The Role of Zinc in Modulating Acid-Sensing Ion Channel Function
Abstract
1. Introduction
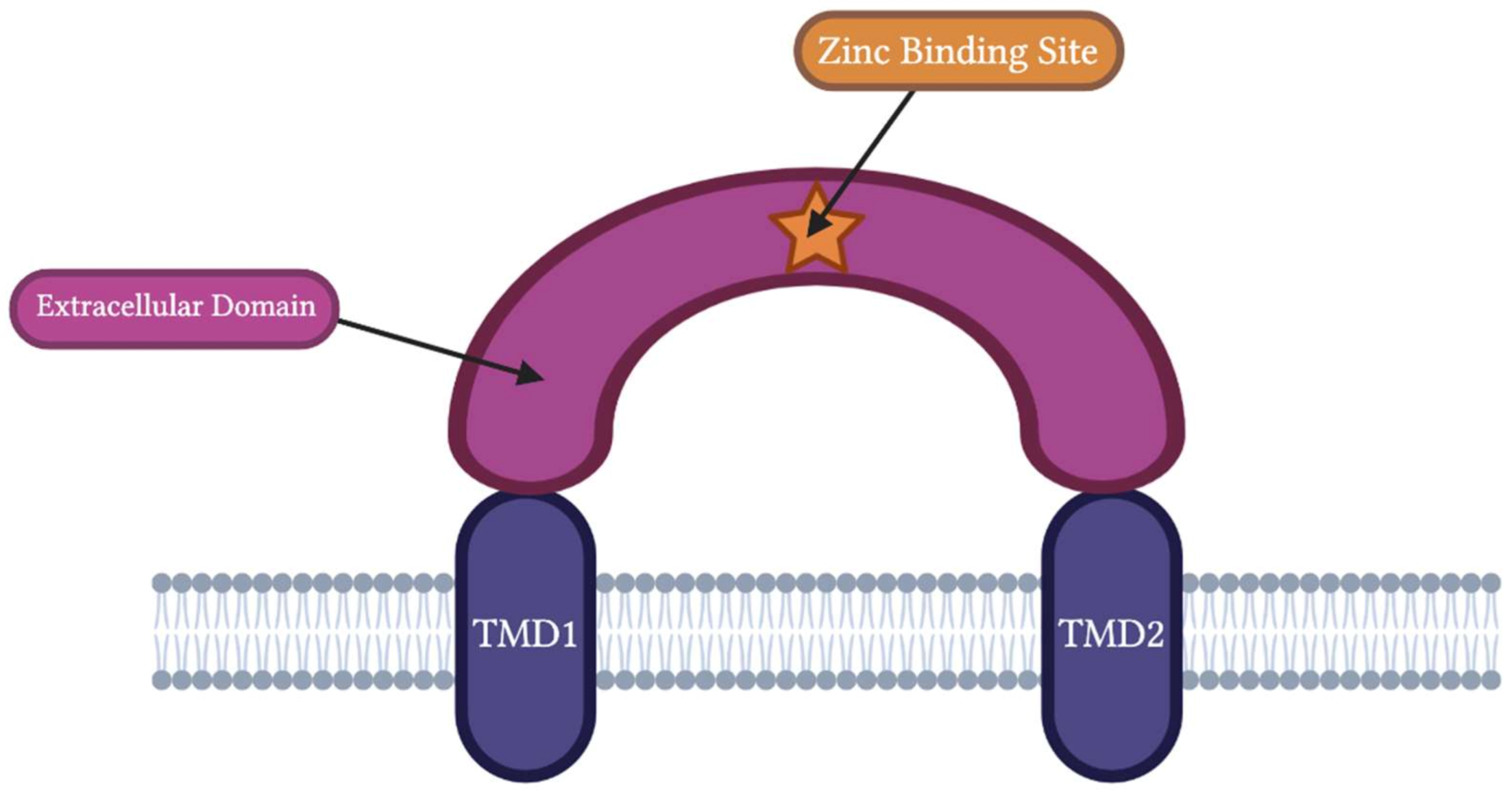
1.1. ASIC Structure
1.2. Zinc Physiology
1.3. Zinc and Disease
1.4. Zinc and Ion Channel Regulation
2. Zinc’s Effects on Different Types of ASICs
2.1. Zinc and ASIC1a
2.2. Zinc and ASIC1b
2.3. Zinc and ASIC1a/3
2.4. Zinc and ASIC1a/2b
2.5. Zinc and ASIC1a/2a
2.6. Zinc and ASIC2a
2.7. Zinc and ASIC2a/3
2.8. Zinc and ASIC3
3. Zinc Regulation of ASICs in Neurological and Psychological Diseases
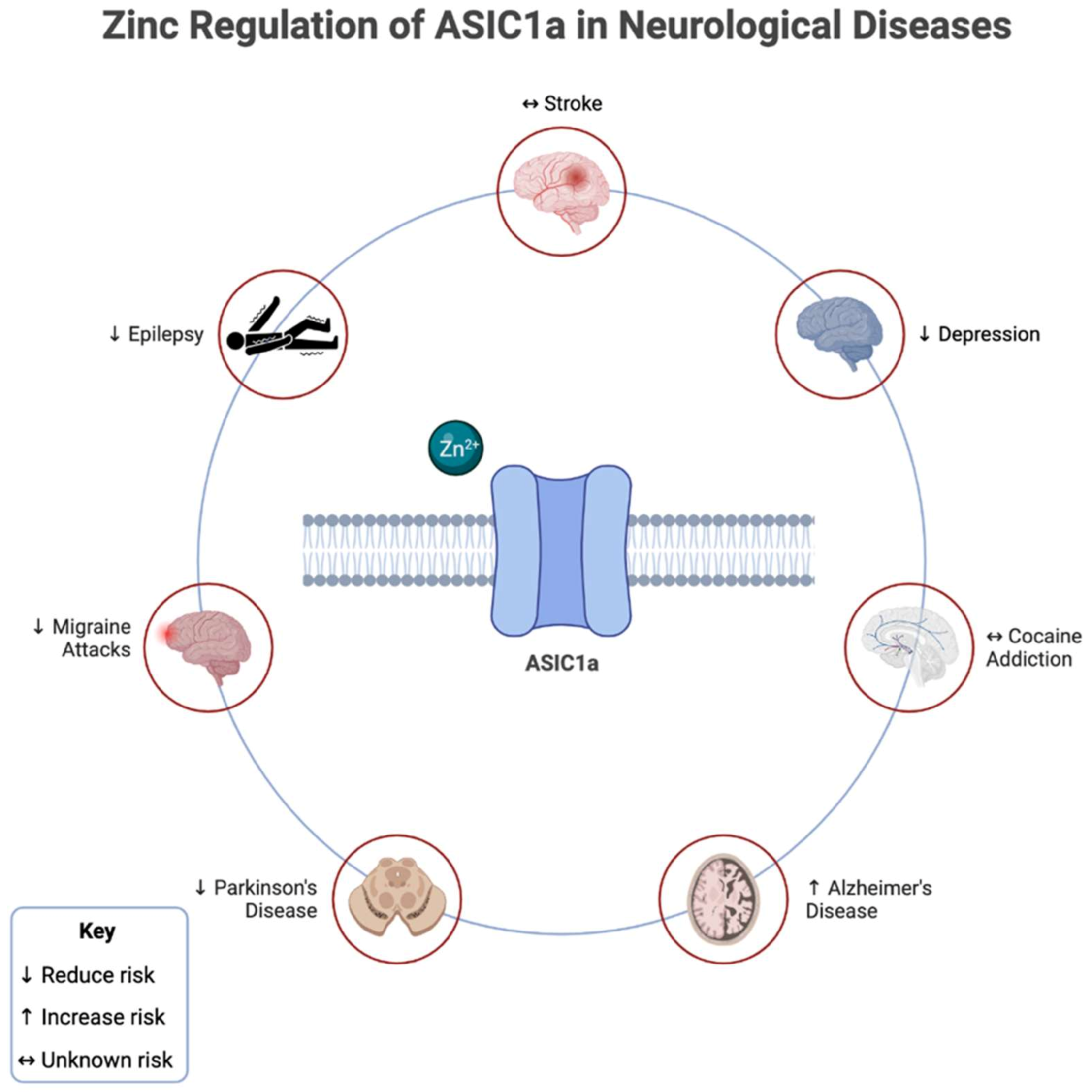
3.1. ASIC1a
3.1.1. Epilepsy
3.1.2. Migraines
3.1.3. Alzheimer’s Disease
3.1.4. Parkinson’s Disease
3.1.5. Depression
3.1.6. Stroke
3.1.7. Cocaine Addiction
3.2. ASIC1b
3.3. ASIC1a/3
3.4. ASIC1a/2b
3.5. ASIC1a/2a
3.6. ASIC2a
3.7. ASIC2a/3
3.8. ASIC3
3.9. ASIC4
3.10. ASIC5
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Waldmann, R.; Champigny, G.; Bassilana, F.; Heurteaux, C.; Lazdunski, M. A proton-gated cation channel involved in acid-sensing. Nature 1997, 386, 173–177. [Google Scholar] [CrossRef]
- Ruan, N.; Tribble, J.; Peterson, A.M.; Jiang, Q.; Wang, J.Q.; Chu, X.P. Acid-Sensing Ion Channels and Mechanosensation. Int. J. Mol. Sci. 2021, 22, 4810. [Google Scholar] [CrossRef]
- Storozhuk, M.; Cherninskyi, A.; Maximyuk, O.; Isaev, D.; Krishtal, O. Acid-Sensing Ion Channels: Focus on Physiological and Some Pathological Roles in the Brain. Curr. Neuropharmacol. 2021, 19, 1570–1589. [Google Scholar]
- Jiang, Q.; Peterson, A.M.; Chu, Y.; Yao, X.; Zha, X.M.; Chu, X.P. Histidine Residues Are Responsible for Bidirectional Effects of Zinc on Acid-Sensing Ion Channel 1a/3 Heteromeric Channels. Biomolecules 2020, 10, 1264. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Deng, S.M.; Chen, H.W.; Tsao, C.H.; Chen, W.T.; Cheng, S.J.; Huang, H.S.; Tan, B.C.; Matzuk, M.M.; Flint, J.; et al. Follistatin mediates learning and synaptic plasticity via regulation of Asic4 expression in the hippocampus. Proc. Natl. Acad. Sci. USA 2021, 118, e2109040118. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, V.; Friedrich, K.; Polleichtner, G.; Gründer, S. Acid-sensing ion channel (ASIC) 4 predominantly localizes to an early endosome-related organelle upon heterologous expression. Sci. Rep. 2015, 5, 18242. [Google Scholar] [CrossRef] [PubMed]
- Hoshikawa, M.; Kato, A.; Hojo, H.; Shibata, Y.; Kumamoto, N.; Watanabe, M.; Ugawa, S. Distribution of ASIC4 transcripts in the adult wild-type mouse brain. Neurosci. Lett. 2017, 651, 57–64. [Google Scholar] [CrossRef]
- Zhou, R.; Leng, T.; Yang, T.; Chen, F.; Hu, W.; Xiong, Z.G. β-Estradiol protects against acidosis-mediated and ischemic neuronal injury by promoting ASIC1a (acid-sensing ion channel 1a) protein degradation. Stroke 2019, 50, 2902–2911. [Google Scholar] [CrossRef]
- Arias, R.L.; Sung, M.L.; Vasylyev, D.; Zhang, M.Y.; Albinson, K.; Kubek, K.; Kagan, N.; Beyer, C.; Lin, Q.; Dwyer, J.M.; et al. Amiloride is neuroprotective in an MPTP model of Parkinson’s disease. Neurobiol. Dis. 2008, 31, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Kweon, H.J.; Suh, B.C. Acid-sensing ion channels (ASICs): Therapeutic targets for neurological diseases and their regulation. BMB Rep. 2013, 46, 295–304. [Google Scholar] [CrossRef]
- Kreple, C.J.; Lu, Y.; Taugher, R.J.; Schwager-Gutman, A.L.; Du, J.; Stump, M.; Wang, Y.; Ghobbeh, A.; Fan, R.; Cosme, C.V.; et al. Acid-sensing ion channels contribute to synaptic transmission and inhibit cocaine-evoked plasticity. Nat. Neurosci. 2014, 17, 1083–1091. [Google Scholar] [CrossRef]
- Jiang, Q.; Zha, X.M.; Chu, X.P. Inhibition of human acid-sensing ion channel 1b by zinc. Int. J. Physiol. Pathophysiol. Pharmacol. 2012, 4, 84–93. [Google Scholar]
- Chu, X.P.; Papasian, C.J.; Wang, J.Q.; Xiong, Z.G. Modulation of acid-sensing ion channels: Molecular mechanisms and therapeutic potential. Int. J. Physiol. Pathophysiol. Pharmacol. 2011, 3, 288–309. [Google Scholar] [PubMed]
- Sivils, A.; Yang, F.; Wang, J.Q.; Chu, X.P. Acid-Sensing Ion Channel 2: Function and Modulation. Membranes 2022, 12, 113. [Google Scholar] [CrossRef]
- Gonzales, E.B.; Kawate, T.; Gouaux, E. Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature 2009, 460, 599–604. [Google Scholar] [CrossRef]
- Cristofori-Armstrong, B.; Rash, L.D. Acid-sensing ion channel (ASIC) structure and function: Insights from spider, snake and sea anemone venoms. Neuropharmacology 2017, 127, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Jasti, J.; Furukawa, H.; Gonzales, E.B.; Gouaux, E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature 2007, 449, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Baron, A.; Schaefer, L.; Lingueglia, E.; Champigny, G.; Lazdunski, M. Zn2+ and H+ are coactivators of acid-sensing ion channels. J. Biol. Chem. 2001, 276, 35361–35367. [Google Scholar] [CrossRef]
- Hassan, A.; Sada, K.K.; Ketheeswaran, S.; Dubey, A.K.; Bhat, M.S. Role of Zinc in Mucosal Health and Disease: A Review of Physiological, Biochemical, and Molecular Processes. Cureus 2020, 12, 8197. [Google Scholar] [CrossRef]
- Doboszewska, U.; Młyniec, K.; Wlaź, A.; Poleszak, E.; Nowak, G.; Wlaź, P. Zinc signaling and epilepsy. Pharmacol. Ther. 2019, 193, 156–177. [Google Scholar] [CrossRef]
- Mezzaroba, L.; Alfieri, D.F.; Colado Simão, A.N.; Vissoci Reiche, E.M. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kumar, A.; Singh, K.; Avasthi, K.; Kim, J.J. Neurobiology of zinc and its role in neurogenesis. Eur. J. Nutr. 2021, 60, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Sauer, A.K.; Vela, H.; Vela, G.; Stark, P.; Barrera-Juarez, E.; Grabrucker, A.M. Zinc Deficiency in Men Over 50 and Its Implications in Prostate Disorders. Front. Oncol. 2020, 10, 1293. [Google Scholar] [CrossRef]
- Christudoss, P.; Selvakumar, R.; Fleming, J.J.; Gopalakrishnan, G. Zinc status of patients with benign prostatic hyperplasia and prostate carcinoma. Ind. J. Urol. 2011, 27, 14–18. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B. A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch. Biochem. Biophys. 2016, 611, 100–112. [Google Scholar] [CrossRef]
- Portbury, S.D.; Adlard, P.A. Zinc Signal in Brain Diseases. Int. J. Mol. Sci. 2017, 18, 2506. [Google Scholar] [CrossRef]
- Bu, S.; Lv, Y.; Liu, Y.; Qiao, S.; Wang, H. Zinc Finger Proteins in Neuro-Related Diseases Progression. Front. Neurosci. 2021, 15, 760567. [Google Scholar] [CrossRef]
- Mammadova-Bach, E.; Braun, A. Zinc Homeostasis in Platelet-Related Diseases. Int. J. Mol. Sci. 2019, 20, 5258. [Google Scholar] [CrossRef] [PubMed]
- Kefauver, J.M.; Ward, A.B.; Patapoutian, A. Discoveries in structure and physiology of mechanically activated ion channels. Nature 2020, 587, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.L.; Clark, A.J.; Huang, J.; Waxman, S.G.; Dib-Hajj, S.D. The Role of Voltage-Gated Sodium Channels in Pain Signaling. Physiol. Rev. 2019, 99, 079–1151. [Google Scholar] [CrossRef]
- Peralta, F.A.; Huidobro-Toro, J.P. Zinc as Allosteric Ion Channel Modulator: Ionotropic Receptors as Metalloproteins. Int. J. Mol. Sci. 2016, 17, 1059. [Google Scholar] [CrossRef]
- Noh, S.; Lee, S.R.; Jeong, Y.J.; Ko, K.S.; Rhee, B.D.; Kim, N.; Han, J. The direct modulatory activity of zinc toward ion channels. Integr. Med. Res. 2015, 4, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Q.; Purhonen, P.; Hebert, H. Structure of potassium channels. Cell. Mol. Life Sci. 2015, 72, 3677–3693. [Google Scholar] [CrossRef]
- Niday, Z.; Tzingounis, A.V. Potassium Channel Gain of Function in Epilepsy: An Unresolved Paradox. Neuroscientist 2018, 24, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wan, J.Q.; Tong, X.P. Potassium channel dysfunction in neurons and astrocytes in Huntington’s disease. CNS Neurosci. Ther. 2018, 24, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Wie, J.; Liu, Z.; Song, H.; Tropea, T.F.; Yang, L.; Wang, H.; Liang, Y.; Cang, C.; Aranda, K.; Lohmann, J.; et al. A growth-factor-activated lysosomal K+ channel regulates Parkinson’s pathology. Nature 2021, 591, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Nguyen, H.M.; Malovic, E.; Luo, J.; Langley, M.; Palanisamy, B.N.; Singh, N.; Manne, S.; Neal, M.; Gabrielle, M.; et al. Kv1.3 modulates neuroinflammation and neurodegeneration in Parkinson’s disease. J. Clin. Investig. 2020, 130, 4195–4212. [Google Scholar] [CrossRef] [PubMed]
- Hering, S.; Zangerl-Plessl, E.M.; Beyl, S.; Hohaus, A.; Andranovits, S.; Timin, E.N. Calcium channel gating. Pflug. Arch. 2018, 470, 1291–1309. [Google Scholar] [CrossRef]
- Nanou, E.; Catterall, W.A. Calcium Channels, Synaptic Plasticity, and Neuropsychiatric Disease. Neuron 2018, 98, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, B.S. Glutamate as a neurotransmitter in the brain: Review of physiology and pathology. J. Nutr. 2000, 130, 1007S–1015S. [Google Scholar] [CrossRef]
- Cuestas Torres, D.M.; Cardenas, F.P. Synaptic plasticity in Alzheimer’s disease and healthy aging. Rev. Neurosci. 2020, 31, 245–268. [Google Scholar] [CrossRef]
- Nakazawa, K.; Sapkota, K. The origin of NMDA receptor hypofunction in schizophrenia. Pharmacol. Ther. 2020, 205, 107426. [Google Scholar] [CrossRef] [PubMed]
- Babaei, P. NMDA and AMPA receptors dysregulation in Alzheimer’s disease. Eur. J. Pharmacol. 2021, 908, 174310. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Hughes, T.E.T.; Moiseenkova-Bell, V.Y. Transient Receptor Potential (TRP) Channels. Subcell Biochem. 2018, 87, 141–165. [Google Scholar] [PubMed]
- Zhu, S.; Noviello, C.M.; Teng, J.; Walsh, R.M., Jr.; Kim, J.J.; Hibbs, R.E. Structure of a human synaptic GABAA receptor. Nature 2018, 559, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.P.; Wemmie, J.A.; Wang, W.Z.; Zhu, X.M.; Saugstad, J.A.; Price, M.P.; Simon, R.P.; Xiong, Z.G. Subunit-Dependent High-Affinity Zinc Inhibition of Acid-Sensing Ion Channels. J. Neurosci. 2004, 24, 8678–8689. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Chien, Y.C.; Chiang, W.W.; Liu, Y.Z.; Lien, C.C.; Chen, C.C. Genetic mapping of ASIC4 and contrasting phenotype to ASIC1a in modulating innate fear and anxiety. Eur. J. Neurosci. 2015, 41, 1553–1568. [Google Scholar] [CrossRef]
- Sun, C.; Wang, S.; Hu, W. Acid-sensing ion channel 1a mediates acid-induced inhibition of matrix metabolism of rat articular chondrocytes via the MAPK signaling pathway. Mol. Cell. Biochem. 2018, 443, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Pignataro, G.; Simon, R.P.; Xiong, Z.G. Prolonged activation of ASIC1a and the time window for neuroprotection in cerebral ischaemia. Brain A J. Neurol. 2007, 130, 151158. [Google Scholar] [CrossRef]
- Sherwood, T.W.; Lee, K.G.; Gormley, M.G.; Askwith, C.C. Heteromeric acid-sensing ion channels (ASICs) composed of ASIC2b and ASIC1a display novel channel properties and contribute to acidosis-induced neuronal death. J. Neurosci. 2011, 31, 9723–9734. [Google Scholar] [CrossRef]
- Jiang, Q.; Inoue, K.; Wu, X.; Papasian, C.J.; Wang, J.Q.; Xiong, Z.G.; Chu, X.P. Cysteine 149 in the extracellular finger domain of acid-sensing ion channel 1b subunit is critical for zinc-mediated inhibition. Neuroscience 2011, 193, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Papasian, C.J.; Wang, J.Q.; Xiong, Z.G.; Chu, X.P. Inhibitory regulation of acid-sensing ion channel 3 by zinc. Neuroscience 2010, 169, 574–583. [Google Scholar] [CrossRef]
- Zaremba, M.; Ruiz-Velasco, V. Opioid-mediated modulation of acid-sensing ion channel currents in adult rat sensory neurons. Mol. Pharmacol. 2019, 95, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Chen, C.C. Roles of ASICs in nociception and proprioception. Adv. Exp. Med. Biol. 2018, 1099, 37–47. [Google Scholar]
- Chen, X.; Kalbacher, H.; Grunder, S. The tarantula toxin psalmotoxin 1 inhibits acid-sensing ion channel (ASIC) 1a by increasing its apparent H+ affinity. J. Gen. Physiol. 2005, 126, 71–79. [Google Scholar] [CrossRef]
- Babinski, K.; Catarsi, S.; Biagini, G.; Séguéla, P. Mammalian ASIC2a and ASIC3 subunits co-assemble into heteromeric proton-gated channels sensitive to Gd3+. J. Biol. Chem. 2000, 275, 28519–28525. [Google Scholar] [CrossRef]
- Kweon, H.J.; Cho, J.H.; Jang, I.S.; Suh, B.C. ASIC2a-dependent increase of ASIC3 surface expression enhances the sustained component of the currents. BMB Rep. 2016, 49, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Heusser, S.A.; Pless, S.A. Acid-sensing ion channels as potential therapeutic targets. Trends Pharmacol. Sci. 2021, 42, 1035–1050. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gao, G.; Zhang, Y.; Sun, Y.; Li, H.; Dong, S.; Ma, W.; Liu, B.; Wang, W.; Wu, H.; et al. Glucose Deficiency Elevates Acid-Sensing Ion Channel 2a Expression and Increases Seizure Susceptibility in Temporal Lobe Epilepsy. Sci. Rep. 2017, 7, 5870. [Google Scholar] [CrossRef]
- Yagi, J.; Wenk, H.N.; Naves, L.A.; McCleskey, E.W. Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ. Res. 2006, 99, 501–509. [Google Scholar] [CrossRef]
- González-Garrido, A.; Vega, R.; Mercado, F.; López, I.A.; Soto, E. Acid-Sensing Ion Channels Expression, Identity and Role in the Excitability of the Cochlear Afferent Neurons. Front. Cell. Neurosci. 2015, 9, 483. [Google Scholar] [CrossRef]
- Goldie, I.; Nachemson, A. Synovial pH in rheumatoid knee-joints I. The effect of synovectomy. Acta Orthop. 1969, 40, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Decker, Y.; Németh, E.; Schomburg, R.; Chemla, A.; Fülöp, L.; Menger, M.D.; Liu, Y.; Fassbender, K. Decreased pH in the aging brain and Alzheimer’s disease. Neurobiol. Aging 2021, 101, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Dulai, J.S.; Smith, E.S.J.; Rahman, T. Acid-sensing ion channel 3: An analgesic target. Channels 2021, 15, 94–127. [Google Scholar] [CrossRef]
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J., Jr.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE official report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef]
- Amar, M.; Perin-Dureau, F.; Neyton, J. High-affinity Zn block in recombinant N-methyl-D-aspartate receptors with cysteine substitutions at the Q/R/N site. Biophys. J. 2001, 81, 107–116. [Google Scholar] [CrossRef]
- Hey, J.G.; Chu, X.P.; Seeds, J.; Simon, R.P.; Xiong, Z.G. Extracellular zinc protects against acidosis-induced injury of cells expressing Ca2+-permeable acid-sensing ion channels. Stroke 2007, 38 (Suppl. 2), 670–673. [Google Scholar] [CrossRef]
- Koh, J.Y.; Choi, D.W. Zinc toxicity on cultured cortical neurons: Involvement of N-methyl-d-aspartate receptors. Neuroscience 1994, 60, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef]
- Azoulay, I.S.; Qi, X.; Rozenfeld, M.; Liu, F.; Hu, Q.; Ben-Kasus Nissim, T.; Stavsky, A.; Zhu, M.X.; Xu, T.L.; Sekler, I. ASIC1a senses lactate uptake to regulate metabolism in neurons. Redox Biol. 2022, 51, 102253. [Google Scholar] [CrossRef]
- Verkest, C.; Piquet, E.; Diochot, S.; Dauvois, M.; Lanteri-Minet, M.; Lingueglia, E.; Baron, A. Effects of systemic inhibitors of acid-sensing ion channels 1 (ASIC1) against acute and chronic mechanical allodynia in a rodent model of migraine. Br. J. Pharmacol. 2018, 175, 4154–4166. [Google Scholar] [CrossRef]
- Ahmadi, H.; Mazloumi-Kiapey, S.S.; Sadeghi, O.; Nasiri, M.; Khorvash, F.; Mottaghi, T.; Askari, G. Zinc supplementation affects favorably the frequency of migraine attacks: A double-blind randomized placebo-controlled clinical trial. Nutr. J. 2020, 19, 101. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, M.; Aghdam, A.M.; Heidari, M.; Zarrin, R. Assessing the Effect of Zinc Supplementation on the Frequency of Migraine Attack, Duration, Severity, Lipid Profile and hs-CRP in Adult Women. Clin. Nutr. Res. 2021, 10, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Schytz, H.W.; Hargreaves, R. Ashina, M. Challenges in developing drugs for primary headaches. Prog. Neurobiol. 2017, 152, 70–88. [Google Scholar] [CrossRef] [PubMed]
- Lipton, R.B.; Munjal, S.; Buse, D.C.; Bennett, A.; Fanning, K.M.; Burstein, R.; Reed, M.L. Allodynia Is Associated With Initial and Sustained Response to Acute Migraine Treatment: Results from the American Migraine Prevalence and Prevention Study. Headache 2017, 57, 1026–1040. [Google Scholar] [CrossRef]
- Chapman, P.F.; White, G.L.; Jones, M.W.; Cooper-Blacketer, D.; Marshall, V.J.; Irizarry, M.; Younkin, L.; Good, M.A.; Bliss, T.V.; Hyman, B.T.; et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat. Neurosci. 1999, 2, 271–276. [Google Scholar] [CrossRef]
- Mango, D.; Nisticò, R. Acid-Sensing ion channel 1a is involved in N-Methyl D-aspartate receptor-dependent long-term depression in the hippocampus. Front. Pharmacol. 2019, 10, 555. [Google Scholar] [CrossRef]
- Bush, A.I.; Pettingell, W.H.; Multhaup, G.; Paradis, M.; Vonsattel, J.P.; Gusella, J.F.; Beyreuther, K.; Masters, C.L.; Tanzi, R.E. Rapid induction of Alzheimer A beta amyloid formation by zinc. Science 1994, 265, 1464–1467. [Google Scholar] [CrossRef]
- Lashley, T.; Schott, J.M.; Weston, P.; Murray, C.E.; Wellington, H.; Keshavan, A.; Foti, S.C.; Foiani, M.; Toombs, J.; Rohrer, J.D.; et al. Molecular biomarkers of Alzheimer’s disease: Progress and prospects. Dis. Model. Mech. 2018, 11, dmm031781. [Google Scholar] [CrossRef]
- Lee, M.C.; Yu, W.C.; Shih, Y.H.; Chen, C.Y.; Guo, Z.H.; Huang, S.J.; Chan, J.C.C.; Chen, Y.R. Zinc ion rapidly induces toxic, off-pathway amyloid-beta oligomers distinct from amyloid-beta derived diffusible ligands in Alzheimer’s disease. Sci. Rep. 2018, 8, 4772. [Google Scholar] [CrossRef]
- James, S.A.; Churches, Q.I.; de Jonge, M.D.; Birchall, I.E.; Streltsov, V.; McColl, G.; Adlard, P.A.; Hare, D.J. Iron, copper, and zinc concentration in abeta plaques in the APP/PS1 mouse model of Alzheimer’s disease correlates with metal levels in the surrounding neuropil. ACS Chem. Neurosci. 2017, 8, 629–637. [Google Scholar] [CrossRef]
- Ayton, S.; Wang, Y.; Diouf, I.; Schneider, J.A.; Brockman, J.; Morris, M.C.; Bush, A.I. Brain iron is associated with accelerated cognitive decline in people with Alzheimer pathology. Mol. Psychiatry 2019, 45, 358–367. [Google Scholar] [CrossRef]
- Datki, Z.; Galik-Olah, Z.; Janosi-Mozes, E.; Szegedi, V.; Kalman, J.; Hunya, A.G.; Fulop, L.; Tamano, H.; Takeda, A.; Adlard, P.A.; et al. Alzheimer risk factors age and female sex induce cortical Aβ aggregation by raising extracellular zinc. Mol. Psychiatry 2020, 25, 2728–2741. [Google Scholar] [CrossRef]
- Kepp, K.P. Alzheimer’s disease: How metal ions define β-amyloid function. Coord. Chem. Rev. 2017, 351, 127–157. [Google Scholar] [CrossRef]
- Zhang, H.L.; Wang, X.C.; Liu, R. Zinc in Regulating Protein Kinases and Phosphatases in Neurodegenerative Diseases. Biomolecules 2022, 12, 785. [Google Scholar] [CrossRef] [PubMed]
- Squitti, R.; Pal, A.; Picozza, M.; Avan, A.; Ventriglia, M.; Rongioletti, M.C.; Hoogenraad, T. Zinc Therapy in Early Alzheimer’s Disease: Safety and Potential Therapeutic Efficacy. Biomolecules 2020, 10, 1164. [Google Scholar] [CrossRef]
- Gelders, G.; Baekelandt, V.; Van der Perren, A. Linking Neuroinflammation and Neurodegeneration in Parkinson’s Disease. J. Immunol. Res. 2018, 2018, 4784268. [Google Scholar] [CrossRef]
- Granzotto, A.; Sensi, S.L. Intracellular zinc is a critical intermediate in the excitotoxic cascade. Neurobiol. Dis. 2015, 81, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, P.; Di Filippo, M.; Gallina, A.; Wang, Y.; Stankowski, J.N.; Picconi, B.; Dawson, V.L.; Dawson, T.M. New synaptic and molecular targets for neuroprotection in Parkinson’s disease. Mov. Disord. 2013, 28, 51–60. [Google Scholar] [CrossRef]
- Ahmed, S.S.; Santosh, W. Metallomic profiling and linkage map analysis of early Parkinson’s disease: A new insight to aluminum marker for the possible diagnosis. PLoS ONE 2010, 5, e11252. [Google Scholar] [CrossRef]
- Hegde, M.L.; Shanmugavelu, P.; Vengamma, B.; Rao, T.S.; Menon, R.B.; Rao, R.V.; Rao, K.S. Serum trace element levels and the complexity of inter-element relations in patients with Parkinson’s disease. J. Trace Elem. Med. Biol. 2004, 18, 163–171. [Google Scholar] [CrossRef]
- Ajjimaporn, A.; Phansuwan-Pujito, P.; Ebadi, M.; Govitrapong, P. Zinc protects SK-N-SH cells from methamphetamine-induced alpha-synuclein expression. Neurosci. Lett. 2007, 419, 59–63. [Google Scholar] [CrossRef]
- Ajjimaporn, A.; Shavali, S.; Ebadi, M.; Govitrapong, P. Zinc rescues dopaminergic SK-N-SH cell lines from methamphetamine-induced toxicity. Brain Res. Bull. 2008, 77, 361–366. [Google Scholar] [CrossRef]
- Hussain, S.; Ali, S.F. Zinc potentiates 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced dopamine depletion in caudate nucleus of mice brain. Neurosci. Lett. 2002, 335, 25–28. [Google Scholar] [CrossRef]
- Raj, K.; Kaur, P.; Gupta, G.D.; Singh, S. Metals associated neurodegeneration in Parkinson’s disease: Insight to physiological, pathological mechanisms and management. Neurosci. Lett. 2021, 753, 135383. [Google Scholar] [CrossRef]
- Kitada, T.; Asakawa, S.; Hattori, N.; Matsumine, H.; Yamamura, Y.; Minoshima, S.; Yokochi, M.; Mizuno, Y.; Shimizu, N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 1998, 392, 605–608. [Google Scholar] [CrossRef]
- Joch, M.; Ase, A.R.; Chen, C.X.; MacDonald, P.A.; Kontogiannea, M.; Corera, A.T.; Brice, A.; Séguéla, P.; Fon, E.A. Parkin-mediated monoubiquitination of the PDZ protein PICK1 regulates the activity of acid-sensing ion channels. Mol. Biol. Cell 2007, 18, 3105–3118. [Google Scholar] [CrossRef]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]
- Siodłak, D.; Nowak, G.; Mlyniec, K. Interaction between zinc, the GPR39 zinc receptor and the serotonergic system in depression. Brain Res. Bull. 2021, 170, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, G.L.; Mayer, M.L. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature 1987, 328, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Assaf, S.Y.; Chung, S.H. Release of endogenous Zn2+ from brain tissue during activity. Nature 1984, 308, 734–736. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Zinc deficiency. Bmj 2003, 326, 409–410. [Google Scholar] [CrossRef] [PubMed]
- Jokinen, J.; Nordström, P. HPA axis hyperactivity and attempted suicide in young adult mood disorder inpatients. J. Affect. Disord. 2009, 116, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Młyniec, K.; Gaweł, M.; Librowski, T.; Reczyński, W.; Bystrowska, B.; Holst, B. Investigation of the GPR39 zinc receptor following inhibition of monoaminergic neurotransmission and potentialization of glutamatergic neurotransmission. Brain Res. Bull. 2015, 115, 23–29. [Google Scholar] [CrossRef]
- Satała, G.; Duszyńska, B.; Lenda, T.; Nowak, G.; Bojarski, A.J. Allosteric Inhibition of Serotonin 5-HT7 Receptors by Zinc Ions. Mol. Neurobiol. 2018, 55, 2897–2910. [Google Scholar] [CrossRef]
- Mo, F.; Tang, Y.; Du, P.; Shen, Z.; Yang, J.; Cai, M.; Zhang, Y.; Li, H.; Shen, H. GPR39 protects against corticosterone-induced neuronal injury in hippocampal cells through the CREB-BDNF signaling pathway. J. Affect. Disord. 2020, 272, 474–484. [Google Scholar] [CrossRef]
- Suh, S.W.; Won, S.J.; Hamby, A.M.; Yoo, B.H.; Fan, Y.; Sheline, C.T.; Tamano, H.; Takeda, A.; Liu, J. Decreased brain zinc availability reduces hippocampal neurogenesis in mice and rats. J. Cereb. Blood Flow Metab. 2009, 29, 1579–1588. [Google Scholar] [CrossRef]
- Wang, J.; Um, P.; Dickerman, B.A.; Liu, J. Zinc, Magnesium, Selenium and Depression: A Review of the Evidence, Potential Mechanisms and Implications. Nutrients 2018, 10, 584. [Google Scholar] [CrossRef]
- Malykhin, N.; Coupland, N. Hippocampal neuroplasticity in major depressive disorder. Neuroscience 2015, 309, 200–213. [Google Scholar] [CrossRef]
- Taugher, R.J.; Lu, Y.; Fan, R.; Ghobbeh, A.; Kreple, C.J.; Faraci, F.M.; Wemmie, J.A. ASIC1A in neurons is critical for fear-related behaviors. Genes Brain Behav. 2017, 16, 745–755. [Google Scholar] [CrossRef]
- Coryell, M.W.; Wunsch, A.M.; Haenfler, J.M.; Allen, J.E.; Schnizler, M.; Ziemann, A.E.; Cook, M.N.; Dunning, J.P.; Price, M.P.; Rainier, J.D.; et al. Acid-sensing ion channel-1a in the amygdala, a novel therapeutic target in depression-related behavior. J. Neurosci. 2009, 29, 5381–5388. [Google Scholar] [CrossRef] [PubMed]
- Wemmie, J.A.; Askwith, C.C.; Lamani, E.; Cassell, M.D.; Freeman, J.H., Jr.; Welsh, M.J. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J. Neurosci. 2003, 23, 5496–5502. [Google Scholar] [CrossRef]
- Zhu, H.; Hu, S.; Li, Y.; Sun, Y.; Xiong, X.; Hu, X.; Chen, J.; Qiu, S. Interleukins and Ischemic Stroke. Front. Immunol. 2022, 13, 828447. [Google Scholar] [CrossRef] [PubMed]
- Maida, C.D.; Norrito, R.L.; Daidone, M.; Tuttolomondo, A.; Pinto, A. Neuroinflammatory Mechanisms in Ischemic Stroke: Focus on Cardioembolic Stroke, Background, and Therapeutic Approaches. Int. J. Mol. Sci. 2020, 21, 6454. [Google Scholar] [CrossRef]
- Mammadova-Bach, E.; Nagy, M.; Heemskerk, J.W.M.; Nieswandt, B.; Braun, A. Store-operated calcium entry in thrombosis and thrombo-inflammation. Cell Calcium 2019, 77, 39–48. [Google Scholar] [CrossRef]
- Lee, J.M.; Zipfel, G.J.; Park, K.H.; He, Y.Y.; Hsu, C.Y.; Choi, D.W. Zinc translocation accelerates infarction after mild transient focal ischemia. Neuroscience 2002, 115, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Helal, G.K. Systemic administration of Zn2+ during the reperfusion phase of transient cerebral ischaemia protects rat hippocampus against iron-catalysed postischaemic injury. Clin. Exp. Pharmacol. Physiol. 2008, 35, 775–781. [Google Scholar] [CrossRef]
- Aquilani, R.; Baiardi, P.; Scocchi, M.; Iadarola, P.; Verri, M.; Sessarego, P.; Boschi, F.; Pasini, E.; Pastoris, O.; Viglio, S. Normalization of zinc intake enhances neurological retrieval of patients suffering from ischemic strokes. Nutr. Neurosci. 2009, 12, 219–225. [Google Scholar] [CrossRef]
- Xiong, Z.G. Neuroprotection in ischemia: Blocking calcium-permeable acid-sensing ion channels. Cell 2004, 118, 687–698. [Google Scholar] [CrossRef]
- O’Bryant, Z.; Vann, K.T.; Xiong, Z.G. Translational strategies for neuroprotection in ischemic stroke--focusing on acid-sensing ion channel 1a. Transl. Stroke Res. 2014, 5, 59–68. [Google Scholar] [CrossRef]
- Gao, J.; Duan, B.; Wang, D.G.; Deng, X.H.; Zhang, G.Y.; Xu, L.; Xu, T.L. Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron 2005, 48, 635–646. [Google Scholar] [CrossRef]
- Gutman, A.L.; Cosme, C.V.; Noterman, M.F.; Worth, W.R.; Wemmie, J.A.; LaLumiere, R.T. Overexpression of ASIC1A in the nucleus accumbens of rats potentiates cocaine-seeking behavior. Addict. Biol. 2020, 25, e12690. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.L.; Bonaventura, J.; Keighron, J.; Wright, K.M.; Marable, D.L.; Rodriguez, L.A.; Lam, S.; Carlton, M.L.; Ellis, R.J.; Jordan, C.J.; et al. Synaptic Zn2+ potentiates the effects of cocaine on striatal dopamine neurotransmission and behavior. Transl. Psychiatry 2021, 11, 570. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Li, M.H.; Papasian, C.J.; Branigan, D.; Xiong, Z.G.; Wang, J.Q.; Chu, X.P. Characterization of acid-sensing ion channels in medium spiny neurons of mouse striatum. Neuroscience 2009, 162, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Kreple, C.J.; Lu, Y.; LaLumiere, R.T.; Wemmie, J.A. Drug abuse and the simplest neurotransmitter. ACS Chem. Neurosci. 2014, 5, 746–748. [Google Scholar] [CrossRef]
- Hennigar, S.R.; Kelley, A.M.; Anderson, B.J.; Armstrong, N.J.; McClung, H.L.; Berryman, C.E.; Karl, J.P.; McClung, J.P. Sensitivity and reliability of zinc transporter and metallothionein gene expression in peripheral blood mononuclear cells as indicators of zinc status: Responses to ex vivo zinc exposure and habitual zinc intake in humans. Br. J. Nutr. 2021, 125, 361–368. [Google Scholar] [CrossRef]
- Dursun, N.; Erenmemisoglu, A.; Cem, S.; Gogusten, B. The effect of zinc deficiency on morphine antinociception. Res. Commun. Alcohol. Subst. Abus. 1995, 16, 47–52. [Google Scholar]
- Malcangio, M. Role of the immune system in neuropathic pain. Scand. J. Pain 2019, 20, 33–37. [Google Scholar] [CrossRef]
- Li, L.; Chen, C.; Chiang, C.; Xiao, T.; Chen, Y.; Zhao, Y.; Zheng, D. The Impact of TRPV1 on Cancer Pathogenesis and Therapy: A Systematic Review. Int. J. Biol. Sci. 2021, 17, 2034–2049. [Google Scholar] [CrossRef]
- Safieh-Garabedian, B.; Poole, S.; Allchorne, A.; Kanaan, S.; Saade, N.; Woolf, C.J. Zinc reduces the hyperalgesia and upregulation of NGF and IL-1 beta produced by peripheral inflammation in the rat. Neuropharmacology 1996, 35, 599–603. [Google Scholar] [CrossRef]
- Larson, A.A.; Kitto, K.F. Manipulations of zinc in the spinal cord, by intrathecal injection of zinc chloride, disodium-calcium-EDTA, or dipicolinic acid, alter nociceptive activity in mice. J. Pharmacol. Exp. Ther. 1997, 282, 1319–1325. [Google Scholar] [PubMed]
- Chang, C.T.; Fong, S.W.; Lee, C.H.; Chuang, Y.C.; Lin, S.H.; Chen, C.C. Involvement of Acid-Sensing Ion Channel 1b in the Development of Acid-Induced Chronic Muscle Pain. Front. Neurosci. 2019, 13, 1247. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Bavencoffe, A.; Yang, P.; Feng, J.; Yin, S.; Qian, A.; Yu, W.; Liu, S.; Gong, X.; Cai, T.; et al. Zinc Inhibits TRPV1 to Alleviate Chemotherapy-Induced Neuropathic Pain. J. Neurosci. 2018, 38, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.M.; Danscher, G.; Schrøder, H.D.; Suh, S.W. Depletion of vesicular zinc in dorsal horn of spinal cord causes increased neuropathic pain in mice. Biometals 2008, 21, 151–158. [Google Scholar] [CrossRef]
- Hoagland, E.N.; Sherwood, T.W.; Lee, K.G.; Walker, C.J.; Askwith, C.C. Identification of a calcium permeable human acid-sensing ion channel 1 transcript variant. J. Biol. Chem. 2010, 285, 41852–41862. [Google Scholar] [CrossRef]
- Dubé, G.R.; Lehto, S.G.; Breese, N.M.; Baker, S.J.; Wang, X.; Matulenko, M.A.; Honoré, P.; Stewart, A.O.; Moreland, R.B.; Brioni, J.D. Electrophysiological and in vivo characterization of A-317567, a novel blocker of acid sensing ion channels. Pain 2005, 117, 88–96. [Google Scholar] [CrossRef]
- Gautam, M.; Benson, C.J. Acid-sensing ion channels (ASICs) in mouse skeletal muscle afferents are heteromers composed of ASIC1a, ASIC2, and ASIC3 subunits. FASEB J. 2013, 27, 793–802. [Google Scholar] [CrossRef]
- Gregory, N.S.; Gautam, M.; Benson, C.J.; Sluka, K.A. Acid Sensing Ion Channel 1a (ASIC1a) Mediates Activity-induced Pain by Modulation of Heteromeric ASIC Channel Kinetics. Neuroscience 2018, 386, 166–174. [Google Scholar] [CrossRef]
- Ugarte, M.; Osborne, N.N.; Brown, L.A.; Bishop, P.N. Iron, zinc, and copper in retinal physiology and disease. Surv. Ophthalmol. 2013, 58, 585–609. [Google Scholar] [CrossRef]
- Gilbert, R.; Peto, T.; Lengyel, I.; Emri, E. Zinc Nutrition and Inflammation in the Aging Retina. Mol. Nutr. Food Res. 2019, 63, 15. [Google Scholar] [CrossRef]
- Blasiak, J.; Pawlowska, E.; Chojnacki, J.; Szczepanska, J.; Chojnacki, C.; Kaarniranta, K. Zinc and Autophagy in Age-Related Macular Degeneration. Int. J. Mol. Sci. 2020, 21, 4994. [Google Scholar] [CrossRef]
- Evans, J.R.; Lawrenson, J.G. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst. Rev. 2017, 7, CD000254. [Google Scholar] [CrossRef]
- Márquez, A.G.; Salazar, V.; Lima, L.P. Consequences of zinc deficiency on zinc localization, taurine transport, and zinc transporters in rat retina. Microsc. Res. Tech. 2022, 85, 3382–3390. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.J.; Duan, B.; Mei, Y.D.; Gao, J.; Chen, J.G.; Zhuo, M.; Xu, L.; Wu, M.; Xu, T.L. Characterization of acid-sensing ion channels in dorsal horn neurons of rat spinal cord. J. Biol. Chem. 2004, 279, 43716–43724. [Google Scholar] [CrossRef]
- Papalampropoulou-Tsiridou, M.; Labrecque, S.; Godin, A.G.; De Koninck, Y.; Wang, F. Differential Expression of Acid—Sensing Ion Channels in Mouse Primary Afferents in Naïve and Injured Conditions. Front. Cell. Neurosci. 2020, 14, 103. [Google Scholar] [CrossRef]
- Zha, X.M.; Costa, V.; Harding, A.M.S.; Reznikov, L.; Benson, C.J.; Welsh, M.J. ASIC2 Subunits target acid-sensing ion channels to the synapse via an association with PSD-95. J. Neurosci. 2009, 29, 8438–8446. [Google Scholar] [CrossRef]
- Jiang, N.; Wu, J.; Leng, T.; Yang, T.; Zhou, Y.; Jiang, Q.; Wang, B.; Hu, Y.; Ji, Y.; Simon, R.P.; et al. Region specific contribution of ASIC2 to acidosis-and ischemia-induced neuronal injury. J. Cereb. Blood Flow Metab. 2017, 37, 528–540. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, N.; Li, J.; Ji, Y.H.; Xiong, Z.G.; Zha, X.M. Two aspects of ASIC function: Synaptic plasticity and neuronal injury. Neuropharmacology. 2015, 94, 42–48. [Google Scholar] [CrossRef]
- Bartoi, T.; Augustinowski, K.; Polleichtner, G.; Gründer, S.; Ulbrich, M.H. Acid-sensing ion channel (ASIC) 1a/2a heteromers have a flexible 2:1/1:2 stoichiometry. Proc. Natl. Acad. Sci. USA 2014, 111, 8281–8286. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Liu, S.Q.; Inoue, K.; Lan, J.; Simon, R.P.; Xiong, Z.G. Acid-sensing ion channels in mouse olfactory bulb M/T neurons. J. Gen. Physiol. 2014, 143, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Saini, N.; Schaffner, W. Zinc supplement greatly improves the condition of parkin mutant Drosophila. Biol. Chem. 2010, 391, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Askwith, C.C.; Wemmie, J.A.; Price, M.P.; Rokhlina, T.; Welsh, M.J. Acid-sensing ion channel 2 (ASIC2) modulates ASIC1 H+-activated currents in hippocampal neurons. J. Biol. Chem. 2004, 279, 18296–18305. [Google Scholar] [CrossRef] [PubMed]
- Ettaiche, M.; Guy, N.; Hofman, P.; Lazdunski, M.; Waldmann, R. Acid-sensing ion channel 2 is important for retinal function and protects against light-induced retinal degeneration. J. Neurosci. 2004, 24, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhuo, Y.; Li, Y. Effects of Iron and Zinc on Mitochondria: Potential Mechanisms of Glaucomatous Injury. Front. Cell. Dev. Biol. 2021, 9, 720288. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Andereggen, L.; Yuki, K.; Omura, K.; Yin, Y.; Gilbert, H.Y.; Erdogan, B.; Asdourian, M.S.; Shrock, C.; de Lima, S.; et al. Mobile zinc increases rapidly in the retina after optic nerve injury and regulates ganglion cell survival and optic nerve regeneration. Proc. Natl. Acad. Sci. USA 2017, 114, E209–E218. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Chen, J.; Harding, A.M.; Price, M.P.; Lu, Y.; Abboud, F.M.; Benson, C.J. ASIC2a and ASIC3 heteromultimerize to form pH-sensitive channels in mouse cardiac dorsal root ganglia neurons. Circ. Res. 2009, 105, 279–286. [Google Scholar] [CrossRef]
- Choi, S.; Liu, X.; Pan, Z. Zinc deficiency and cellular oxidative stress: Prognostic implications in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1120–1132. [Google Scholar] [CrossRef]
- Li, L.; Niu, P.; Wang, X.; Bing, F.; Tan, W.; Huo, Y. Short-Term Inhalation of Ultrafine Zinc Particles Could Alleviate Cardiac Dysfunctions in Rats of Myocardial Infarction. Front. Bioeng. Biotechnol. 2021, 9, 646533. [Google Scholar] [CrossRef]
- Gonçalves, A.F.; Polegato, B.F.; Fernandes, A.A.; Ishikawa, L.L.; Okoshi, K.; Bazan, S.G.Z.; Minicucci, M.F.; Azevedo, P.S.; Ikoma, M.R.; Penitenti, M.; et al. Zinc Supplementation Attenuates Cardiac Remodeling After Experimental Myocardial Infarction. Cell. Physiol. Biochem. 2018, 50, 353–362. [Google Scholar] [CrossRef]
- Abdel-Hady, E.; Mohamed, F.; Ahmed, M.; Abdel-Salam, M.; Ayobe, M. Supplementation of Lipoic Acid, Zinc and Clopidogrel Reduces Mortality Rate and Incidence of Ventricular Arrhythmia in Experimental Myocardial Infarction. Front Physiol. 2021, 12, 582223. [Google Scholar] [CrossRef]
- Wu, W.L.; Cheng, C.F.; Sun, W.H.; Wong, C.W.; Chen, C.C. Targeting ASIC3 for pain, anxiety, and insulin resistance. Pharmacol. Ther. 2012, 134, 127–138. [Google Scholar] [CrossRef]
- Karczewski, J.; Spencer, R.H.; Garsky, V.M.; Liang, A.; Leitl, M.D.; Cato, M.J.; Cook, S.P.; Kane, S.; Urban, M.O. Reversal of acid-induced and inflammatory pain by the selective ASIC3 inhibitor, APETx2. Br. J. Pharmacol. 2010, 161, 950–960. [Google Scholar] [CrossRef]
- Montalbetti, N.; Rooney, J.G.; Marciszyn, A.L.; Carattino, M.D. ASIC3 fine-tunes bladder sensory signaling. Am. J. Physiol. Renal. Physiol. 2018, 315, F870–F879. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.R.; Jiang, B.Y.; Chen, C.C. Acid-sensing ion channels: Dual function proteins for chemo-sensing and mechano-sensing. J. Biomed Sci. 2018, 25, 46. [Google Scholar] [CrossRef]
- Akbari, G. Role of Zinc Supplementation on Ischemia/Reperfusion Injury in Various Organs. Biol. Trace Elem. Res. 2020, 196, 1–9. [Google Scholar] [CrossRef]
- Chen, X.; Polleichtner, G.; Kadurin, I.; Gründer, S. Zebrafish acid-sensing ion channel (ASIC) 4, characterization of homo- and heteromeric channels, and identification of regions important for activation by H+. J. Biol. Chem. 2007, 282, 30406–30413. [Google Scholar] [CrossRef]
- Paukert, M.; Sidi, S.; Russell, C.; Siba, M.; Wilson, S.W.; Nicolson, T.; Gründer, S. A family of acid-sensing ion channels from the zebrafish: Widespread expression in the central nervous system suggests a conserved role in neuronal communication. J. Biol. Chem. 2004, 279, 18783–18791. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, C.M.T.; Diakov, A.; Haerteis, S.; Korbmacher, C.; Gründer, S.; Wiemuth, D. Pharmacological and electrophysiological characterization of the human bile acid-sensitive ion channel (hBASIC). Pflug. Arch.–Eur. J. Physiol. 2014, 466, 253–263. [Google Scholar] [CrossRef]
- Wiemuth, D.; Assmann, M.; Gründer, S. The bile acid-sensitive ion channel (BASIC), the ignored cousin of ASICs and ENaC. Channels 2014, 8, 29–34. [Google Scholar] [CrossRef]
- Kreko-Pierce, T.; Boiko, N.; Harbidge, D.G.; Marcus, D.C.; Stockand, J.D.; Pugh, J.R. Cerebellar Ataxia Caused by Type II Unipolar Brush Cell Dysfunction in the Asic5 Knockout Mouse. Sci. Rep. 2020, 10, 2168. [Google Scholar] [CrossRef] [PubMed]
| ASIC Subtype | Zinc Binding Site | Zinc Effect | EC50/IC50 | Binding State of Channel | References |
|---|---|---|---|---|---|
| ASIC1a | K133↑ | Inhibitory | IC50: 7.0 ± 0.35 nM | Closed | [46] |
| ASIC1b | C149↓ | Inhibitory | IC50: 36.5 ± 1.5 μM | Closed | [12,51] |
| ASIC1a/3 | H72↓, H73↓, H83↓ | Excitatory* [μM] Inhibitory# [1–250 μM] Excitatory# [> 250 μM] | EC50*: 26 µM EC50# [1–100 µM]: 24 µM EC50# [100–250 µM]: 128 µM IC50# [> 250 µM]: 206 µM | Closed1,2 Open1 | [4] |
| ASIC1a/2b | Unknown | Inhibitory | Unknown | Open | [50] |
| ASIC1a/2a | H339↓,1, K133↑,2 | Excitatory [μM] Inhibitory [1–250 μM] | EC50: 111 μM IC50: 10.04 ± 1.23 nM | Open | [18,46] |
| ASIC2a | H339↓, H162↓ | Excitatory | EC50: 120 μM | Open | [18] |
| ASIC2a/3 | H339↓, H162↓ | Excitatory | Unknown | Open | [18] |
| ASIC3 | Unknown↓ | Inhibitory | IC50: 61 ± 3.2 μM | Closed | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, A.W.; Wu, M.H.; Vijayalingam, M.; Wacker, M.J.; Chu, X.-P. The Role of Zinc in Modulating Acid-Sensing Ion Channel Function. Biomolecules 2023, 13, 229. https://doi.org/10.3390/biom13020229
Sun AW, Wu MH, Vijayalingam M, Wacker MJ, Chu X-P. The Role of Zinc in Modulating Acid-Sensing Ion Channel Function. Biomolecules. 2023; 13(2):229. https://doi.org/10.3390/biom13020229
Chicago/Turabian StyleSun, Amber W., Michelle H. Wu, Madhumathi Vijayalingam, Michael J. Wacker, and Xiang-Ping Chu. 2023. "The Role of Zinc in Modulating Acid-Sensing Ion Channel Function" Biomolecules 13, no. 2: 229. https://doi.org/10.3390/biom13020229
APA StyleSun, A. W., Wu, M. H., Vijayalingam, M., Wacker, M. J., & Chu, X.-P. (2023). The Role of Zinc in Modulating Acid-Sensing Ion Channel Function. Biomolecules, 13(2), 229. https://doi.org/10.3390/biom13020229









