Abstract
Corneal nerve homeostasis is essential for the functional integrity of the ocular surface. Vitamin D deficiency (VDD) and vitamin D receptor knockout (VDR KO) have been found to reduce corneal nerve density in diabetic mice. This is the first study to comprehensively examine the influence of vitamin D on nerve regeneration following corneal epithelial injury in diabetic mice. Corneal nerve regeneration was significantly retarded by diabetes, VDR KO, and VDD, and it was accelerated following topical 1,25 Vit D and 24,25 Vit D administration. Furthermore, topical 1,25 Vit D and 24,25 Vit D increased nerve growth factor, glial cell line-derived neurotropic factor, and neurotropin-3 protein expression, and it increased secretion of GDNF protein from human corneal epithelial cells. CD45+ cells and macrophage numbers were significantly decreased, and vitamin D increased CD45+ cell and macrophage recruitment in these wounded diabetic mouse corneas. The accelerated nerve regeneration observed in these corneas following topical 1,25 Vit D and 24,25 Vit D administration may be related to the vitamin D-stimulated expression, secretion of neurotrophic factors, and recruitment of immune cells.
1. Introduction
The cornea is one of the most densely innervated tissues in the human body, containing 19,000 to 44,000 small nerve fiber afferents. It exhibits a complex nerve architecture, distribution, and structural organization [1]. The outermost layer of the cornea consists of a stratified squamous, non-keratinized epithelium. Cornea nerve fibers originate from cell bodies residing in the trigeminal ganglion, losing their myelin as they enter the cornea and terminating on the superficial surface of the cornea between the basal epithelium and Bowman’s layer. In addition to their important sensory functions, corneal nerves help to maintain epithelial homeostasis, which is essential for the functional integrity of the ocular surface [2]. Loss of corneal nerves in diabetic corneas and following corneal wounding, particularly in diabetic corneas, can contribute to diabetic keratitis and poor wound healing.
Diabetic keratopathy, a condition that causes progressive damage to corneal epithelial cells and sensory nerves, affects up to 50% of patients with diabetes mellitus (DM) [3,4,5]. Nerve changes in diabetic corneas have been well described and include reduced nerve density, thin nerve fibers, and impaired nerve migration [6,7,8,9]. The degree of nerve loss increases with the duration of DM [10,11], and the accompanying reduction of neurotrophic stimuli can result in the thinning of the epithelial layer and recurrent corneal erosions [11]. The interactions between epithelium and intraepithelial nerves are critical for nerve regeneration and nerve maintenance. Only a few studies have examined nerve regeneration following epithelial injury in diabetic corneas.
In addition to neuro–epithelial interactions, interactions between nerves and immune cells are also critical to corneal nerve maintenance and regeneration [12,13]. Resident immune cells distributed throughout the corneal epithelium and stroma include dendritic cells, macrophages, mast cells, and innate lymphoid cells [14,15]. Large numbers of CD45+ cells invade the stroma after mouse cornea epithelial debridement, including monocytes, macrophages, neutrophils, lymphocytes, and stem cells [16,17,18,19]. Stromal macrophages were found to promote nerve remodeling after epithelial abrasion [20] and to contribute to corneal homeostasis and pathology [21].
Vitamin D is both a hormone, as it can be synthesized from 7-dehydrocholestrol, and a vitamin, given that most populations do not synthesize enough vitamin D through the de novo pathway. Endogenous vitamin D is synthesized from 7-dehydrocholesterol in skin following UVB exposure, although vitamin D can also be activated and metabolized in the cornea [22]. The classical systemic route for vitamin D activation is initial hepatic 25-hydroxylation to 25-hydroxy vitamin D3, followed by renal conversion to the active 1,25-dihydroxy vitamin D3 (1,25 Vit D) by cytoplasmic 1α-hydroxylase. The conversion of 25(OH)D to 24,25-dihydroxy vitamin D3 (24,25 Vit D) is facilitated by 24(R)-hydroxylase, which is strongly induced by 1,25 Vit D [23,24].
Low serum vitamin D concentrations and serum vitamin D-binding protein levels have consistently been detected in patients with type 1 and type 2 diabetes mellitus (DM) [25,26,27], and there is a highly consistent inverse association between 25-hydroxy vitamin D concentration and incident diabetes [28]. Our group recently determined that corneal nerve density is significantly decreased in vitamin D-deficient diabetic mice (VDD) and vitamin D receptor knockout (VDR KO) diabetic mice [29]. Low corneal nerve density has also been documented in patients with DM [30]. In several neurodegenerative disorders, 1,25 Vit D has been shown to have neuroprotective effects, and VDR is present in neurons and glial cells [31,32]. Recognized for its positive effects on bone, 24,25 Vit D is active and likely physiologically significant in the corneal epithelium (in some cases more so than 1,25 Vit D) [33,34]. The current study examined the effects of vitamin D deficiency on nerve density in diabetic corneas. In addition, the expression and secretion of nerve growth factor (NGF), neurotropin-3 (NTF3), and glial cell line-derived neurotropic factor (GDNF) in corneal epithelial cells after exposure to 1,25 Vit D and 24,25 Vit D was measured. Corneal CD45+ cells and macrophage infiltration in diabetic mouse corneas following epithelial abrasion and subsequent treatment with topical 1,25 Vit D and 24,25 Vit D was also measured. Given the high prevalence of vitamin D deficiency and diabetes, the links between these two conditions and low nerve density in the cornea and other tissues, and the importance of cornea innervation to cornea function, it is important to determine if topical vitamin D treatment can positively influence reinnervation following corneal wounding.
2. Material and Methods
2.1. Materials
The 1,25 Vit D and 24,25 Vit D included in our study were purchased from Enzo Life Sciences (Catalog # BML-DM200-0050, BML-DM300-0050, Farmingdale, NY, USA). Antibodies for β-tubilin and NGF were purchased from ABCAM (Catalog # ab18207, ab52918, Cambridge, MA, USA). GDNF and NTF3 antibody were purchased from ThermoFisher Scientific (Catalog # MA5-33142 and PA5-102315, Waltham, MA, USA). Pre-stained protein markers were obtained from Bio-Rad (Catalog # LC5699, Hercules, CA, USA). Polyvinylidene difluoride (PVDF) membrane and the enhanced chemiluminescence (ECL) detection system were obtained from Bio-Rad (Catalog # 1704272, 1705060).
2.2. Animal Experiments
All animal studies were approved by the Augusta University IACUC, and the animals were treated according to the ARVO statement for the Use of Animals in Ophthalmic and Visual Research: https://www.arvo.org/About/policies/arvo-statement-for-the-use-of-animals-in-ophthalmic-and-vision-research/ (accessed on 30 November 2023). All the animals were housed in standard conditions with a 12 h dark–light cycle. The mice were euthanized via CO2 inhalation and cervical dislocation at the end of the experiments.
The low-dose streptozotocin (STZ) injection method was used to induce diabetes. Briefly, five sequential daily intraperitoneal injections of a freshly prepared solution of STZ in 0.1 mol/L citrate buffer (pH 4.5) at a dosage of 60 mg/kg body weight were administered to 4-week-old mice. Blood glucose was measured 1 week after the final STZ injection. We limited the mice receiving corneal epithelial wounds to those with blood glucose levels >249 and <651 mg/dL [35].
Male and female C57BL/6 mice were used in our studies. Table 1 lists all of the mouse groups used in this study along with their abbreviations. VDD mice were fed a vitamin D-deficient diet (TD.89123 Diet; Envigo, Tampa, FL, USA) for 4 weeks. The control group for the VDD mice was fed a TD.89124 vitamin D control diet (Envigo, Tampa, FL, USA). VDR KO Ca++ mice were fed a supplemental diet high in calcium, lactose, and phosphate (20% lactose, 2% calcium, 1.25% phosphate) (TD.96348 Diet; Envigo, Tampa, FL, USA) that has previously been shown to alleviate many of the VDR KO phenotypical features [36]. We previously documented serum 25-hydroxy vitamin D levels in diabetic and diabetic VDD mice and serum calcium levels in diabetic and diabetic VDD and VDR KO mice, along with VDR KO mice fed the supplemental diet [37].

Table 1.
Mouse groups and their abbreviations.
2.3. Corneal Injury
Mice were anesthetized via intraperitoneal injection with a mixture of ketamine (200 mg/kg) and xylazine (50 mg/kg). A central section of the cornea was demarcated using a 2 mm trephine, and the epithelium and superficial anterior stroma were gently removed under a dissecting microscope using an Algerbrush II (Alger Equipment Co., Lago Vista, TX, USA) [38]. The mice were examined pre- and post-operatively to exclude ocular surface disease or excessive tissue removal, and they were removed from the study if inflammation or infection occurred. The mice were sacrificed and their eyes were enucleated 10 weeks after the corneal abrasion procedure to analyze their nerves.
2.4. Immunostaining for CD 45+ Cells and Macrophages
CD45+ cells and macrophages in the basal epithelium were immunostained after the corneas were wounded as described above and treated with 1,25 Vit D or 24,25 Vit D three times over 8 h. CD45 and F4/80 antibodies were used to immunolabel hematopoietic-derived immune cells and macrophages, respectively [19,39,40]. The eyes were fixed with Zamboni fixative (American MasterTech Scientific, Lodi, CA, USA) for 75 min and then washed three times with PBS. The corneas were carefully excised along the sclera–corneal border and subjected to a rehydration series with increasing concentrations of Triton X-100 in PBS. To block nonspecific binding, corneas were incubated with 10% normal goat serum with 0.1% Triton X-100 solution in PBS for 60 min at room temperature. The tissues were then incubated with primary anti-CD45 (Catalog# ab10558, Abcam, Cambridge, MA, USA), or anti-F4/80 (Catalog# ab18207; AbcamA) (1:200) antibody in PBS, containing 5% goat serum with 0.1% Triton X-100 for 24 h at 4 °C and constantly shaken. After washing with PBS three times for 10 min each, the corneas were incubated with the secondary antibody (1:1000) Alexa Fluor 594 goat anti-rabbit IgG (H + L) and Alexa Fluor 488 donkey anti-rat IgG (H + L) (Catalog# A-11012, A-21206, ThermoFisher Scientific, Norcross, GA, USA) for 24 h at 4 °C and washed thoroughly with PBS. Immunolabelled cells in the basal epithelial layer and macrophages in the stroma were quantified using the Imaris image processing software Spots package version 10.0.0 (Oxford Instruments, Abingdon, UK).
2.5. Vitamin D Administration and Treatment
The 1,25 Vit D and 24,25 Vit D were dissolved in 14.1 mM dimethyl sulfoxide (DMSO). The mice were treated with topical 40 nM 1,25 Vit D, 200 nM 24,25 Vit D, or with a vehicle control. The treatment consisted of one drop three times per day for 4 weeks, starting the day of corneal epithelial debridement.
Human corneal epithelial cells from de-identified eye bank corneal rims and mouse primary corneal epithelial cells were isolated and cultured as previously described [41] and treated with 1,25 Vit D (20 nM) or 24,25 Vit D (100 nM). The control groups were treated only with the DMSO vehicle. The vitamin D treatment dosages were chosen based on previously measured vitamin D metabolite concentrations in the eye [22] and previous work from our laboratory using these dosages (e.g., [41]).
2.6. Nerve Immunofluorescence Labeling and Imaging
Cornea nerve labeling and density measurements were made as previously described [8,29]. Briefly, the corneas were processed as described above for immunostaining. The corneas were then incubated with primary rabbit polyclonal anti-β III tubulin (1:500) antibody in PBS containing 5% goat serum with 0.1% Triton X-100 for 24 h at 4 °C. The secondary antibody was Alexa Fluor 488 goat anti-rabbit IgG (H + L) (Catalog# A-32731, ThermoFisher, Waltham, MA, USA). To image corneas, four radial cuts were made on each cornea, and the tissue was mounted flat on a slide with the epithelium side up. Images were taken using a Leica STELLARIS Confocal Microscope (Danaher Corporation, Washington, DC, USA).
To calculate nerve density, confocal images were taken just below the central cornea epithelium (four pictures relative to the center: left, right, top, bottom). The most central half of each photo was traced in ImageJ (version 1.53a). The average percentage of pixels traced, among the four central images, was defined as the nerve density value of the image [8,29].
2.7. Protein Extraction from Culture Medium
Human corneal epithelial cells were cultured with 1.5 mL DMEM medium (1% FBS) in a 35 mm dish (ThermoFisher Scientific, Catalog# 12-565-91), and were treated with 20 nM 1,25 Vit D or 100 nM 24,25 Vit D for 48 or 72 h. Cultured medium samples were collected and centrifuged at 800× g for 5 min. Trichloroacetic acid (TCA) was added at a final concentration of 12% to the post-centrifuged supernatants, and these were allowed to precipitate for at least 1 h on ice. The proteins were then pelleted via centrifugation at 16,000× g for 20 min at 4 °C and subsequently washed three times with cold acetone (4 °C). The samples were air dried and solubilized in lysis buffer (Cell Signaling Technology, Catalog# 2881S, Danvers, MA, USA) with protease inhibitor (Sigma, Catalog# P8340-1ML, Ann Arbor, MI, USA). Proteins were then characterized using Western blotting.
2.8. Western Blot Analysis
Proteins were isolated from the culture medium or from human corneal epithelial cells grown on 35 mm dishes. The cultured cells were washed in PBS at 4 °C and treated with lysis buffer. Cell lysates were collected, and Western blotting was performed as previously described [34,42]. The membranes were incubated with primary antibodies of NGF (ABCAM, Catalog# ab52918, Cambridge, MA, USA), NTF3 and GDNF (ThermoFisher Scientific, Catalog# MA5-33142 and PA5-102315, Waltham, MA, USA) at a dilution of 1:1000 in Tris-buffered saline with 0.1% Tween 20 (TBST) and 10% non-fat milk at 4 °C overnight. Removal of excess primary antibody was carried out by washing the membranes in TBST three times for 10 min each. The secondary goat anti-rabbit IgG antibody (H + L) (ThermoFisher Scientific, Catalog# 31460) was diluted 1:3000 and incubated with the membrane in TBST with 10% non-fat dry milk for one 1 hr at room temperature. Detection and quantification of band intensities was conducted using Image Lab 5.2.1 software (Bio-Rad). The bands were normalized by dividing the intensity of the band by the intensity of the total protein from the same sample on the blot using the Bio-Rad Stain-Free method, as we have previously described [34]. The Western blot data are provided as the mean ± SE of at least three experiments.
2.9. Statistical Analysis
Comparisons between multiple groups were analyzed using an ANOVA. Data comparisons between only 2 groups were analyzed using a student’s unpaired t-test comparing experimental groups against controls. p < 0.05 was considered statistically significant. GraphPad Prism 8.4.3 was used for the data analysis (GraphPad Software, La Jolla, CA, USA).
3. Results
3.1. Diabetic Nerve Regeneration
Sub-basal nerves were lost following epithelial cell and superficial stroma removal. After 2 weeks, clusters of regenerated sub-basal nerves were observed in wounded normoglycemic and diabetic corneas (Figure 1A,B). In normoglycemic mice at 4 weeks, the sub-basal nerves were more developed, and 10 weeks after wounding, a developing whorl-like pattern was observed (Figure 1C). In diabetic mice, the sub-basal intraepithelial nerves regenerated more slowly, and the whorl-like pattern was only partially developed (Figure 1B,D). Sub-basal nerve density over the whole cornea and separately in the central cornea was measured 10 weeks after wounding (Figure 1E). The WND CTRL whole cornea nerve density was significantly lower than CTRL (8.3 ± 0.16% vs. 9.5 ± 0.23%, p < 0.05), and the WND Dia corneas had a significantly lower density than the Dia corneas (7.0 ± 0.57% vs. 8.9 ± 0.58%, respectively). The WND Dia whole cornea nerve density was significantly lower than the WND CTRL density.
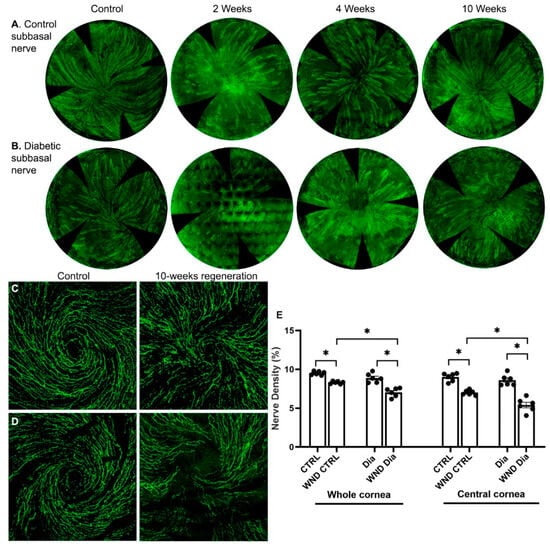
Figure 1.
Nerve regeneration in control and diabetic mice. (A) Representative sub-basal whole cornea nerve regeneration in (A) control and (B) diabetic mice 2, 4, and 10 weeks after corneal abrasion. (C,D) Representative sub-basal nerve regeneration in the central cornea of control (C) and diabetic mice (D) 10 weeks after corneal abrasion. (E) Sub-basal nerve density quantification of whole and central corneas of control and diabetic mice 10 weeks following corneal abrasion. Individual data points graphed as mean ± SE, n = 6, * p < 0.05.
Limiting the density measurement to the central cornea (Figure 1E), which regenerates more slowly the peripheral cornea, 10 weeks after wounding, the WND CTRL central cornea nerve density was significantly lower than the CTRL (7.0 ± 0.32% vs. 9.0 ± 0.53%), and the nerve density of the WND Dia central corneas was significantly lower than the Dia central corneas (5.4 ± 0.88% vs. 8.6 ± 0.72%). The central density of the WND Dia corneas was also significantly lower than that for the WND CTRL corneas.
3.2. Diabetic Vitamin D Deficiency Nerve Regeneration
10 weeks after wounding, the whorl-like pattern of the sub-basal nerve plexus did not develop in the VDD or Dia VDD mice (Figure 2A,B). Figure 2C shows whole and central cornea nerve density quantification for the VDD groups. The WND VDD whole cornea nerve density was significantly lower than the VDD nerve density (5.6 ± 0.65% vs. 8.8 ± 0.83%, respectively), and was also lower than the WND CTRL density (p < 0.05). The WND Dia VDD mouse corneal nerve density was significantly lower than the Dia VDD density (2.7 ± 0.81% vs. 6.3 ± 0.88%, respectively), and it was also significantly lower than the WND VDD density. Similar significant changes were observed when examining the central cornea nerve densities of the same VDD groups.
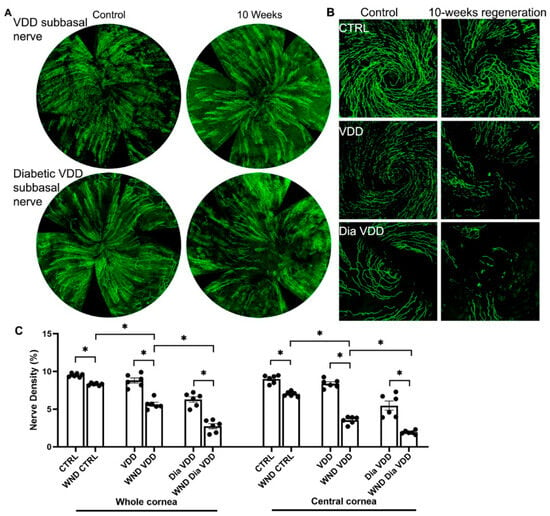
Figure 2.
Nerve regeneration in VDD and diabetic VDD mice. (A) Representative sub-basal whole cornea nerve regeneration in VDD and diabetic VDD mice 10 weeks after corneal abrasion. (B) Representative sub-basal nerve regeneration in the central cornea of VDD and diabetic VDD mice 10 weeks after corneal abrasion. (C) Sub-basal nerve density quantification of whole and central corneas of VDD and diabetic VDD mouse corneas 10 weeks following corneal abrasion. Individual data points graphed as mean ± SE, n = 6, * p < 0.05.
3.3. Diabetic VDR KO and VDD Nerve Regeneration
Initiation of the sub-basal nerve plexus whorl-like pattern was observed in VDR KO mouse corneas but not in the Dia VDR KO mouse corneas 10 weeks after wounding (Figure 3A). Figure 3B shows whole and central cornea nerve density quantification for the VDR KO groups. The whole cornea sub-basal nerve density was significantly lower in the WND VDR KO mice vs. the WND CNTRL and VDR KO mice (7.2 ± 0.43% vs. 8.3 ± 0.16% vs. 8.4 ± 0.36%, respectively), and the WND Dia VDR KO nerve density was significantly lower than that of the Dia VDR KO mice (4.4 ± 0.69% vs. 6.3 ± 0.81%, respectively). The WND Dia VDR KO density was also significantly lower than the WND VDR KO density. There was no significant difference between the whole cornea WT and VDR KO nerve densities; however, the WND VDR KO mice had significantly lower nerve densities compared with WND CTRL mice. Similar significant changes were observed when examining central cornea nerve densities. Comparing the densities of the Dia WT mice (from Figure 1E) with the Dia VDR KO mice, the Dia VDR KO mice had significantly lower nerve densities (4.4 ± 0.69% vs. 7.0 ± 0.57%, respectively). The Dia VDD mouse nerve density was lower than that of the Dia VDR KO mice, although the difference was not significant (p = 0.07).
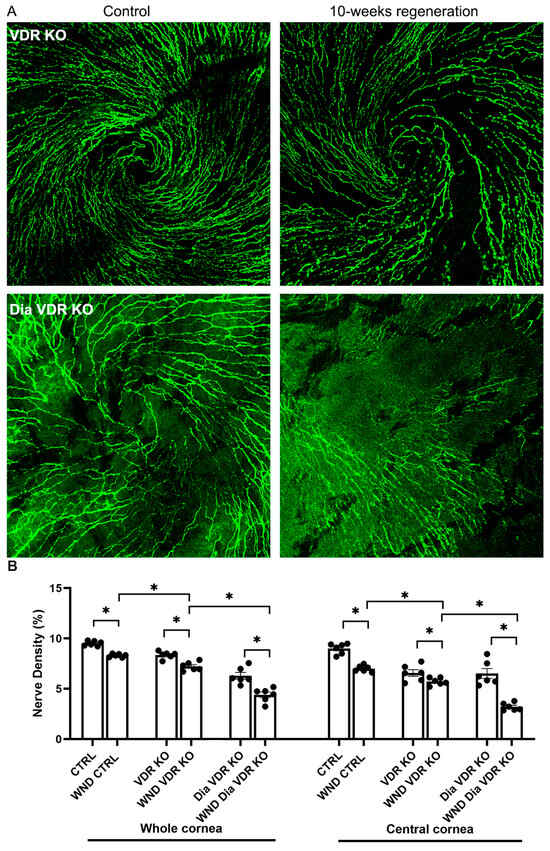
Figure 3.
Nerve regeneration in VDR KO and diabetic VDR KO mice. (A) Representative sub-basal nerve regeneration in the central cornea of VDR KO and diabetic VDR KO mice 10 weeks after corneal abrasion. (B) Sub-basal nerve density quantification of VDR KO and diabetic VDR KO mouse corneas 10 weeks following corneal abrasion. Individual data points graphed as mean ± SE, n = 6, * p < 0.05.
3.4. Nerve Regeneration in Dia VDR KO Mice Fed the Supplemental Diet
Corneas were wounded in the Dia VDR KO mice, which were then fed a supplemental diet enriched in Ca++ to determine if reductions in serum Ca++ brought on by VDR KO is linked to changes in nerve regeneration. The whole cornea nerve density was increased in diabetic VDR KO mice fed the supplemental diet for 10 weeks after wounding, although not to unwounded levels (Figure 4A,B). Interestingly, unlike the Dia VDR KO mice fed the routine diet, the sub-basal nerve whorl-like pattern was partially restored in the wounded diabetic VDR KO mice fed the supplemental diet (Figure 4A compared to Figure 3A (bottom right)).
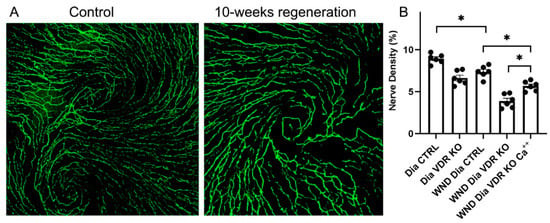
Figure 4.
Dia VDR KO mice with central corneal abrasions that were fed the supplemental diet significantly increased whole corneal nerve density, but not to control levels. (A) Representative sub-basal nerves in the central cornea of unwounded (left) and wounded (right) diabetic VDR KO mice fed the supplemental diet for 10 weeks. (B) Nerve density measurements. Individual data points graphed as mean ± SE, n = 6, * p < 0.05.
3.5. Nerve Regeneration in Wounded Normoglycemic Mouse Corneas Following Topical Vit D Treatment
The wounded mouse corneas were treated with 1,25 Vit D or 24,25 Vit D drops 3 times daily for 4 weeks following epithelial abrasion. Whole cornea sub-basal nerve density was significantly higher in the 1,25 Vit D and 24,25 Vit D-treated mice vs. the WND CTRL mice (6.6 ± 0.89% vs. 6.1 ± 0.84% vs. 3.7 ± 0.31%, respectively) (Figure 5).
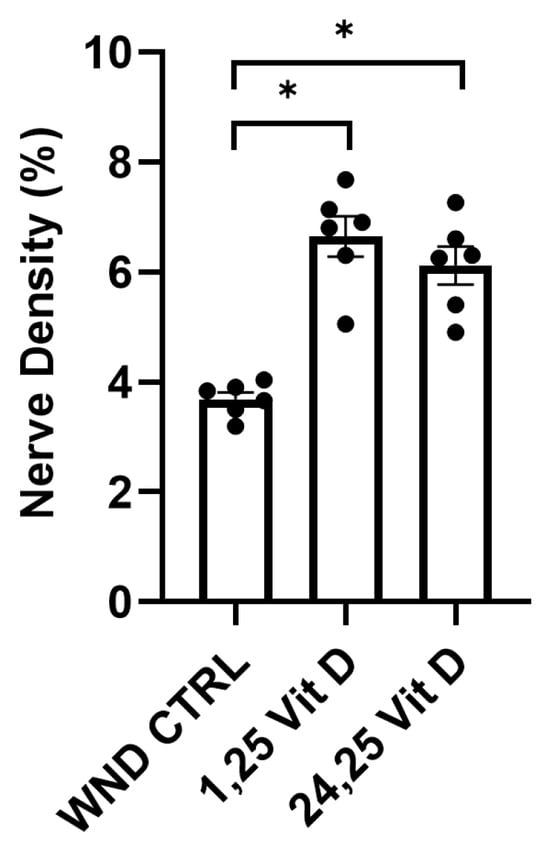
Figure 5.
Topical vitamin D3 increases whole corneal nerve regeneration in control mouse corneas following epithelial abrasion. Nerve density measurements of normoglycemic mouse corneas treated with 40 nM 1,25 Vit D or 200 nM 24,25 Vit D for 4 weeks. Individual data points graphed as mean ± SE, n = 6, * p < 0.05.
3.6. Nerve Regeneration in Diabetic and Dia VDD Mouse Corneas Following Topical Vit D Treatment
Topical 1,25 Vit D application significantly enhanced whole-cornea sub-basal reinnervation in the WND Dia and WND Dia VDD mice compared to the untreated WND Dia and WND Dia VDD mice (5.2 ± 0.64% vs. 2.5 ± 0.82% and 3.5 ± 0.4% vs. 2.6 ± 0.28%, respectively) (Figure 6). Topical 24,25 Vit D application also significantly increased reinnervation in the WND Dia and WND Dia VDD mice (4.9 ± 0.59% vs. 2.5 ± 0.82%, 3.6 ± 0.48% vs. 2.6 ± 0.28%, respectively) (Figure 6).
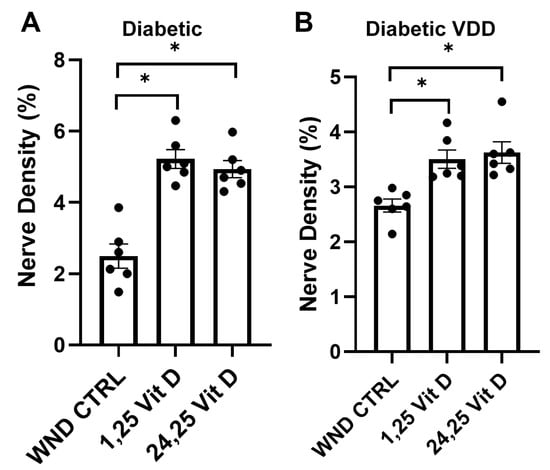
Figure 6.
Topical vitamin D3 increases whole corneal nerve regeneration in diabetic and diabetic VDD mouse corneas following epithelial abrasion. Nerve density measurements of (A) diabetic and (B) diabetic VDD mouse corneas treated with 40 nM 1,25 Vit D or 200 nM 24,25 Vit D for 4 weeks. Individual data points graphed as mean ± SE, n = 6, * p < 0.05.
3.7. Effects of 1,25 Vit D and 24,25 Vit D on NGF Protein Expression
NGF protein expression was increased in the cultured human corneal epithelial cells (HCECs) and the mouse corneal epithelial cells (MCECs) 24 h and 48 h after 1,25 Vit D and 24,25 Vit D exposure (Figure 7; p < 0.05).
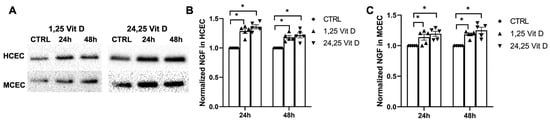
Figure 7.
1,25 Vit D and 24,25 Vit D increase NGF protein expression in human and mouse primary cornea epithelium. (A) Representative Western blots from primary epithelial cells treated with 1,25 and 24,25 Vit D. (B,C) Corresponding average blot density graphs with total protein normalization from (B) human and (C) mouse primary corneal epithelial cells treated with 1,25 and 24,25 Vit D. Individual data points graphed as mean ± SE, n = 5, * p < 0.05. Points at value of 1.0 are normalized controls. Original images for blots are shown in Supplementary Figure S1.
3.8. Effects of 1,25 Vit D and 24,25 Vit D on NTF3 Protein Expression
NTF3 protein expression was significantly increased in the HCECs 48 h after 1,25 Vit D and 24,25 Vit D exposure (p < 0.05 and p < 0.01, respectively), but not at 24 h. MCEC NTF3 protein expression was significantly increased 24 h after 24,25 Vit D exposure and 48 h after 1,25 Vit D and 24,25 Vit D exposure (Figure 8; p < 0.05).
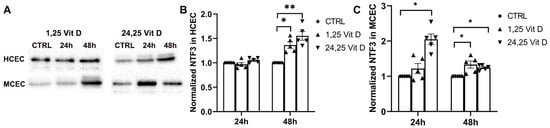
Figure 8.
1,25 Vit D and 24,25 Vit D increase NTF3 protein expression in human and mouse primary cornea epithelium. (A) Representative Western blots from primary epithelial cells treated with 1,25 and 24,25 Vit D. (B,C) Corresponding average blot density graphs with total protein normalization from (B) human and (C) mouse primary corneal epithelial cells treated with 1,25 and 24,25 Vit D. Individual data points graphed as mean ± SE, n = 5, * p < 0.05, ** < 0.01. Points at value of 1.0 are normalized controls. Original images for blots are shown in Supplementary Figure S2.
3.9. Effects of 1,25 Vit D and 24,25 Vit D on GDNF Protein Expression
HCEC GDNF protein expression was significantly increased 24 h and 48 h after 1,25 Vit D and 24,25 Vit D exposure. MCEC GDNF protein expression was significantly increased by 1,25 Vit D and 24,25 Vit D after 24 h (p < 0.05), and by 1,25 Vit D after 48 h exposure (Figure 9).
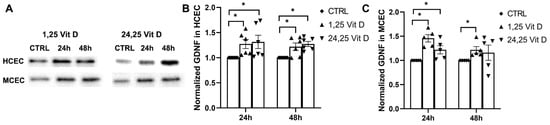
Figure 9.
1,25 Vit D and 24,25 Vit D increase GDNF protein expression in human and mouse primary cornea epithelium. (A) Representative Western blots from primary epithelial cells treated with 1,25 and 24,25 Vit D. (B,C) Corresponding average blot density graphs with total protein normalization from (B) human and (C) mouse primary corneal epithelial cells treated with 1,25 and 24,25 Vit D. Individual data points graphed as mean ± SE, n = 5, * p < 0.05. Points at value of 1.0 are normalized controls. Original images for blots are shown in Supplementary Figure S3.
3.10. Effects of 1,25 Vit D and 24,25 Vit D on HCEC NGF, NTF3, and GDNF Protein Secretion
Baseline NGF, NTF3, and GDNF secretion into the medium of the cultured HCECs was observed. GDNF secretion was significantly increased in the HCECs exposed to 24,25 Vit D at 48 and 72 h (Figure 10B) and following 1,25 Vit D exposure at 72 h. NGF and NTF3 secretion was not significantly increased in the HCECs treated with 1,25 Vit D or 24,25 Vit D (Figure 10C,D).
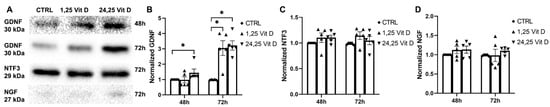
Figure 10.
1,25 and 24,25 Vit D increase GDNF but not NGF and NTF3 protein secretion in HCECs. (A) Representative GDNF, NTF3, and NGF Western blots from conditioned medium of HCECs treated with 1,25 and 24,25 Vit D. (B–D) Corresponding average blot density graphs with total protein normalization for (B) GDNF, (C) NTF3, and (D) NGF from HCECs treated with 1,25 and 24,25 Vit D. Individual data points graphed as mean ± SE, n = 5, * p < 0.05. Points at values of 1.0 are normalized controls. Original images for blots are shown in Supplementary Figure S4.
3.11. CD45+ Cell Recruitment Following 1,25 Vit D and 24,25 Vit D Treatment
Hematopoietic-derived immune cells in the basal epithelium were detected using CD45 immunostaining 8 h after wounding of the diabetic mouse corneas (10 weeks diabetic duration). Figure 11 illustrates significantly reduced CD45+ cell numbers in the basal epithelium of diabetic (Figure 11C) vs. normoglycemic mice (Figure 11A). When treated with topical 24,25 Vit D, CD45+ cells numbers were significantly increased in the basal epithelium of the diabetic mice (Figure 11E,F), with no significant change following 1,25 Vit D treatment (Figure 11D,E). Most of the cells had a dendriform morphology, as illustrated in (Figure 11B).
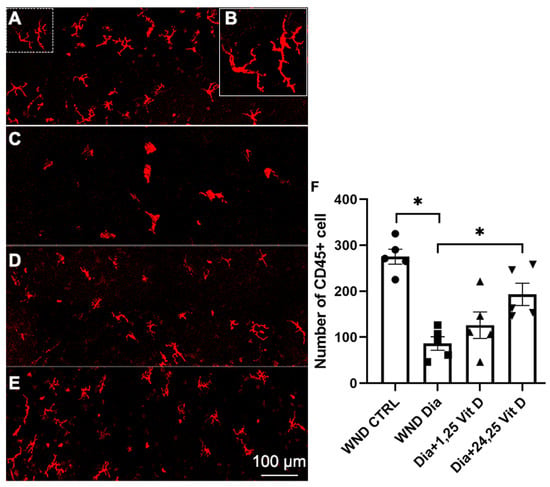
Figure 11.
Representative dendritic cells labeling (CD45+) in the basal epithelium of (A) control, (C) diabetic, (D) diabetic plus 1,25 Vit D, (E) diabetic plus 24,25 Vit D corneas, and (F) quantification of cell numbers for each group in (A–E). (B) Magnified image of dendritic-shaped cells from the dashed area in the upper left of (A). The diabetic corneas had fewer dendritic cells than the normoglycemic corneas following cornea abrasion, and topical 24,25 Vit D significantly increased the number of dendritic cells in the diabetic corneas. Individual data points graphed as mean ± SE, n = 5, * p < 0.05.
3.12. Macrophage Recruitment Following 1,25 Vit D and 24,25 Vit D Treatment
Macrophages in the anterior stroma were identified using F4/80 immunostaining 8 h after wounding of the mouse corneas (10 weeks diabetic duration). Figure 12 illustrates significantly reduced macrophage numbers in the anterior stroma of the diabetic (Figure 12B) vs. normoglycemic mice (Figure 12A). When treated with topical 24,25 Vit D, the macrophage numbers were significantly increased in the anterior stroma of these diabetic mice, although not to pre-treatment levels (Figure 12D,E), with no significant change following 1,25 Vit D treatment (Figure 12C,E).
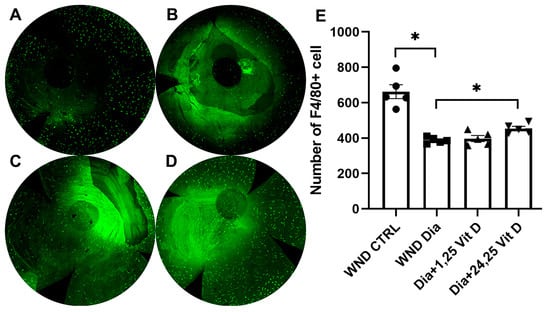
Figure 12.
Representative macrophage labeling (F4/80) in the anterior stroma of (A) control, (B) diabetic, (C) diabetic plus 1,25 Vit D, and (D) diabetic plus 24,25 Vit D corneas. (E) The diabetic corneas had fewer macrophages than the normoglycemic corneas following cornea abrasion, and topical 24,25 Vit D significantly increased the number of macrophages in the diabetic corneas. Individual data points graphed as mean ± SE, n = 5, * p < 0.05.
4. Discussion
Previous studies have found that corneal sensation recovery following laser in situ keratomileusis begins within 4 weeks, with preoperative levels reached after 6 months in the majority of studies [43,44]. Full nerve density regeneration (as opposed to sensation) can take up to 12 or 24 months [45]. The effects of vitamin D on the nervous system have been studied extensively for the past two decades, leading to an accumulation of evidence regarding its roles in the regulation of neurotropic factor synthesis and its neuro-protective actions [46]. However, few papers have explored the effects of vitamin D3 on corneal nerve regeneration, and few commercial eye vitamin formulations include vitamin D3.
Vitamin D3 deficiency is common in diabetic patients. Corneal nerve fiber density, branch density, and nerve fiber length have been shown to be significantly different in diabetic patients relative to controls [3,13]. Previous studies in diabetic mice have determined that the density of sensory nerve fibers was significantly decreased compared to normoglycemic mice [9,13], and a recent report from our group determined that uninjured diabetic VDD and VDR knockout mouse corneas have significantly decreased nerve densities [29]. The current study demonstrates slower corneal nerve regeneration following corneal injury in diabetic mice compared to normoglycemic mice. We also found that vitamin D3 deficiency and VDR KO slow nerve regeneration in both diabetic and normoglycemic mice.
The current study determined that nerve regeneration following corneal injury in diabetic VDR KO mice was increased by the high calcium supplemental diet, although not to WT levels. This is consistent with our previous work demonstrating that unwounded diabetic VDR KO mouse corneas have lower nerve densities than wild-type diabetic mouse corneas, with the same high calcium diet partially restoring this nerve density, but not to the diabetic wild-type value [29]. This indicates that the reduced nerve regeneration observed in the wounded VDR KO mice is likley associated with, but not entirely due to, hypocalcemia induced by VDR KO.
It is generally believed that 24,25 Vit D is an inactive form of vitamin D3. However, previous work from our laboratory has demonstrated that 24,25 Vit D stimulates HCEC proliferation and migration [41]. We also found that 24,25 Vit D is a positive regulator of connexin proteins and gap junction communication in the corneal epithelium [37]. In addition, 24,25 Vit D increases plectin and integrin β4 protein expression in mouse corneas and positively influences cornea desmosome and hemidesmosome junction formation/regulation [33]. Furthermore, in corneal fibroblasts, 24,25 Vit D was found to increase expression of the vitamin D-associated metabolic enzymes 24-hydroxylase and 1α-hydroxylase in the absence VDR KO mice, and thus is likely involved in fibroblast vitamin D metabolism regulation independent of 1,25 Vit D or VDR [34]. The current study adds to the list of potential benefits of 24,25 Vit D for corneal health, demonstrating that nerve regeneration is significantly increased in control and diabetic VDD mice treated topically with 24,25 Vit D.
Complementing the nerve regeneration data and the positive influence of 24,25 Vit D on corneal function, this study determined that 1,25 Vit D and 24,25 Vit D both increase NGF, NTF3, and GDNF protein expression in mouse and human corneal epithelial cells, although GDNF was the only factor found to have its secretion level increased by Vit D (both 1,25 and 24,25). This is in agreement with previous studies showing 1,25 Vit D to be a potent inducer of NGF, GDNF, and NTF3 protein expression [47,48]. In addition, expression levels of NGF and GDNF were found to be reduced in VDD newborn rats [49], and gene expression of NGF is reduced in the brains of VDD rats [50,51]. These results indicate that 1,25 Vit D and 24,25 Vit D-induced synthesis of endogenous neurotropic factors can, at least in part, explain the observed vitamin D stimulation of corneal nerve regeneration following corneal injury.
Previous studies have found that nerve growth factor expression is reduced in the skin and muscle of diabetic patients [52,53]. In addition, a recent study in a diabetic rat model found that oral administration of a vitamin D3 derivative, CB1093, acted as a highly potent inducer of NGF gene expression that prevented depletion of nerve growth factor and the products of its neuronal target genes [54]. It is possible that vitamin D has the potential to act similarly in the cornea of diabetic patients.
Vitamin D has been shown to be an immunomodulatory agent, exerting immunologic activities on multiple components of the innate and adaptive immune system [55]. The current study found that CD45+ cells with a dendriform morphology were significantly decreased in the basal epithelial layer of wounded diabetic mouse corneas compared to normoglycemic mice, with topical 24,25 Vit D increasing their numbers. These results are in agreement with an earlier study from our laboratory in which the corneas received only an epithelial abrasion with no stromal damage, and cells were counted at the level of the highest cell density, as opposed to the basal epithelium, where corneal nerves terminate [19]. Pertinant to the current study, it has been shown that CD45+ cells and macrophages play a pivotal role in the mechanisms involved in peripheral nerve regeneration and degeneration [56]. It is interesting that 24,25 Vit D increased the number of CD45+ cells in the diabetic mice corneas and increased nerve regeneration 8 h after wounding. While not examined in this study, it has been postulated that immune cells can mediate corneal nerve innervation and regeneration through ciliary neurotrophic factor release from immune cells in the cornea [9].
The current study also found a significant decrease in the number of F4/80+ cells in diabetic corneas compared to normoglycemic mouse corneas, with 24,25 Vit D increasing F4/80+ cell numbers in the diabetic mouse corneas 8 h after wounding. Although there are some exceptions, F4/80+ cells in the cornea are generally considered to be macrophages. Cornea macrophages reside throughout the anterior and posterior regions of the corneal stroma [57]. Stromal macrophages contribute to corneal homeostasis and pathology [58], although data demonstrating their precise role in normal corneal homeostasis are still rare [15]. Relevant to this study, corneal macrophages have been shown to promote nerve remodelling after epithelial abrasion [20]. In addition, it was previously determined that macrophages can increase corneal nerve regeneration by stimulating the secretion of neurotrophins, including nerve growth factor (NGF) and neurotrophin 3 (NTF3) [59].
This study has a number of limitations. Western blotting, a semi-quantitative method, was used to measure proteins. To bring this more into the quantitative realm, protein levels were quantified using total protein normalization rather than quantification using a single a housekeeping protein. Total protein quantification takes into account the intensities of all proteins in the lane, sample loading variations, variations during electrophoresis, and variations that occur during transfer. This resolves the inherent difficulties with linearity in the immunodetection of both target and control proteins, resulting in more accurate quantification of the target protein [60]. In addition, while measuring CD45+ and F4/80+ immune cell infiltration in the different mouse models describes the infiltration of two different classes of immune cells, it does not describe specific immune cell type infiltration.
5. Conclusions
This study found that nerve regeneration following corneal injury is significantly delayed by diabetes, VDD, and VDR KO, with compounded delays found in diabetic VDD and VDR KO mice. In addition, nerve regeneration in the VDR KO mice is partially calcium-dependent. Corneal sub-basal nerve regeneration in diabetic and VDD mice was significantly increased following administration of topical 1,25 Vit D and 24,25 Vit D. Topical vitamin D also increased NGF, NTFS, and GDNF corneal epithelial cell protein expression along with secretion of GDNF. Post-injury, CD45+ and F4/80+ cell numbers were significantly decreased 8 h after wounding, and 24,25 Vit D significantly increased the numbers of both cell types, although not to unwounded levels. This increase in immune cell numbers along with the increased expression of neurotrophic factors may contribute to the neural regenerative properties of vitamin D. This study demonstrates the positive role of vitamin D in corneal nerve regeneration and the potential of exogenous vitamin D treatment to improve corneal wound healing outcomes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biom13121754/s1, Figure S1: Uncropped images of Figure 7 western blots; Figure S2: Uncropped images of Figure 8 western blots; Figure S3: Uncropped images of Figure 9 western blots; Figure S4: Uncropped images of Figure 10 western blot.
Author Contributions
X.L. performed experiments, data analysis, and wrote the manuscript, including figure preparation. Z.C. and J.L. performed experiments, data analysis, and manuscript editing. M.A.W. was responsible for the study design, data analysis, and manuscript editing. M.A.W.’s NIH grant, along with the Augusta University’s Vision Discovery Institute P30 award, funded the work performed in this study. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by NIH National Eye Institute grants R01EY021747 and P30 EY031631.
Institutional Review Board Statement
The Augusta University IRB has deemed this study exempt based on the sole use of deidentified corneal tissue obtained from Eye Banks. The animal studies were approved by the Augusta University IACUC (protocol 2013-0581 approved 9 January 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available from the corresponding authors upon request.
Acknowledgments
We are grateful to Amy Estes (Department of Ophthalmology, Medical College of Georgia at Augusta University, Augusta, GA) and The Eye Guys, Eye Physicians, and Surgeons of Augusta, GA for providing donor corneal rims.
Conflicts of Interest
No potential conflicts of interest relevant to this article were reported.
References
- Muller, L.J.; Marfurt, C.F.; Kruse, F.; Tervo, T.M. Corneal nerves: Structure, contents and function. Exp. Eye Res. 2003, 76, 521–542. [Google Scholar] [CrossRef] [PubMed]
- Beuerman, R.W.; Schimmelpfennig, B. Sensory denervation of the rabbit cornea affects epithelial properties. Exp. Neurol. 1980, 69, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, I.N.; Alam, U.; Fadavi, H.; Asghar, O.; Green, P.; Ponirakis, G.; Marshall, A.; Boulton, A.J.; Tavakoli, M.; Malik, R.A. Corneal nerve loss detected with corneal confocal microscopy is symmetrical and related to the severity of diabetic polyneuropathy. Diabetes Care 2013, 36, 3646–3651. [Google Scholar] [CrossRef] [PubMed]
- Bikbova, G.; Oshitari, T.; Baba, T.; Bikbov, M.; Yamamoto, S. Diabetic corneal neuropathy: Clinical perspectives. Clin. Ophthalmol. 2018, 12, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, I.N.; Ponirakis, G.; Ferdousi, M.; Azmi, S.; Kalteniece, A.; Khan, A.; Gad, H.; Bashir, B.; Marshall, A.; Boulton, A.J.M.; et al. Corneal Confocal Microscopy: A Biomarker for Diabetic Peripheral Neuropathy. Clin. Ther. 2021, 43, 1457–1475. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.S.; Yin, J.; Lee, P.; Hwang, F.S.; McDermott, M. Sensory nerve regeneration after epithelium wounding in normal and diabetic cornea. Expert Rev. Ophthalmol. 2015, 10, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.; Pritchard, N.; Poole, C.; Dehghani, C.; Al Rashah, K.; Russell, A.; Malik, R.A.; Efron, N. Development of a Novel Technique to Measure Corneal Nerve Migration Rate. Cornea 2016, 35, 700–705. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Pham, T.L.; Kakazu, A.; Bazan, H.E.P. Recovery of Corneal Sensitivity and Increase in Nerve Density and Wound Healing in Diabetic Mice after PEDF Plus DHA Treatment. Diabetes 2017, 66, 2511–2520. [Google Scholar] [CrossRef]
- Gao, N.; Yan, C.; Lee, P.; Sun, H.; Yu, F.S. Dendritic cell dysfunction and diabetic sensory neuropathy in the cornea. J. Clin. Investig. 2016, 126, 1998–2011. [Google Scholar] [CrossRef]
- Cousen, P.; Cackett, P.; Bennett, H.; Swa, K.; Dhillon, B. Tear production and corneal sensitivity in diabetes. J. Diabetes Complicat. 2007, 21, 371–373. [Google Scholar] [CrossRef]
- Rosenberg, M.E.; Tervo, T.M.; Immonen, I.J.; Muller, L.J.; Gronhagen-Riska, C.; Vesaluoma, M.H. Corneal structure and sensitivity in type 1 diabetes mellitus. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2915–2921. [Google Scholar]
- Hamrah, P.; Seyed-Razavi, Y.; Yamaguchi, T. Translational Immunoimaging and Neuroimaging Demonstrate Corneal Neuroimmune Crosstalk. Cornea 2016, 35 (Suppl. S1), S20–S24. [Google Scholar] [CrossRef]
- Yu, F.X.; Lee, P.S.Y.; Yang, L.; Gao, N.; Zhang, Y.; Ljubimov, A.V.; Yang, E.; Zhou, Q.; Xie, L. The impact of sensory neuropathy and inflammation on epithelial wound healing in diabetic corneas. Prog. Retin. Eye Res. 2022, 89, 101039. [Google Scholar] [CrossRef]
- Liu, J.; Li, Z. Resident Innate Immune Cells in the Cornea. Front. Immunol. 2021, 12, 620284. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Hill, L.J.; Downie, L.E.; Chinnery, H.R. Neuroimmune crosstalk in the cornea: The role of immune cells in corneal nerve maintenance during homeostasis and inflammation. Prog. Retin. Eye Res. 2022, 91, 101105. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E.; Sampaio, L.P.; Shiju, T.M.; de Oliveira, R.C. Fibroblastic and bone marrow-derived cellularity in the corneal stroma. Exp. Eye Res. 2021, 202, 108303. [Google Scholar] [CrossRef] [PubMed]
- Brissette-Storkus, C.S.; Reynolds, S.M.; Lepisto, A.J.; Hendricks, R.L. Identification of a novel macrophage population in the normal mouse corneal stroma. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2264–2271. [Google Scholar]
- Lassance, L.; Marino, G.K.; Medeiros, C.S.; Thangavadivel, S.; Wilson, S.E. Fibrocyte migration, differentiation and apoptosis during the corneal wound healing response to injury. Exp. Eye Res. 2018, 170, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, Z.; Lu, J.; Watsky, M.A. Effects of Topical 1,25 and 24,25 Vitamin D on Diabetic, Vitamin D Deficient and Vitamin D Receptor Knockout Mouse Corneal Wound Healing. Biomolecules 2023, 13, 1065. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xue, Y.; Dong, D.; Xiao, C.; Lin, C.; Wang, H.; Song, F.; Fu, T.; Wang, Z.; Chen, J.; et al. CCR2(-) and CCR2(+) corneal macrophages exhibit distinct characteristics and balance inflammatory responses after epithelial abrasion. Mucosal Immunol. 2017, 10, 1145–1159. [Google Scholar] [CrossRef]
- Chinnery, H.R.; McMenamin, P.G.; Dando, S.J. Macrophage physiology in the eye. Pflugers Arch. 2017, 469, 501–515. [Google Scholar] [CrossRef]
- Lin, Y.; Ubels, J.L.; Schotanus, M.P.; Yin, Z.; Pintea, V.; Hammock, B.D.; Watsky, M.A. Enhancement of vitamin D metabolites in the eye following vitamin D3 supplementation and UV-B irradiation. Curr. Eye Res. 2012, 37, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, P.P.; Gao, X.H.; Tan, J.C.; Evdokiou, A.; Ferrante, A.; Morris, H.A.; May, B.K.; Hii, C.S. A role for the phosphatidylinositol 3-kinase–protein kinase C zeta–Sp1 pathway in the 1,25-dihydroxyvitamin D3 induction of the 25-hydroxyvitamin D3 24-hydroxylase gene in human kidney cells. Cell. Signal. 2010, 22, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Strugnell, S.A.; DeLuca, H.F. Current understanding of the molecular actions of vitamin D. Physiol. Rev. 1998, 78, 1193–1231. [Google Scholar] [CrossRef] [PubMed]
- Grammatiki, M.; Rapti, E.; Karras, S.; Ajjan, R.A.; Kotsa, K. Vitamin D and diabetes mellitus: Causal or casual association? Rev. Endocr. Metab. Disord. 2017, 18, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Norris, J.M.; Lee, H.S.; Frederiksen, B.; Erlund, I.; Uusitalo, U.; Yang, J.; Lernmark, A.; Simell, O.; Toppari, J.; Rewers, M.; et al. Plasma 25-Hydroxyvitamin D Concentration and Risk of Islet Autoimmunity. Diabetes 2018, 67, 146–154. [Google Scholar] [CrossRef]
- Van Belle, T.L.; Gysemans, C.; Mathieu, C. Vitamin D and diabetes: The odd couple. Trends Endocrinol. Metab. 2013, 24, 561–568. [Google Scholar] [CrossRef]
- Angellotti, E.; Pittas, A.G. The Role of Vitamin D in the Prevention of Type 2 Diabetes: To D or Not to D? Endocrinology 2017, 158, 2013–2021. [Google Scholar] [CrossRef]
- Lu, X.; Vick, S.; Chen, Z.; Chen, J.; Watsky, M.A. Effects of Vitamin D Receptor Knockout and Vitamin D Deficiency on Corneal Epithelial Wound Healing and Nerve Density in Diabetic Mice. Diabetes 2020, 69, 1042–1051. [Google Scholar] [CrossRef]
- Calik Basaran, N.; Kiragi, D.; Tan, C.; Ozisik, L.; Cagdas Ayvaz, N.D.; Kocabeyoglu, S.; Oz, S.G.; Irkec, M.; Tezcan, F.I. Ocular Changes and Tear Cytokines in Individuals with Low Serum Vitamin D Levels: A Cross-Sectional, Controlled Study. Ocul. Immunol. Inflamm. online ahead of print. 2023. [Google Scholar] [CrossRef]
- Garcion, E.; Wion-Barbot, N.; Montero-Menei, C.N.; Berger, F.; Wion, D. New clues about vitamin D functions in the nervous system. Trends Endocrinol. Metab. 2002, 13, 100–105. [Google Scholar] [CrossRef]
- Landel, V.; Annweiler, C.; Millet, P.; Morello, M.; Feron, F. Vitamin D, Cognition and Alzheimer’s Disease: The Therapeutic Benefit is in the D-Tails. J. Alzheimer’s Dis. 2016, 53, 419–444. [Google Scholar] [CrossRef]
- Lu, X.; Watsky, M.A. Influence of Vitamin D on Corneal Epithelial Cell Desmosomes and Hemidesmosomes. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4074–4083. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, Z.; Watsky, M.A. Effects of 1,25 and 24,25 Vitamin D on Corneal Fibroblast VDR and Vitamin D Metabolizing and Catabolizing Enzymes. Curr. Eye Res. 2021, 46, 1271–1282. [Google Scholar] [CrossRef]
- Yamakawa, I.; Kojima, H.; Terashima, T.; Katagi, M.; Oi, J.; Urabe, H.; Sanada, M.; Kawai, H.; Chan, L.; Yasuda, H.; et al. Inactivation of TNF-alpha ameliorates diabetic neuropathy in mice. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E844–E852. [Google Scholar] [CrossRef] [PubMed]
- Kollenkirchen, U.; Fox, J.; Walters, M.R. Normocalcemia without Hyperparathyroidism in Vitamin-D-Deficient Rats. J. Bone Miner. Res. 1991, 6, 273–278. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Z.; Vick, S.; Watsky, M.A. Vitamin D receptor and metabolite effects on corneal epithelial cell gap junction proteins. Exp. Eye Res. 2019, 187, 107776. [Google Scholar] [CrossRef]
- Pham, T.L.; Kakazu, A.; He, J.; Bazan, H.E.P. Mouse strains and sexual divergence in corneal innervation and nerve regeneration. FASEB J. 2019, 33, 4598–4609. [Google Scholar] [CrossRef]
- Jiao, H.; Naranjo Golborne, C.; Dando, S.J.; McMenamin, P.G.; Downie, L.E.; Chinnery, H.R. Topographical and Morphological Differences of Corneal Dendritic Cells during Steady State and Inflammation. Ocul. Immunol. Inflamm. 2020, 28, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Yamagami, S.; Yokoo, S.; Usui, T.; Yamagami, H.; Amano, S.; Ebihara, N. Distinct populations of dendritic cells in the normal human donor corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4489–4494. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Z.; Mylarapu, N.; Watsky, M.A. Effects of 1,25 and 24,25 Vitamin D on Corneal Epithelial Proliferation, Migration and Vitamin D Metabolizing and Catabolizing Enzymes. Sci. Rep. 2017, 7, 16951. [Google Scholar] [CrossRef]
- Yin, Z.; Pintea, V.; Lin, Y.; Hammock, B.D.; Watsky, M.A. Vitamin D enhances corneal epithelial barrier function. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7359–7364. [Google Scholar] [CrossRef]
- Reinstein, D.Z.; Archer, T.J.; Gobbe, M.; Bartoli, E. Corneal sensitivity after small-incision lenticule extraction and laser in situ keratomileusis. J. Cataract. Refract. Surg. 2015, 41, 1580–1587. [Google Scholar] [CrossRef]
- Medeiros, C.S.; Santhiago, M.R. Corneal nerves anatomy, function, injury and regeneration. Exp. Eye Res. 2020, 200, 108243. [Google Scholar] [CrossRef] [PubMed]
- Ishii, R.; Shimizu, K.; Igarashi, A.; Kobashi, H.; Kamiya, K. Influence of femtosecond lenticule extraction and small incision lenticule extraction on corneal nerve density and ocular surface: A 1-year prospective, confocal, microscopic study. J. Refract. Surg. 2015, 31, 10–15. [Google Scholar] [CrossRef]
- Filipovic, N.; Ferhatovic, L.; Marelja, I.; Puljak, L.; Grkovic, I. Increased vitamin D receptor expression in dorsal root ganglia neurons of diabetic rats. Neurosci. Lett. 2013, 549, 140–145. [Google Scholar] [CrossRef]
- Naveilhan, P.; Neveu, I.; Wion, D.; Brachet, P. 1,25-Dihydroxyvitamin D3, an inducer of glial cell line-derived neurotrophic factor. Neuroreport 1996, 7, 2171–2175. [Google Scholar] [CrossRef]
- Wion, D.; MacGrogan, D.; Neveu, I.; Jehan, F.; Houlgatte, R.; Brachet, P. 1,25-Dihydroxyvitamin D3 is a potent inducer of nerve growth factor synthesis. J. Neurosci. Res. 1991, 28, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.; Brown, J.; Mackay-Sim, A.; McGrath, J.; Feron, F. Vitamin D3 and brain development. Neuroscience 2003, 118, 641–653. [Google Scholar] [CrossRef]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef]
- Feron, F.; Burne, T.H.; Brown, J.; Smith, E.; McGrath, J.J.; Mackay-Sim, A.; Eyles, D.W. Developmental Vitamin D3 deficiency alters the adult rat brain. Brain Res. Bull. 2005, 65, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Hellweg, R.; Hartung, H.D. Endogenous levels of nerve growth factor (NGF) are altered in experimental diabetes mellitus: A possible role for NGF in the pathogenesis of diabetic neuropathy. J. Neurosci. Res. 1990, 26, 258–267. [Google Scholar] [CrossRef]
- Fernyhough, P.; Diemel, L.T.; Brewster, W.J.; Tomlinson, D.R. Altered neurotrophin mRNA levels in peripheral nerve and skeletal muscle of experimentally diabetic rats. J. Neurochem. 1995, 64, 1231–1237. [Google Scholar] [CrossRef]
- Riaz, S.; Malcangio, M.; Miller, M.; Tomlinson, D.R. A vitamin D(3) derivative (CB1093) induces nerve growth factor and prevents neurotrophic deficits in streptozotocin-diabetic rats. Diabetologia 1999, 42, 1308–1313. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Chen, P.; Piao, X.; Bonaldo, P. Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol. 2015, 130, 605–618. [Google Scholar] [CrossRef]
- Sosnova, M.; Bradl, M.; Forrester, J.V. CD34+ corneal stromal cells are bone marrow-derived and express hemopoietic stem cell markers. Stem Cells 2005, 23, 507–515. [Google Scholar] [CrossRef]
- Chinnery, H.R.; Ruitenberg, M.J.; Plant, G.W.; Pearlman, E.; Jung, S.; McMenamin, P.G. The chemokine receptor CX3CR1 mediates homing of MHC class II-positive cells to the normal mouse corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1568–1574. [Google Scholar] [CrossRef]
- Liu, J.; Wu, M.; He, J.; Xiao, C.; Xue, Y.; Fu, T.; Lin, C.; Dong, D.; Li, Z. Antibiotic-Induced Dysbiosis of Gut Microbiota Impairs Corneal Nerve Regeneration by Affecting CCR2-Negative Macrophage Distribution. Am. J. Pathol. 2018, 188, 2786–2799. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Du, S.; Yu, J.; Yang, X.; Yang, C.; Zhou, D.; Wang, Q.; Qin, S.; Yan, X.; He, L.; et al. Common housekeeping proteins are upregulated in colorectal adenocarcinoma and hepatocellular carcinoma, making the total protein a better “housekeeper”. Oncotarget 2016, 7, 66679–66688. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).