From Retrograde Menstruation to Endometrial Determinism and a Brave New World of “Root Treatment” of Endometriosis: Destiny or a Fanciful Utopia?
Abstract
1. Introduction
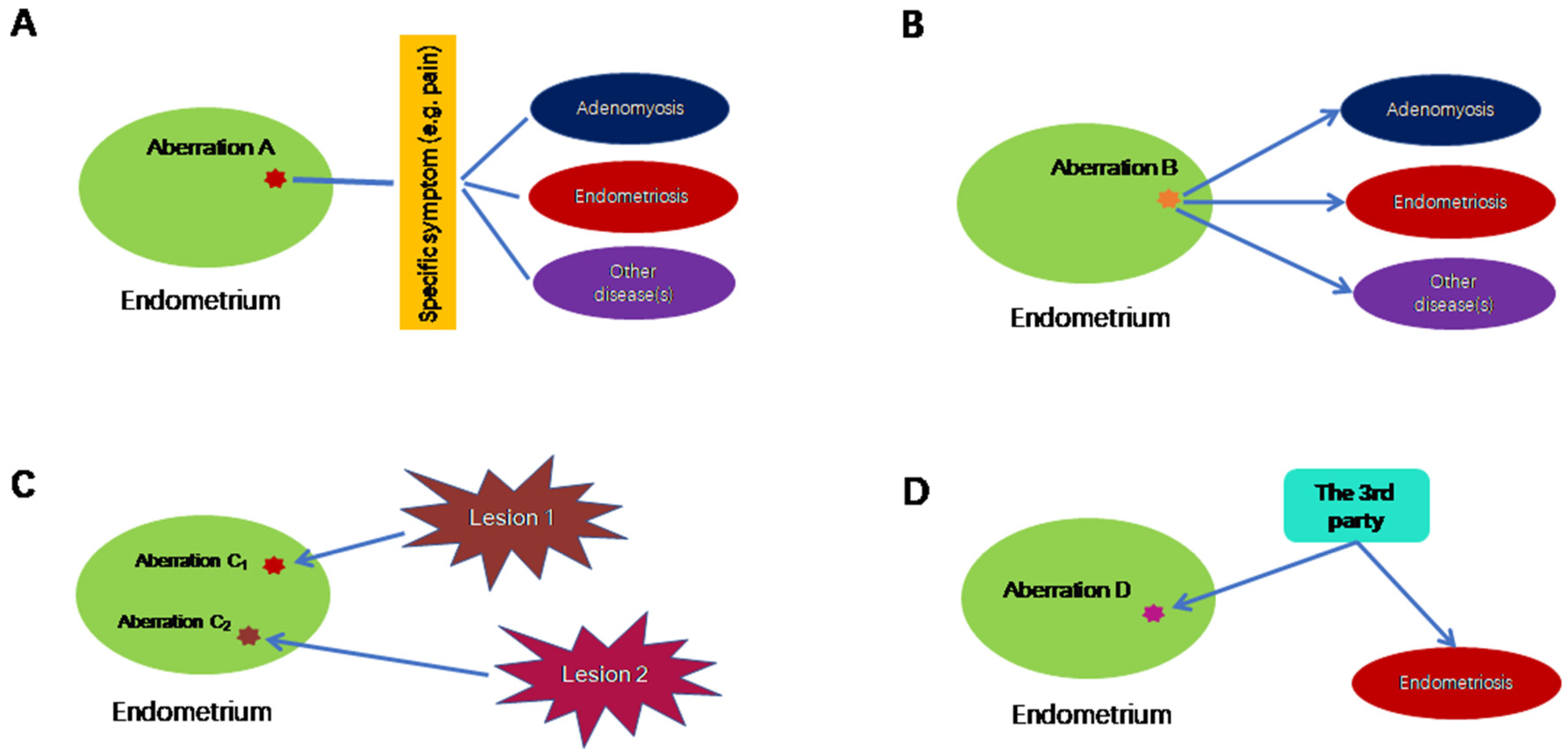
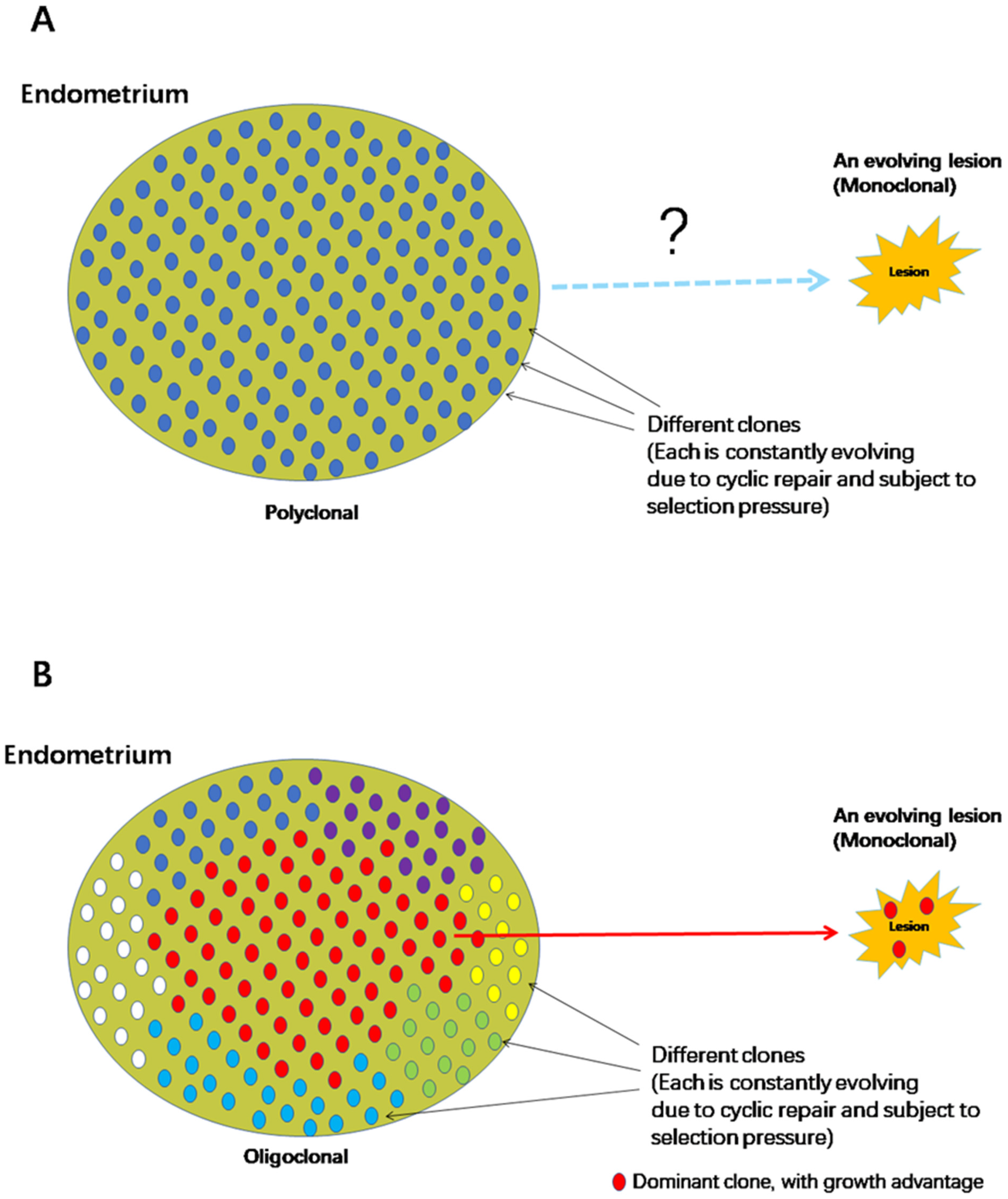
2. Aberrations in Eutopic Endometrium: Cause or Consequence of Endometriosis?
3. Aberrations in the Eutopic Endometrium Are Secondary to the Establishment of Ectopic Foci
4. The Mandate to Establish a Phylogenetic and Causal Relationship
5. The “Root Treatment”: Practicality and Biological Plausibility Concerns
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sampson, J.A. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am.J. Obstet Gynecol. 1927, 14, 422–469. [Google Scholar]
- Te Linde, R.W.; Scott, R.B. Experimental endometriosis. Am.J. Obstet Gynecol. 1950, 60, 1147–1173. [Google Scholar] [CrossRef]
- Ridley, J.H.; Edwards, I.K. Experimental endometriosis in the human. Am.J. Obstet Gynecol. 1958, 76, 783–789. [Google Scholar] [CrossRef] [PubMed]
- D’Hooghe, T.M.; Bambra, C.S.; Raeymaekers, B.M.; De Jonge, I.; Lauweryns, J.M.; Koninckx, P.R. Intrapelvic injection of menstrual endometrium causes endometriosis in baboons (Papio cynocephalus and Papio anubis). Am.J. Obstet Gynecol. 1995, 173, 125–134. [Google Scholar] [CrossRef]
- Fazleabas, A.T. A baboon model for inducing endometriosis. Methods Mol. Med. 2006, 121, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.T.; Hitchcock, A. Endometriosis: Its association with retrograde menstruation, dysmenorrhoea and tubal pathology. Br.J. Obstet Gynaecol. 1986, 93, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Vinatier, D.; Cosson, M.; Dufour, P. Is endometriosis an endometrial disease? Eur.J. Obstet Gynecol. Reprod. Biol. 2000, 91, 113–125. [Google Scholar] [CrossRef]
- Lang, J.H. Cornerstone of study on endometriosis. Zhonghua Fu Chan Ke Za Zhi 2005, 40, 3–4. [Google Scholar]
- Lang, J.H. Promotion and enhancement of research on endometriosis. Zhonghua Fu Chan Ke Za Zhi 2010, 45, 241–242. [Google Scholar]
- Lang, J. Endometriosis: Its re-acquaintance and significance. Chin. Eng. Sci. 2009, 11, 137–142. (In Chinese) [Google Scholar] [CrossRef]
- Lang, J. Facilitating endometriosis research based on the principles of evidence-based medicine. J. Int. Obstet. Gynecol. 2011, 38, 261–262. (In Chinese) [Google Scholar]
- Leng, J.o.b.o.t.E.S.; Gynecologists and Obstetrician Branch; Association, C.P. [Consensus among Chinese Experts on the long-term management of endometriosis.]. Chin.J. Obstet. Gynecol. 2018, 53, 836–840. (In Chinese) [Google Scholar]
- Suda, K.; Nakaoka, H.; Yoshihara, K.; Ishiguro, T.; Tamura, R.; Mori, Y.; Yamawaki, K.; Adachi, S.; Takahashi, T.; Kase, H.; et al. Clonal Expansion and Diversification of Cancer-Associated Mutations in Endometriosis and Normal Endometrium. Cell Rep. 2018, 24, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Yoshida, E.; Fukui, Y.; Ueno, T.; Kawazu, M.; Takeyama, R.; Ikemura, M.; Osuga, Y.; Terao, Y.; Hirota, Y.; et al. KRAS mutations in uterine endometrium are associated with gravidity and parity. Cell Death Dis. 2020, 11, 347. [Google Scholar] [CrossRef]
- Canon, J.; Rex, K.; Saiki, A.Y.; Mohr, C.; Cooke, K.; Bagal, D.; Gaida, K.; Holt, T.; Knutson, C.G.; Koppada, N.; et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 2019, 575, 217–223. [Google Scholar] [CrossRef]
- Kao, L.C.; Germeyer, A.; Tulac, S.; Lobo, S.; Yang, J.P.; Taylor, R.N.; Osteen, K.; Lessey, B.A.; Giudice, L.C. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology 2003, 144, 2870–2881. [Google Scholar] [CrossRef]
- Burney, R.O.; Talbi, S.; Hamilton, A.E.; Vo, K.C.; Nyegaard, M.; Nezhat, C.R.; Lessey, B.A.; Giudice, L.C. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 2007, 148, 3814–3826. [Google Scholar] [CrossRef]
- Burney, R.O.; Hamilton, A.E.; Aghajanova, L.; Vo, K.C.; Nezhat, C.N.; Lessey, B.A.; Giudice, L.C. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Mol. Hum. Reprod 2009, 15, 625–631. [Google Scholar] [CrossRef]
- Fassbender, A.; Verbeeck, N.; Bornigen, D.; Kyama, C.M.; Bokor, A.; Vodolazkaia, A.; Peeraer, K.; Tomassetti, C.; Meuleman, C.; Gevaert, O.; et al. Combined mRNA microarray and proteomic analysis of eutopic endometrium of women with and without endometriosis. Hum. Reprod. 2012, 27, 2020–2029. [Google Scholar] [CrossRef]
- Laudanski, P.; Charkiewicz, R.; Tolwinska, A.; Szamatowicz, J.; Charkiewicz, A.; Niklinski, J. Profiling of Selected MicroRNAs in Proliferative Eutopic Endometrium of Women with Ovarian Endometriosis. BioMed Res. Int. 2015, 2015, 760698. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Yang, Z.; Liu, K.; Wang, D. Genome-Wide Microarray Analysis of Long Non-Coding RNAs in Eutopic Secretory Endometrium with Endometriosis. Cell Physiol. Biochem. 2015, 37, 2231–2245. [Google Scholar] [CrossRef]
- Herndon, C.N.; Aghajanova, L.; Balayan, S.; Erikson, D.; Barragan, F.; Goldfien, G.; Vo, K.C.; Hawkins, S.; Giudice, L.C. Global Transcriptome Abnormalities of the Eutopic Endometrium From Women With Adenomyosis. Reprod. Sci. 2016, 23, 1289–1303. [Google Scholar] [CrossRef] [PubMed]
- Kukushkina, V.; Modhukur, V.; Suhorutsenko, M.; Peters, M.; Magi, R.; Rahmioglu, N.; Velthut-Meikas, A.; Altmae, S.; Esteban, F.J.; Vilo, J.; et al. DNA methylation changes in endometrium and correlation with gene expression during the transition from pre-receptive to receptive phase. Sci. Rep. 2017, 7, 3916. [Google Scholar] [CrossRef] [PubMed]
- Barjaste, N.; Shahhoseini, M.; Afsharian, P.; Sharifi-Zarchi, A.; Masoudi-Nejad, A. Genome-wide DNA methylation profiling in ectopic and eutopic of endometrial tissues. J. Assist. Reprod. Genet. 2019, 36, 1743–1752. [Google Scholar] [CrossRef]
- Gupta, D.; Hull, M.L.; Fraser, I.; Miller, L.; Bossuyt, P.M.; Johnson, N.; Nisenblat, V. Endometrial biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016, 4, CD012165. [Google Scholar] [CrossRef]
- Da Broi, M.G.; Meola, J.; Placa, J.R.; Peronni, K.C.; Rocha, C.V.; Silva, W.A.; Ferriani, R.A.; Navarro, P.A. Is the profile of transcripts altered in the eutopic endometrium of infertile women with endometriosis during the implantation window? Hum. Reprod. 2019, 34, 2381–2390. [Google Scholar] [CrossRef]
- Tokushige, N.; Markham, R.; Russell, P.; Fraser, I.S. High density of small nerve fibres in the functional layer of the endometrium in women with endometriosis. Hum. Reprod. 2006, 21, 782–787. [Google Scholar] [CrossRef]
- Tokushige, N.; Markham, R.; Russell, P.; Fraser, I.S. Effects of hormonal treatment on nerve fibers in endometrium and myometrium in women with endometriosis. Fertil. Steril. 2008, 90, 1589–1598. [Google Scholar] [CrossRef]
- Tokushige, N.; Markham, R.; Russell, P.; Fraser, I.S. Different types of small nerve fibers in eutopic endometrium and myometrium in women with endometriosis. Fertil. Steril. 2007, 88, 795–803. [Google Scholar] [CrossRef]
- Al-Jefout, M.; Dezarnaulds, G.; Cooper, M.; Tokushige, N.; Luscombe, G.M.; Markham, R.; Fraser, I.S. Diagnosis of endometriosis by detection of nerve fibres in an endometrial biopsy: A double blind study. Hum. Reprod. 2009, 24, 3019–3024. [Google Scholar] [CrossRef] [PubMed]
- Bokor, A.; Kyama, C.M.; Vercruysse, L.; Fassbender, A.; Gevaert, O.; Vodolazkaia, A.; De Moor, B.; Fulop, V.; D’Hooghe, T. Density of small diameter sensory nerve fibres in endometrium: A semi-invasive diagnostic test for minimal to mild endometriosis. Hum. Reprod. 2009, 24, 3025–3032. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, B.; Huang, X.; Xu, H.; Zhou, C.; Lin, J. Endometrial nerve fibers in women with endometriosis, adenomyosis, and uterine fibroids. Fertil. Steril. 2009, 92, 1799–1801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, B.; Huang, X.; Xu, H.; Zhou, C.; Lin, J. Innervation of endometrium and myometrium in women with painful adenomyosis and uterine fibroids. Fertil. Steril. 2010, 94, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Newman, T.A.; Bailey, J.L.; Stocker, L.J.; Woo, Y.L.; Macklon, N.S.; Cheong, Y.C. Expression of neuronal markers in the endometrium of women with and those without endometriosis. Hum. Reprod. 2013, 28, 2502–2510. [Google Scholar] [CrossRef] [PubMed]
- Ellett, L.; Readman, E.; Newman, M.; McIlwaine, K.; Villegas, R.; Jagasia, N.; Maher, P. Are endometrial nerve fibres unique to endometriosis? A prospective case-control study of endometrial biopsy as a diagnostic test for endometriosis in women with pelvic pain. Hum. Reprod. 2015, 30, 2808–2815. [Google Scholar] [CrossRef] [PubMed]
- Liutkeviciene, R.; Mecejus, G.; Zilovic, D.; Bumbuliene, Z. Endometrial biopsy and density of nerve fibers in eutopic endometrium. Looking for easier ways to diagnose endometriosis. Gynecol. Endocrinol. 2019, 35, 1107–1110. [Google Scholar] [CrossRef]
- Gronemeyer, H.; Meyer, M.E.; Bocquel, M.T.; Kastner, P.; Turcotte, B.; Chambon, P. Progestin receptors: Isoforms and antihormone action. J. Steroid Biochem. Mol. Biol. 1991, 40, 271–278. [Google Scholar] [CrossRef]
- McKinnon, B.; Mueller, M.; Montgomery, G. Progesterone Resistance in Endometriosis: An Acquired Property? Trends Endocrinol. Metab. 2018, 29, 535–548. [Google Scholar] [CrossRef]
- Attia, G.R.; Zeitoun, K.; Edwards, D.; Johns, A.; Carr, B.R.; Bulun, S.E. Progesterone receptor isoform A but not B is expressed in endometriosis. J.Clin. Endocrinol. Metab. 2000, 85, 2897–2902. [Google Scholar] [CrossRef]
- Bukulmez, O.; Hardy, D.B.; Carr, B.R.; Word, R.A.; Mendelson, C.R. Inflammatory status influences aromatase and steroid receptor expression in endometriosis. Endocrinology 2008, 149, 1190–1204. [Google Scholar] [CrossRef] [PubMed]
- Bedaiwy, M.A.; Dahoud, W.; Skomorovska-Prokvolit, Y.; Yi, L.; Liu, J.H.; Falcone, T.; Hurd, W.W.; Mesiano, S. Abundance and Localization of Progesterone Receptor Isoforms in Endometrium in Women With and Without Endometriosis and in Peritoneal and Ovarian Endometriotic Implants. Reprod. Sci. 2015, 22, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Strawn, E.; Basir, Z.; Halverson, G.; Guo, S.W. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics 2006, 1, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Jichan, N.; Xishi, L.; Guo, S.W. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in adenomyosis and its rectification by a histone deacetylase inhibitor and a demethylation agent. Reprod. Sci. 2010, 17, 995–1005. [Google Scholar] [CrossRef]
- Wu, Y.; Starzinski-Powitz, A.; Guo, S.W. Prolonged stimulation with tumor necrosis factor-alpha induced partial methylation at PR-B promoter in immortalized epithelial-like endometriotic cells. Fertil. Steril. 2008, 90, 234–237. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, X.; Guo, S.W. The knockdown of progesterone receptor isoform B (PR-B) promotes proliferation in immortalized endometrial stromal cells. Fertil. Steril. 2008, 90, 1320–1323. [Google Scholar] [CrossRef]
- Igarashi, T.M.; Bruner-Tran, K.L.; Yeaman, G.R.; Lessey, B.A.; Edwards, D.P.; Eisenberg, E.; Osteen, K.G. Reduced expression of progesterone receptor-B in the endometrium of women with endometriosis and in cocultures of endometrial cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fertil. Steril. 2005, 84, 67–74. [Google Scholar] [CrossRef]
- Shen, F.; Yan, C.; Liu, M.; Feng, Y.; Chen, Y. Decreased expression of mucin-1 in endometriosis endometrium correlated with progesterone receptor B involved in infertility. Arch. Gynecol. Obstet. 2015, 291, 439–445. [Google Scholar] [CrossRef]
- Wolfler, M.M.; Kuppers, M.; Rath, W.; Buck, V.U.; Meinhold-Heerlein, I.; Classen-Linke, I. Altered expression of progesterone receptor isoforms A and B in human eutopic endometrium in endometriosis patients. Ann. Anat. 2016, 206, 1–6. [Google Scholar] [CrossRef]
- Hou, Z.; Mamillapalli, R.; Taylor, H.S. Predictive biomarkers may allow precision therapy of endometriosis. J. Endometr. Pelvic Pain Disord. 2017, 9, 279–285. [Google Scholar] [CrossRef]
- Pei, T.; Liu, C.; Liu, T.; Xiao, L.; Luo, B.; Tan, J.; Li, X.; Zhou, G.; Duan, C.; Huang, W. miR-194-3p Represses the Progesterone Receptor and Decidualization in Eutopic Endometrium From Women With Endometriosis. Endocrinology 2018, 159, 2554–2562. [Google Scholar] [CrossRef] [PubMed]
- Colon-Caraballo, M.; Garcia, M.; Mendoza, A.; Flores, I. Human Endometriosis Tissue Microarray Reveals Site-specific Expression of Estrogen Receptors, Progesterone Receptor, and Ki67. Appl. Immunohistochem. Mol. Morphol. 2018, 27, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Junior, C.V.; Da Broi, M.G.; Miranda-Furtado, C.L.; Navarro, P.A.; Ferriani, R.A.; Meola, J. Progesterone Receptor B ( PGR-B) Is Partially Methylated in Eutopic Endometrium From Infertile Women With Endometriosis. Reprod. Sci. 2019, 26, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A.; Randall, B.J.; Weddell, A.; McGill, A.; Henry, L.; Horne, C.H.; Thomas, E.J. Ovarian steroid receptor expression in endometriosis and in two potential parent epithelia: Endometrium and peritoneal mesothelium. Hum. Reprod. 1992, 7, 1318–1325. [Google Scholar] [CrossRef]
- Broi, M.G.D.; Rocha, C.V.J.; Meola, J.; Martins, W.P.; Carvalho, F.M.; Ferriani, R.A.; Navarro, P.A. Expression of PGR, HBEGF, ITGAV, ITGB3 and SPP1 genes in eutopic endometrium of infertile women with endometriosis during the implantation window: A pilot study. JBRA Assist. Reprod. 2017, 21, 196–202. [Google Scholar] [CrossRef]
- Gentilini, D.; Vigano, P.; Vignali, M.; Busacca, M.; Panina-Bordignon, P.; Caporizzo, E.; Di Blasio, A.M. Endometrial stromal progesterone receptor-A/progesterone receptor-B ratio: No difference between women with and without endometriosis. Fertil. Steril. 2010, 94, 1538–1540. [Google Scholar] [CrossRef]
- Marquardt, R.M.; Kim, T.H.; Shin, J.H.; Jeong, J.W. Progesterone and Estrogen Signaling in the Endometrium: What Goes Wrong in Endometriosis? Int.J. Mol. Sci. 2019, 20, 3822. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Critchley, H.; Fu, Z.; Guo, S.-W. How does the extent of fibrosis in adenomyosis lesions contribute to heavy menstrual bleeding? Reprod. Med. Biol. 2022, 21, e12442. [Google Scholar] [CrossRef]
- Nie, J.; Lu, Y.; Liu, X.; Guo, S.W. Immunoreactivity of progesterone receptor isoform B, nuclear factor kappaB, and IkappaBalpha in adenomyosis. Fertil. Steril. 2009, 92, 886–889. [Google Scholar] [CrossRef]
- Kumar, P.V.; Esfahani, F.N. Cytopathology of peritoneal endometriosis caused by ruptured ovarian cysts. Acta Cytol. 1988, 32, 523–526. [Google Scholar]
- Critchley, H.O.; Wang, H.; Kelly, R.W.; Gebbie, A.E.; Glasier, A.F. Progestin receptor isoforms and prostaglandin dehydrogenase in the endometrium of women using a levonorgestrel-releasing intrauterine system. Hum. Reprod. 1998, 13, 1210–1217. [Google Scholar] [CrossRef]
- Wu, Y.; Halverson, G.; Basir, Z.; Strawn, E.; Yan, P.; Guo, S.W. Aberrant methylation at HOXA10 may be responsible for its aberrant expression in the endometrium of patients with endometriosis. Am. J. Obstet. Gynecol. 2005, 193, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Taylor, H.S.; Lu, Z.; Ladhani, O.; Hastings, J.M.; Jackson, K.S.; Wu, Y.; Guo, S.W.; Fazleabas, A.T. Altered expression of HOXA10 in endometriosis: Potential role in decidualization. Mol. Hum. Reprod. 2007, 13, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Du, H.; Taylor, H.S. Experimental murine endometriosis induces DNA methylation and altered gene expression in eutopic endometrium. Biol. Reprod. 2009, 80, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Szczepanska, M.; Wirstlein, P.; Luczak, M.; Jagodzinski, P.P.; Skrzypczak, J. Reduced expression of HOXA10 in the midluteal endometrium from infertile women with minimal endometriosis. Biomed. Pharmacother. 2010, 64, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Fambrini, M.; Sorbi, F.; Bussani, C.; Cioni, R.; Sisti, G.; Andersson, K.L. Hypermethylation of HOXA10 gene in mid-luteal endometrium from women with ovarian endometriomas. Acta Obstet. Gynecol. Scand. 2013, 92, 1331–1334. [Google Scholar] [CrossRef]
- Kulp, J.L.; Mamillapalli, R.; Taylor, H.S. Aberrant HOXA10 Methylation in Patients With Common Gynecologic Disorders: Implications for Reproductive Outcomes. Reprod. Sci. 2016, 23, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Nie, J.; Liu, X.; Guo, S.W. Reduced expression and concomitant promoter hypermethylation of HOXA10 in endometrium from women wearing intrauterine devices. Fertil. Steril. 2010, 94, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ferrer, M.L.; Mendiola, J.; Jimenez-Velazquez, R.; Canovas-Lopez, L.; Corbalan-Biyang, S.; Hernandez-Penalver, A.I.; Carmona-Barnosi, A.; Maldonado-Carceles, A.B.; Prieto-Sanchez, M.T.; Machado-Linde, F.; et al. Investigation of anogenital distance as a diagnostic tool in endometriosis. Reprod. Biomed. Online 2017, 34, 375–382. [Google Scholar] [CrossRef]
- Dinsdale, N.L.; Crespi, B.J. Endometriosis and polycystic ovary syndrome are diametric disorders. Evol. Appl. 2021, 14, 1693–1715. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Kennedy, S.H.; Barlow, D.H. Endometriotic disease: The role of peritoneal fluid. Hum. Reprod. Update 1998, 4, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, H.; Mamillapalli, R.; Krikun, G.; Taylor, H.S. Endometriosis Located Proximal to or Remote From the Uterus Differentially Affects Uterine Gene Expression. Reprod. Sci. 2016, 23, 186–191. [Google Scholar] [CrossRef]
- Goetz, T.G.; Mamillapalli, R.; Taylor, H.S. Low Body Mass Index in Endometriosis Is Promoted by Hepatic Metabolic Gene Dysregulation in Mice. Biol. Reprod. 2016, 95, 115. [Google Scholar] [CrossRef]
- Shah, D.K.; Correia, K.F.; Vitonis, A.F.; Missmer, S.A. Body size and endometriosis: Results from 20 years of follow-up within the Nurses’ Health Study II prospective cohort. Hum. Reprod. 2013, 28, 1783–1792. [Google Scholar] [CrossRef] [PubMed]
- Mamillapalli, R.; Toffoloni, N.; Habata, S.; Qunhua, H.; Atwani, R.; Stachenfeld, N.; Taylor, H.S. Endometriosis promotes atherosclerosis in a murine model. Am. J. Obstet. Gynecol. 2022, 227, 248.e1–248.e8. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Yoo, J.Y.; Choi, K.C.; Shin, J.H.; Leach, R.E.; Fazleabas, A.T.; Young, S.L.; Lessey, B.A.; Yoon, H.G.; Jeong, J.W. Loss of HDAC3 results in nonreceptive endometrium and female infertility. Sci. Transl. Med. 2019, 11, eaaf7533. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, J.R.; Hastings, J.M.; Jackson, K.S.; Mavrogianis, P.A.; Sharkey, A.M.; Fazleabas, A.T. The endometrial response to chorionic gonadotropin is blunted in a baboon model of endometriosis. Endocrinology 2010, 151, 4982–4993. [Google Scholar] [CrossRef]
- Lessey, B.A. Endometrial integrins and the establishment of uterine receptivity. Hum. Reprod. 1998, 13 (Suppl. S3), 247–258. [Google Scholar] [CrossRef]
- Gui, Y.; Zhang, J.; Yuan, L.; Lessey, B.A. Regulation of HOXA-10 and its expression in normal and abnormal endometrium. Mol. Hum. Reprod. 1999, 5, 866–873. [Google Scholar] [CrossRef]
- Taylor, H.S.; Bagot, C.; Kardana, A.; Olive, D.; Arici, A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum. Reprod. 1999, 14, 1328–1331. [Google Scholar] [CrossRef]
- Fazleabas, A.T.; Brudney, A.; Gurates, B.; Chai, D.; Bulun, S. A modified baboon model for endometriosis. Ann. N. Y. Acad. Sci. 2002, 955, 308–317. [Google Scholar]
- Wu, Y.; Basir, Z.; Kajdacsy-Balla, A.; Strawn, E.; Macias, V.; Montgomery, K.; Guo, S.W. Resolution of clonal origins for endometriotic lesions using laser capture microdissection and the human androgen receptor (HUMARA) assay. Fertil. Steril. 2003, 79 (Suppl. S1), 710–717. [Google Scholar] [PubMed]
- Tanaka, M.; Kyo, S.; Kanaya, T.; Yatabe, N.; Nakamura, M.; Maida, Y.; Okabe, M.; Inoue, M. Evidence of the monoclonal composition of human endometrial epithelial glands and mosaic pattern of clonal distribution in luminal epithelium. Am. J. Pathol. 2003, 163, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, S.W. Reconstructing cellular lineages in endometrial cells. Fertil. Steril. 2008, 89, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Praetorius, T.H.; Leonova, A.; Lac, V.; Senz, J.; Tessier-Cloutier, B.; Nazeran, T.M.; Kobel, M.; Grube, M.; Kraemer, B.; Yong, P.J.; et al. Molecular analysis suggests oligoclonality and metastasis of endometriosis lesions across anatomically defined subtypes. Fertil. Steril. 2022, 118, 524–534. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Nakaoka, H.; Suda, K.; Yoshihara, K.; Ishiguro, T.; Yachida, N.; Saito, K.; Ueda, H.; Sugino, K.; Mori, Y.; et al. Spatiotemporal dynamics of clonal selection and diversification in normal endometrial epithelium. Nat. Commun. 2022, 13, 943. [Google Scholar] [CrossRef]
- Greenman, C.; Stephens, P.; Smith, R.; Dalgliesh, G.L.; Hunter, C.; Bignell, G.; Davies, H.; Teague, J.; Butler, A.; Stevens, C.; et al. Patterns of somatic mutation in human cancer genomes. Nature 2007, 446, 153–158. [Google Scholar] [CrossRef]
- Martincorena, I.; Raine, K.M.; Gerstung, M.; Dawson, K.J.; Haase, K.; Van Loo, P.; Davies, H.; Stratton, M.R.; Campbell, P.J. Universal Patterns of Selection in Cancer and Somatic Tissues. Cell 2017, 171, 1029–1041.e1021. [Google Scholar] [CrossRef]
- Martincorena, I.; Roshan, A.; Gerstung, M.; Ellis, P.; Van Loo, P.; McLaren, S.; Wedge, D.C.; Fullam, A.; Alexandrov, L.B.; Tubio, J.M.; et al. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science 2015, 348, 880–886. [Google Scholar] [CrossRef]
- Martincorena, I.; Fowler, J.C.; Wabik, A.; Lawson, A.R.J.; Abascal, F.; Hall, M.W.J.; Cagan, A.; Murai, K.; Mahbubani, K.; Stratton, M.R.; et al. Somatic mutant clones colonize the human esophagus with age. Science 2018, 365, 911–917. [Google Scholar] [CrossRef]
- Anglesio, M.S.; Papadopoulos, N.; Ayhan, A.; Nazeran, T.M.; Noe, M.; Horlings, H.M.; Lum, A.; Jones, S.; Senz, J.; Seckin, T.; et al. Cancer-Associated Mutations in Endometriosis without Cancer. N. Engl. J. Med. 2017, 376, 1835–1848. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.; Leongamornlert, D.; Coorens, T.H.H.; Sanders, M.A.; Ellis, P.; Dentro, S.C.; Dawson, K.J.; Butler, T.; Rahbari, R.; Mitchell, T.J.; et al. The mutational landscape of normal human endometrial epithelium. Nature 2020, 580, 640–646. [Google Scholar] [CrossRef]
- Lac, V.; Nazeran, T.M.; Tessier-Cloutier, B.; Aguirre-Hernandez, R.; Albert, A.; Lum, A.; Khattra, J.; Praetorius, T.; Mason, M.; Chiu, D.; et al. Oncogenic mutations in histologically normal endometrium: The new normal? J. Pathol. 2019, 249, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Househam, J.; Heide, T.; Cresswell, G.D.; Spiteri, I.; Kimberley, C.; Zapata, L.; Lynn, C.; James, C.; Mossner, M.; Fernandez-Mateos, J.; et al. Phenotypic plasticity and genetic control in colorectal cancer evolution. Nature 2022, 611, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.W. Cancer-associated mutations in endometriosis: Shedding light on the pathogenesis and pathophysiology. Hum. Reprod. Update 2020, 26, 423–449. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Banno, K.; Kobayashi, Y.; Adachi, M.; Yanokura, M.; Tominaga, E.; Kosaki, K.; Aoki, D. Mutations of RAS genes in endometrial polyps. Oncol. Rep. 2019, 42, 2303–2308. [Google Scholar] [CrossRef]
- Siegmund, K.D.; Marjoram, P.; Woo, Y.J.; Tavare, S.; Shibata, D. Inferring clonal expansion and cancer stem cell dynamics from DNA methylation patterns in colorectal cancers. Proc. Natl. Acad. Sci. USA 2009, 106, 4828–4833. [Google Scholar] [CrossRef]
- Gabbutt, C.; Schenck, R.O.; Weisenberger, D.J.; Kimberley, C.; Berner, A.; Househam, J.; Lakatos, E.; Robertson-Tessi, M.; Martin, I.; Patel, R.; et al. Fluctuating methylation clocks for cell lineage tracing at high temporal resolution in human tissues. Nat. Biotechnol. 2022, 40, 720–730. [Google Scholar] [CrossRef]
- Yokoyama, A.; Kakiuchi, N.; Yoshizato, T.; Nannya, Y.; Suzuki, H.; Takeuchi, Y.; Shiozawa, Y.; Sato, Y.; Aoki, K.; Kim, S.K.; et al. Age-related remodelling of oesophageal epithelia by mutated cancer drivers. Nature 2019, 565, 312–317. [Google Scholar] [CrossRef]
- Liu, E.; Nisenblat, V.; Farquhar, C.; Fraser, I.; Bossuyt, P.M.; Johnson, N.; Hull, M.L. Urinary biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2015, 2015, CD012019. [Google Scholar] [CrossRef]
- Nisenblat, V.; Bossuyt, P.M.; Farquhar, C.; Johnson, N.; Hull, M.L. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016, 2, CD009591. [Google Scholar] [CrossRef] [PubMed]
- Nisenblat, V.; Bossuyt, P.M.; Shaikh, R.; Farquhar, C.; Jordan, V.; Scheffers, C.S.; Mol, B.W.; Johnson, N.; Hull, M.L. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016, 2016, CD012179. [Google Scholar] [CrossRef] [PubMed]
- Nisenblat, V.; Prentice, L.; Bossuyt, P.M.; Farquhar, C.; Hull, M.L.; Johnson, N. Combination of the non-invasive tests for the diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016, 7, CD012281. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.-W.; Habiba, M.; Benagiano, G. From Retrograde Menstruation to Endometrial Determinism and a Brave New World of “Root Treatment” of Endometriosis: Destiny or a Fanciful Utopia? Biomolecules 2023, 13, 336. https://doi.org/10.3390/biom13020336
Guo S-W, Habiba M, Benagiano G. From Retrograde Menstruation to Endometrial Determinism and a Brave New World of “Root Treatment” of Endometriosis: Destiny or a Fanciful Utopia? Biomolecules. 2023; 13(2):336. https://doi.org/10.3390/biom13020336
Chicago/Turabian StyleGuo, Sun-Wei, Marwan Habiba, and Giuseppe Benagiano. 2023. "From Retrograde Menstruation to Endometrial Determinism and a Brave New World of “Root Treatment” of Endometriosis: Destiny or a Fanciful Utopia?" Biomolecules 13, no. 2: 336. https://doi.org/10.3390/biom13020336
APA StyleGuo, S.-W., Habiba, M., & Benagiano, G. (2023). From Retrograde Menstruation to Endometrial Determinism and a Brave New World of “Root Treatment” of Endometriosis: Destiny or a Fanciful Utopia? Biomolecules, 13(2), 336. https://doi.org/10.3390/biom13020336






