Integrin α6β4 Confers Doxorubicin Resistance in Cancer Cells by Suppressing Caspase-3–Mediated Apoptosis: Involvement of N-Glycans on β4 Integrin Subunit
Abstract
:1. Introduction
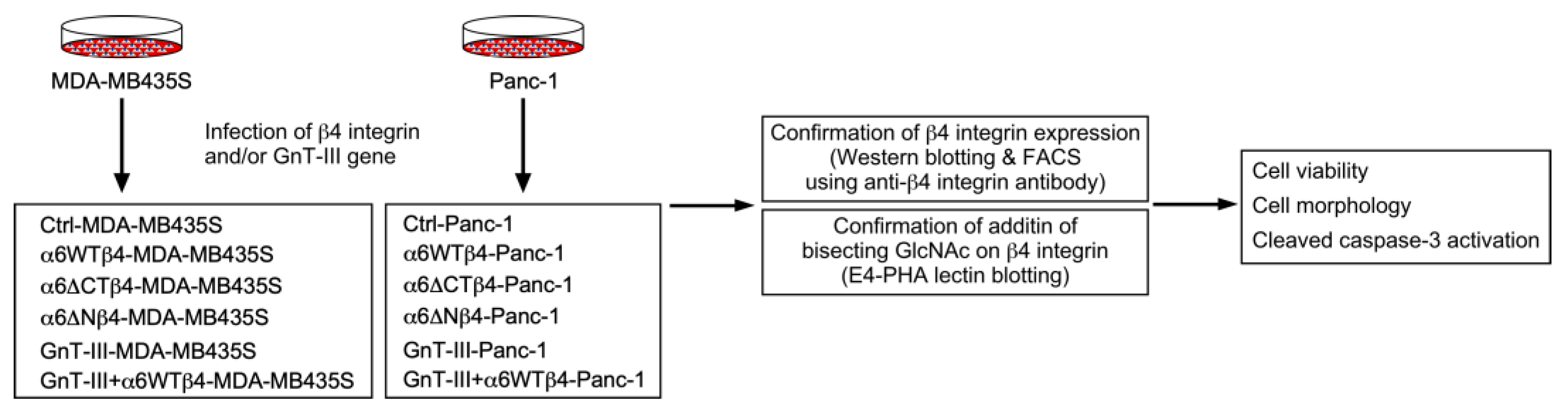
2. Materials and Methods
2.1. Plasmids and Reagents
2.2. Cell Culture
2.3. Immunoprecipitation and Western Blotting
2.4. Cell Viability Assay
2.5. FACS Analysis
2.6. Statistics and Reproducibility
3. Results
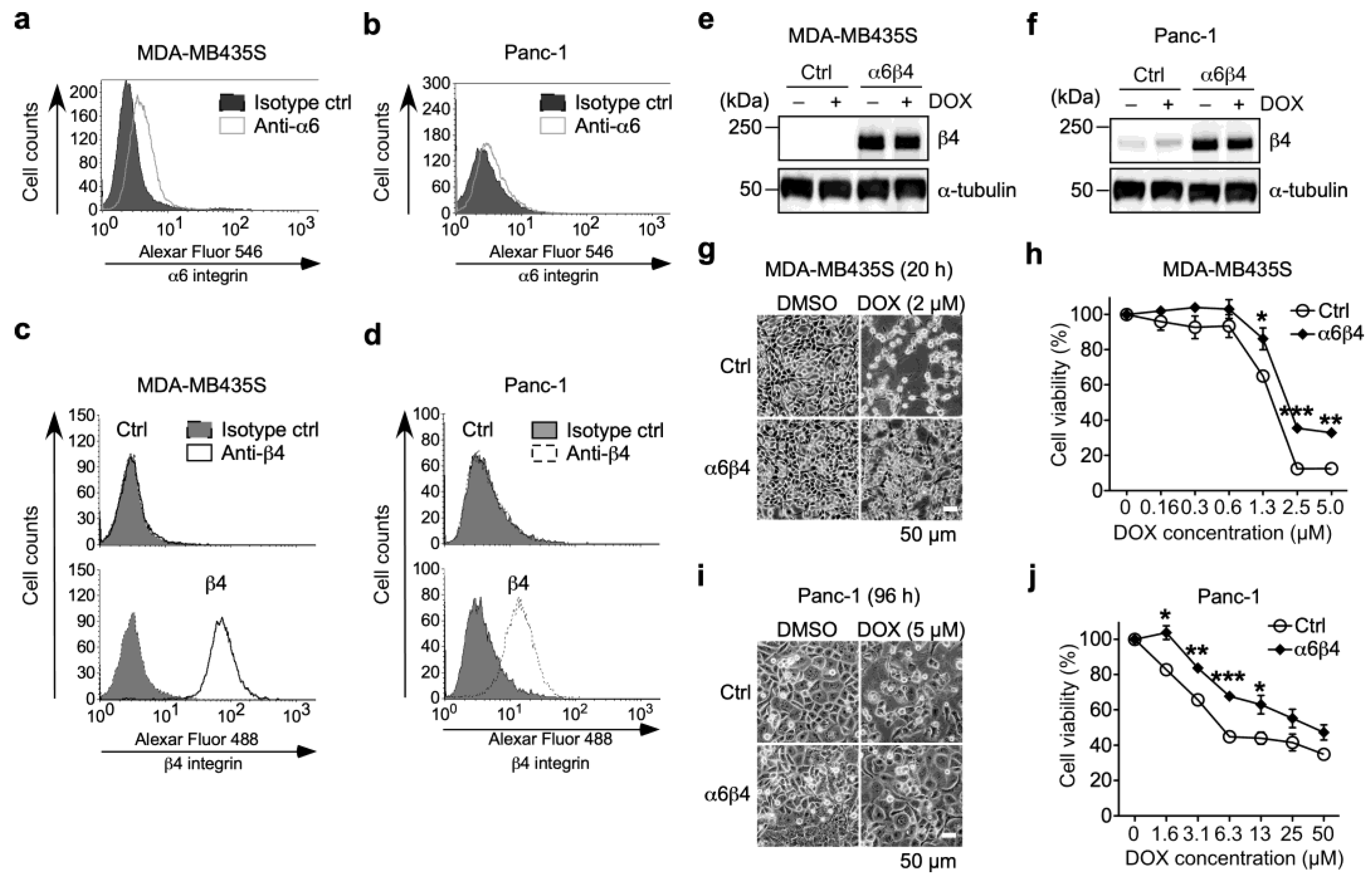
3.1. α6β4 Integrin Expression Is Involved in the Acquisition of DOX Resistance
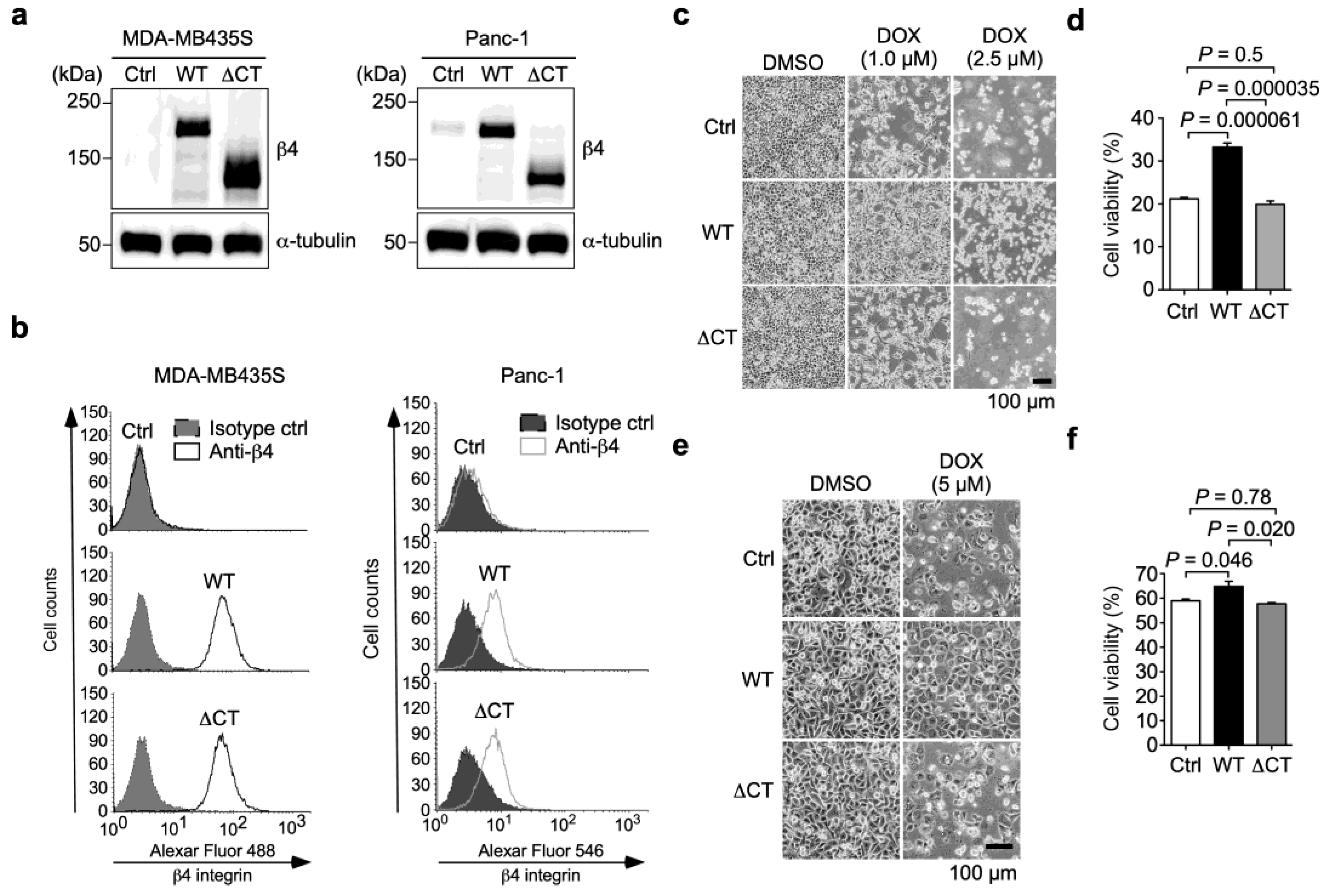
3.2. α6β4 Integrin Signaling Is Involved in the DOX Resistance Acquired by α6β4 Integrin Expression
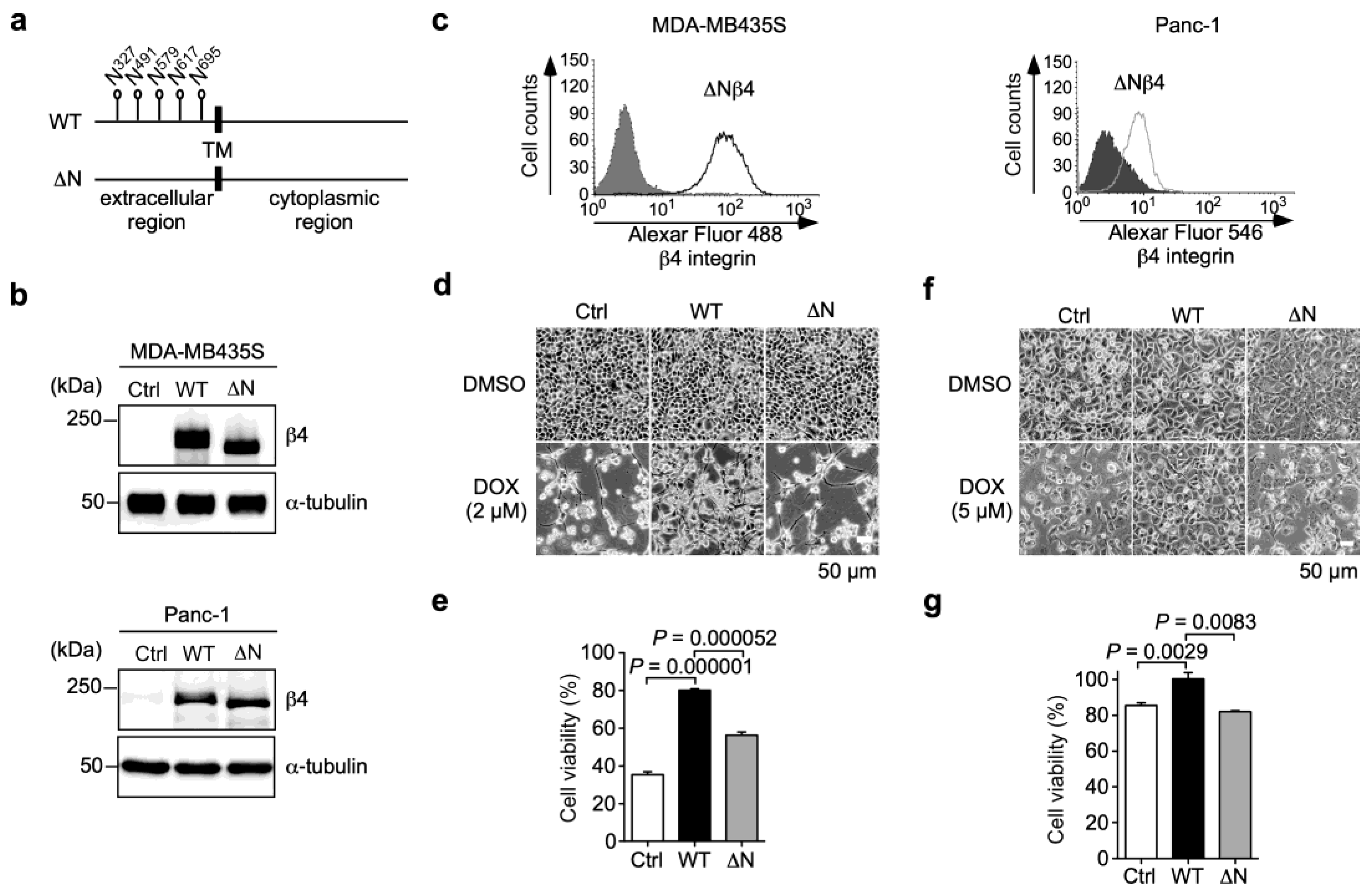
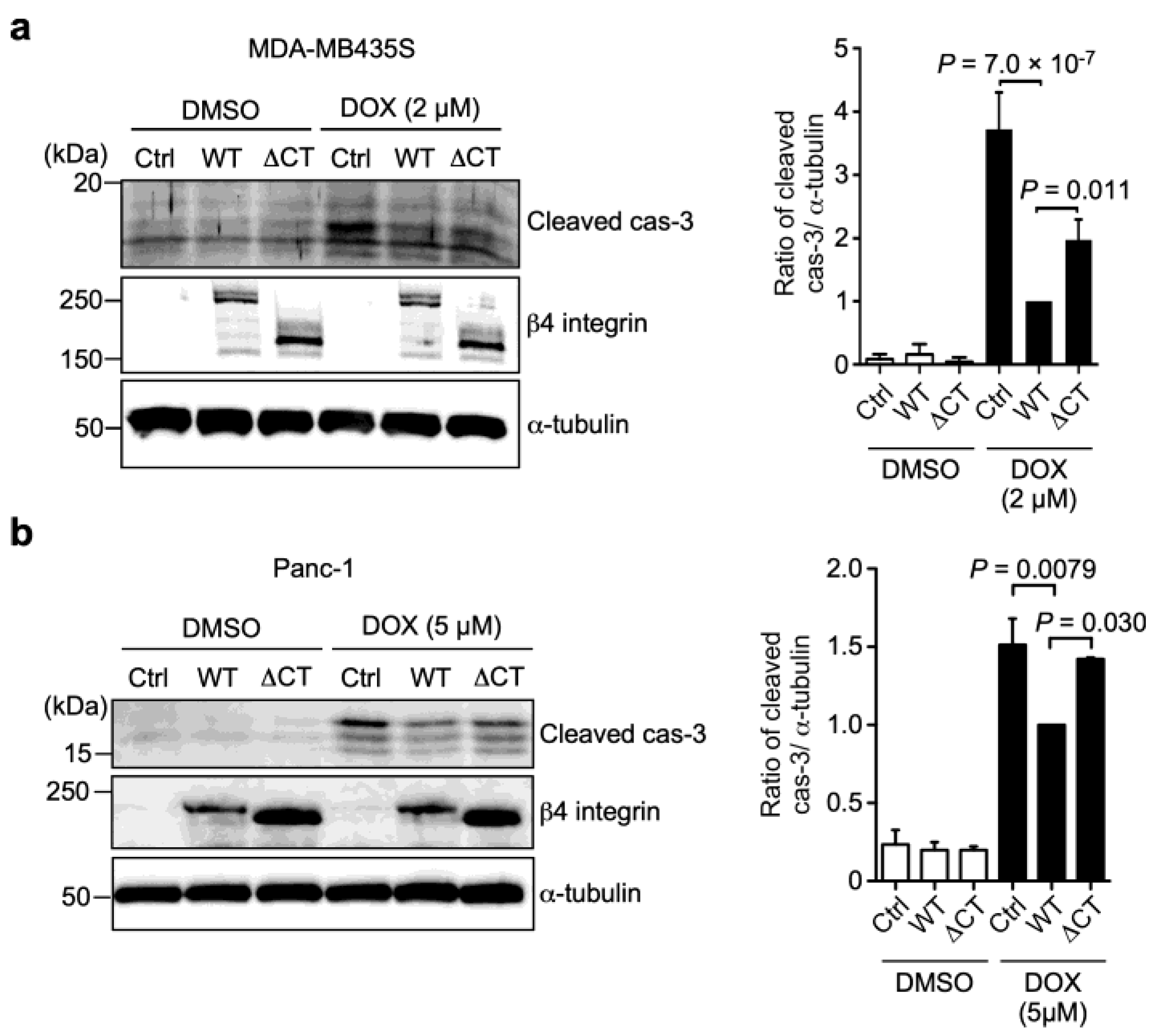
3.3. N-Glycans on β4 Integrin Are Involved in α6β4 Integrin-Mediated DOX Resistance
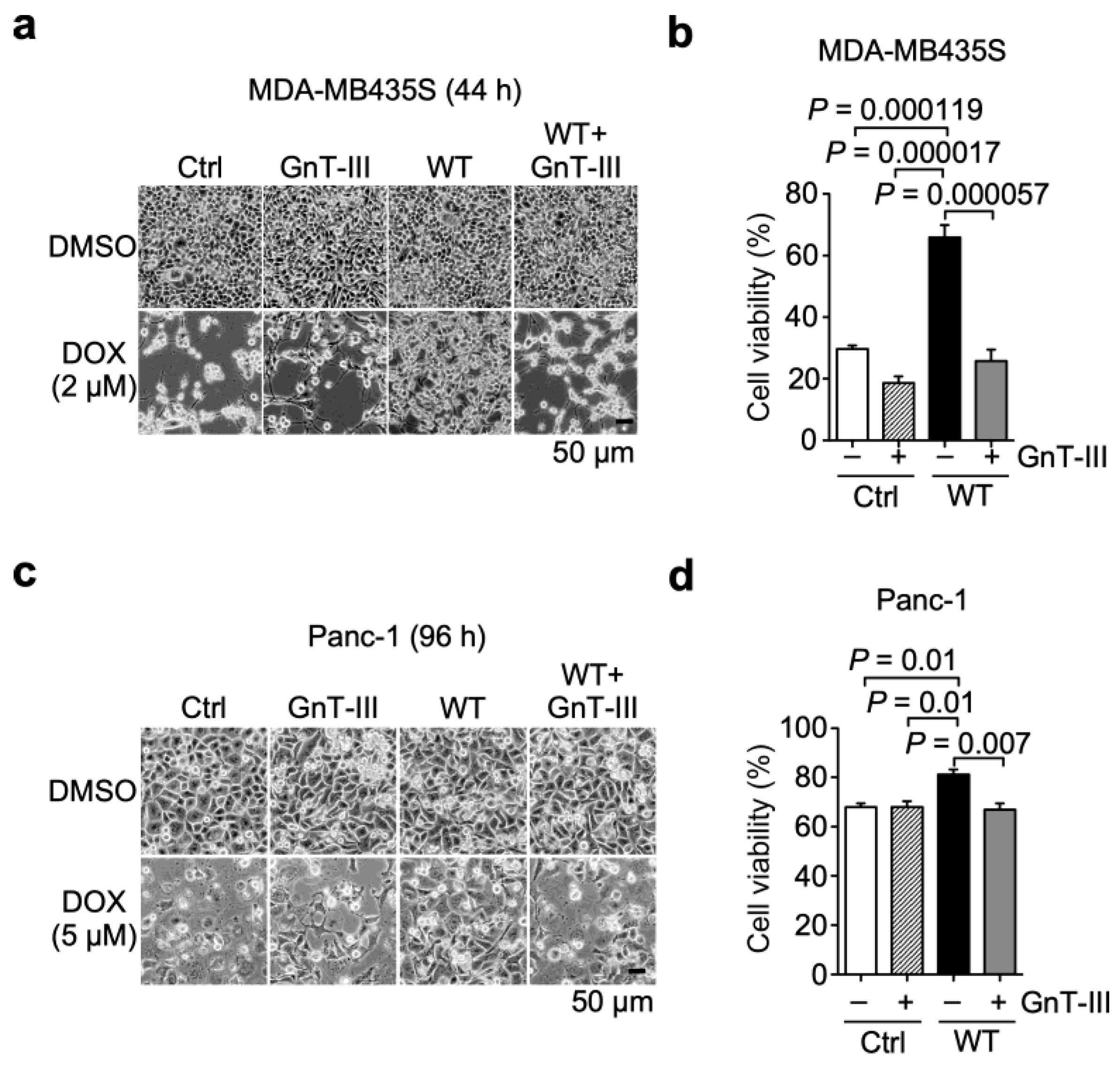
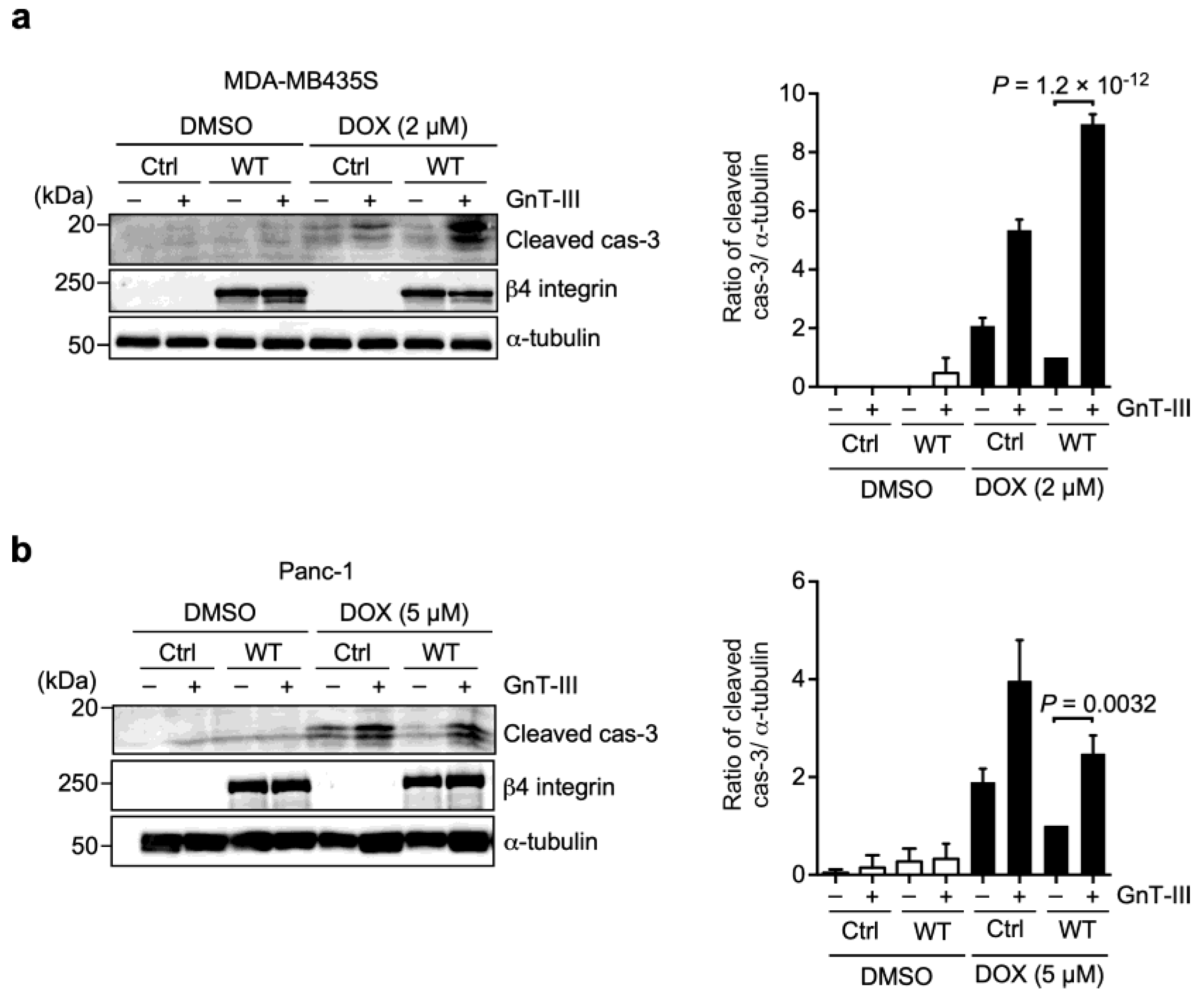
3.4. α6β4 Integrin-Mediated DOX Resistance Is Abolished by the Addition of Bisecting GlcNAc to β4 Integrin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meads, M.B.; Gatenby, R.A.; Dalton, W.S. Environment-mediated drug resistance: A major contributor to minimal residual disease. Nat. Rev. Cancer 2009, 9, 665–674. [Google Scholar] [CrossRef]
- Carabias, P.; Espelt, M.V.; Bacigalupo, M.L.; Rojas, P.; Sarrias, L.; Rubin, A.; Saffioti, N.A.; Elola, M.T.; Rossi, J.P.; Wolfenstein-Todel, C.; et al. Galectin-1 confers resistance to doxorubicin in hepatocellular carcinoma cells through modulation of P-glycoprotein expression. Cell Death Dis. 2022, 13, 79. [Google Scholar] [CrossRef]
- Govaere, O.; Wouters, J.; Petz, M.; Vandewynckel, Y.P.; Van den Eynde, K.; Van den Broeck, A.; Verhulst, S.; Dolle, L.; Gremeaux, L.; Ceulemans, A.; et al. Laminin-332 sustains chemoresistance and quiescence as part of the human hepatic cancer stem cell niche. J. Hepatol. 2016, 64, 609–617. [Google Scholar] [CrossRef]
- Longley, D.B.; Johnston, P.G. Molecular mechanisms of drug resistance. J. Pathol. 2005, 205, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.; Kukkurainen, S.; Hytonen, V.P.; Wehrle-Haller, B. Cell Adhesion by Integrins. Physiol. Rev. 2019, 99, 1655–1699. [Google Scholar] [CrossRef]
- Pang, X.; He, X.; Qiu, Z.; Zhang, H.; Xie, R.; Liu, Z.; Gu, Y.; Zhao, N.; Xiang, Q.; Cui, Y. Targeting integrin pathways: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 1. [Google Scholar] [CrossRef]
- Hosen, N. Integrins in multiple myeloma. Inflamm. Regen. 2020, 40, 4. [Google Scholar] [CrossRef] [PubMed]
- Damiano, J.S.; Cress, A.E.; Hazlehurst, L.A.; Shtil, A.A.; Dalton, W.S. Cell adhesion mediated drug resistance (CAM-DR): Role of integrins and resistance to apoptosis in human myeloma cell lines. Blood 1999, 93, 1658–1667. [Google Scholar] [CrossRef]
- Iyoda, T.; Nagamine, Y.; Nakane, Y.; Tokita, Y.; Akari, S.; Otsuka, K.; Fujita, M.; Itagaki, K.; Takizawa, Y.; Orita, H.; et al. Coadministration of the FNIII14 Peptide Synergistically Augments the Anti-Cancer Activity of Chemotherapeutic Drugs by Activating Pro-Apoptotic Bim. PLoS ONE 2016, 11, e0162525. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, Y.; Lei, Z.; Liu, T.; Cai, T.; Wang, A.; Du, W.; Zeng, Y.; Zhu, J.; Liu, Z.; et al. Abnormally activated OPN/integrin αVβ3/FAK signalling is responsible for EGFR-TKI resistance in EGFR mutant non-small-cell lung cancer. J. Hematol. Oncol. 2020, 13, 169. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Gao, Z.F.; Dong, G.H.; Li, X.; Wu, Y.; Li, G.; Wang, A.L.; Li, H.L.; Yin, D.L. Suppression of alphavbeta6 downregulates P-glycoprotein and sensitizes multidrug-resistant breast cancer cells to anticancer drugs. Neoplasma 2020, 67, 379–388. [Google Scholar] [CrossRef]
- Huang, W.; Fan, L.; Tang, Y.; Chi, Y.; Li, J. A Pan-Cancer Analysis of the Oncogenic Role of Integrin Beta4 (ITGB4) in Human Tumors. Int. J. Gen. Med. 2021, 14, 9629–9645. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xu, Z.; Peng, Y.; Wang, J.; Xiang, Y. Integrin beta4 as a Potential Diagnostic and Therapeutic Tumor Marker. Biomolecules 2021, 11, 1197. [Google Scholar] [CrossRef] [PubMed]
- Kariya, Y.; Kariya, Y.; Gu, J. Roles of Integrin α6β4 Glycosylation in Cancer. Cancers 2017, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Kariya, Y.; Oyama, M.; Hashimoto, Y.; Gu, J.; Kariya, Y. beta4-Integrin/PI3K Signaling Promotes Tumor Progression through the Galectin-3-N-Glycan Complex. Mol. Cancer Res. 2018, 16, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Kariya, Y.; Oyama, M.; Ohtsuka, M.; Kikuchi, N.; Hashimoto, Y.; Yamamoto, T. Quantitative analysis of β1,6GlcNAc-branched N-glycans on β4 integrin in cutaneous squamous cell carcinoma. Fukushima J. Med. Sci. 2020, 66, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef]
- Schjoldager, K.T.; Narimatsu, Y.; Joshi, H.J.; Clausen, H. Global view of human protein glycosylation pathways and functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 729–749. [Google Scholar] [CrossRef]
- Tang, K.; Nie, D.; Cai, Y.; Honn, K.V. The beta4 integrin subunit rescues A431 cells from apoptosis through a PI3K/Akt kinase signaling pathway. Biochem. Biophys. Res. Commun. 1999, 264, 127–132. [Google Scholar] [CrossRef]
- Weaver, V.M.; Lelievre, S.; Lakins, J.N.; Chrenek, M.A.; Jones, J.C.; Giancotti, F.; Werb, Z.; Bissell, M.J. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell 2002, 2, 205–216. [Google Scholar] [CrossRef]
- Lipscomb, E.A.; Simpson, K.J.; Lyle, S.R.; Ring, J.E.; Dugan, A.S.; Mercurio, A.M. The alpha6beta4 integrin maintains the survival of human breast carcinoma cells in vivo. Cancer Res. 2005, 65, 10970–10976. [Google Scholar] [CrossRef]
- Huafeng, J.; Deqing, Z.; Yong, D.; Yulian, Z.; Ailing, H. A cross-talk between integrin beta4 and epidermal growth factor receptor induces gefitinib chemoresistance to gastric cancer. Cancer Cell Int. 2018, 18, 50. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Pylayeva, Y.; Pepe, A.; Yoshioka, T.; Muller, W.J.; Inghirami, G.; Giancotti, F.G. Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell 2006, 126, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Pullar, C.E.; Baier, B.S.; Kariya, Y.; Russell, A.J.; Horst, B.A.; Marinkovich, M.P.; Isseroff, R.R. beta4 integrin and epidermal growth factor coordinately regulate electric field-mediated directional migration via Rac1. Mol. Biol. Cell 2006, 17, 4925–4935. [Google Scholar] [CrossRef] [PubMed]
- Kariya, Y.; Gu, J. N-glycosylation of β4 integrin controls the adhesion and motility of keratinocytes. PLoS ONE 2011, 6, e27084. [Google Scholar] [CrossRef]
- Jacque, J.M.; Triques, K.; Stevenson, M. Modulation of HIV-1 replication by RNA interference. Nature 2002, 418, 435–438. [Google Scholar] [CrossRef]
- Song, W.; Liang, C.; Sun, Y.; Morii, S.; Yomogida, S.; Isaji, T.; Fukuda, T.; Hang, Q.; Hara, A.; Nakano, M.; et al. Expression of GnT-III decreases chemoresistance via negatively regulating P-glycoprotein expression: Involvement of the TNFR2-NF-κB signaling pathway. J. Biol. Chem. 2023, 299, 103051. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca, L.M.; Calvalhan, D.M.; Previato, J.O.; Mendonca Previato, L.; Freire-de-Lima, L. Resistance to paclitaxel induces glycophenotype changes and mesenchymal-to-epithelial transition activation in the human prostate cancer cell line PC-3. Tumour Biol. 2020, 42, 1010428320957506. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Tang, J.; Qin, S.; Shen, X.; He, S.; Ju, S. CAM-DR: Mechanisms, Roles and Clinical Application in Tumors. Front. Cell Dev. Biol. 2021, 9, 698047. [Google Scholar] [CrossRef]
- Shukla, A.; Hillegass, J.M.; MacPherson, M.B.; Beuschel, S.L.; Vacek, P.M.; Pass, H.I.; Carbone, M.; Testa, J.R.; Mossman, B.T. Blocking of ERK1 and ERK2 sensitizes human mesothelioma cells to doxorubicin. Mol. Cancer 2010, 9, 314. [Google Scholar] [CrossRef]
- Lee, E.R.; Kim, J.Y.; Kang, Y.J.; Ahn, J.Y.; Kim, J.H.; Kim, B.W.; Choi, H.Y.; Jeong, M.Y.; Cho, S.G. Interplay between PI3K/Akt and MAPK signaling pathways in DNA-damaging drug-induced apoptosis. Biochim. Biophys. Acta 2006, 1763, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Stanislovas, J.; Kermorgant, S. c-Met-integrin cooperation: Mechanisms, tumorigenic effects, and therapeutic relevance. Front. Cell Dev. Biol. 2022, 10, 994528. [Google Scholar] [CrossRef] [PubMed]
- Gambaletta, D.; Marchetti, A.; Benedetti, L.; Mercurio, A.M.; Sacchi, A.; Falcioni, R. Cooperative signaling between alpha(6)beta(4) integrin and ErbB-2 receptor is required to promote phosphatidylinositol 3-kinase-dependent invasion. J. Biol. Chem. 2000, 275, 10604–10610. [Google Scholar] [CrossRef] [PubMed]
- Kariya, Y.; Kawamura, C.; Tabei, T.; Gu, J. Bisecting GlcNAc residues on laminin-332 down-regulate galectin-3-dependent keratinocyte motility. J. Biol. Chem. 2010, 285, 3330–3340. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Miyoshi, E.; Kameyama, M.; Ishikawa, O.; Kabuto, T.; Sasaki, Y.; Hiratsuka, M.; Ohigashi, H.; Ishiguro, S.; Ito, S.; et al. Expression of N-acetylglucosaminyltransferase V in colorectal cancer correlates with metastasis and poor prognosis. Clin. Cancer Res. 2000, 6, 1772–1777. [Google Scholar]
- Gagnoux-Palacios, L.; Dans, M.; van’t Hof, W.; Mariotti, A.; Pepe, A.; Meneguzzi, G.; Resh, M.D.; Giancotti, F.G. Compartmentalization of integrin alpha6beta4 signaling in lipid rafts. J. Cell Biol. 2003, 162, 1189–1196. [Google Scholar] [CrossRef]
- Yague, E.; Armesilla, A.L.; Harrison, G.; Elliott, J.; Sardini, A.; Higgins, C.F.; Raguz, S. P-glycoprotein (MDR1) expression in leukemic cells is regulated at two distinct steps, mRNA stabilization and translational initiation. J. Biol. Chem. 2003, 278, 10344–10352. [Google Scholar] [CrossRef]
- van der Zanden, S.Y.; Qiao, X.; Neefjes, J. New insights into the activities and toxicities of the old anticancer drug doxorubicin. FEBS J. 2021, 288, 6095–6111. [Google Scholar] [CrossRef]
- Nakahara, S.; Miyoshi, E.; Noda, K.; Ihara, S.; Gu, J.; Honke, K.; Inohara, H.; Kubo, T.; Taniguchi, N. Involvement of oligosaccharide changes in alpha5beta1 integrin in a cisplatin-resistant human squamous cell carcinoma cell line. Mol. Cancer Ther. 2003, 2, 1207–1214. [Google Scholar]
- Bejarano, L.; Jordao, M.J.C.; Joyce, J.A. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discov. 2021, 11, 933–959. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergun, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2019, 6, 160. [Google Scholar] [CrossRef] [PubMed]
- Kariya, Y.; Kariya, Y. Osteopontin in Cancer: Mechanisms and Therapeutic Targets. Int. J. Trans. Med. 2022, 2, 419–447. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kariya, Y.; Gu, J.; Kariya, Y. Integrin α6β4 Confers Doxorubicin Resistance in Cancer Cells by Suppressing Caspase-3–Mediated Apoptosis: Involvement of N-Glycans on β4 Integrin Subunit. Biomolecules 2023, 13, 1752. https://doi.org/10.3390/biom13121752
Kariya Y, Gu J, Kariya Y. Integrin α6β4 Confers Doxorubicin Resistance in Cancer Cells by Suppressing Caspase-3–Mediated Apoptosis: Involvement of N-Glycans on β4 Integrin Subunit. Biomolecules. 2023; 13(12):1752. https://doi.org/10.3390/biom13121752
Chicago/Turabian StyleKariya, Yoshinobu, Jianguo Gu, and Yukiko Kariya. 2023. "Integrin α6β4 Confers Doxorubicin Resistance in Cancer Cells by Suppressing Caspase-3–Mediated Apoptosis: Involvement of N-Glycans on β4 Integrin Subunit" Biomolecules 13, no. 12: 1752. https://doi.org/10.3390/biom13121752
APA StyleKariya, Y., Gu, J., & Kariya, Y. (2023). Integrin α6β4 Confers Doxorubicin Resistance in Cancer Cells by Suppressing Caspase-3–Mediated Apoptosis: Involvement of N-Glycans on β4 Integrin Subunit. Biomolecules, 13(12), 1752. https://doi.org/10.3390/biom13121752






