Myostatin and the Heart
Abstract
1. Background
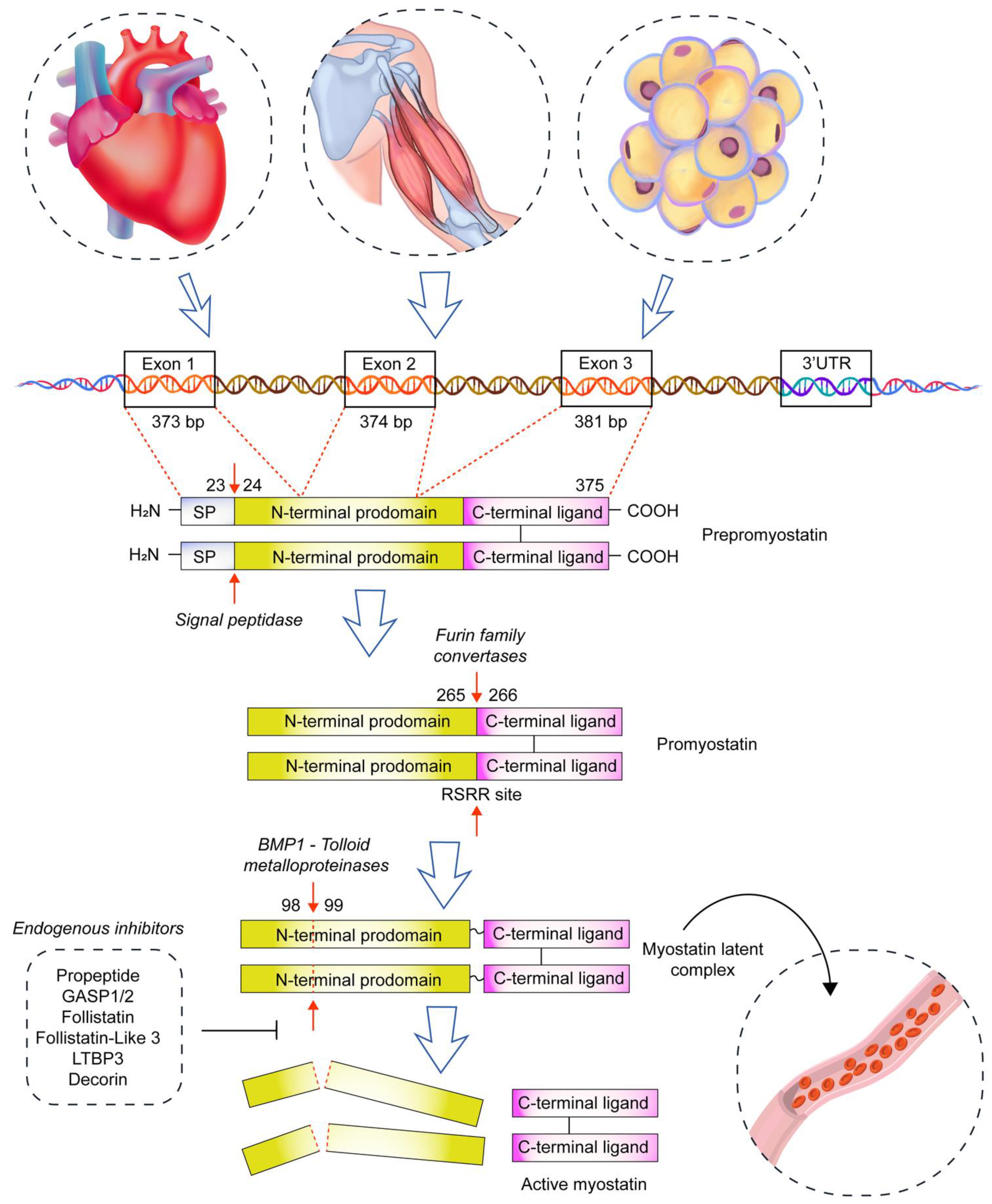
2. Myostatin and Skeletal Muscles
3. Myostatin and Heart Physiology
3.1. Myostatin and Heart Morphology
3.2. Regulation of Heart Myostatin Expression
3.3. Myostatin and Heart Function
3.4. Myostatin and Heart Metabolism
3.5. Myostatin and Heart Pathology
3.6. Myocardial Infarction
3.7. Myocardial Hypertrophy
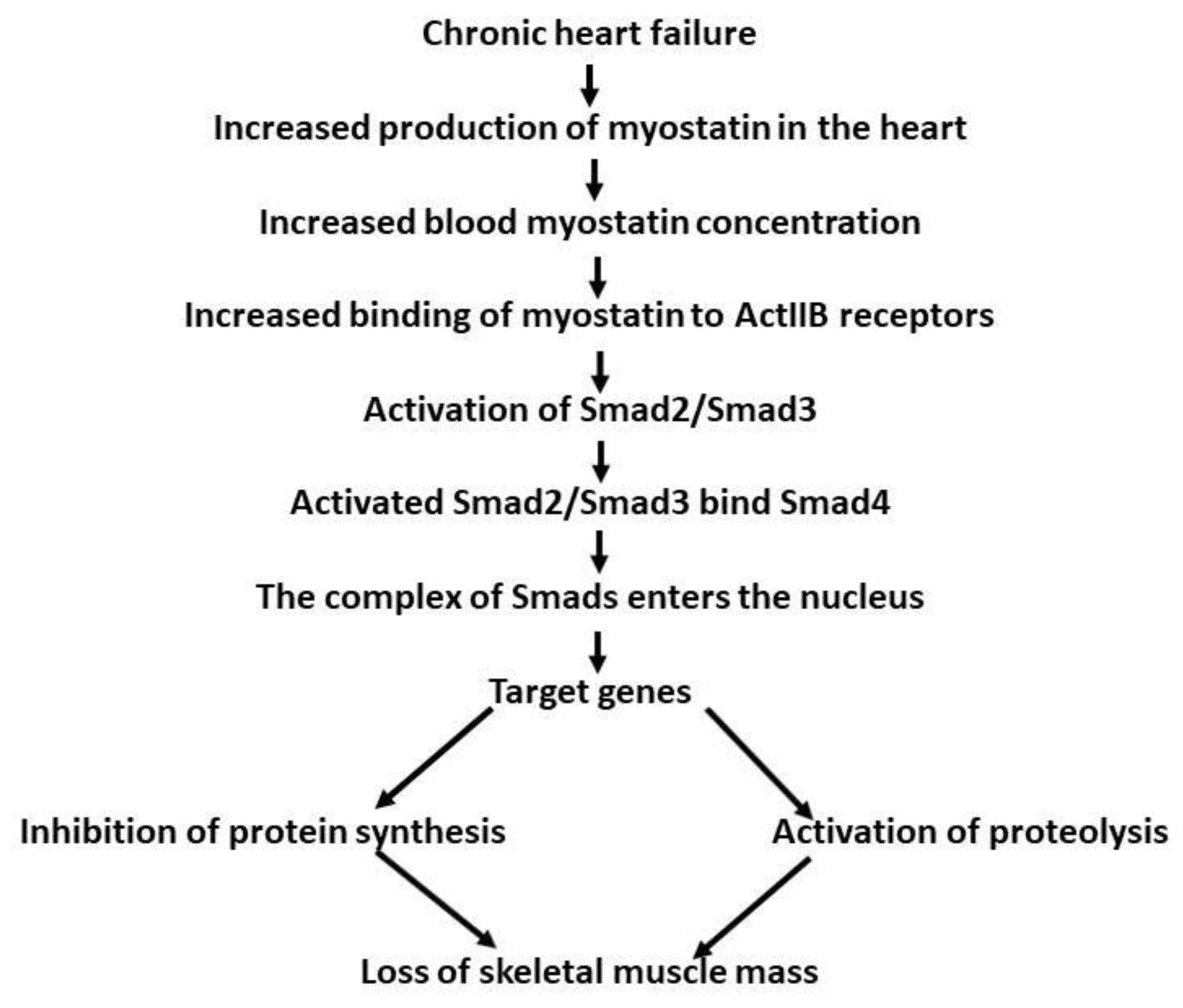
3.8. Chronic Heart Failure in Humans
3.9. Cardiac Cachexia
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Severinsen, M.C.K.; Pedersen, B.K. Muscle–Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, K.; Machida, T.; Hirafuji, M. Skeletal muscle is an endocrine organ. J. Pharmacol. Sci. 2014, 125, 125–131. [Google Scholar] [CrossRef] [PubMed]
- McPherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Kambadur, R.; Matthews, K.G.; Somers, W.G.; Devlin, G.P.; Conaglen, J.V.; Fowke, P.J.; Bass, J.J. Myostatin, a transforming growth factor-β superfamily member, is expressed in heart muscle and is upregulated in cardiomyocytes after infarct. J. Cell. Physiol. 1999, 180, 1–9. [Google Scholar] [CrossRef]
- Matsakas, A.; Bozzo, C.; Cacciani, N.; Caliaro, F.; Reggiani, C.; Mascarello, F.; Patruno, M. Effect of swimming on myostatin expression in white and red gastrocnemius muscle and in cardiac muscle of rats. Exp. Physiol. 2006, 91, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.H.; Ahmad, K.; Moon, J.S.; Park, S.-Y.; Ho Lim, J.; Chun, H.J.; Qadri, A.F.; Hwang, Y.C.; Jan, A.T.; Ahmad, S.S.; et al. Myostatin and its Regulation: A Comprehensive Review of Myostatin Inhibiting Strategies. Front. Physiol. 2022, 13, 876078. [Google Scholar] [CrossRef] [PubMed]
- Kostyunina, D.S.; Ivanova, A.D.; Smirnova, O.V. Myostatin: Twenty Years Later. Hum. Physiol. 2018, 44, 88–101. [Google Scholar] [CrossRef]
- Esposito, P.; Picciotto, D.; Battaglia, Y.; Costigliolo, F.; Viazzi, F.; Verzola, D. Myostatin: Basic biology to clinical application. In Advances in Clinical Chemistry; Elsevier Inc.: Amsterdam, Netherlands, 2022; Volume 106, pp. 181–234. ISBN 9780323988377. [Google Scholar]
- Hill, J.J.; Davies, M.V.; Pearson, A.A.; Wang, J.H.; Hewick, R.M.; Wolfman, N.M.; Qiu, Y. The myostatin propeptide and the follistatin-related gene are inhibitory binding proteins of myostatin in normal serum. J. Biol. Chem. 2002, 277, 40735–40741. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, Y.S.; Zimmers, T.A.; Soleimani, A.; Matzuk, M.M.; Tsuchida, K.; Cohn, R.D.; Barton, E.R. Regulation of muscle mass by follistatin and activins. Mol. Endocrinol. 2010, 24, 1998–2008. [Google Scholar] [CrossRef]
- Amthor, H.; Nicholas, G.; McKinnell, I.; Kemp, C.F.; Sharma, M.; Kambadur, R.; Patel, K. Follistatin complexes Myostatin and antagonises Myostatin-mediated inhibition of myogenesis. Dev. Biol. 2004, 270, 19–30. [Google Scholar] [CrossRef]
- Dasarathy, S.; McCullough, A.J.; Muc, S.; Schneyer, A.; Bennett, C.D.; Dodig, M.; Kalhan, S.C. Sarcopenia associated with portosystemic shunting is reversed by follistatin. J. Hepatol. 2011, 54, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J. TGFβ signalling in context. Nat. Rev. Mol. Cell Biol. 2012, 13, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961, 9, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Shadrach, J.L.; Wagers, A.J. Stem cells for skeletal muscle repair. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Fukada, S.; Higashimoto, T.; Kaneshige, A. Differences in muscle satellite cell dynamics during muscle hypertrophy and regeneration. Skelet. Muscle 2022, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.J.; Sattler, K.M.; Lepper, C. Molecular regulation of satellite cells via intercellular signaling. Gene 2023, 858, 147172. [Google Scholar] [CrossRef] [PubMed]
- McCroskery, S.; Thomas, M.; Maxwell, L.; Sharma, M.; Kambadur, R. Myostatin negatively regulates satellite cell activation and self-renewal. J. Cell Biol. 2003, 162, 1135–1147. [Google Scholar] [CrossRef]
- Wang, Q.; McPherron, A.C. Myostatin inhibition induces muscle fibre hypertrophy prior to satellite cell activation. J. Physiol. 2012, 590, 2151–2165. [Google Scholar] [CrossRef]
- Ariano, M.A.; Armstrong, R.B.; Edgerton, V.R. Hindlimb muscle fiber populations of five mammals. J. Histochem. Cytochem. 1973, 21, 51–55. [Google Scholar] [CrossRef]
- Sullivan, T.E.; Armstrong, R.B. Rat locomotory muscle fiber activity during trotting and galloping. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1978, 44, 358–363. [Google Scholar] [CrossRef]
- Artaza, J.N.; Bhasin, S.; Mallidis, C.; Taylor, W.; Ma, K.; Gonzalez-Cadavid, N.F. Endogenous expression and localization of myostatin and its relation to myosin heavy chain distribution in C2C12 skeletal muscle cells. J. Cell. Physiol. 2002, 190, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Kalds, P.; Zhou, S.; Huang, S.; Gao, Y.; Wang, X.; Chen, Y. When Less Is More: Targeting the Myostatin Gene in Livestock for Augmenting Meat Production. J. Agric. Food Chem. 2023, 71, 4216–4227. [Google Scholar] [CrossRef] [PubMed]
- Schuelke, M.; Wagner, K.R.; Stolz, L.E.; Hübner, C.; Riebel, T.; Kömen, W.; Braun, T.; Tobin, J.F.; Lee, S.-J. Myostatin Mutation Associated with Gross Muscle Hypertrophy in a Child. N. Engl. J. Med. 2004, 350, 2682–2688. [Google Scholar] [CrossRef] [PubMed]
- Reisz-Porszasz, S.; Bhasin, S.; Artaza, J.N.; Shen, R.; Sinha-Hikim, I.; Hogue, A.; Fielder, T.J.; Gonzalez-Cadavid, N.F. Lower skeletal muscle mass in male transgenic mice with muscle-specific overexpression of myostatin. Am. J. Physiol.-Endocrinol. Metab. 2003, 285, E876–E888. [Google Scholar] [CrossRef]
- Zimmers, T.A.; Davies, M.V.; Koniaris, L.G.; Haynes, P.; Esquela, A.F.; Tomkinson, K.N.; McPherron, A.C.; Wolfman, N.M.; Lee, S.J. Induction of cachexia in mice by systemically administered myostatin. Science 2002, 296, 1486–1488. [Google Scholar] [CrossRef] [PubMed]
- Girgenrath, S.; Song, K.; Whittemore, L.A. Loss of myostatin expression alters fiber-type distribution and expression of myosin heavy chain isoforms in slow- and fast-type skeletal muscle. Muscle Nerve 2005, 31, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, M.; Ding, F.; Gu, X. Expression of myostatin RNA transcript and protein in gastrocnemius muscle of rats after sciatic nerve resection. J. Muscle Res. Cell Motil. 2006, 27, 37–44. [Google Scholar] [CrossRef]
- Kazemi, F. The correlation of resistance exercise-induced myostatin with insulin resistance and plasma cytokines in healthy young men. J. Endocrinol. Investig. 2016, 39, 383–388. [Google Scholar] [CrossRef]
- Raue, U.; Slivka, D.; Jemiolo, B.; Hollon, C.; Trappe, S. Myogenic gene expression at rest and after a bout of resistance exercise in young (18–30 yr) and old (80–89 yr) women. J. Appl. Physiol. 2006, 101, 53–59. [Google Scholar] [CrossRef]
- Louis, E.; Raue, U.; Yang, Y.; Jemiolo, B.; Trappe, S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J. Appl. Physiol. 2007, 103, 1744–1751. [Google Scholar] [CrossRef]
- Harber, M.P.; Crane, J.D.; Dickinson, J.M.; Jemiolo, B.; Raue, U.; Trappe, T.A.; Trappe, S.W. Protein synthesis and the expression of growth-related genes are altered by running in human vastus lateralis and soleus muscles. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 296, R708–R714. [Google Scholar] [CrossRef] [PubMed]
- Kollias, H.D.; McDermott, J.C. Transforming growth factor-β and myostatin signaling in skeletal muscle. J. Appl. Physiol. 2008, 104, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Cross, J.M.; Bamman, M.M. Impact of resistance loading on myostatin expression and cell cycle regulation in young and older men and women. Am. J. Physiol.-Endocrinol. Metab. 2005, 288, E1110–E1119. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.M.; Martel, G.F.; Ferrell, R.E.; Metter, E.J.; Hurley, B.F.; Rogers, M.A. Myostatin gene expression is reduced in humans with heavy-resistance strength training: A brief communication. Exp. Biol. Med. 2003, 228, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.S.; Kambadur, R.; Sharma, M.; Smith, H.K. Resistance Training Alters Plasma Myostatin but not IGF-1 in Healthy Men. Med. Sci. Sports Exerc. 2004, 36, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, D.S. Effects of Heavy Resistance Training on Myostatin mRNA and Protein Expression. Med. Sci. Sports Exerc. 2004, 36, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Zachwieja, J.J.; Smith, S.R.; Sinha-Hikim, I.; Gonzalez-Cadavid, N.; Bhasin, S. Plasma myostatin-immunoreactive protein is increased after prolonged bed rest with low-dose T3 administration. J. Gravit. Physiol. 1999, 6, 11–15. [Google Scholar]
- Willoughby, D.S.; Sultemeire, S.; Brown, M. Human muscle disuse atrophy after 28 days of immobilization in alower-limbwalking boot: A case study. J. Exerc. Physiol. Online 2003, 6, 88–95. [Google Scholar]
- Carlson, C.J.; Booth, F.W.; Gordon, S.E. Skeletal muscle myostatin mRNA expression is fiber-type specific and increases during hindlimb unloading. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1999, 277, R601–R606. [Google Scholar] [CrossRef]
- Lalani, R.; Bhasin, S.; Byhower, F.; Tarnuzzer, R.; Grant, M.; Shen, R.; Asa, S.; Ezzat, S.; Gonzalez-Cadavid, N.F. Myostatin and insulin-like growth factor-I and -II expression in the muscle of rats exposed to the microgravity environment of the neurolab space shuttle flight. J. Endocrinol. 2000, 167, 417–428. [Google Scholar] [CrossRef]
- Ma, K.; Mallidis, C.; Bhasin, S.; Mahabadi, V.; Artaza, J.; Gonzalez-Cadavid, N.; Arias, J.; Salehian, B. Glucocorticoid-induced skeletal muscle atrophy is associated with upregulation of myostatin gene expression. Am. J. Physiol.-Endocrinol. Metab. 2003, 285, E363–E371. [Google Scholar] [CrossRef] [PubMed]
- Vingren, J.L.; Kraemer, W.J.; Ratamess, N.A.; Anderson, J.M.; Volek, J.S.; Maresh, C.M. Testosterone Physiology in Resistance Exercise and Training. Sports Med. 2010, 40, 1037–1053. [Google Scholar] [CrossRef] [PubMed]
- Gilson, H.; Schakman, O.; Combaret, L.; Lause, P.; Grobet, L.; Attaix, D.; Ketelslegers, J.M.; Thissen, J.P. Myostatin gene deletion prevents glucocorticoid-induced muscle atrophy. Endocrinology 2007, 148, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Dubois, V.; Laurent, M.R.; Sinnesael, M.; Cielen, N.; Helsen, C.; Clinckemalie, L.; Spans, L.; Gayan-Ramirez, G.; Deldicque, L.; Hespel, P.; et al. A satellite cell-specific knockout of the androgen receptor reveals myostatin as a direct androgen target in skeletal muscle. FASEB J. 2014, 28, 2979–2994. [Google Scholar] [CrossRef] [PubMed]
- Hittel, D.S.; Berggren, J.R.; Shearer, J.; Boyle, K.; Houmard, J.A. Increased secretion and expression of myostatin in skeletal muscle from extremely obese women. Diabetes 2009, 58, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Berggren, J.R.; Hulver, M.W.; Houmard, J.A.; Hoffman, E.P. GRB14, GPD1, and GDF8 as potential network collaborators in weight loss-induced improvements in insulin action in human skeletal muscle. Physiol. Genom. 2006, 27, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Dutra, D.B.; Bueno, P.G.; Silva, R.N.; Nakahara, N.H.; Selistre-Araújo, H.S.; Nonaka, K.O.; Leal, A.M.O. Expression of myostatin, myostatin receptors and follistatin in diabetic rats submitted to exercise. Clin. Exp. Pharmacol. Physiol. 2012, 39, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Bullough, W.S. The control of mitotic activity in adult mammalian tissues. Biol. Rev. Camb. Philos. Soc. 1962, 37, 307–342. [Google Scholar] [CrossRef]
- Lee, S.-J. Myostatin: A Skeletal Muscle Chalone. Annu. Rev. Physiol. 2023, 85, 269–291. [Google Scholar] [CrossRef]
- Artaza, J.N.; Reisz-Porszasz, S.; Dow, J.S.; Kloner, R.A.; Tsao, J.; Bhasin, S.; Gonzalez-Cadavid, N.F. Alterations in myostatin expression are associated with changes in cardiac left ventricular mass but not ejection fraction in the mouse. J. Endocrinol. 2007, 194, 63–76. [Google Scholar] [CrossRef]
- Rodgers, B.D.; Interlichia, J.P.; Garikipati, D.K.; Mamidi, R.; Chandra, M.; Nelson, O.L.; Murry, C.E.; Santana, L.F. Myostatin represses physiological hypertrophy of the heart and excitation-contraction coupling. J. Physiol. 2009, 587, 4873–4886. [Google Scholar] [CrossRef] [PubMed]
- Cohn, R.D.; Liang, H.Y.; Shetty, R.; Abraham, T.; Wagner, K.R. Myostatin does not regulate cardiac hypertrophy or fibrosis. Neuromuscul. Disord. 2007, 17, 290–296. [Google Scholar] [CrossRef] [PubMed]
- McKoy, G.; Bicknell, K.A.; Patel, K.; Brooks, G. Developmental expression of myostatin in cardiomyocytes and its effect on foetal and neonatal rat cardiomyocyte proliferation. Cardiovasc. Res. 2007, 74, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, A.T.; Torrado, M.; Iglesias, R.; Nespereira, B. Identification of candidate genes potentially relevant to chamber-specific remodeling in postnatal ventricular myocardium. J. Biomed. Biotechnol. 2010, 2010, 603159. [Google Scholar] [CrossRef]
- Bish, L.T.; Morine, K.J.; Sleeper, M.M.; Sweeney, H.L. Myostatin is upregulated following stress in an Erk-dependent manner and negatively regulates cardiomyocyte growth in culture and in a mouse model. PLoS ONE 2010, 5, e10230. [Google Scholar] [CrossRef] [PubMed]
- Morissette, M.R.; Cook, S.A.; Foo, S.; McKoy, G.; Ashida, N.; Novikov, M.; Scherrer-Crosbie, M.; Li, L.; Matsui, T.; Brooks, G.; et al. Myostatin regulates cardiomyocyte growth through modulation of Akt signaling. Circ. Res. 2006, 99, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Shyu, K.G.; Ko, W.H.; Yang, W.S.; Wang, B.W.; Kuan, P. Insulin-like growth factor-1 mediates stretch-induced upregulation of myostatin expression in neonatal rat cardiomyocytes. Cardiovasc. Res. 2005, 68, 405–414. [Google Scholar] [CrossRef]
- Rashidlamir, A.; Attarzadeh Hosseini, S.R.; Hejazi, K.; Motevalli Anberani, S.M. The effect of eight weeks resistance and aerobic training on myostatin and follistatin expression in cardiac muscle of rats. J. Cardiovasc. Thorac. Res. 2016, 8, 164–169. [Google Scholar] [CrossRef]
- Wang, B.W.; Chang, H.; Kuan, P.; Shyu, K.G. Angiotensin II activates myostatin expression in cultured rat neonatal cardiomyocytes via p38 MAP kinase and myocyte enhance factor 2 pathway. J. Endocrinol. 2008, 197, 85–93. [Google Scholar] [CrossRef]
- Callis, T.E.; Pandya, K.; Hee, Y.S.; Tang, R.H.; Tatsuguchi, M.; Huang, Z.P.; Chen, J.F.; Deng, Z.; Gunn, B.; Shumate, J.; et al. MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J. Clin. Investig. 2009, 119, 2772–2786. [Google Scholar] [CrossRef]
- Lenk, K.; Schur, R.; Linke, A.; Erbs, S.; Matsumoto, Y.; Adams, V.; Schuler, G. Impact of exercise training on myostatin expression in the myocardium and skeletal muscle in a chronic heart failure model. Eur. J. Heart Fail. 2009, 11, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Castillero, E.; Akashi, H.; Wang, C.; Najjar, M.; Ji, R.; Kennel, P.J.; Sweeney, H.L.; Schulze, P.C.; George, I. Cardiac myostatin upregulation occurs immediately after myocardial ischemia and is involved in skeletal muscle activation of atrophy. Biochem. Biophys. Res. Commun. 2015, 457, 106–111. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oliveira, P.G.S.; Schwed, J.F.; Chiuso-Minicucci, F.; Duarte, S.R.S.; Nascimento, L.M.; Dorna, M.S.; Costa, N.A.; Okoshi, K.; Okoshi, M.P.; Azevedo, P.S.; et al. Association Between Serum Myostatin Levels, Hospital Mortality, and Muscle Mass and Strength Following ST-Elevation Myocardial Infarction. Heart Lung Circ. 2022, 31, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Damatto, R.L.; Lima, A.R.R.; Martinez, P.F.; Cezar, M.D.M.; Okoshi, K.; Okoshi, M.P. Myocardial myostatin in spontaneously hypertensive rats with heart failure. Int. J. Cardiol. 2016, 215, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Shyu, K.G.; Lu, M.J.; Wang, B.W.; Sun, H.Y.; Chang, H. Myostatin expression in ventricular myocardium in a rat model of volume-overload heart failure. Eur. J. Clin. Investig. 2006, 36, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Furihata, T.; Kinugawa, S.; Fukushima, A.; Takada, S.; Homma, T.; Masaki, Y.; Abe, T.; Yokota, T.; Oba, K.; Okita, K.; et al. Serum myostatin levels are independently associated with skeletal muscle wasting in patients with heart failure. Int. J. Cardiol. 2016, 220, 483–487. [Google Scholar] [CrossRef]
- Chen, P.; Liu, Z.; Luo, Y.; Chen, L.; Li, S.; Pan, Y.; Lei, X.; Wu, D.; Xu, D. Predictive value of serum myostatin for the severity and clinical outcome of heart failure. Eur. J. Intern. Med. 2019, 64, 33–40. [Google Scholar] [CrossRef]
- Gruson, D.; Ahn, S.A.; Ketelslegers, J.M.; Rousseau, M.F. Increased plasma myostatin in heart failure. Eur. J. Heart Fail. 2011, 13, 734–736. [Google Scholar] [CrossRef]
- Chiang, J.Y.; Lin, L.; Wu, C.C.; Hwang, J.J.; Yang, W.S.; Wu, Y.W. Serum myostatin level is associated with myocardial scar burden by SPECT myocardial perfusion imaging. Clin. Chim. Acta 2022, 537, 9–15. [Google Scholar] [CrossRef]
- Ishida, J.; Konishi, M.; Saitoh, M.; Anker, M.; Anker, S.D.; Springer, J. Myostatin signaling is up-regulated in female patients with advanced heart failure. Int. J. Cardiol. 2017, 238, 37–42. [Google Scholar] [CrossRef]
- Schnee, J. Angiotensin II, adhesion, and cardiac fibrosis. Cardiovasc. Res. 2000, 46, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Gaussin, V.; Depre, C. Myostatin, the cardiac chalone of insulin-like growth factor-1. Cardiovasc. Res. 2005, 68, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Ren, J.; Ba, L.; Song, C.; Zhang, Q.; Cao, Y.; Shi, P.; Fu, B.; Liu, Y.; Sun, H. MSTN Attenuates Cardiac Hypertrophy through Inhibition of Excessive Cardiac Autophagy by Blocking AMPK/mTOR and miR-128/PPARγ/NF-κB. Mol. Ther.-Nucleic Acids 2020, 19, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Butcher, J.T.; Ali, M.I.; Ma, M.W.; McCarthy, C.G.; Islam, B.N.; Fox, L.G.; Mintz, J.D.; Larion, S.; Fulton, D.J.; Stepp, D.W. Effect of myostatin deletion on cardiac and microvascular function. Physiol. Rep. 2017, 5, e13525. [Google Scholar] [CrossRef] [PubMed]
- Biesemann, N.; Mendler, L.; Wietelmann, A.; Hermann, S.; Schäfers, M.; Krüger, M.; Boettger, T.; Borchardt, T.; Braun, T. Myostatin regulates energy homeostasis in the heart and prevents heart failure. Circ. Res. 2014, 115, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Biesemann, N.; Mendler, L.; Kostin, S.; Wietelmann, A.; Borchardt, T.; Braun, T. Myostatin induces interstitial fibrosis in the heart via TAK1 and p38. Cell Tissue Res. 2015, 361, 779–787. [Google Scholar] [CrossRef] [PubMed]
- McPherron, A.C.; Huynh, T.V.; Lee, S.J. Redundancy of myostatin and growth/differentiation factor 11 function. BMC Dev. Biol. 2009, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Kolwicz, S.C.; Tian, R. Glucose metabolism and cardiac hypertrophy. Cardiovasc. Res. 2011, 90, 194–201. [Google Scholar] [CrossRef]
- Arad, M.; Seidman, C.E.; Seidman, J.G. AMP-Activated Protein Kinase in the Heart. Circ. Res. 2007, 100, 474–488. [Google Scholar] [CrossRef]
- Manfredi, L.H.; Paula-Gomes, S.; Zanon, N.M.; Kettelhut, I.C. Myostatin promotes distinct responses on protein metabolism of skeletal and cardiac muscle fibers of rodents. Braz. J. Med. Biol. Res. 2017, 50, e6733. [Google Scholar] [CrossRef]
- Lim, S.; McMahon, C.D.; Matthews, K.G.; Devlin, G.P.; Elston, M.S.; Conaglen, J.V. Absence of Myostatin Improves Cardiac Function Following Myocardial Infarction. Heart Lung Circ. 2018, 27, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Meloux, A.; Rochette, L.; Maza, M.; Bichat, F.; Tribouillard, L.; Cottin, Y.; Zeller, M.; Vergely, C. Growth differentiation factor-8 (GDF8)/myostatin is a predictor of troponin i peak and a marker of clinical severity after acute myocardial infarction. J. Clin. Med. 2020, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Solà, J.; Borrisser-Pairó, F.; Antúnez, E.; Tobías, E. Myostatin and insulin-like growth factor-1 in hypertensive heart disease. J. Hypertens. 2015, 33, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Baán, J.A.; Varga, Z.V.; Leszek, P.; Kuśmierczyk, M.; Baranyai, T.; Dux, L.; Ferdinandy, P.; Braun, T.; Mendler, L. Myostatin and IGF-I signaling in end-stage human heart failure: A qRT-PCR study. J. Transl. Med. 2015, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mikłosz, A.; Łukaszuk, B.; Baranowski, M.; Chabowski, A.; Górski, J. Assessment of the main compounds of the lipolytic system in treadmill running rats: Different response patterns between the right and left ventricle. Int. J. Mol. Sci. 2019, 20, 2556. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, B.; Miklosz, A.; Zabielski, P.; Chabowski, A.; Gorski, J. Effect of tachycardia on mRNA and protein expression of the principal components of the lipolytic system in the rat’s heart ventricles. J. Physiol. Pharmacol. 2017, 68, 731–736. [Google Scholar] [PubMed]
- George, I.; Bish, L.T.; Kamalakkannan, G.; Petrilli, C.M.; Oz, M.C.; Naka, Y.; Sweeney, H.L.; Maybaum, S. Myostatin activation in patients with advanced heart failure and after mechanical unloading. Eur. J. Heart Fail. 2010, 12, 444–453. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Anker, S.D.; Ponikowski, P.; Varney, S.; Chua, T.P.; Clark, A.L.; Webb-Peploe, K.M.; Harrington, D.; Kox, W.J.; Poole-Wilson, P.A.; Coats, A.J.S. Wasting as independent risk factor for mortality in chronic heart failure. Lancet 1997, 349, 1050–1053. [Google Scholar] [CrossRef]
- von Haehling, S. The wasting continuum in heart failure: From sarcopenia to cachexia. Proc. Nutr. Soc. 2015, 74, 367–377. [Google Scholar] [CrossRef]
- Suzuki, T.; Palus, S.; Springer, J. Skeletal muscle wasting in chronic heart failure. ESC Heart Fail. 2018, 5, 1099–1107. [Google Scholar] [CrossRef]
- Lima, A.R.R.; Martinez, P.F.; Okoshi, K.; Guizoni, D.M.; Zornoff, L.A.M.; Campos, D.H.S.; Oliveira, S.A.; Bonomo, C.; Pai-Silva, M.D.; Okoshi, M.P. Myostatin and follistatin expression in skeletal muscles of rats with chronic heart failure. Int. J. Exp. Pathol. 2010, 91, 54–62. [Google Scholar] [CrossRef]
- Breitbart, A.; Auger-Messier, M.; Molkentin, J.D.; Heineke, J. Myostatin from the heart: Local and systemic actions in cardiac failure and muscle wasting. Am. J. Physiol.-Heart Circ. Physiol. 2011, 300, H1973. [Google Scholar] [CrossRef]
- Heineke, J.; Auger-Messier, M.; Xu, J.; Sargent, M.; York, A.; Welle, S.; Molkentin, J.D. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation 2010, 121, 419–425. [Google Scholar] [CrossRef]
- Lee, S.J. Targeting the myostatin signaling pathway to treat muscle loss and metabolic dysfunction. J. Clin. Investig. 2021, 131, e148372. [Google Scholar] [CrossRef]
- Nielsen, T.L.; Vissing, J.; Krag, T.O. Antimyostatin treatment in health and disease: The story of great expectations and limited success. Cells 2021, 10, 533. [Google Scholar] [CrossRef] [PubMed]
| Factor | Tissue/Cell | Serum | Reference | |
|---|---|---|---|---|
| mRNA | Protein | |||
| Cyclic stretch, iv | ↑ | ↓ | - | [58] |
| Exercise training, rat | N | N | - | [59] |
| Exercise training, rat | ↑ | - | - | [5] |
| Hypokinesia, man | ↑ | ↑ | ↑ | [38,39] |
| rat | ↑ | ↑ | - | [40,41] |
| Angiotensin, iv | ↑ | ↑ | - | [60] |
| IGF-1, iv | - | ↑ | - | [58] |
| miRNA8 overexpression, mice | N | ↓ | - | [61] |
| miRNA8 deletion, mice | N | ↑ | - | [61] |
| Phenylephrine, iv | - | ↑ | - | [57] |
| Myocardial infarction, sheep | - | ↑ | - | [4] |
| rat | - | ↑ | - | [62] |
| rat | - | ↑ | ↑ | [63] |
| human | - | - | ↓ | [64] |
| Hypertension, rat | - | ↓ | - | [65] |
| Hypertrophy, rat | ↑ | ↑ | - | [66] |
| Chronic heart failure, human | - | - | ↓ | [67] |
| human | - | - | ↑ | [68,69,70] |
| human-F | - | ↑ | - | [71] |
| human-M | - | N | - | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knapp, M.; Supruniuk, E.; Górski, J. Myostatin and the Heart. Biomolecules 2023, 13, 1777. https://doi.org/10.3390/biom13121777
Knapp M, Supruniuk E, Górski J. Myostatin and the Heart. Biomolecules. 2023; 13(12):1777. https://doi.org/10.3390/biom13121777
Chicago/Turabian StyleKnapp, Małgorzata, Elżbieta Supruniuk, and Jan Górski. 2023. "Myostatin and the Heart" Biomolecules 13, no. 12: 1777. https://doi.org/10.3390/biom13121777
APA StyleKnapp, M., Supruniuk, E., & Górski, J. (2023). Myostatin and the Heart. Biomolecules, 13(12), 1777. https://doi.org/10.3390/biom13121777








