Plasma Neutrophil Gelatinase-Associated Lipocalin Associates with New-Onset Chronic Kidney Disease in the General Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Measurements of NGAL
2.4. Study Outcomes and Definitions
2.5. Statistical Analyses
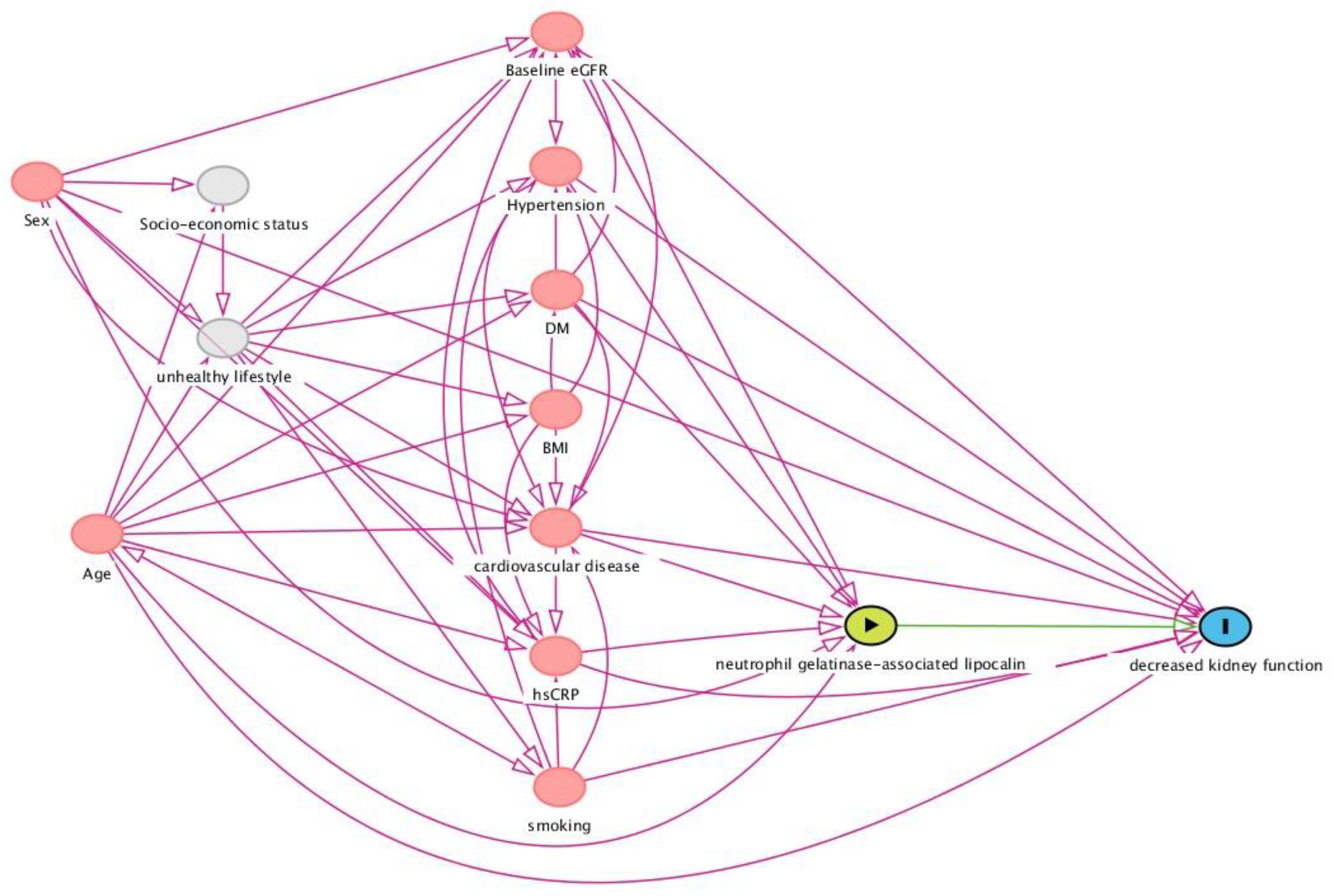
2.6. Selection of Potentially Confounding Variables: Directed Acyclic Graph (DAG)
3. Results
3.1. Baseline Study Population Characteristics
3.2. Plasma NGAL Concentrations and New-Onset CKD
3.3. Stratified Analyses
3.4. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kidney disease: Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Shlipak, M.G.; Day, E.C. Biomarkers for incident CKD: A new framework for interpreting the literature. Nat. Rev. Nephrol. 2013, 9, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Gansevoort, R.T.; de Jong, P.E. The case for using albuminuria in staging chronic kidney disease. J. Am. Soc. Nephrol. 2009, 20, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Cowland, J.B.; Borregaard, N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics 1997, 45, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Banai, A.; Rozenfeld, K.-L.; Levit, D.; Merdler, I.; Loewenstein, I.; Banai, S.; Shacham, Y. Neutrophil gelatinase-associated lipocalin (NGAL) for the prediction of acute kidney injury in chronic kidney disease patients treated with primary percutaneous coronary intervention. Int. J. Cardiol. Heart Vasc. 2020, 32, 100695. [Google Scholar] [CrossRef] [PubMed]
- Lumlertgul, N.; Amprai, M.; Tachaboon, S.; Dinhuzen, J.; Peerapornratana, S.; Kerr, S.J.; Srisawat, N. Urine Neutrophil Gelatinase-associated Lipocalin (NGAL) for Prediction of Persistent AKI and Major Adverse Kidney Events. Sci. Rep. 2020, 10, 8718. [Google Scholar] [CrossRef]
- Cruz, D.N.; De Cal, M.; Garzotto, F.; Perazella, M.A.; Lentini, P.; Corradi, V.; Piccinni, P.; Ronco, C. Plasma neutrophil gelatinase-associated lipocalin is an early biomarker for acute kidney injury in an adult ICU population. Intensiv. Care Med. 2010, 36, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Dent, C.; Tarabishi, R.; Mitsnefes, M.M.; Ma, Q.; Kelly, C.; Ruff, S.M.; Zahedi, K.; Shao, M.; Bean, J.; et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005, 365, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Kremer, D.; Post, A.; Gomes-Neto, A.W.; Groothof, D.; Kunutsor, S.K.; Nilsen, T.; Hidden, C.; Sundrehagen, E.; Eisenga, M.F.; Navis, G.; et al. Plasma neutrophil gelatinase-associated lipocalin and kidney graft outcome. Clin. Kidney J. 2021, 15, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Bolignano, D.; Lacquaniti, A.; Coppolino, G.; Donato, V.; Campo, S.; Fazio, M.R.; Nicocia, G.; Buemi, M. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Bolignano, D.; Coppolino, G.; Campo, S.; Aloisi, C.; Nicocia, G.; Frisina, N.; Buemi, M. Urinary neutrophil gelatinase-associated lipocalin (NGAL) is associated with severity of renal disease in proteinuric patients. Nephrol. Dial. Transplant. 2008, 23, 414–416. [Google Scholar] [CrossRef] [PubMed]
- Mitsnefes, M.M.; Kathman, T.S.; Mishra, J.; Kartal, J.; Khoury, P.R.; Nickolas, T.L.; Barasch, J.; Devarajan, P. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in children with chronic kidney disease. Pediatr. Nephrol. 2007, 22, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; He, Y.; Li, K.; Yang, J.; Li, X.; Lu, R.; Gao, W. Urinary neutrophil gelatinase-associated lipocalin (NGAL) is an early biomarker for renal tubulointerstitial injury in IgA nephropathy. Clin. Immunol. 2017, 123, 227–234. [Google Scholar] [CrossRef]
- Mori, K.; Nakao, K. Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int. 2007, 71, 967–970. [Google Scholar] [CrossRef]
- Bolignano, D.; Donato, V.; Coppolino, G.; Campo, S.; Buemi, A.; Lacquaniti, A.; Buemi, M. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am. J. Kidney Dis. 2008, 52, 595–605. [Google Scholar] [CrossRef]
- Bhavsar, N.A.; Köttgen, A.; Coresh, J.; Astor, B.C. Neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule 1 (KIM-1) as predictors of incident CKD stage 3: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Kidney Dis. 2012, 60, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Hillege, H.L.; Janssen, W.M.T.; Bak, A.A.A.; Diercks, G.F.H.; Grobbee, D.E.; Crijns, H.J.G.M.; Van Gilst, W.H.; De Zeeuw, D.; De Jong, P.E. Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J. Intern. Med. 2001, 249, 519–526. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef]
- Post, A.; Kremer, D.; Swarte, J.C.; Sokooti, S.; Vogelpohl, F.A.; Groothof, D.; Kema, I.; Garcia, E.; Connelly, M.A.; Wallimann, T.; et al. Plasma creatine concentration is associated with incident hypertension in a cohort enriched for the presence of high urinary albumin concentration: The Prevention of Renal and Vascular Endstage Disease study. J. Hypertens. 2021, 40, 229–239. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Bourgonje, M.F.; Post, A.; Gemert, S.L.B.-V.; Kieneker, L.M.; Bulthuis, M.L.; Gordijn, S.J.; Gansevoort, R.T.; Bakker, S.J.L.; Mulder, D.J.; et al. Systemic oxidative stress associates with new-onset hypertension in the general population. Free. Radic. Biol. Med. 2022, 187, 123–131. [Google Scholar] [CrossRef]
- Kappelle, P.J.W.H.; Gansevoort, R.T.; Hillege, J.L.; Wolffenbuttel, B.H.R.; Dullaart, R.P.F. PREVEND study group. Apolipoprotein B/A-I and total cholesterol/high-density lipoprotein cholesterol ratios both predict cardiovascular events in the general population independently of nonlipid risk factors, albuminuria and C-reactive protein. J. Intern. Med. 2011, 269, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Borggreve, S.E.; Hillege, H.L.; Wolffenbuttel, B.H.R.; De Jong, P.E.; Bakker, S.J.L.; Van Der Steege, G.; Van Tol, A.; Dullaart, R.P.F. The effect of cholesteryl ester transfer protein -629C->A promoter polymorphism on high-density lipoprotein cholesterol is dependent on serum triglycerides. J. Clin. Endocrinol. Metab. 2005, 90, 4198–4204. [Google Scholar] [CrossRef] [PubMed]
- Grubb, A.; Blirup-Jensen, S.; Lindström, V.; Schmidt, C.; Althaus, H.; Zegers, I. IFCC Working Group on Standardisation of Cystatin C (WG-SCC). First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin. Chem. Lab. Med. 2010, 48, 1619–1621. [Google Scholar] [CrossRef] [PubMed]
- Blirup-Jensen, S.; Johnson, A.M.; Larsen, M. IFCC Committee on Plasma Proteins. Protein standardization V: Value transfer. A practical protocol for the assignment of serum protein values from a Reference Material to a Target Material. Clin. Chem. Lab. Med. 2008, 46, 1470–1479. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Ferrari, A.; Gelati, M.; Brocco, G.; Lippi, G. Analytical validation of Gentian NGAL particle-enhanced enhanced turbidimetric immunoassay (PETIA). Pract. Lab. Med. 2017, 8, 60–64. [Google Scholar] [CrossRef]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef]
- Abbasi, A.; Corpeleijn, E.; Gansevoort, R.T.; Gans, R.; Hillege, H.L.; Stolk, R.; Navis, G.; Bakker, S.J.L.; Dullaart, R.P.F. Role of HDL cholesterol and estimates of HDL particle composition in future development of type 2 diabetes in the general population: The PREVEND study. J. Clin. Endocrinol. Metab. 2013, 98, E1352–E1359. [Google Scholar] [CrossRef]
- Gemert, S.L.B.-V.; Heuvel, E.V.D. Exploring causal hypotheses: Breaking with long-standing research traditions. Dev. Med. Child Neurol. 2013, 55, 975–976. [Google Scholar] [CrossRef]
- Leopold, J.A. The Central Role of Neutrophil Gelatinase–Associated Lipocalin in Cardiovascular Fibrosis. Hypertension 2015, 66, 20–22. [Google Scholar] [CrossRef]
- Lindberg, S.; Jensen, J.S.; Hoffmann, S.; Iversen, A.Z.; Pedersen, S.H.; Biering-Sørensen, T.; Galatius, S.; Flyvbjerg, A.; Mogelvang, R.; Magnusson, N.E. Plasma Neutrophil Gelatinase-Associated Lipocalin Reflects Both Inflammation and Kidney Function in Patients with Myocardial Infarction. Cardiorenal Med. 2016, 6, 180–190. [Google Scholar] [CrossRef]
- Mosialou, I.; Shikhel, S.; Luo, N.; Petropoulou, P.I.; Panitsas, K.; Bisikirska, B.; Rothman, N.J.; Tenta, R.; Cariou, B.; Wargny, M.; et al. Lipocalin-2 counteracts metabolic dysregulation in obesity and diabetes. J. Exp. Med. 2020, 217, e20191261. [Google Scholar] [CrossRef] [PubMed]
- Moschen, A.R.; Adolph, T.E.; Gerner, R.R.; Wieser, V.; Tilg, H. Lipocalin-2: A Master Mediator of Intestinal and Metabolic Inflammation. Trends Endocrinol. Metab. 2017, 28, 388–397. [Google Scholar] [CrossRef]
- Iqbal, N.; Choudhary, R.; Chan, J.; Wentworth, B.; Higginbotham, E.; Maisel, A.S. Neutrophil gelatinase-associated lipocalin as diagnostic and prognostic tool for cardiovascular disease and heart failure. Expert Opin. Med Diagn. 2013, 7, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Rodriguez, E.; Fernandez-Prado, R.; Martin-Cleary, C.; Pizarro-Sánchez, M.S.; Sanchez-Niño, M.D.; Sanz, A.B.; Fernandez-Fernandez, B.; Ortiz, A. Kidney Injury Marker 1 and Neutrophil Gelatinase-Associated Lipocalin in Chronic Kidney Disease. Nephron 2017, 136, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.J.H.; Prince, R.L.; Thompson, P.L.; Thavapalachandran, S.; Ooi, E.; Devine, A.; Lim, E.E.M.; Byrnes, E.; Wong, G.; Lim, W.H.; et al. Association Between Plasma Neutrophil Gelatinase-Associated Lipocalin and Cardiac Disease Hospitalizations and Deaths in Older Women. J. Am. Heart Assoc. 2019, 8, e011028. [Google Scholar] [CrossRef]
- Kuwabara, T.; Mori, K.; Mukoyama, M.; Kasahara, M.; Yokoi, H.; Saito, Y.; Yoshioka, T.; Ogawa, Y.; Imamaki, H.; Kusakabe, T.; et al. Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int. 2009, 75, 285–294. [Google Scholar] [CrossRef]
- Haase, M.; Bellomo, R.; Devarajan, P.; Schlattmann, P.; Haase-Fielitz, A. NGAL Meta-analysis Investigator Group. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: A systematic review and meta-analysis. Am. J. Kidney Dis. 2009, 54, 1012–1024. [Google Scholar] [CrossRef]
- Haase-Fielitz, A.; Haase, M. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury: A critical evaluation of current status. Ann. Clin. Biochem. 2014, 51, 335–351. [Google Scholar] [CrossRef]
- Yavas, H.; Sahin, O.Z.; Ersoy, R.; Taşlı, F.; Genek, D.G.; Uzum, A.; Cirit, M. Prognostic value of NGAL staining in patients with IgA nephropathy. Ren. Fail. 2013, 35, 472–476. [Google Scholar] [CrossRef]
- Damman, K.; Van Veldhuisen, D.J.; Navis, G.; Voors, A.A.; Hillege, H.L. Urinary neutrophil gelatinase associated lipocalin (NGAL), a marker of tubular damage, is increased in patients with chronic heart failure. Eur. J. Heart Fail. 2008, 10, 997–1000. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Flores-Guerrero, J.L.; Kieneker, L.M.; Nilsen, T.; Hidden, C.; Sundrehagen, E.; Seidu, S.; Dullaart, R.P.; Bakker, S.J. Plasma neutrophil gelatinase-associated lipocalin and risk of cardiovascular disease: Findings from the PREVEND prospective cohort study. Clin. Chim. Acta 2018, 486, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Daniels, L.B.; Barrett-Connor, E.; Clopton, P.; Laughlin, G.A.; Ix, J.H.; Maisel, A.S. Plasma neutrophil gelatinase-associated lipocalin is independently associated with cardiovascular disease and mortality in community-dwelling older adults: The Rancho Bernardo Study. J. Am. Coll. Cardiol. 2012, 59, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Bolignano, D.; Coppolino, G.; Campo, S.; Aloisi, C.; Nicocia, G.; Frisina, N.; Buemi, M. Neutrophil gelatinase-associated lipocalin in patients with autosomal-dominant polycystic kidney disease. Am. J. Nephrol. 2007, 27, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Bolignano, D.; Lacquaniti, A.; Coppolino, G.; Campo, S.; Arena, A.; Buemi, M. Neutrophil gelatinase-associated lipocalin reflects the severity of renal impairment in subjects affected by chronic kidney disease. Kidney Blood Press. Res. 2008, 31, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Eagan, T.M.; Damås, J.K.; Ueland, T.; Voll-Aanerud, M.; Mollnes, T.E.; Hardie, J.A.; Bakke, P.S.; Aukrust, P. Neutrophil gelatinase-associated lipocalin: A biomarker in COPD. Chest 2010, 138, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Rubin, J.; Han, W.; Venge, P.; Xu, S. The origin of multiple molecular forms in urine of HNL/NGAL. Clin. J. Am. Soc. Nephrol. 2010, 5, 2229–2235. [Google Scholar] [CrossRef]


| Total | T1 | T2 | T3 | p-Value | |
|---|---|---|---|---|---|
| ♂: <89.9 μg/L ♀: <85.7 μg/L | ♂: 89.9–113.9 μg/L ♀: 85.7–111.1μg/L | ♂: >113.9 μg/L ♀: >111.1 μg/L | |||
| Plasma NGAL (μg/L) | 104.0 (34.7) | 71.0 (13.6) | 99.6 (7.2) | 141.4 (29.4) | <0.001 |
| Demographics | |||||
| Age (years) | 50.0 [42.2–58.8] | 50.5 [42.1–58.3] | 50.3 [42.6–59.0] | 49.2 [42.0–59.2] | 0.653 |
| Female, n (%) | 2513 (53.9) | 896 (57.7) | 817 (52.6) | 800 (51.5) | 0.001 |
| Race, n (%) | <0.001 | ||||
| White, n (%) | 4439 (96.0) | 1438 (93.1) | 1487 (96.6) | 1514 (98.2) | |
| Black, n (%) | 42 (0.9) | 24 (1.6) | 9 (0.6) | 9 (0.6) | |
| Asian, n (%) | 94 (2.0) | 61 (3.9) | 23 (1.5) | 10 (0.6) | |
| Other, n (%) | 51 (1.1) | 22 (1.4) | 20 (1.3) | 9 (0.6) | |
| Anthropometrics | |||||
| BMI (kg/m2) | 25.7 [23.4–28.4] | 25.8 [23.3–28.5] | 25.7 [23.4–28.4] | 25.7 [23.4–28.4] | 0.992 |
| Waist circumference (cm) | 90 [81–98] | 89 [80–98] | 90 [82–98] | 90 [82–99] | 0.003 |
| Cardiovascular risk factors | |||||
| SBP (mmHg) | 120 [111–133] | 121 [111–133] | 121 [111–134] | 120 [110–132] | 0.339 |
| DBP (mmHg) | 72 [66–78] | 72 [66–78] | 72 [66–78] | 71 [66–77] | 0.129 |
| Heart rate (bpm) | 68 [62–74] | 67 [61–73] | 68 [62–74] | 68 [62–75] | <0.001 |
| Smoking | <0.001 | ||||
| Never, n (%) | 1449 (31.5) | 552 (35.9) | 499 (32.6) | 398 (25.9) | |
| Current, n (%) | 1283 (27.9) | 329 (21.4) | 399 (26.1) | 555 (36.2) | |
| Former, n (%) | 1870 (40.6) | 656 (42.7) | 632 (41.3) | 582 (37.9) | |
| History of CVD, n (%) | 123 (97.1) | 37 (2.4) | 41 (2.6) | 45 (2.9) | 0.672 |
| Diabetes, n (%) | 68 (1.5) | 24 (1.6) | 21 (1.4) | 23 (1.5) | 0.900 |
| Hypertension, n (%) | 1166 (25.0) | 398 (25.6) | 390 (25.1) | 378 (24.3) | 0.705 |
| Medication | |||||
| Antihypertensive drugs, n (%) | 624 (13.8) | 220 (14.5) | 201 (13.4) | 203 (13.4) | 0.361 |
| Lipid-lowering drugs, n (%) | 272 (5.8) | 95 (6.1) | 79 (5.1) | 98 (6.3) | 0.296 |
| Glucose-lowering drugs, n (%) | 39 (1.0) | 15 (1.1) | 12 (0.9) | 12 (0.9) | 0.774 |
| Laboratory measurements | |||||
| Total cholesterol (mmol/L) | 5.36 [4.72–6.09] | 5.43 [4.79–6.19] | 5.31 [4.68–6.05] | 5.33 [4.69–6.06] | 0.002 |
| hs-CRP (mg/L) | 1.16 [0.56–2.65] | 0.91 [0.42–1.84] | 1.09 [0.53–2.40] | 1.77 [0.80–3.85] | <0.001 |
| eGFR (mL/min/1.73 m2) | 96.1 [85.3–105.8] | 99.3 [88.7–108.6] | 96.0 [85.2–105.7] | 93.0 [80.8–102.3] | <0.001 |
| UAE (mg/L) | 7.7 [5.8–11.2] | 7.8 [5.9–11.0] | 7.7 [5.8–11.4] | 7.5 [5.6–11.2] | 0.409 |
| Serum creatinine (μmol/l) | 81.1 [72.9–90.4] | 79.1 [70.8–88.3] | 81.1 [73.9–90.4] | 84.2 [74.9–93.4] | <0.001 |
| Urine creatinine (mmol/24-h) | 11.9 [9.9–14.5] | 11.7 [9.8–14.7] | 12.0 [10.1–14.5] | 11.9 [9.9–14.4] | 0.317 |
| Study outcomes | |||||
| CKD (eGFR < 60 mL/min/1.73 m2), n (%) | 151 (3.3) | 33 (2.1) | 51 (3.3) | 67 (4.4) | 0.002 |
| CKD (UAE >30 mg/24-h), n (%) | 349 (7.6) | 109 (7.1) | 115 (7.4) | 125 (8.2) | 0.483 |
| CKD (combined), n (%) | 467 (10.1) | 132 (8.6) | 161 (10.4) | 174 (11.4) | 0.029 |
| HR per Doubling | T1 | T2 | T3 | |
|---|---|---|---|---|
| <87.6 μg/L | 87.6–112.6 μg/L | >112.6 μg/L | ||
| A. CKD (composite outcome) (n = 467) | ||||
| Model 1 | 1.35 [1.11–1.63], p = 0.002 | 1.00 (reference) | 1.24 [0.99–1.57], p = 0.063 | 1.44 [1.15–1.81], p = 0.002 |
| Model 2 | 1.36 [1.12–1.65], p = 0.002 | 1.00 (reference) | 1.18 [0.94–1.49], p = 0.150 | 1.46 [1.16–1.83], p = 0.001 |
| Model 3 | 1.36 [1.12–1.66], p = 0.002 | 1.00 (reference) | 1.22 [0.96–1.53], p = 0.099 | 1.50 [1.19–1.88], p < 0.001 |
| Model 4 | 1.37 [1.09–1.73], p = 0.007 | 1.00 (reference) | 1.19 [0.92–1.55], p = 0.191 | 1.51 [1.16–1.96], p = 0.002 |
| Model 5 | 1.09 [0.86–1.37], p = 0.490 | 1.00 (reference) | 1.05 [0.80–1.37], p = 0.726 | 1.16 [0.89–1.53], p = 0.274 |
| B. CKD (eGFR < 60 mL/min/1.73 m2) (n = 151) | ||||
| Model 1 | 2.07 [1.47–2.91], p < 0.001 | 1.00 (reference) | 1.56 [1.01–2.42], p = 0.046 | 2.20 [1.45–3.34], p < 0.001 |
| Model 2 | 2.42 [1.70–3.46], p < 0.001 | 1.00 (reference) | 1.50 [0.96–2.32], p = 0.073 | 2.48 [1.63–3.78], p < 0.001 |
| Model 3 | 2.35 [1.66–3.34], p < 0.001 | 1.00 (reference) | 1.54 [0.99–2.39], p = 0.056 | 2.49 [1.63–3.80], p < 0.001 |
| Model 4 | 2.54 [1.69–3.80], p < 0.001 | 1.00 (reference) | 1.54 [0.95–2.50], p = 0.079 | 2.55 [1.60–4.06], p < 0.001 |
| Model 5 | 1.05 [0.69–1.59], p = 0.828 | 1.00 (reference) | 0.89 [0.54–1.45], p = 0.633 | 0.98 [0.61–1.58], p = 0.929 |
| C. CKD (UAE > 30 mg/24-h) (n = 349) | ||||
| Model 1 | 1.21 [0.98–1.50], p = 0.080 | 1.00 (reference) | 1.08 [0.83–1.40], p = 0.573 | 1.27 [0.98–1.64], p = 0.068 |
| Model 2 | 1.15 [0.93–1.43], p = 0.210 | 1.00 (reference) | 1.02 [0.79–1.33], p = 0.814 | 1.22 [0.94–1.57], p = 0.132 |
| Model 3 | 1.15 [0.92–1.43], p = 0.214 | 1.00 (reference) | 1.05 [0.81–1.37], p = 0.710 | 1.23 [0.95–1.59], p = 0.114 |
| Model 4 | 1.07 [0.83–1.37], p = 0.609 | 1.00 (reference) | 1.02 [0.75–1.39], p = 0.892 | 1.18 [0.87–1.60], p = 0.296 |
| Model 5 | 1.07 [0.82–1.40], p = 0.604 | 1.00 (reference) | 1.03 [0.76–1.40], p = 0.864 | 1.19 [0.87–1.64], p = 0.279 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourgonje, A.R.; Abdulle, A.E.; Bourgonje, M.F.; Kieneker, L.M.; la Bastide-van Gemert, S.; Gordijn, S.J.; Hidden, C.; Nilsen, T.; Gansevoort, R.T.; Mulder, D.J.; et al. Plasma Neutrophil Gelatinase-Associated Lipocalin Associates with New-Onset Chronic Kidney Disease in the General Population. Biomolecules 2023, 13, 338. https://doi.org/10.3390/biom13020338
Bourgonje AR, Abdulle AE, Bourgonje MF, Kieneker LM, la Bastide-van Gemert S, Gordijn SJ, Hidden C, Nilsen T, Gansevoort RT, Mulder DJ, et al. Plasma Neutrophil Gelatinase-Associated Lipocalin Associates with New-Onset Chronic Kidney Disease in the General Population. Biomolecules. 2023; 13(2):338. https://doi.org/10.3390/biom13020338
Chicago/Turabian StyleBourgonje, Arno R., Amaal E. Abdulle, Martin F. Bourgonje, Lyanne M. Kieneker, Sacha la Bastide-van Gemert, Sanne J. Gordijn, Clara Hidden, Tom Nilsen, Ron T. Gansevoort, Douwe J. Mulder, and et al. 2023. "Plasma Neutrophil Gelatinase-Associated Lipocalin Associates with New-Onset Chronic Kidney Disease in the General Population" Biomolecules 13, no. 2: 338. https://doi.org/10.3390/biom13020338
APA StyleBourgonje, A. R., Abdulle, A. E., Bourgonje, M. F., Kieneker, L. M., la Bastide-van Gemert, S., Gordijn, S. J., Hidden, C., Nilsen, T., Gansevoort, R. T., Mulder, D. J., Dullaart, R. P. F., de Borst, M. H., Bakker, S. J. L., & van Goor, H. (2023). Plasma Neutrophil Gelatinase-Associated Lipocalin Associates with New-Onset Chronic Kidney Disease in the General Population. Biomolecules, 13(2), 338. https://doi.org/10.3390/biom13020338







