep. 2018, 8, 12964–12978. [Google Scholar] [CrossRef]
- Bratoeva, K.; Nikolova, S.; Merdzhanova, A.; Stoyanov, G.S.; Dimitrova, E.; Kashlov, J.; Conev, N.; Radanova, M. Association between serum CK-18 levels and the degree of liver damage in fructose-induced metabolic syndrome. Metab. Syndr. Relat. Disord. 2018, 16, 350–357. [Google Scholar] [CrossRef]
- Chomphoo, S.; Kunprom, W.; Thithuan, K.; Sorin, S.; Khawkhiaw, K.; Kamkaew, A.; Phoomak, C.; Chiu, C.F.; Saengboonmee, C. Hyperglycemia Alters O-GlcNAcylation Patterns of Hepatocytes in Mice Treated with Hepatoxic Carcinogen. In Vivo 2023, 37, 685–695. [Google Scholar] [CrossRef]
- Ma, J.; Hart, G.W. Protein O-GlcNAcylation in diabetes and diabetic complications. Expert Rev. Proteom. 2013, 10, 365–380. [Google Scholar] [CrossRef]
- Srikanth, B.; Vaidya, M.M.; Kalraiya, R.D. O-GlcNAcylation determines the solubility, filament organization, and stability of keratins 8 and 18. J. Biol. Chem. 2010, 285, 34062–34071. [Google Scholar] [CrossRef]
- Ku, N.O.; Toivola, D.M.; Strnad, P.; Omary, M.B. Cytoskeletal keratin glycosylation protects epithelial tissue from injury. Nat. Cell Biol. 2010, 12, 876–885. [Google Scholar] [CrossRef]
- Alam, C.M.; Baghestani, S.; Pajari, A.; Omary, M.B.; Toivola, D.M. Keratin 7 is a constituent of the keratin network in mouse pancreatic islets and is upregulated in experimental diabetes. Int. J. Mol. Sci. 2021, 22, 7784. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Kim, H.T.; Lee, S.; Lee, Y.S.; Choi, U.K.; Kang, J.H.; Choi, S.Y.; Kang, T.C.; Choi, M.S.; Kwon, O.S. Differential expression of intermediate filaments in the process of developing hepatic steatosis. Proteomics 2011, 11, 2777–2789. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, I.; Cho, W.; Oh, G.T.; Park, Y.M. Vimentin deficiency prevents high-fat diet-induced obesity and insulin resistance in mice. Diabetes Metab. J. 2021, 45, 97–108. [Google Scholar] [CrossRef]
- Shen, W.J.; Patel, S.; Eriksson, J.E.; Kraemer, F.B. Vimentin is a functional partner of hormone sensitive lipase and facilitates lipolysis. J. Proteome Res. 2010, 9, 1786–1794. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Hosaka, T.; Iwata, T.; Le, C.T.K.; Jambaldorj, B.; Teshigawara, K.; Harada, N.; Sakaue, H.; Sakai, T.; Yoshimoto, K.; et al. Vimentin binds IRAP and is involved in GLUT4 vesicle trafficking. Biochem. Biophys. Res. Commun. 2011, 405, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Lam, K.S.L.; Xu, A. The therapeutic potential of FGF21 in metabolic diseases: From bench to clinic. Nat. Rev. Endocrinol. 2020, 16, 654–667. [Google Scholar] [CrossRef]
- Winn, N.C.; Liu, Y.; Rector, R.S.; Parks, E.J.; Ibdah, J.A.; Kanaley, J.A. Energy-matched moderate and high intensity exercise training improves nonalcoholic fatty liver disease risk independent of changes in body mass or abdominal adiposity—A randomized trial. Metabolism 2018, 78, 128–140. [Google Scholar] [CrossRef]
- Mana, M.F.; Parisi, M.C.R.; Correa-Giannella, M.L.; Neto, A.M.; Yamanaka, A.; Cunha-Silva, M.; Cavaleiro, A.M.; Dos Santos, C.R.; Pavan, C.R.; Sevá-Pereira, T.; et al. Non-Alcoholic Fatty Liver Disease in Long-Term Type 2 Diabetes: Role of rs738409 PNPLA3 and rs499765 FGF21 Polymorphisms and Serum Biomarkers. Molecules 2022, 27, 3193. [Google Scholar] [CrossRef]
- Berzigotti, A.; Tsochatzis, E.; Boursier, J.; Castera, L.; Cazzagon, N.; Friedrich-Rust, M. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis–2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Chalasani, N. Non-invasive assessment of non-alcoholic fatty liver disease: Clinical prediction rules and blood-based biomarkers. J. Hepatol. 2018, 68, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Samms, R.J.; Murphy, M.; Fowler, M.J.; Cooper, S.; Emmerson, P.; Coskun, T.; Adams, A.C.; Kharitonenkov, A.; Ebling, F.J.; Tsintzas, K. Dual effects of fibroblast growth factor 21 on hepatic energy metabolism. J. Endocrinol. 2015, 227, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Puengel, T.; Lefere, S.; Hundertmark, J.; Kohlhepp, M.; Penners, C.; Van de Velde, F.; Lapauw, B.; Hoorens, A.; Devisscher, L.; Geerts, A.; et al. Combined Therapy with a CCR2/CCR5 Antagonist and FGF21 Analogue Synergizes in Ameliorating Steatohepatitis and Fibrosis. Int. J. Mol. Sci. 2022, 23, 6696. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zeng, J.; Xing, L.; Li, C. Extra-and intra-cellular mechanisms of hepatic stellate cell activation. Biomedicines 2021, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Marques, P.; Francisco, V.; Martínez-Arenas, L.; Carvalho-Gomes, Â.; Domingo, E.; Piqueras, L.; Berenguer, M.; Sanz, M.J. Overview of Cellular and Soluble Mediators in Systemic Inflammation Associated with Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2023, 24, 2313. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Itoh, Y.; Yokomizo, C.; Nishimura, T.; Niimi, T.; Fujii, H.; Okanoue, T.; Yoshikawa, T. Blockade of interleukin-6 signaling enhances hepatic steatosis but improves liver injury in methionine choline-deficient diet-fed mice. Lab. Investig. 2010, 90, 1169–1178. [Google Scholar] [CrossRef]
- Valva, P.; Rios, D.; Casciato, P.; Gadano, A.; Galdame, O.; Mullen, E.; Bertot, G.; de Matteo, E.; Preciado, M.V. Nonalcoholic fatty liver disease: Biomarkers as diagnostic tools for liver damage assessment in adult patients from Argentina. Eur. J. Gastroenterol. Hepatol. 2018, 30, 637–644. [Google Scholar] [CrossRef]
- Houghton, D.; Hallsworth, K.; Thoma, C.; Cassidy, S.; Hardy, T.; Heaps, S. Effects of Exercise on Liver Fat and Metabolism in Alcohol Drinkers. Clin. Gastroenterol. Hepatol. 2017, 15, 1596–1603.e3. [Google Scholar] [CrossRef]
- Cheng, Y.; Qin, K.; Huang, N.; Zhou, Z.; Xiong, H.; Zhao, J.; Zhang, Y.; Yu, S. Cytokeratin 18 regulates the transcription and alternative splicing of apoptotic-related genes and pathways in HeLa cells. Oncol. Rep. 2019, 42, 301–312. [Google Scholar] [CrossRef]
- Dai, G.; Tan, Y.; Liu, J.; Yuan, B.; Song, Q.; Liu, J.; He, S. The significance of IL-28B and CK-18 M30 levels in the diagnosis of non-alcoholic steatohepatitis in SD rats. Pathol. Res. Pract. 2020, 216, 152901. [Google Scholar] [CrossRef]
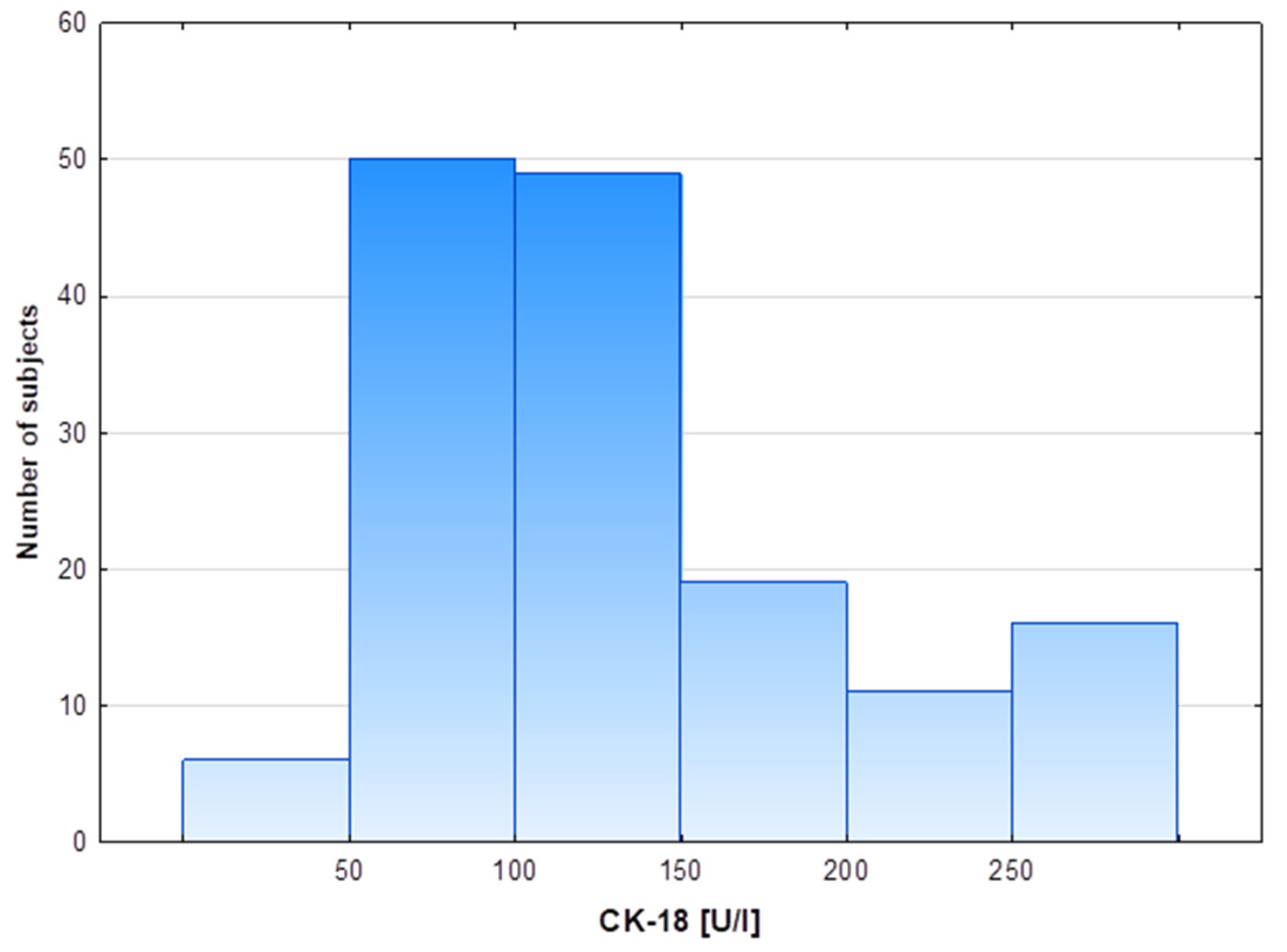

| All n = 151 | Low CK-18 n = 105 | High CK-18 n = 46 | p-Value | |
|---|---|---|---|---|
| Age | 47 | 49 | 46 | |
| [years] | (39–57) | (39–58) | (38–57) | 0.2934 |
| Sex | 109 | 84 | 25 | |
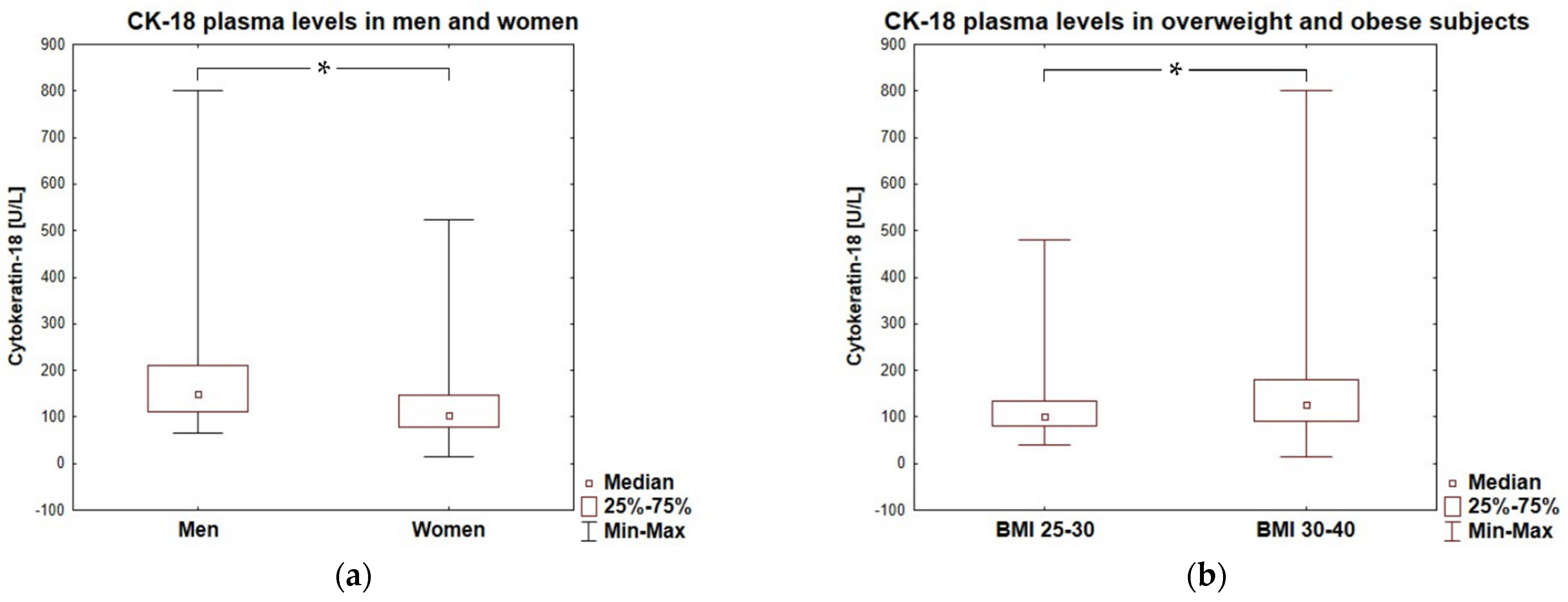
| [% female] | (72%) | (80%) | (54%) | 0.0012 |
| Anthropometrics | ||||
| Body weight | 91.5 | 88 | 100.6 | |
| [kg] | (81–103.9) | (79–99) | (86.8–111.1) | 0.0013 |
| BMI | 32.45 | 32.01 | 33.83 | |
| [kg/m2] | (30.02–35.30) | (29.31–35.09) | (31.68–35.69) | 0.0134 |
| Body fat | 38.30 | 38.9 | 35.4 | |
| [%] | (32.90–42.20) | (33.9–42.6) | (31.25–41.33) | 0.1006 |
| Waist circumference | 102.0 | 99.5 | 110.0 | |
| [cm] | (95.0–111.5) | (93.8–108.0) | (99.5–118.0) | 0.0005 |
| WHR | 0.89 | 0.85 | 0.96 | |
| (0.82–0.97) | (0.82–0.92) | (0.87–1.01) | 0.0004 | |
| Systolic BP | 129 | 125 | 130 | |
| [mmHg] | (120–140) | (120–132) | (120–142) | 0.0591 |
| Diastolic BP | 84 | 82 | 87.5 | |
| [mmHg] | (80–90) | (80–90) | (80–90) | 0.3284 |
| Glucose metabolism | ||||
| Glucose | 5.15 | 5.10 | 5.30 | |
| [mmol/L] | (4.80–5.60) | (4.80–5.53) | (4.90–5.70) | 0.0724 |
| Glucose AUC OGTT | 3474 | 3342 | 3891 | |
| [mmol/L·min] | (2922–4188) | (2919–3855) | (3372–4415) | 0.0078 |
| Insulin | 13.05 | 12.35 | 16.10 | |
| [mU/L] | (9.70–19.10) | (9.58–16.58) | (12.05–24.83) | 0.0078 |
| Insulin AUC OGTT | 39462 | 35724 | 45498 | |
| [mmol/L·min] | (27138–60696) | (25473–55901) | (32247–78528) | 0.0138 |
| HOMA-IR | 2.94 | 2.81 | 3.50 | |
| (2.20–4.56) | (2.15–3.92) | (2.65–6.08) | 0.0194 | |
| Lipid metabolism | ||||
| Total cholesterol | 5.39 | 5.34 | 5.57 | |
| [mmol/L] | (4.80–6.15) | (4.82–5.99) | (4.79–6.34) | 0.3683 |
| HDL cholesterol | 1.29 | 1.29 | 1.28 | |
| [mmol/L] | (1.13–1.46) | (1.14–1.46) | (1.12–1.46) | 0.5375 |
| LDL cholesterol | 3.45 | 3.42 | 3.65 | |
| [mmol/L] | (2.90–4.14) | (2.96–4.07) | (2.70–4.44) | 0.5463 |
| Fasting triglycerides | 1.31 | 1.21 | 1.48 | |
| [mmol/L] | (0.95–1.78) | (0.89–1.68) | (1.21–2.25) | 0.0096 |
| Postprandial triglycerides AUC | 3482 | 3216 | 3900 | |
| [mmol/L·min] | (2494–4884) | (2314–4573) | (3356–5291) | 0.009 |
| Fasting NEFAs | 0.69 | 0.67 | 0.79 | |
| [mmol/L] | (0.53–0.97) | (0.51–0.94) | (0.59–1.03) | 0.0678 |
| All n = 151 | LOW CK-18 n = 105 | HIGH CK-18 n = 46 | p | |
|---|---|---|---|---|
| Liver markers | ||||
| CK-18 [U/L] | 118.6 (87.5–156.7) | 95.6 (73.8–121.03) | 220.6 (160.7–322.9) | <0.0001 |
| ALT [U/L] | 16.0 (12.0–23.0) | 14.0 (11.0–19.0) | 23.5 (16.0–34.8) | <0.0001 |
| GGT [U/L] | 19.0 (12.5–32.0) | 16.0 (12.0–27.0) | 25.0 (17.0–41.5) | 0.0004 |
| FLI | 73.01 (45.49–87.54) | 62.27 (43.31–81.83) | 86.78 (66.42–93.84) | 0.0001 |
| FGF-19 [pg/mL] | 112.60 (69.64–188.36) | 112.60 (69.64–185.19) | 106.36 (71.22–207.57) | 0.8556 |
| FGF-21 [pg/mL] | 212.83 (127.83–303.99) | 191.29 (119.74–273.04) | 263.17 (148.91–387.26) | 0.0231 |
| Adipokines | ||||
| Leptin [pg/mL] | 30,726 (19,740–46,988) | 32,190.2 (21,683–50,153) | 27,307.35 (17,732–41,835) | 0.3091 |
| Adiponectin [ng/mL] | 6251 (4293–8899) | 6533 (4376–9614) | 5542 (3693–7462) | 0.0518 |
| Visfatin [ng/mL] | 0.90 (0.60–1.35) | 0.89 (0.60–1.26) | 1.03 (0.63–1.48) | 0.2613 |
| Resistin [ng/mL] | 9.20 (7.39–11.67) | 9.5 (7.44–11.73) | 8.74 (7.53–11.15) | 0.4401 |
| Inflammatory markers | ||||
| CRP [mg/L] | 1.60 (0.68–3.88) | 1.60 (0.64–3.79) | 1.69 (0.84–3.89) | 0.6588 |
| IL-6 [pg/mL] | 1.24 (0.87–1.74) | 1.28 (0.86–1.89) | 1.11 (0.88–1.56) | 0.4645 |
| sE-Selectin [ng/mL] | 39.02 (29.79–48.98) | 38.1 (29.7–47.45) | 40.65 (31.92–52.42) | 0.1751 |
| sVCAM [ng/mL] | 599.22 (511.22–711.46) | 584.37 (520.41–700.75) | 619.13 (512.79–749.47) | |
| MCP-1 [pg/mL] | 338.37 (271.31–415.88) | 330.55 (257.63–400.42) | 351.54 (308.94–421.96) | 0.0469 |
| sPECAM [ng/mL] | 70.10 (59.95–84.77) | 70.90 (61.15–84.66) | 68.50 (56.48–86.07) | 0.4071 |
| VEGF [pg/mL] | 312.7 (200.2–501.7) | 348.4 (206.8–529.6) | 276.1 (189.1–465.9) | 0.1736 |
| R | p | |
|---|---|---|
| ALT | 0.45 | <0.0001 |
| FLI | 0.36 | <0.0001 |
| GGT | 0.34 | <0.0001 |
| Waist circumference | 0.33 | <0.0001 |
| Body weight | 0.33 | <0.0001 |
| WHR | 0.30 | 0.0005 |
| BMI | 0.23 | 0.0055 |
| Fasting insulin | 0.25 | 0.0036 |
| Insulin AUC OGTT | 0.23 | 0.0079 |
| Fasting glucose | 0.16 | 0.0472 |
| Glucose AUC OGTT | 0.23 | 0.0058 |
| Fasting TG | 0.16 | 0.0489 |
| Postprandial TG | 0.20 | 0.0126 |
| FGF-21 | 0.18 | 0.0245 |
| Adiponectin | −0.23 | 0.0049 |
| MCP1 | 0.20 | 0.0209 |
| Model 1 | Model 2 | |
|---|---|---|
| β (95% CI) | β (95% CI) | |
| ALT | 0.41 (0.20–0.61) | 0.40 (0.19–0.61) |
| Insulin AUC OGTT | 0.13 (−0.06–0.32) | 0.12 (−0.08–0.32) |
| Fasting triglycerides | −0.11 (−0.29–0.08) | −0.11 (−0.29–0.08) |
| MCP1 | 0.05 (−0.14–0.23) | 0.05 (−0.14–2.23) |
| Adiponectin | −0.08 (−0.28–0.12) | −0.09 (−0.29–0.12) |
| Model 1 | p | Model 2 | p | |
|---|---|---|---|---|
| β (95% CI) | β (95% CI) | |||
| ALT | 1.13 (1.05–1.22) | 0.0017 | 1.13 (1.04–1.22) | 0.0024 |
| Insulin AUC OGTT | 1.00 (1.00–1.00) | 0.2891 | 1.00 (1.00–1.00) | 0.4851 |
| Fasting triglycerides | 0.99 (0.51–1.91) | 0.9744 | 0.98 (0.5–1.89) | 0.9425 |
| Fasting NEFA | 0.39 (0.07–2.12) | 0.2733 | 0.35 (0.06–1.98) | 0.2354 |
| MCP1 | 1.00 (1.00–1.01) | 0.5877 | 1.00 (1.00–1.01) | 0.6866 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goralska, J.; Razny, U.; Gruca, A.; Zdzienicka, A.; Micek, A.; Dembinska-Kiec, A.; Solnica, B.; Malczewska-Malec, M. Plasma Cytokeratin-18 Fragment Level Reflects the Metabolic Phenotype in Obesity. Biomolecules 2023, 13, 675. https://doi.org/10.3390/biom13040675
Goralska J, Razny U, Gruca A, Zdzienicka A, Micek A, Dembinska-Kiec A, Solnica B, Malczewska-Malec M. Plasma Cytokeratin-18 Fragment Level Reflects the Metabolic Phenotype in Obesity. Biomolecules. 2023; 13(4):675. https://doi.org/10.3390/biom13040675
Chicago/Turabian StyleGoralska, Joanna, Urszula Razny, Anna Gruca, Anna Zdzienicka, Agnieszka Micek, Aldona Dembinska-Kiec, Bogdan Solnica, and Malgorzata Malczewska-Malec. 2023. "Plasma Cytokeratin-18 Fragment Level Reflects the Metabolic Phenotype in Obesity" Biomolecules 13, no. 4: 675. https://doi.org/10.3390/biom13040675
APA StyleGoralska, J., Razny, U., Gruca, A., Zdzienicka, A., Micek, A., Dembinska-Kiec, A., Solnica, B., & Malczewska-Malec, M. (2023). Plasma Cytokeratin-18 Fragment Level Reflects the Metabolic Phenotype in Obesity. Biomolecules, 13(4), 675. https://doi.org/10.3390/biom13040675







