Abstract
In our research on sphingolipids from marine invertebrates, a mixture of phytoceramides was isolated from the sponge Monanchora clathrata (Western Australia). Total ceramide, ceramide molecular species (obtained by RP-HPLC, high-performance liquid chromatography on reversed-phase column) and their sphingoid/fatty acid components were analyzed by NMR (nuclear magnetic resonance) spectroscopy and mass spectrometry. Sixteen new (1b, 3a, 3c, 3d, 3f, 3g, 5c, 5d, 5f, 5g, 6b–g) and twelve known (2b, 2e, 2f, 3b, 3e, 4a–c, 4e, 4f, 5b, 5e) compounds were shown to contain phytosphingosine-type backbones i-t17:0 (1), n-t17:0 (2), i-t18:0 (3), n-t18:0 (4), i-t19:0 (5), or ai-t19:0 (6), N-acylated with saturated (2R)-2-hydroxy C21 (a), C22 (b), C23 (c), i-C23 (d), C24 (e), C25 (f), or C26 (g) acids. The used combination of the instrumental and chemical methods permitted the more detailed investigation of the sponge ceramides than previously reported. It was found that the cytotoxic effect of crambescidin 359 (alkaloid from M. clathrata) and cisplatin decreased after pre-incubation of MDA-MB-231 and HL-60 cells with the investigated phytoceramides. In an in vitro paraquat model of Parkinson’s disease, the phytoceramides decreased the neurodegenerative effect and ROS (reactive oxygen species) formation induced by paraquat in neuroblastoma cells. In general, the preliminary treatment (for 24 or 48 h) of the cells with the phytoceramides of M. clathrata was necessary for their cytoprotective functions, otherwise the additive damaging effect of these sphingolipids and cytotoxic compounds (crambescidin 359, cisplatin or paraquat) was observed.
1. Introduction
Ceramide is a complex lipid, which consists of a sphingoid base attached to a fatty acid via an amide bond. Ceramides are present in membranes in small amounts only, but they serve as central mediators, regulating many fundamental cellular responses [1,2,3]. These sphingolipids trigger a number of tumor suppressive and anti-proliferative cellular programs, for example, apoptosis, autophagy, senescence, and necroptosis [4,5]. Ceramide-dependent effects may hold implications for the progression of many diseases including cancer, diabetes, atherosclerosis, Alzheimer’s and Parkinson’s diseases [1].
Ceramides vary appreciably in the compositions of both long-chain alkyl (sphingoid and fatty acid) components, depending on their biological origins. In particular, phytoceramides, consisting of phytosphingosine-type backbones N-acylated with 2-hydroxy fatty acids, are common for marine sponges [6]. In our research on sphingolipids from marine invertebrates, phytoceramides, mainly containing iso-methyl-branched chains, were found in the sponge Monanchora clathrata, collected in Australian waters.
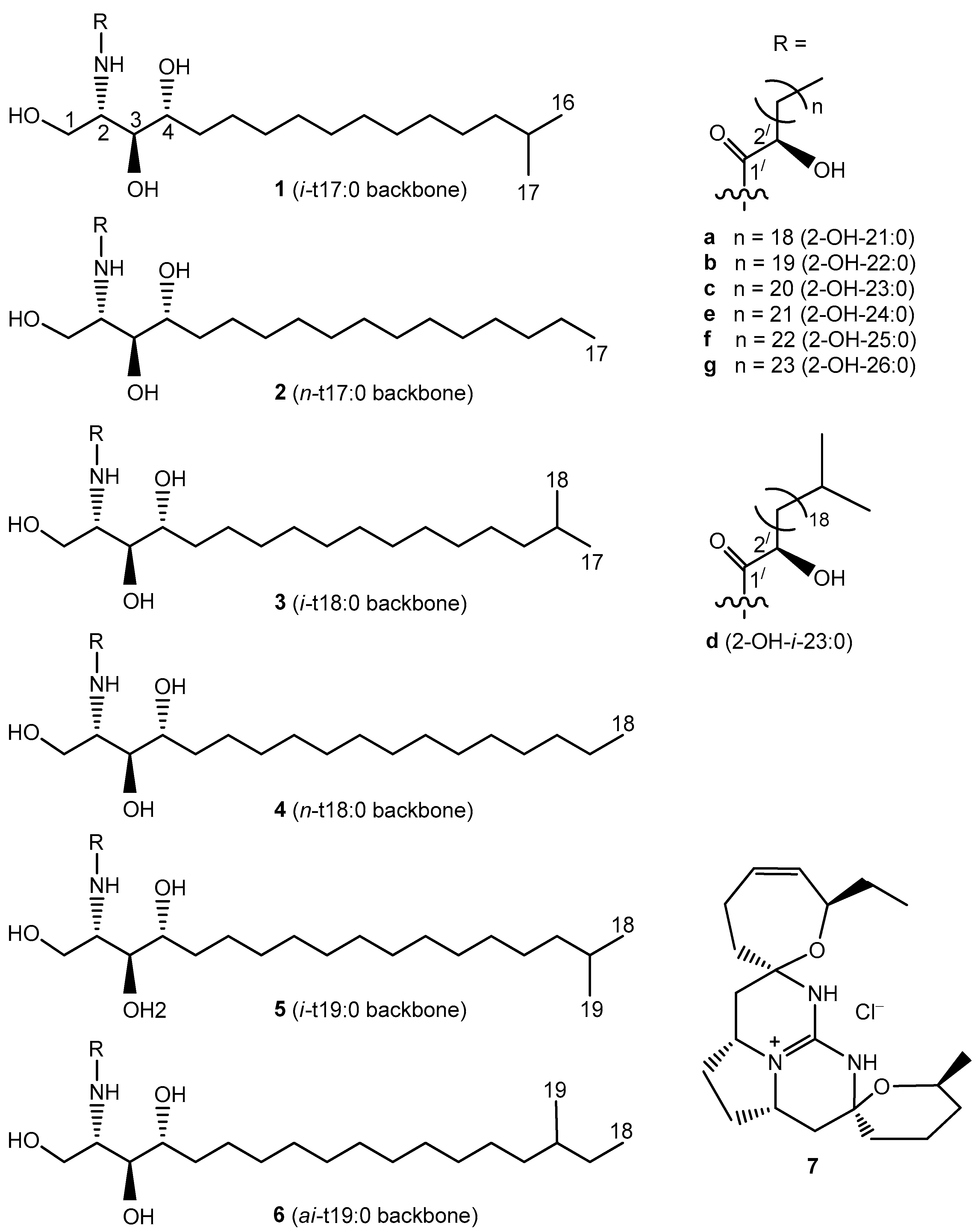
The phytoceramides of the sponge M. clathrata have been the subjects of a study reporting the structures of four new compounds [7]. However, since the MS/MS (tandem mass spectrometry) technique was not used in the previous study, it remained possible that the ceramide composition of M. clathrata was more complex than that reported. The present work was undertaken in order to provide more detailed information on the ceramide composition of M. clathrata by applying a combination of chemical and instrumental methods including RP-HPLC (high-performance liquid chromatography on reversed-phase column), NMR (nuclear magnetic resonance), ESI-MS (electrospray ionization mass spectrometry), ESI-MS/MS, and GC-MS (gas chromatography-mass spectrometry). The phytoceramides of M. clathrata were analyzed as constituents of multi-component RP-HPLC fractions, and the structures of 16 new (1b, 3a, 3c, 3d, 3f, 3g, 5c, 5d, 5f, 5g, 6b–g) and 12 known (2b, 2e, 2f, 3b, 3e, 4a–c, 4e, 4f, 5b, 5e) compounds were elucidated (Figure 1).
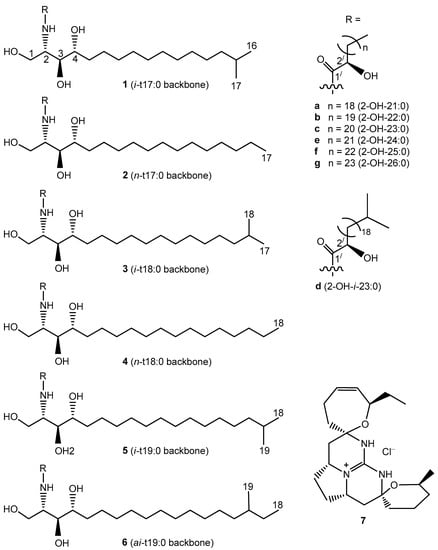
Figure 1.
Phytoceramides (1b, 2b, 2e, 2f, 3a–g, 4a–c, 4e, 4f, 5b–g, 6b–g) and crambescidin 359 (7) from the sponge Monanchora clathrata. Shorthand designation [8] was used: for example, iso-methyl-branched and normal-chain trihydroxy C17 sphingoid bases were denoted as i-t17:0 and n-t17:0, respectively; anteiso-methyl-branched trihydroxy C19 sphingoid base was denoted as ai-t19:0.
In the previous study of the phytoceramides of M. clathrata, these compounds were shown to be cytotoxic to MES-SA, MCF-7, and HK-2 cell lines [7]. The results from other studies suggest that phytoceramides may help to prevent neurodegeneration in vitro and in vivo [9,10]. The neuroprotective effect of phytoceramides is consistent with a possible therapeutic role of these compounds in managing cognitive impairment, associated, in the first turn, with Alzheimer’s disease. We aimed to investigate mainly the cytoprotective effects of the phytoceramides from M. clathrata, in particular their effect on paraquat-induced neurotoxicity using in vitro model of Parkinson’s disease. The neuroprotective activity of phytoceramides in Parkinson’s disease has not been studied, although this pathology is the second most common neurodegenerative disorder behind Alzheimer’s disease [11]. Alkaloid crambescidin 359 (7), previously isolated from the sample of M. clathrata analyzed here [12], was also used in our bioassays. The cytotoxic and/or cytoprotective effects of total ceramide, crambescidin 359 and their combinations were tested on MDA-MB-231 (breast adenocarcinoma) and HL-60 (leukemia) cells. The cytotoxic effect of cisplatin in combinations with the phytoceramides of M. clathrata was also tested on these cells. Cisplatin is employed for the treatment of various tumors, and there is an urgent need for therapeutic agents reducing cisplatin-induced toxicity. Based on the results of the present study, it is concluded that, depending on the time of cell pre-treatment by phytoceramides, these sphingolipids can increase or decrease the cytotoxicity of crambescidin 359, cisplatin or paraquat.
2. Materials and Methods
2.1. General Procedures
1H-, 13C-NMR, 1H,1H-COSY and HSQC spectra (in C5D5N or CDCl3) were recorded on Bruker Avance III HD 500 and Bruker Avance III 700 spectrometers (Bruker BioSpin, Bremen, Germany) at 125 MHz (13C), 500 (1H), and 700 (1H) MHz. A Bruker Impact II Q-TOF mass spectrometer (Bruker Daltonik GmbH, Bremen, Germany) equipped with ESI ionization source was employed to record MS and MS/MS spectra. The operating parameters for ESI-MS were the following: a capillary voltage of 4.0 kV, nebulization with nitrogen at 0.4 bar, and a dry gas flow of 4 L/min at a temperature of 200 °C. GC analyses were performed on an Agilent 6850 Series GC System chromatograph (Agilent Technologies, Santa Clara, CA, USA) equipped with an DB-1 (J&W Scientific, Folsom, CA, USA) capillary column (30 m × 0.32 mm), the carrier gas was helium (flow rate 1.7 mL/min), and the detector temperature was 300 °C. GC-MS analyses were carried out on a Hewlett-Packard HP6890 GC System (Hewlett-Packard Company, Palo Alto, CA, USA) with an HP-5MS (J&W Scientific, Folsom, CA, USA) capillary column (30.0 m × 0.25 mm), helium as the carrier gas, and 70 eV ionizing potential. The GC and GC-MS analyses of fatty acid esters and peracetylated sphingoid bases were performed using the injector temperature of 270 °C and the temperature program 100 °C (1 min) − 10 °C/min − 280 °C (30 min). Optical rotation was measured on a Perkin–Elmer polarimeter, model 343 (Perkin-Elmer GmbH, Überlingen, Germany). Column chromatography was performed using silica gel (50/100 μm, Sorbpolimer, Krasnodar, Russia). RP-HPLC separations were performed using a Du Pont Series 8800 Instrument (DuPont, Wilmington, DE, USA) with a RIDK-102 refractometer (Laboratorni pristroje, Praha, Czechoslovakia). An Agilent ZORBAX Eclipse XDB-C8 column (4 × 150 mm; Agilent Technologies, Santa Clara, CA, USA) was used for the HPLC.
2.2. Animal Material
The sponge Monanchora clathrata (phylum Porifera, class Demospongiae, order Poecilosclerida, family Crambeidae) was collected by divers from 7 m depth near the Western Australian coast (26°09.8/S, 113°12.8/E) during a cruise onboard the r/v “Akademik Oparin” in September, 1987. The species was identified by V.B. Krasokhin (G.B. Elyakov Pacific Institute of Bioorganic Chemistry, Russia).
2.3. Extraction and Isolation
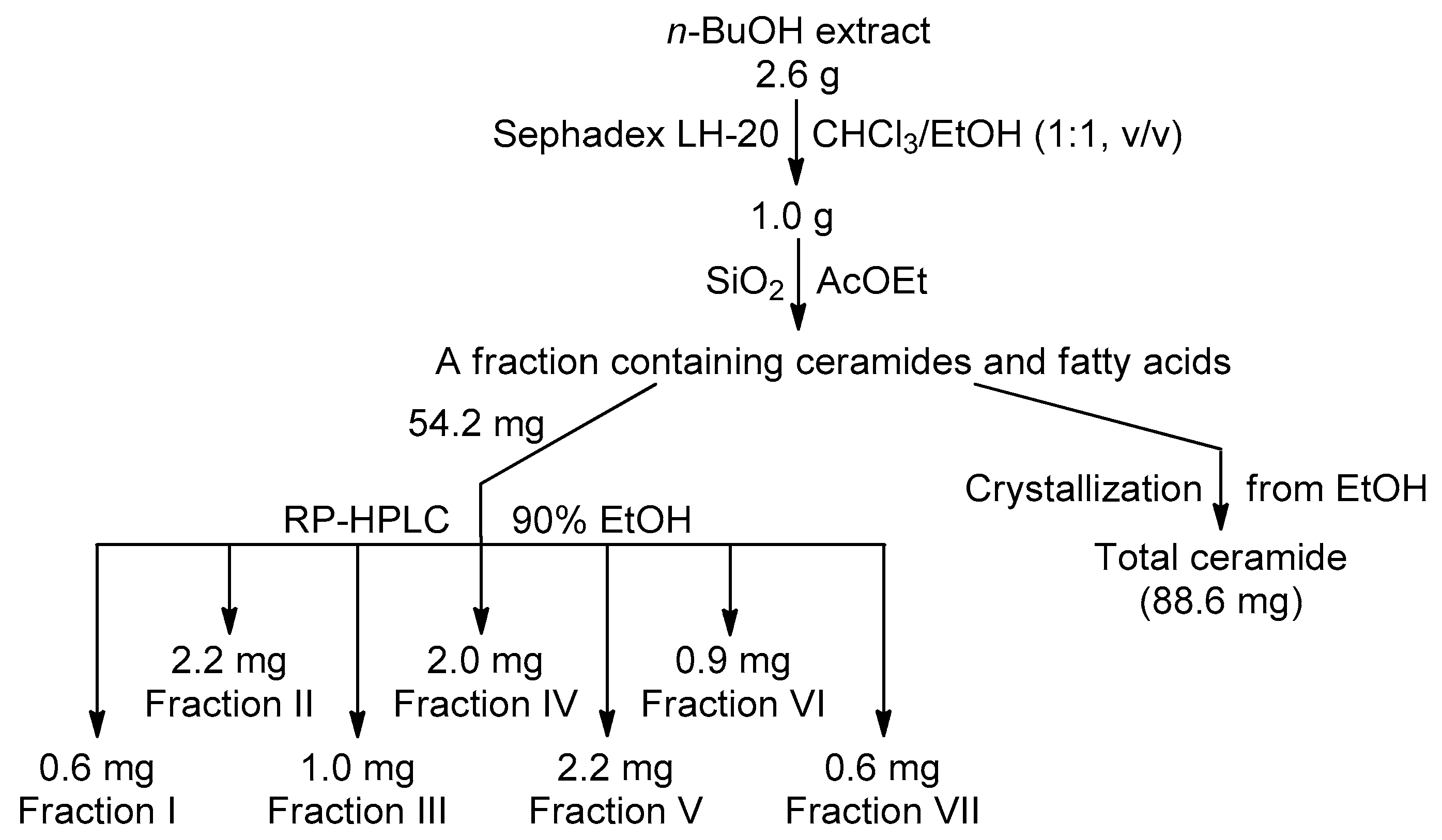
The collected sponge was cut, lyophilized, stored at −15 °C, and then extracted with EtOH at room temperature. After evaporation in vacuo, EtOH extract (4.6 g) was partitioned between H2O (200 mL) and n-BuOH (150 mL). The dry n-BuOH extract was separated to give total ceramide and ceramide HPLC fractions (Scheme 1). Total ceramide: amorphous solid; 1H- and 13C-NMR (500 MHz, C5D5N): see Table 1; high resolution ESI-MS in negative ion mode (HR-(–)ESI-MS): 640.5886 ([M − H]− of 1b, 2b, 3a, and 4a, C39H78NO5−; calc. 640.5885); 654.6045 ([M − H]− of 3b and 4b, C40H80NO5−; calc. 654.6042); 668.6196 ([M − H]− of 2e, 3c, 3d, 4d, 5b, and 6b, C41H82NO5−; calc. 668.6198); 682.6354 ([M − H]− of 2f, 3e, 4e, 5c, 5d, 6c, and 6d, C42H84NO5−; calc. 682.6355); 696.6510 ([M − H]− of 3f, 4f, 5e, and 6e, C43H86NO5−; calc. 696.6511); 710.6665 ([M − H]− of 3g, 5f, and 6f, C44H88NO5−; calc. 710.6668); and 724.6828 ([M − H]− of 5g and 6g, C45H90NO5−; calc. 724.6824).
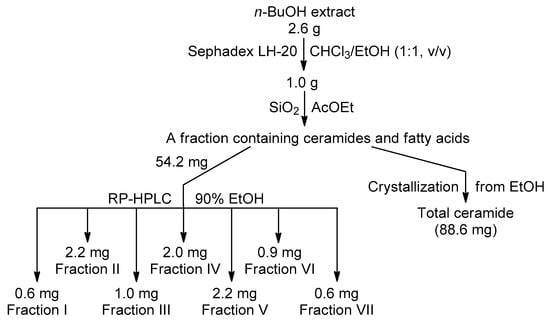
Scheme 1.
Isolation of total ceramide and ceramide RP-HPLC Fractions I–VII.

Table 1.
1H- (700 MHz) and 13C- (125 MHz) NMR data * for the total ceramide of M. clathrata (C5D5N).
HCl-Catalyzed hydrolysis was performed according to the procedure of Aveldaño and Horrocks [13]. A part (7.0 mg) of the total ceramide was hydrolyzed in MeCN (0.45 mL) and 5 N HCl (0.05 mL) for 4 h at 70 °C. The reaction mixture was concentrated in vacuo and acetylated with Ac2O in pyridine (1:1, v/v, 0.2 mL, overnight). The acetylated material was separated by chromatography on silica gel (column: 4.5 cm × 1.5 cm) using hexane/ethylacetate (5:1 → 2:1 → 1:1, v/v) systems, then CHCl3/EtOH (1:1) system. Elution with hexane/ethylacetate (2:1, v/v, 60 mL) gave tetracetates of sphingoid bases (3.5 mg): [α]22D = +25.5 (c = 0.2, CHCl3); 1H-NMR (700 MHz, CDCl3): 5.96 (d, J = 9.3 Hz, NH), 5.10 (dd, J = 3.1, 8.2 Hz, CH-3), 4.94 (dt, J = 3.1, 10.1 Hz, CH-4), 4.47 (m, CH-2), 4.28 (dd, J = 4.9, 11.6 Hz, CH-1b), 4.005 (dd, J = 3.1, 11.6 Hz, CH-1a), 2.075 (s, −OCOCH3 at C-3), 2.045 (s, −OCOCH3 at C-4 and at C-1), 2.02 (s, −NHCOCH3), 1.63 (m, CH2-5), 1.51 (sep, J = 6.6 Hz, –CH(CH3)2 of iso-methyl-branched components), 1.40 − 1.20 (m, CH2-pool), 1.15 (m, –CH2CH(CH3)2 of iso-methyl-branched components), 0.88 (t, J = 7.0 Hz, terminal –CH3 of minor normal-chain components), 0.86 (d, J = 6.6 Hz, terminal −CH3 of iso-methyl-branched components), 0.85 (t, J = 7.0 Hz, −CH(CH3)CH2CH3 of minor anteiso-methyl-branched components), 0.84 (d, J = 6.0 Hz, −CH(CH3)CH2CH3 of minor anteiso-methyl-branched components); GC-MS: see Section 3.2 below. Elution with CHCl3/EtOH (1:1, 20 mL) gave 2-acetyloxy fatty acids. These acids were treated with 2% H2SO4 in (2R)-octan-2-ol or (2S)-octan-2-ol (0.2 mL) for 4 h at 75 °C in a capped vial [14] to obtain (2R)- and (2S)-oct-2-yl esters of 2-hydroxy fatty acids.
The RP-HPLC purification of the tetracetylated sphingoid bases (3.5 mg) on an Agilent ZORBAX Eclipse XDB-C8 column (70% EtOH) yielded subfractions I (0.1 mg: n-t17:0, 94.4%; i-t17:0, 5.6%), II (1.9 mg: i-t18:0, 98.2%; n-t18:0, 1.8%), III (0.3 mg: i-t18:0, 50.9%; n-t18:0, 32.8%; ai-t19:0, 16.3%), and IV (0.6 mg: i-t19:0, 26.0%; ai-t19:0, 74.0%).
Methanolysis of ceramides (73.6 mg) in MeOH (2.0 mL) and HCl (0.4 mL) for 4 h at 90 °C gave methyl esters of 2-hydroxy acids (19.2 mg): [α]22D = −5.2 (c = 0.2, CHCl3); 1H-NMR (CDCl3, 300 MHz): 4.185 (m, H-2), 3.79 (s, −OCH3), 2.66 (d, J = 5.8 Hz, −OH), 1.78 (m, H-3b), 1.64 (m, H-3a), 1.50−1.20 (m, CH2-pool), 0.88 (t, J = 6.9 Hz, terminal –CH3 of dominant straght-chain compounds) and 0.86 (d, J = 6.6 Hz, terminal –CH3 of minor iso-methyl-branched compound); GC-MS: see Section 3.2 below. The methyl esters of 2-hydroxy acids were converted to DMOX and pyrrolidine derivatives, as described earlier [15].
The acid hydrolysis of ceramide molecular species (Fractions I–VII), obtained by RP-HPLC, was carried out under the same conditions as for total ceramide. Water (0.2 mL) was added to hydrolysate, and MeCN–H2O layer was extracted with hexane (5 × 0.5 mL). Hexane extract was dried in vacuo, acetylated with Ac2O in pyridine (1:1, v/v, 0.2 mL, overnight) and ethylated with N-nitroso-N-ethylurea. The resulting tetraacetates of sphingoid bases and ethyl esters of 2-acetyloxy acids were analyzed by GC-MS. The ethyl esters dominated in the mixture, and their signals and some signals of tetraacetylated sphingoid bases overlapped in GC profiles. To obtain a pure subfraction of the acetates of sphingoid bases, the mixture was separated by chromatography on silica gel (column: 3.0 cm × 1.5 cm) using hexane/ethylacetate (5:1 → 2:1, v/v). Elution with hexane/ethylacetate, 2:1 (60 mL) gave tetraacetates of sphingoid bases that were again analyzed by GC-MS.
2.4. Cytotoxic and Cytoprotective Activities
2.4.1. Reagents
Dulbecco’s Modified Eagle Medium (DMEM), Roswell Park Memorial Institute medium (RPMI 1640), Minimum Essential Medium (MEM), 1% penicillin-streptomycin solution and 10% fetal bovine serum (FBS) were purchased from Biolot (Moscow or St. Petersburg, Russia), penicillin-streptomycin solution, 10 μg/mL, and dimethyl sulfoxide (DMSO) from Sigma-Aldrich, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS reagent) from Promega (Madison, WI, USA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) from Sigma-Aldrich (St. Louis, MO, USA), 2,7-dichlorodihydrofluorescein diacetate solution (10.0 µM H2DCFDA) from Molecular Probes (Eugene, OR, USA), and cisplatin from VeroPharm (Moscow, Russia).
2.4.2. Cell Lines and Culture Conditions
Human breast cancer MDA-MB-231 cells (ATCC® HTB-26™), acute promyelocytic leukemia HL-60 cells (ATCC® CCL-240™), neuroblastoma SH-SY5Y cells (CRL-2266™) and murine neuroblastoma Neuro-2a cells (CCL-131™) were obtained from the American Type Culture Collection (Manassas, VA, USA). The MDA-MB-231 and HL-60 cells were cultured in complete DMEM/10% FBS and RPMI 1640/10% FBS, respectively, containing 1% of penicillin-streptomycin solution. The SH-SY5Y cells were cultured in MEM containing 10% FBS and 1% penicillin-streptomycin solution. The Neuro-2a cells were cultured in DMEM containing 10% FBS and 1% penicillin-streptomycin solution. The cell cultures were incubated at 37 °C in a humidified atmosphere containing 5% (v/v) CO2.
2.4.3. Cell Viability Assay for MDA-MB-231 and HL-60 Cells
The effect of compounds on cell viability was evaluated using reduction of MTS into formazan product. The cells were cultured in 96-well plates (5000 cells/well) with the corresponding medium (100 µL/well, containing 10% FBS) for 12 h. The cells were treated with compounds at various concentrations (0–200 µM in DMSO) for 24 or 48 h. Then, MTS reagent (20 µL) was added into each well, and, after 4 h, MTS reduction was measured spectrophotometrically at 492 and 690 nm as background, using a Power Wave XS microplate reader (BioTek, Winooski, VT, USA). Cisplatin was used as a positive control.
2.4.4. Paraquat Induced In Vitro Model of Neurotoxicity
Crambescidin 359 and ceramide were dissolved in DMSO to obtain stock solutions (10 mM concentrations). Cells SH-SY5Y and Neuro-2a (1 × 104 cells/well) were supplemented with the test compounds (20 µL in PBS) at final concentrations of 0.1, 1.0, or 10.0 µM, and preincubated for 1, 24 or 48 h. Then, the cells were treated with 1.5 mM paraquat. The cells incubated without paraquat were used as a positive control, and the cells incubated with this inducer were used as a negative control. Cell viability was measured after 24 h. For this, the medium with tested substances was changed by 100 μL of fresh culture medium containing 10 μL of MTT solution (5 mg/mL). After that, the microplate was incubated for an additional 4 h. Then, 100 µL of SDS-HCl solution (1 g SDS/10 mL dH2O/17 µL 6N HCl) was added and incubated for 18 h. Dye absorbance was measured using a plate format spectrophotometer at a wavelength of 570 nm (Thermo Scientific, Waltham, MA, USA). All the experiments were carried out three times. The toxic activity of paraquat was expressed as a percentage of control (untreated) cells.
2.4.5. Analysis of ROS (Reactive Oxygen Species) Level
Neuro-2a cells were transferred to a microplate for adhesion for 24 h, then incubated with ceramide or crambescidin 359 at various concentrations for 1h. To increase ROS in cells, paraquat was added at a concentration of 1mM per 3h. In order to study ROS formation, 20 µL of H2DCFDA solution (100 µM concentration) were added to each well (to a final concentration of 10.0 µM), and the microplate with cells was incubated for an additional 10 min at 37 °C. The intensity of fluorescence was measured using PHERAstar FS plate reader (BMG Labtech, Ortenberg, Germany) at λex = 485 nm and λem = 520 nm.
2.4.6. Statistical Analysis
All experiments were carried out in three or more independent experiments. Plot data were presented as mean ± standard deviation (SD). Student’s t-test was performed using SigmaPlot 14.0 (Systat Software Inc., San Jose, CA, USA) to determine statistical significance.
3. Results
3.1. Analysis of the Total Ceramide of M. clathrata
The 1H- and 13C-NMR spectra (C5D5N) of the total ceramide, isolated from M. clathrata, showed the signals of saturated phytosphingosine-type backbones, N-acylated with saturated 2-hydroxy acids (Table 1, Figures S1 and S2). In the 1H,1H-COSY spectrum, the signal of –NH–CO– (δH 8.58, d, J = 9.0 Hz), characteristic of the sphingoid base moieties of ceramides, displayed a cross-peak with CH-2 (δH 5.12, m). The 1H,1H-COSY diagram also indicated that several protons, from CH2-1 (δH 4.44, dd; 4.53, dd) to CH2-6 (δH 1.48, m; 1.72, m), formed a linear spin system of phytosphingosine-type moieties. Another spin system consisted of CH-2/ (δH 4.635, dd), CH2-3/ (δH 2.06, m; 2.25, m), and CH2-4/ (δH 1.77, m) protons of the 2-hydroxy acyls. Accordingly, the signals of one CH2 (δC 61.9, CH2-1) and three CH (δC 72.3, CH2-2/; 72.9, CH2-4; 76.7, CH2-3), bearing –OH groups, and the signals of –C=O (δC 175.1, C-1/) and CH (δC 52.9, CH-2), linked to –NH–, were observed in the 13C-NMR spectrum of the investigated phytoceramides. The 1H- and 13C- NMR spectra of these compounds also showed signals of long hydrocarbon chains with terminal methyl groups belonging to normal-chain, iso- and minor anteiso-methyl branched constituents (Table 1). These spectra resembled the corresponding spectra of monanchoramides A–D, previously isolated from Philippine sample of the sponge M. clathrata [7]. Monanchoramides A–D were reported to contain iso-methyl-branched saturated phytosphingosine-type C18–C21 backbones, N-acylated with saturated normal-chain (2R)-2-hydroxy C22 acid.
The molecular formulae of the phytoceramides from M. clathrata were determined by HR-ESI-MS (high resolution ESI-MS) analyses in negative ion mode (Figure S3). The HR-(−)ESI-MS analysis of total ceramide resulted in a series of peaks, representing seven homologous [M − H]− ions. ESI-MS/MS experiments showed that these molecular species contained C17–C19 sphingoid bases, linked to C21–C26 acids (Table 2).

Table 2.
Ceramide molecular species of M. clathrata.
3.2. Analysis of the 2-Hydroxy Fatty Acids and Sphingoid Bases Obtained from the Total Ceramide of M. clathrata
We applied methanolysis and hydrolysis for the chemical degradation of total ceramide. Methanolysis procedure was used to prepare fatty acid methyl esters. Hydrolysis was mainly used to release sphingoid bases because sphingoid bases could be harmed by vigorous conditions of methanolysis [16].
The 1H-NMR spectrum (CDCl3) of the methyl esters of 2-hydroxy acids displayed characteristic signals at δH 4.185 (m, H-2), 3.79 (s, −OCH3), and 2.66 (d, J = 5.8 Hz, −OH at CH-2). This spectrum also showed signals of methyl groups belonging to dominant normal-chain (δH 0.88, t, J = 6.9 Hz) and minor iso-methyl-branched (δH 0.86, d, J = 6.6 Hz) acyls. In GC-MS analysis, the methyl esters of 2-hydroxy acids fragmented to give diagnostic [MeOCOCH2OH]+ (m/z 90) and [M−59]+ ions [17]. The GC-MS analysis of these fatty acid esters showed the presence of seven compounds, two of which were isomeric (Table 3). Then, the methyl esters of 2-hydroxy acids were converted to 4,4-dimethyloxazoline and pyrrolidine derivatives, the mass spectra of which were used to clarify the structures of six 2-hydroxy normal-chain C21–C26 acids and one iso-methyl-branched C23 acid [15]. (2R)-configurations of 2-hydroxy fatty acids, liberated by hydrolysis of phytoceramides, were determined by GC-MS analyses of the (2R)- and (2S)-oct-2-yl esters of these acids. Each (2R)-oct-2-yl ester eluted before the diastereomeric (2S)-oct-2-yl ester, as described for the elution order of the (2R)/(2S)-oct-2-yl esters of standard (2R)-2-hydroxy fatty acids [14]. The optical rotation value ([α]D22 = −5.2, CHCl3) of the methyl esters of 2-hydroxy acids, obtained from the total ceramide of M. clathrata, also indicated their (2R)-configurations [18,19].

Table 3.
Fatty acid composition of the phytoceramides from M. clathrata *.
The tetraacetylated sphingoid bases (Table 4), obtained in the result of hydrolysis of the total ceramide followed by acetylation, were separated by RP-HPLC and analyzed by GC-MS and 1H-NMR spectroscopy. GC-MS data allowed for the identification of the following six components in the resulting HPLC fractions: major n-t17:0, i-t18:0, i-t19:0, and ai-t19:0 acetates and minor i-t17:0 and n-t18:0 acetates. The 1H-NMR spectra (CDCl3) of all the fractions contained resonances at δH 5.96 (d, J = 9.3 Hz, NH), 5.10 (dd, J = 3.1, 8.2 Hz, CH-3), 4.94 (dt, J = 3.1, 10.1 Hz, CH-4), 4.47 (m, CH-2), 4.28 (dd, J = 4.9, 11.6 Hz, CH-1b), and 4.005 (dd, J = 3.1, 11.6 Hz, CH-1a), typical of tetraacetylated phytosphingosine-type compounds [20]. The 1H-NMR chemical shifts of CH2-1–CH-4 and optical rotation value ([α]D22 = +25.5, CHCl3) of the mixture of the tetraacetylated sphingoid bases indicated their (2S,3S,4R)-configurations [20]. The 1H-NMR spectra of these compounds also showed signals of the methyl groups of major iso-methyl-branched (δH 0.86, d, J = 6.6 Hz), minor anteiso-methyl-branched (δH 0.84, d, J = 6.2 Hz; δH 0.855, t, J = 7.2 Hz), and minor normal-chain (δH 0.88, t, J = 7.0 Hz) forms. The mass spectra of the acetates of sphingoid bases were also used for distinguishing between their terminal structures because the differences in the relative abundances of [M − AcOH − CH3]+ and [M − AcOH − CH2CH3]+ ions reflected the position of methyl branching in iso- and anteiso- forms [21]. In the mass spectrum of tetraacetylated i-t19:0, the gap of 28 amu between the peaks at m/z 396 [M − AcOH − CH(CH3)2]+ and 424 [M − AcOH − CH3]+ reflected cleavage on either side of the carbon, bearing a methyl group (–CH2–CH2-/-CH(CH3)-/-CH3). In the mass spectrum of tetraacetylated ai-t19:0, the significant peaks at m/z 410 [M − AcOH − CH2CH3]+ and 382 [M − AcOH − CH(CH3)CH2CH3]+ and the small peak at m/z 396 were characteristic (according to cleavage –CH2-/-CH(CH3)-/-CH2–CH3).

Table 4.
Sphingoid base composition of the phytoceramides from M. clathrata *.
3.3. Structure Elucidation of the Phytoceramides of M. clathrata
The total ceramide of M. clathrata was separated into seven molecular species by RP-HPLC (Fractions I–VII, Table 5).

Table 5.
Phytoceramides of M. clathrata.
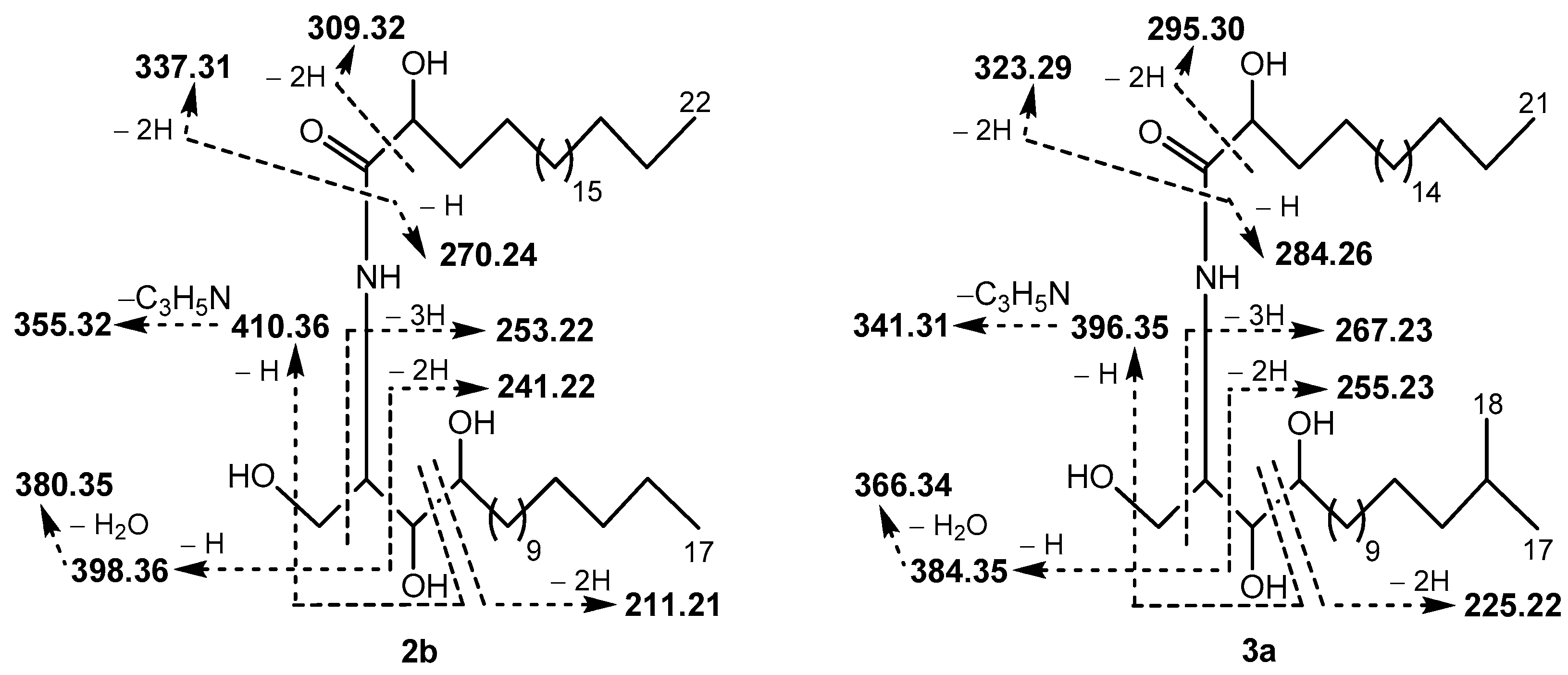
Fraction I (Table 5) was shown to contain isomeric compounds 1b (3.3%), 2b (30.7%), 3a (61.3%), and 4a (4.7%). Their molecular formula C39H79NO5 was established by HR-(−)ESI-MS analysis. To establish the chain length of sphingoid base and 2-hydroxy acid moieties in the phytoceramides, (−)ESI-MS/MS experiments were applied, as exemplified with the MS/MS analysis of two isomers 2b and 3a (Figure 2 and Figure S4). Thus, the ceramides of Fraction I were found to contain saturated C17 and C18 sphingoid bases, N-acylated with saturated 2-hydroxy C22 and C21 fatty acids, respectively (isomeric C17/C22 and C18/C21 structures). Hydrolysis of Fraction I gave the following two normal-chain (2R)-2-hydroxy fatty acids, identified by GC-MS: (2R)-2-hydroxydocosanoic (from 1b and 2b) and (2R)-2-hydroxyheneicosanoic (from 3a and 4a). Additionally, GC-MS analysis allowed for the identification of four phytosphingosine-type compounds in hydrolysate including (2S,3S,4R)-2-amino-15-methyl-hexadecane-1,3,4-triol (i-t17:0, from 1b), (2S,3S,4R)-2-amino-heptadecane-1,3,4-triol (n-t17:0, from 2b), (2S,3S,4R)-2-amino-16-methyl-heptadecane-1,3,4-triol (i-t18:0, from 3a), and (2S,3S,4R)-2-amino-octadecane-1,3,4-triol (n-t18:0, from 4a). The structures of fatty acids and sphingoid bases were determined based on the data obtained for the “building blocks” of the total ceramide (Section 3.2). The sphingoid bases and 2-hydroxy acids were connected according to the (−)ESI-MS/MS data, and, as a result, the structures of two new (1b and 3a) and two known (2b and 4a) ceramides were elucidated.
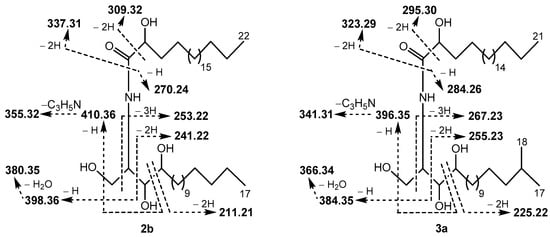
Figure 2.
Fragment ions detected in the (–)ESI-MS/MS spectrum of [M − H]− ion (m/z 640.59) of ceramides 2b (as well as 1b) and 3a (as well as 4a). In addition, [M − H − H2O]− (m/z 622.58), [M − H − H2CO]− (m/z 610.57), [M − H − H2CO − 2H]− (m/z 608.56), [M − H − 2H2O]− (m/z 604.57), and [M − H − H2O − H2CO]− (m/z 574.56) ions were observed in the spectrum. This MS/MS interpretation is consistent with the one described by Hsu [22].
Analogously, other 24 phytoceramides were analyzed as constituents of Fractions II–VII (Table 5, Figures S5–S10).
3.4. Cytoprotective and/or Cytotoxic Effects of the Phytoceramides and Crambescidin 359 from M. clathrata
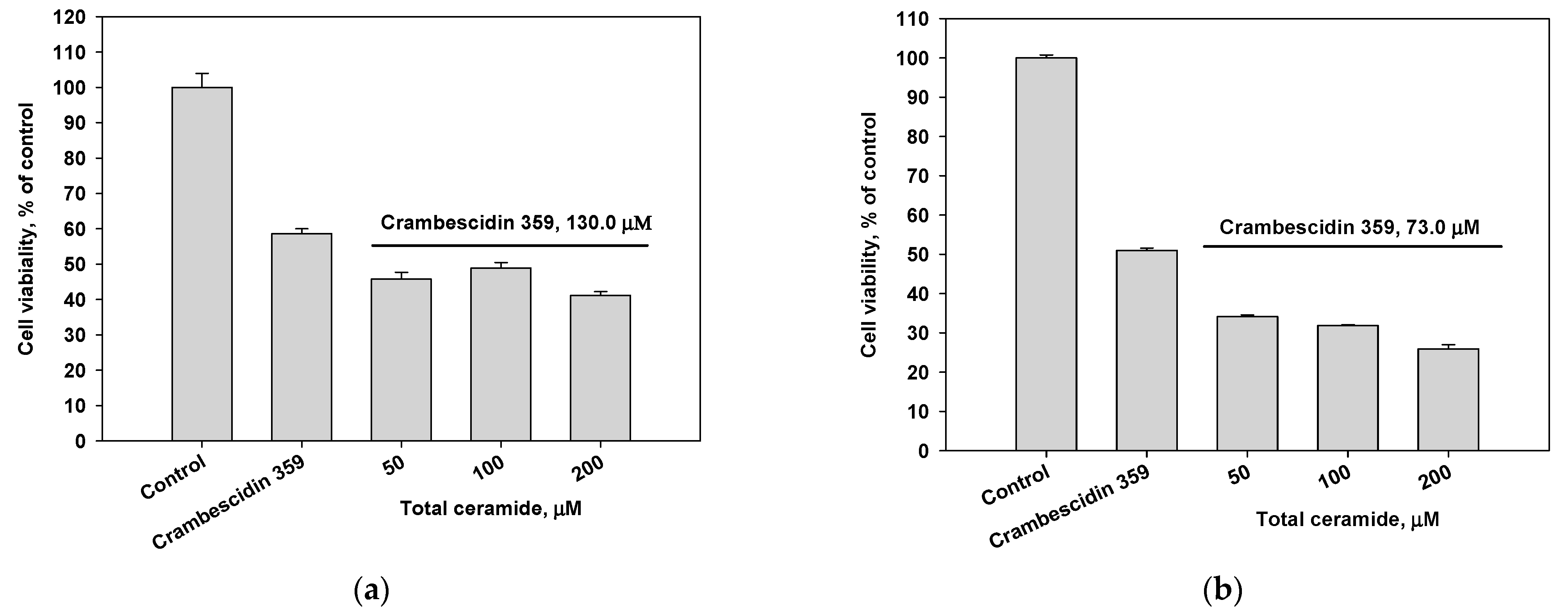
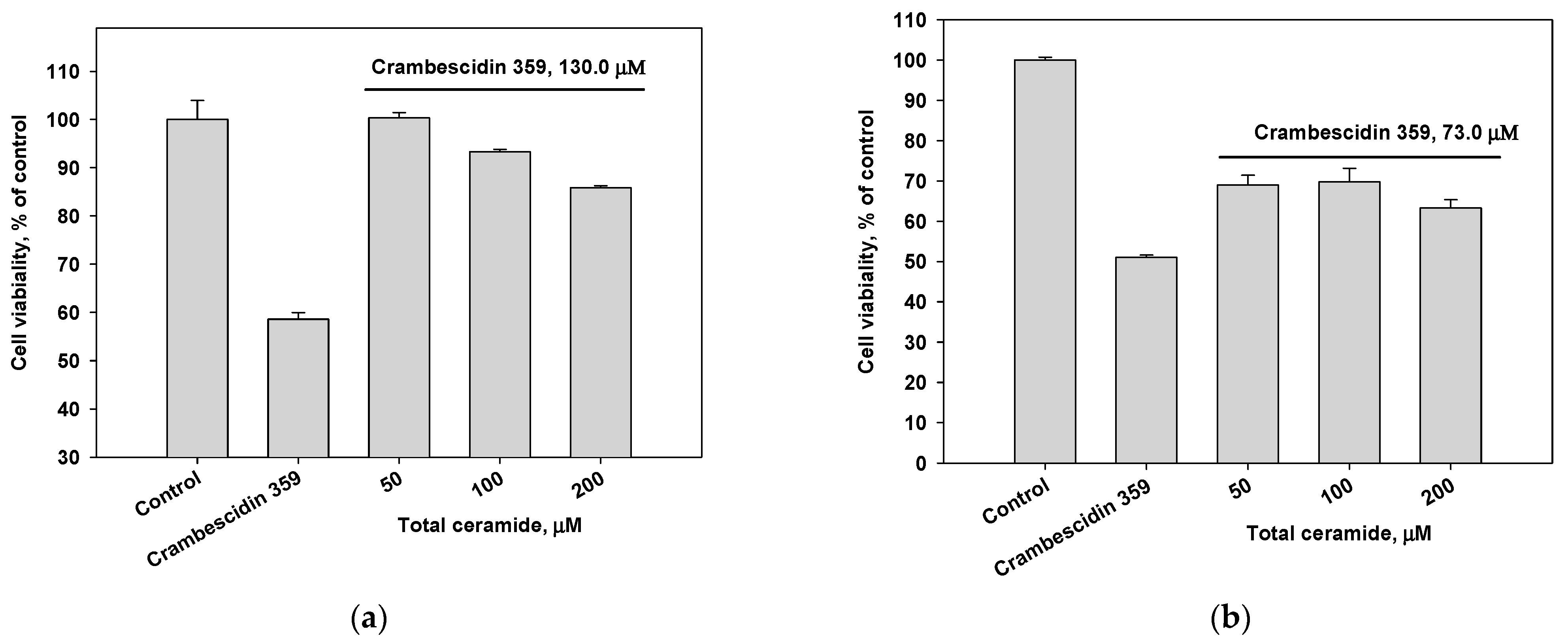
The cytotoxic activity of the total ceramide of M. clathrata against MDA-MB-231 and HL-60 cells was extremely weak (IC50 values ≥ 200 μM, incubation for 48 h; Figure S11). Crambescidin 359 (7) exhibited weak cytotoxic activity against MDA-MB-231 (IC50 130 μM) and HL-60 (IC50 73 μM) cells (incubation for 24 h; Figure S12). Combinations of crambescidin 359 and total ceramide were slightly more cytotoxic for MDA-MB-231 cells (Figure 3a) and significantly inhibited the viability of HL-60 cells (Figure 3b).
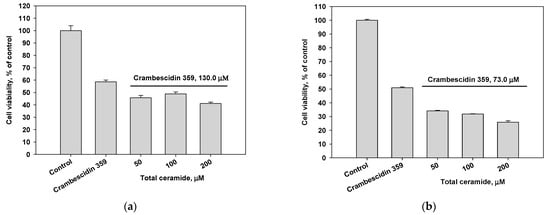
Figure 3.
Cytotoxic effects of crambescidin 359 (IC50 concentration) in combination with total ceramide (various concentrations) on (a) MDA-MB-231 and (b) HL-60 cell lines.
The cytotoxic effect of alkaloid 7 on MDA-MB-231 and HL-60 cells decreased after the pre-incubation of these cells with the phytoceramides from M. clathrata (Figure 4). In particular, the pre-treatment of MDA-MB-231 cells with total ceramide (50 μM concentration) for 24 h prevented cell death induced by crambescidin 359 at the concentration of IC50 (Figure 4a).
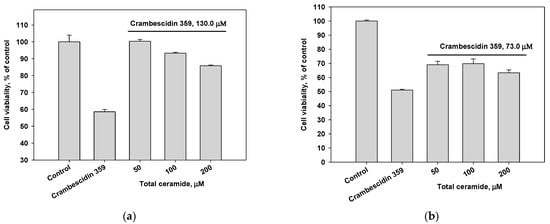
Figure 4.
Cytotoxic effects of crambescidin 359 at the concentration of IC50 on (a) MDA-MB-231 and (b) HL-60 cell lines after 24 h pre-incubation of the cells with total ceramide in various concentrations.
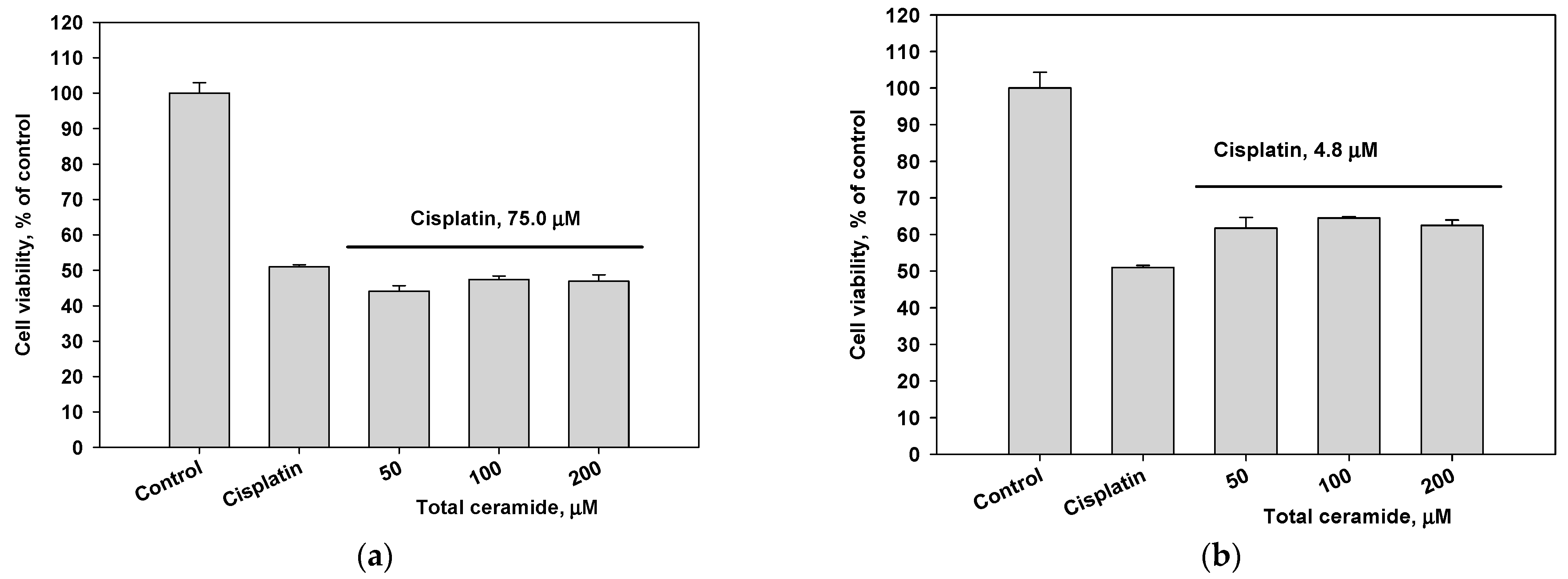
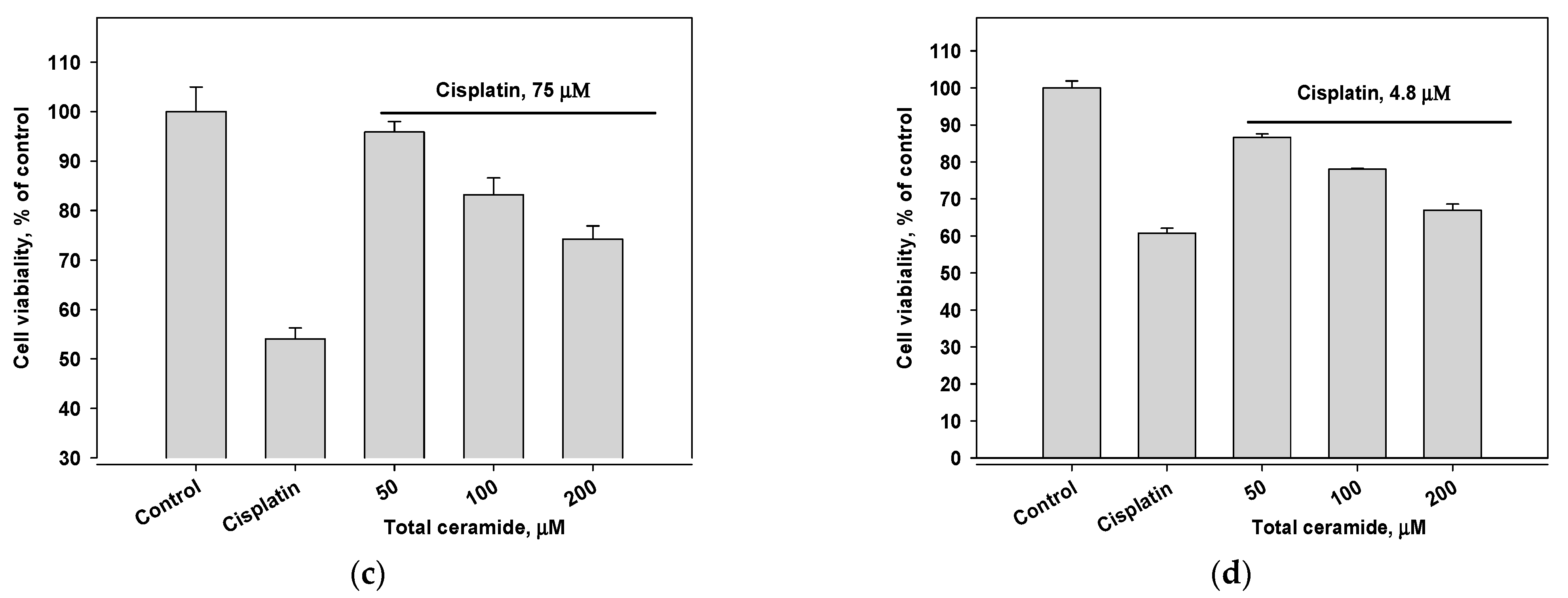
Combinations of cisplatin and total ceramide were slightly more cytotoxic for MDA-MB-231 cells (Figure 5a) than only cisplatin. However, these combinations insignificantly increased the survival of HL-60 cells (Figure 5b). After 24 h pre-incubation of MDA-MB-231 and HL-60 cells with total ceramide (especially, at a concentration of 50 μM), the cytotoxic effect of cisplatin was noticeably reduced (Figure 5c,d).
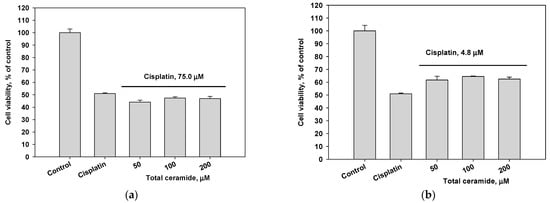
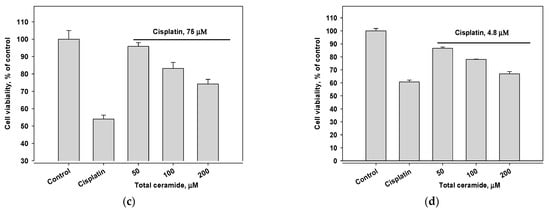
Figure 5.
Cytotoxic effects of cisplatin (IC50 concentration) in combination with total ceramide (various concentrations) on (a) MDA-MB-231 cells, (b) HL-60 cells, (c) MDA-MB-231 cells after their 24 h pre-incubation with total ceramide, and (d) HL-60 cells after their 24 h pre-incubation with total ceramide.
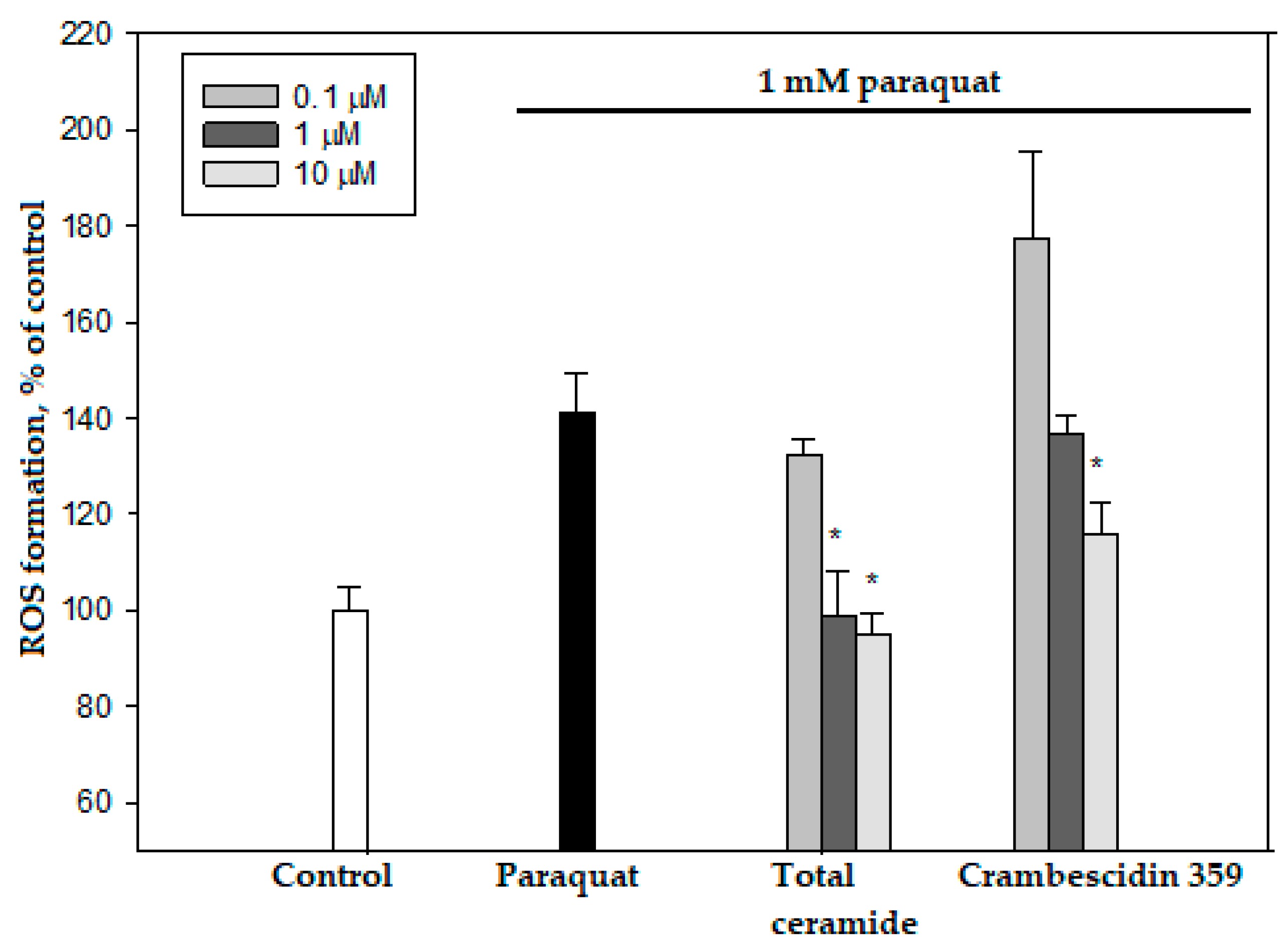
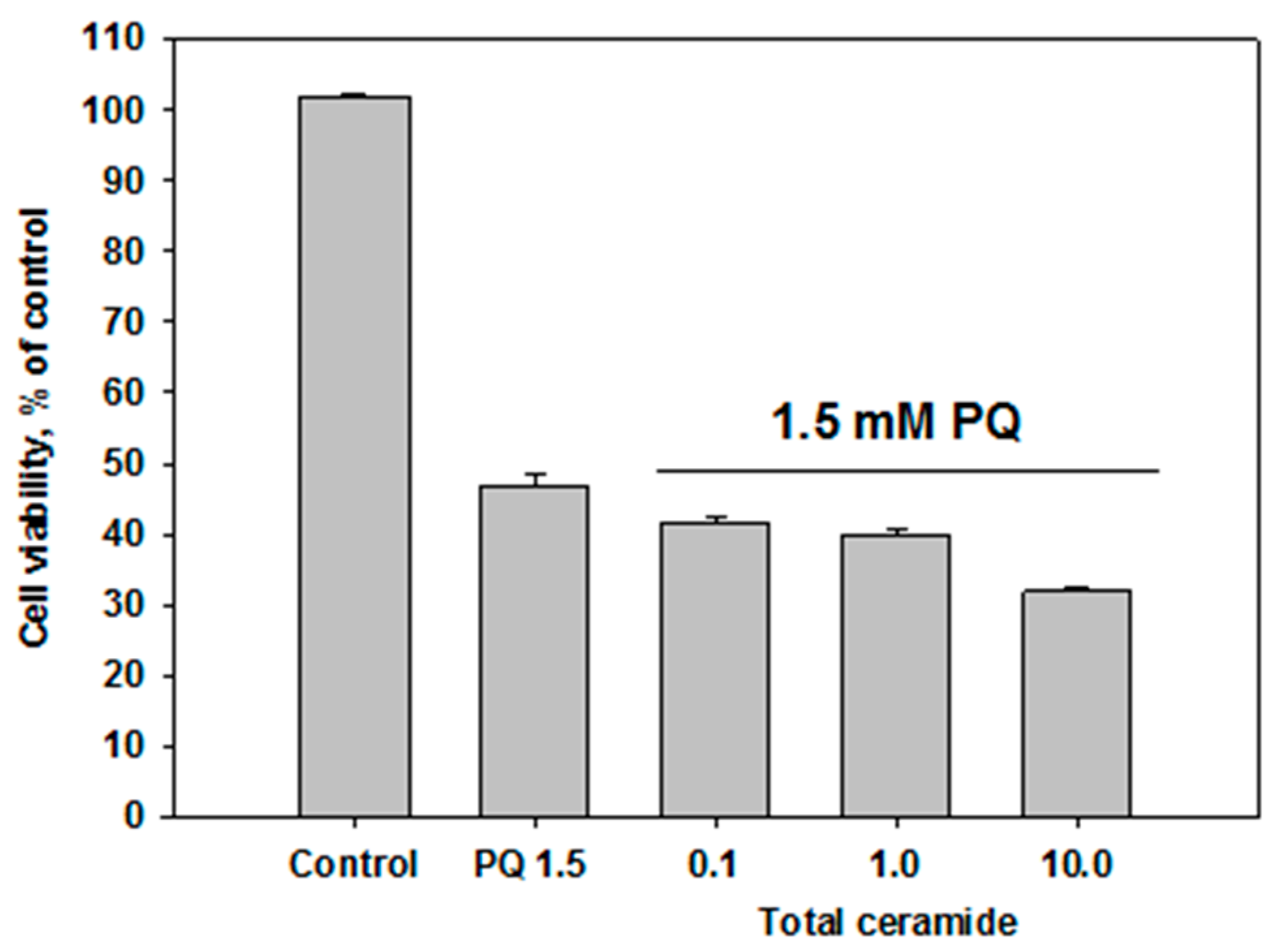
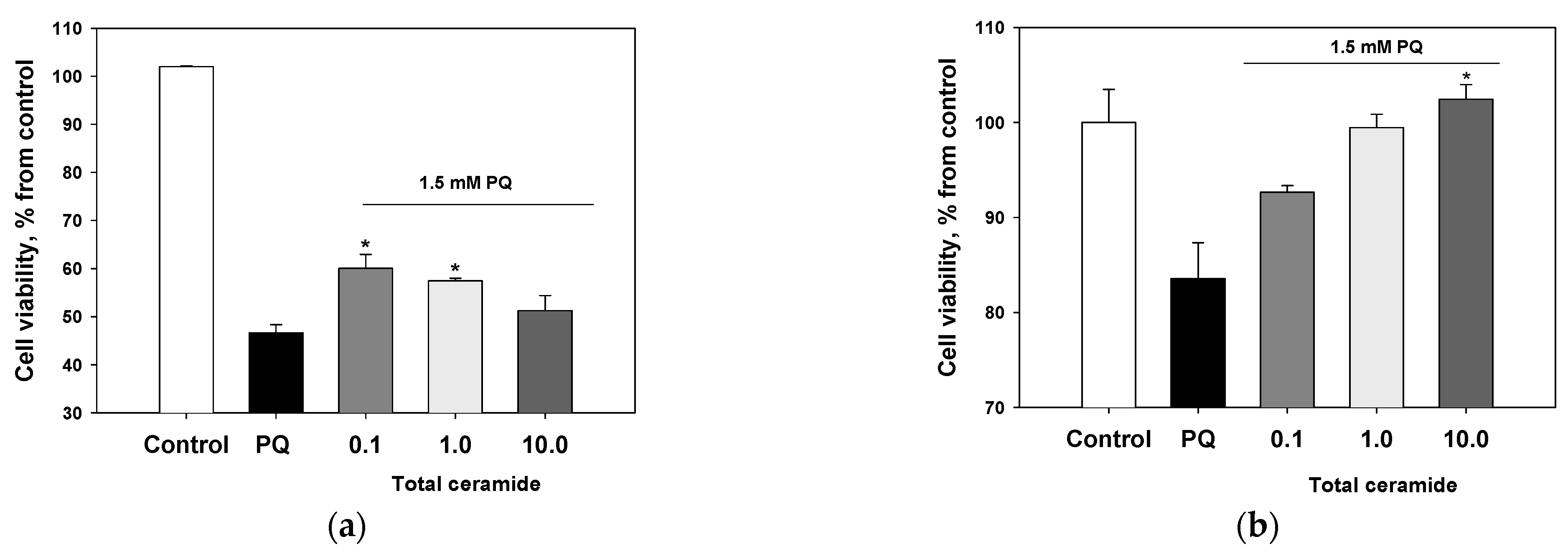
Total ceramide at the concentrations of 1 and 10 μM significantly reduced ROS formation (by 29.8 ± 6.7% and 32.5 ± 3.2%, respectively) in neuroblastoma cells exposed to paraquat (Figure 6). In this experiment, phytoceramides in non-cytotoxic concentrations were incubated with the cells for 1 h before paraquat was introduced into the culture. After analogous short-time pre-treatment (1 h) of the neuroblastoma cells with total ceramide, the neurotoxicity of paraquat unexpectedly increased (Figure 7). In contrast, after a longer pre-treatment (24 or 48 h) of the cells with phytoceramides, the neurotoxic activity of paraquat decreased (Figure 8). For example, total ceramide at the concentrations of 0.1 and 1.0 μM increased the viability of Neuro 2a cells by 28.7 ± 6.3 and 23.1 ± 1.2%, respectively, compared to the control cells exposed to only paraquat (Figure 8a). Furthermore, the pre-treatment (for 48 h) of SH-SY5Y cells with total ceramide at the concentration of 10 μM prevented cell death induced by paraquat (Figure 8b).
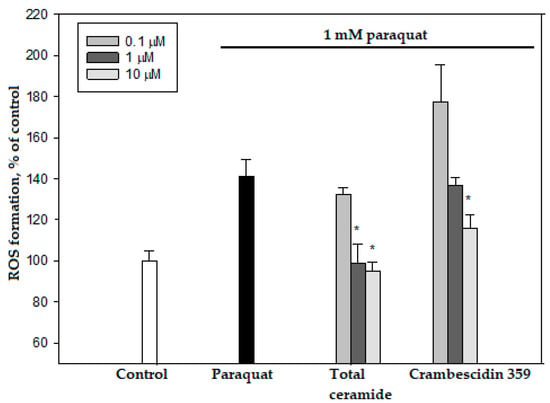
Figure 6.
Effects of total ceramide and crambescidin 359 on ROS formation in Neuro-2a cells. The data are presented as m ± se (n = 3). * p < 0.05 compared to cells exposed to paraquat alone.
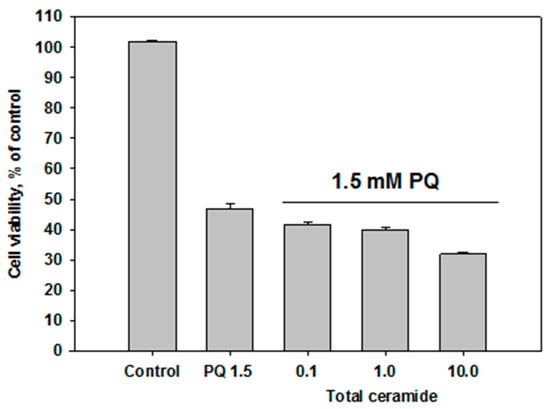
Figure 7.
Effect of total ceramide on neurotoxicity of paraquat (PQ): the impact of 1 h pre-incubation with phytoceramides on the viability of Neuro-2a cells.
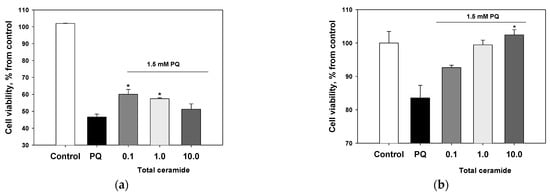
Figure 8.
Protective effects of total ceramide against neurotoxicity of paraquat (PQ): the impact of pre-incubation with phytoceramides on the viability of (a) Neuro-2a cells (24 h pre-incubation) and (b) SH-SY5Y cells (48 h pre-incubation). The data are presented as m ± se (n = 3). * p < 0.05 compared to cells exposed to paraquat alone.
Crambescidin 359 reduced ROS formation (maximum 17.9 ± 4.6%, 10 μM concentration) in Neuro 2a cells exposed to paraquat (Figure 5) and exhibited neuroprotective effects after 1, 24, and 48 h pre-incubation with these cells (Figure S13). However, we did not consider crambescidin 359 a promising antiparkinsonic agent because this alkaloid showed inhibitory activities in electrophysiology experiments on human α7 nicotinic acetylcholine receptors [23]. The stimulation (not inhibition) of these receptors, promoting cognitive functions, may be a perspective strategy for the treatment of Parkinson‘s disease [24].
4. Discussion
We conducted the structural analysis of ceramides using sensitive and specific analytical tools to detect ceramide species, differentiate between them and to elucidate individual structures. The used combination of the instrumental and chemical methods permitted the more detailed investigation of the sponge ceramides than previously reported [7]. As a result, 16 new (1b, 3a, 3c, 3d, 3f, 3g, 5c, 5d, 5f, 5g, 6b–g) and 12 known (2b, 2e, 2f, 3b, 3e, 4a–c, 4e, 4f, 5b, 5e) compounds were found in the complex mixture of the phytoceramides from M. clathrata. These compounds contain phytosphingosine-type backbones i-t17:0 (1), n-t17:0 (2), i-t18:0 (3), n-t18:0 (4), i-t19:0 (5), or ai-t19:0 (6), N-acylated with saturated (2R)-2-hydroxy C21 (a), C22 (b), C23 (c), i-C23 (d), C24 (e), C25 (f), or C26 (g) acids. Phytoceramides 3b, 3e, and 3f, consisting of i-t18:0 backbone and straight-chain C22, C24, and C25 acyls, respectively, are the most abundant in the ceramide mixture. The most remarkable features of this mixture may be considered the large amounts of constituents with methyl-branched sphingoid base moieties (iso-forms: 76.2%, anteiso-forms: 16.6%) and the extremely low amounts of components, containing common unbranched sphingoid bases (4%). Only one methyl-branched acyl (1.9%, iso-methyl-branched 23:0) was detected among the fatty acid residues of the investigated phytoceramides.
Phytoceramides 2b, 2e, 2f, 4a–c, 4e, 4f with unbranched backbones may be found in many organisms, including terrestrial mushrooms, plants, and animals [25]. However, phytoceramides with methyl-branched backbones 1, 3, 5, and 6 are far less common. The compounds of this group were mainly obtained from marine organisms including sponges ([7]: i-t18:0 and i-t19:0; [26]: i-t17:0, i-t18:0, and i-t19:0; [27]: i-t17:0; [28]: i-t19:0; [29]: i-t18:0; [30]: i-t19:0; [31]: i-t19:0 and ai-t19:0), starfish ([32]: ai-t19:0), and sea grass ([33]: i-t17:0 and i-t19:0). All the unknown phytoceramides found in the present study are also characterized by these iso- or anteiso-methyl-branched backbones. Among the new variants of N-acylation of the above-mentioned marine sphingoid bases, compounds 3d, 5d, and 6d from M. clathrata have methyl branching at both the sphingoid and fatty acid (i-C23) chains.
Compounds 3b (monanchoramide B) and 5b (monanchoramide C) have previously been found in the Philippine sample of M. clathrata, along with monanchoramides A (i-t20:0/(2R)-2-OH-22:0) and D (i-t21:0/(2R)-2-OH-22:0) [7], which were not detected in the Australian sample of M. clathrata studied here. However, although monanchoramides do not contain anteiso-methyl-branched chains, the signals of unidentified minor anteiso-forms were found in their 13C-NMR spectra ([7]: Supplementary data). Apparently, the differences in the ceramide compositions of the Philippine and Australian samples of M. clathrata may be connected with geographical and seasonal influences on their fatty acid compositions. Fatty acids are known to serve as precursors in ceramide biosynthesis, and the sensitivity of the fatty acid profiles of sponges to environmentally induced (seasonal and geographical) variations was noted earlier [34].
The sponges of the family Crambeidae and related species were shown to contain several polycyclic guanidine alkaloids including crambescidins ([35] and references cited therein). Co-occurrence of crambescidin-type alkaloids and phytoceramides was found in three crambeids, including M. clathrata studied here. In particular, monanchoramides A–D and alkaloids of the crambescidin group were isolated from the Philippine sample of M. clathrata [7,36]. In addition, ceramide i-t17:0/2-OH-24:0 and crambescidins were obtained from the related sponge Crambe crambe [27]. In our study, the pretreatment of human tumor-derived cells with the phytoceramides from M. clathrata decreased cell death induced by crambescidin 359 (7). We suggest that, similarly, these phytoceramides may help to protect sponge cells against injury, caused by their own cytotoxic crambescidin(s). Most of the crambescidin alkaloids exhibited a wide range of biological activities (including potent cytotoxicity), but little information regarding the true interaction of their polycyclic core with biological targets is known [35]. Therefore, a possible relationship between cytoprotective phytoceramides and cytotoxic crambescidins needs further investigation.
Our work showed a decrease of the cytotoxic effect of cisplatin on MDA-MB-231 and HL-60 cells after their pre-incubation with the phytoceramides of M. clathrata (Figure 5c,d). Whether or not phytoceramides may be helpful for reducing cisplatin-induced toxicity in combination therapy, depends on the further experiments with these compounds. On the other hand, phytoceramides can be not only cytoprotective, but also cytotoxic agents for some tumor cells. The “dual” properties of the phytoceramides should be taken into account in an evaluation of their antitumor potential.
Using an in vitro paraquat model of Parkinson’s disease, we found that the phytoceramides from M. clathrata influenced the neurodegenerative effect induced by paraquat. After a short time of pre-incubation (1 h), these phytoceramides decreased ROS formation and potentiated the neurodegenerative effect of paraquat. This looks contradictory because paraquat is known to exert deleterious effects through oxidative stress ([37] and references cited therein). Thus, the reason for the observed additive damaging effect of phytoceramides and paraquat was unclear. However, we admitted that the absorption of phytoceramides by cells during 1h pre-incubation was insufficient to cause the cytoprotective properties of these compounds, and longer periods of time were required for this, as in the cases with crambescidin 359 and cisplatin (Figure 4 and Figure 5). Indeed, the neuroprotective effect of phytoceramides was then revealed in the result of their 24 and 48 h pre-incubation with neuroblastoma cells (Figure 8). This suggests that phytoceramides, reducing neurodegeneration caused by paraquat, may be potential prophylactic agents for decreasing the risk of Parkinson’s disease.
In general, the long-term preliminary treatment of the cells with the phytoceramides of M. clathrata was necessary for their cytoprotective functions; otherwise, an additive damaging effect of these sphingolipids and cytotoxic compound (crambescidin 359, cisplatin or paraquat) was observed. Therefore, exogenous phytoceramides can exert dual effects on cell survival, but their “delayed” effect may be cytoprotective.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biom13040677/s1, Figure S1: 1H-NMR spectrum of total ceramide (700 MHz, C5D5N); Figure S2: 13C-NMR spectrum of total ceramide (125 MHz, C5D5N); Figure S3: HR-ESI-MS analyses (negative ion mode) of total ceramide; Figure S4: (–)ESI-MS/MS spectrum of ion at m/z 640.59 ([M − H]−); Figure S5: (–)ESI-MS/MS spectrum of ion at m/z 654.60 ([M − H]−), illustrated with fragmentation pattern of compound 3b; Figure S6: (–)ESI-MS/MS spectrum of ion at m/z 668.62 ([M − H]−), illustrated with fragmentation patterns of compounds 2e, 3c, and 6b; Figure S7: (–)ESI-MS/MS spectrum of ion at m/z 682.63 ([M − H]−), illustrated with fragmentation patterns of compounds 2f, 3e, and 6c; Figure S8: (–)ESI-MS/MS spectrum of ion at m/z 696.65 ([M − H]−), illustrated with fragmentation patterns of compounds 3f and 6e; Figure S9: (–)ESI-MS/MS spectrum of ion at m/z 710.665 ([M − H]−), illustrated with fragmentation patterns of compounds 3g and 6f; Figure S10: (–)ESI-MS/MS spectrum of ion at m/z 724.68 ([M − H]−), illustrated with fragmentation patterns of compounds 5g and 6g; Figure S11: Cytotoxicities of total ceramide against (a) MDA-MB-231 and (b) HL-60 cell lines; Figure S12: Cytotoxicities of crambescidin 359 against (a) MDA-MB-231and (b) HL-60 cell lines; Figure S13: Protective effects of crambescidin 359 against neurotoxicity of paraquat (PQ): (a) Neuro-2a cells (24 h pre-incubation with compound 7) and (b) SH-SY5Y cells (48 h pre-incubation with compound 7).
Author Contributions
Conceptualization, methodology, formal analysis, investigation, writing—original draft preparation, review and editing, E.A.S.; investigation, original draft preparation, A.S.K.; investigation, formal analysis, writing—original draft preparation, data curation, E.A.C., E.S.M. and E.A.P.; supervision, validation, formal analysis, P.S.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This study was carried out on the equipment of the Collective Facilities Center “The Far Eastern Center for Structural Molecular Research (NMR/MS) of PIBOC FEB RAS”. The authors express their gratitude to O.P. Moiseenko and L.P. Ponomarenko for their kind help in the GC–MS analyses and N.V. Ivanchina and V.A. Stonik for reading the manuscript and discussing its content. The authors would like to thank A.S. Dmitrenok, N.V. Zvyagintsev, D.V. Denisenko, A.S. Menshov, and A.I. Kalinovsky for NMR analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- The LipidWeb. Ceramides. Available online: https://www.lipidmaps.org/resources/lipidweb/index.php?page=lipids/sphingo/ceramide/index.htm (accessed on 1 November 2022).
- Perry, D.K.; Hannun, Y.A. The role of ceramide in cell signaling. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1998, 1436, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Pettus, B.J.; Chalfant, C.E.; Hannun, Y.A. Ceramide in apoptosis: An overview and current perspectives. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2002, 1585, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Beckman, B.S.; Foroozesh, M. A review of ceramide analogs as potential anticancer agents. Future Med. Chem. 2013, 5, 1405–1421. [Google Scholar] [CrossRef]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Tumor suppressive functions of ceramide: Evidence and mechanisms. Apoptosis 2015, 20, 689–711. [Google Scholar] [CrossRef]
- Muralidhar, P.; Radhika, P.; Krishna, N.; Venkata Rao, D.; Bheemasankara Rao, C. Sphingolipids from marine organisms: A review. Nat. Prod. Sci. 2003, 9, 117–142. [Google Scholar]
- Raslan, A.E.; Radwan, M.M.; Ahmed, S.A.; Nafady, A.M.; Zaki, M.A.; Wanas, A.S.; Abou-Karam, M.; Shier, T.W.; Hassanean, H.A.; ElSohly, M.A. Monanchoramides A–D, ceramides from the marine sponge Monanchora clathrata with cytotoxic activity. Phytochem. Lett. 2018, 23, 83–89. [Google Scholar] [CrossRef]
- The LipidWeb. Long-Chain (Sphingoid) Bases. Available online: https://www.lipidmaps.org/resources/lipidweb/index.php?page=lipids/sphingo/lcb/index.htm (accessed on 1 November 2022).
- Jung, J.-C.; Lee, Y.; Moon, S.; Ryu, J.H.; Oh, S. Phytoceramide shows neuroprotection and ameliorates scopolamine-induced memory impairment. Molecules 2011, 16, 9090–9100. [Google Scholar] [CrossRef]
- Jang, J.Y.; Lee, H.K.; Yoo, H.-S.; Seong, Y.H. Phytoceramide ameliorates β-amyloid protein-induced memory impairment and neuronal death in mice. Arch. Pharm. Res. 2017, 40, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Custodia, A.; Aramburu-Núñez, M.; Correa-Paz, C.; Posado-Fernández, A.; Gómez-Larrauri, A.; Castillo, J.; Gómez-Muñoz, A.; Sobrino, T.; Ouro, A. Ceramide Metabolism and Parkinson’s Disease—Therapeutic Targets. Biomolecules 2021, 11, 945. [Google Scholar] [CrossRef] [PubMed]
- Santalova, E.A. Structural Analysis of Sterols and Cytotoxic Compounds from Some Marine Sponges. Ph.D. Thesis, Pacific Institute of Bioorganic Chemistry, Vladivostok, Russia, 2005. [Google Scholar]
- Aveldaño, M.I.; Horrocks, L.A. Quantitative release of fatty acids from lipids by a simple hydrolysis procedure. J. Lipid Res. 1983, 24, 1101–1105. [Google Scholar] [CrossRef]
- Santalova, E.A.; Denisenko, V.A.; Dmitrenok, P.S.; Drozdov, A.L.; Stonik, V.A. Cerebrosides from a Far-Eastern glass sponge Aulosaccus sp. Lipids 2015, 50, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Santalova, E.A.; Svetashev, V.I. Preparation of 4,4-dimethyloxazoline and pyrrolidine derivatives from fatty acid methyl esters using sodium borohydride: Mild and simple one-pot derivatization procedures for a gas chromatographic–mass spectrometric analysis of fatty acids. Nat. Prod. Commun. 2022, 17, 1934578X221131408. [Google Scholar] [CrossRef]
- AOCS Lipid Library. Preparation of Ester Derivatives of Fatty Acids for Chromatographic Analysis. Available online: https://lipidlibrary.aocs.org/lipid-analysis/selected-topics-in-the-analysis-of-lipids/preparation-of-ester-derivatives-of-fatty-acids-for-chromatographic-analysis (accessed on 12 November 2022).
- The LipidWeb. Mass Spectrometry of Methyl Esters. Hydroxy Fatty Acids (Not Further Derivatized). Available online: https://www.lipidmaps.org/resources/lipidweb/index.php?page=ms/methesters/me-hydroxy-1/index.htm (accessed on 20 November 2022).
- Horn, D.H.S.; Pretorius, Y.Y. Wool wax. Part VI. The synthesis and stereochemistry of the straight-chain α-hydroxy-acids. J. Chem. Soc. 1954, 1954, 1460–1464. [Google Scholar] [CrossRef]
- Koike, K.; Sugimoto, M.; Nakahara, Y.; Ogawa, T. Total synthesis of cerebrosides: (2S,3R,4E)-1-O-β-D-galactopyranosyl-N-(2′R and 2′S)-2′-hydroxytetracosanoylsphingenine. Carbohydr. Res. 1987, 162, 237–246. [Google Scholar] [CrossRef]
- Sugiyama, S.; Honda, M.; Komori, T. Biologically active glycosides from Asteroidea. XV. Asymmetric synthesis of phytosphingosine and phytosphingosine anhydro base: Assignment of the absolute stereochemistry. Liebigs Ann. Chem. 1988, 1988, 619–625. [Google Scholar] [CrossRef]
- Santalova, E.A.; Denisenko, V.A.; Dmitrenok, P.S.; Ha, D.T.; Hung, N.A.; Drozdov, A.L.; Malyarenko, O.S.; Thuy, T.T.T.; Quan, P.M.; Long, P.Q. Occurrence of melibiose-containing glycosphingolipids in a sample of a sponge-coral association (Desmapsamma anchorata/Carijoa riisei). Chem. Biodivers. 2019, 16, e1800401. [Google Scholar] [CrossRef]
- Hsu, F.-F. Complete structural characterization of ceramides as [M–H]– ions by multiple-stage linear ion trap mass spectrometry. Biochimie 2016, 130, 63–75. [Google Scholar] [CrossRef]
- Kudryavtsev, D.; Makarieva, T.; Utkina, N.; Santalova, E.; Kryukova, E.; Methfessel, C.; Tsetlin, V.; Stonik, V.; Kasheverov, I. Marine Natural Products Acting on the Acetylcholine-Binding Protein and Nicotinic Receptors: From Computer Modeling to Binding Studies and Electrophysiology. Mar. Drugs 2014, 12, 1859–1875. [Google Scholar] [CrossRef]
- Makotrova, T.A. A role of alpha-7 nicotinic acetylcholine receptors in pharmacotherapy of neurodegenerative diseases. Zhurnal Nevrol. Psikhiatrii imeni SS Korsakova 2012, 112, 57–59. [Google Scholar]
- Database SciFinder. Available online: https://scifinder-n.cas.org (accessed on 17 January 2023).
- Hirsch, S.; Kashman, Y. New glycosphigolipids from marine organisms. Tetrahedron 1989, 45, 3897–3906. [Google Scholar] [CrossRef]
- Berlinck, R.G.S. Chromatographic approach to polar compounds: Isolation of hydrophilic constituents of the marine sponge Crambe crambe. Quim. Nova 1994, 17, 167–171. [Google Scholar]
- Xu, S.-H.; Yang, K. Three new ceramides from the sponge Spongia suriganensis. Chin. J. Org. Chem. 2006, 26, 56–59. [Google Scholar]
- Lakshmi, V.; Raghubir, R.; Gupta, P. New ceramides from the sponge Cinachyra cavernosa. J. Asian Nat. Prod. Res. 2008, 10, 747–751. [Google Scholar] [CrossRef]
- Xu, S.; Liao, X.; Li, H. Compounds with nitrogen from the sponge Hyatella sp. Tianran Chanwu Yanjiu Yu Kaifa 2009, 21, 63–65. [Google Scholar]
- Abdelhameed, R.; Elgawish, M.S.; Mira, A.; Ibrahim, A.K.; Ahmed, S.A.; Shimizu, K.; Yamada, K. Anti-choline esterase activity of ceramides from the Red Sea marine sponge Mycale euplectellioides. RSC Adv. 2016, 6, 20422–20430. [Google Scholar] [CrossRef]
- Inagaki, M.; Ikeda, Y.; Kawatake, S.; Nakamura, K.; Tanaka, M.; Misawa, E.; Yamada, M.; Higuchi, R. Isolation and structure of four new ceramides from the starfish Luidia maculata. Chem. Pharm. Bull. 2006, 54, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Abdelhameed, R.F.; Ibrahim, A.K.; Yamada, K.; Ahmed, S.A. Cytotoxic and anti-inflammatory compounds from Red Sea grass Thalassodendron ciliatum. Med. Chem. Res. 2018, 27, 1238–1244. [Google Scholar] [CrossRef]
- Lawson, M.P.; Bergquist, P.R.; Cambie, R.C. Fatty acid composition and classification of the Porifera. Biochem. Syst. Ecol. 1984, 12, 375–393. [Google Scholar] [CrossRef]
- Shi, Y.; Moazami, Y.; Pierce, J.G. Structure, synthesis and biological properties of the pentacyclic guanidinium alkaloids. Bioorg. Med. Chem. 2017, 25, 2817–2824. [Google Scholar] [CrossRef]
- Raslan, A.E.; Radwan, M.M.; Ahmed, S.A.; Nafady, A.M.; Metwaly, A.M.; Eissa, I.H.; Jacob, M.R.; Hassanean, H.A.; ElSohly, M.A.; Wanas, A.S. Antifungal guanidine alkaloids from the marine sponges Monanchora clathrata and Monanchora unguiculata. Phytochem. Lett. 2022, 51, 132–139. [Google Scholar] [CrossRef]
- Bove, J.; Prou, D.; Perier, C.; Przedborski, S. Toxin-induced models of Parkinson’s disease. NeuroRX 2005, 2, 484–494. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).