Exploring the Potential Mechanism of Action of Piperine against Candida albicans and Targeting Its Virulence Factors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Common Experimental Procedures
2.2. Phytochemical Extract
2.3. Purification of Piperine by Centrifugal Partition Chromatography (CPC)
2.4. Fungal Strains and Growth Conditions
2.5. Antifungal Activity Assay
2.6. Time-Kill Kinetic Assay
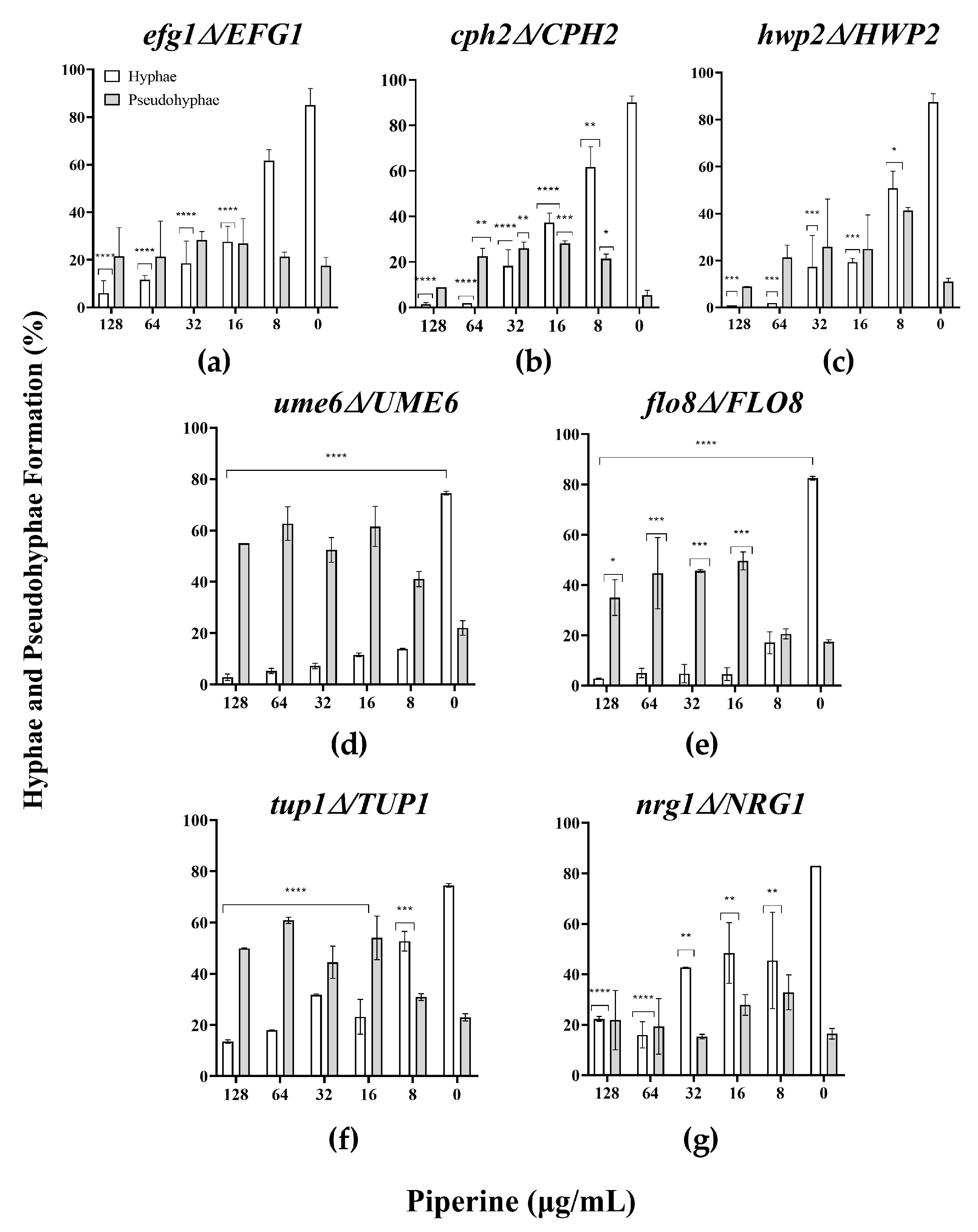
2.7. Hyphal Morphogenesis of Candida albicans
2.8. Formation of C. albicans Biofilm
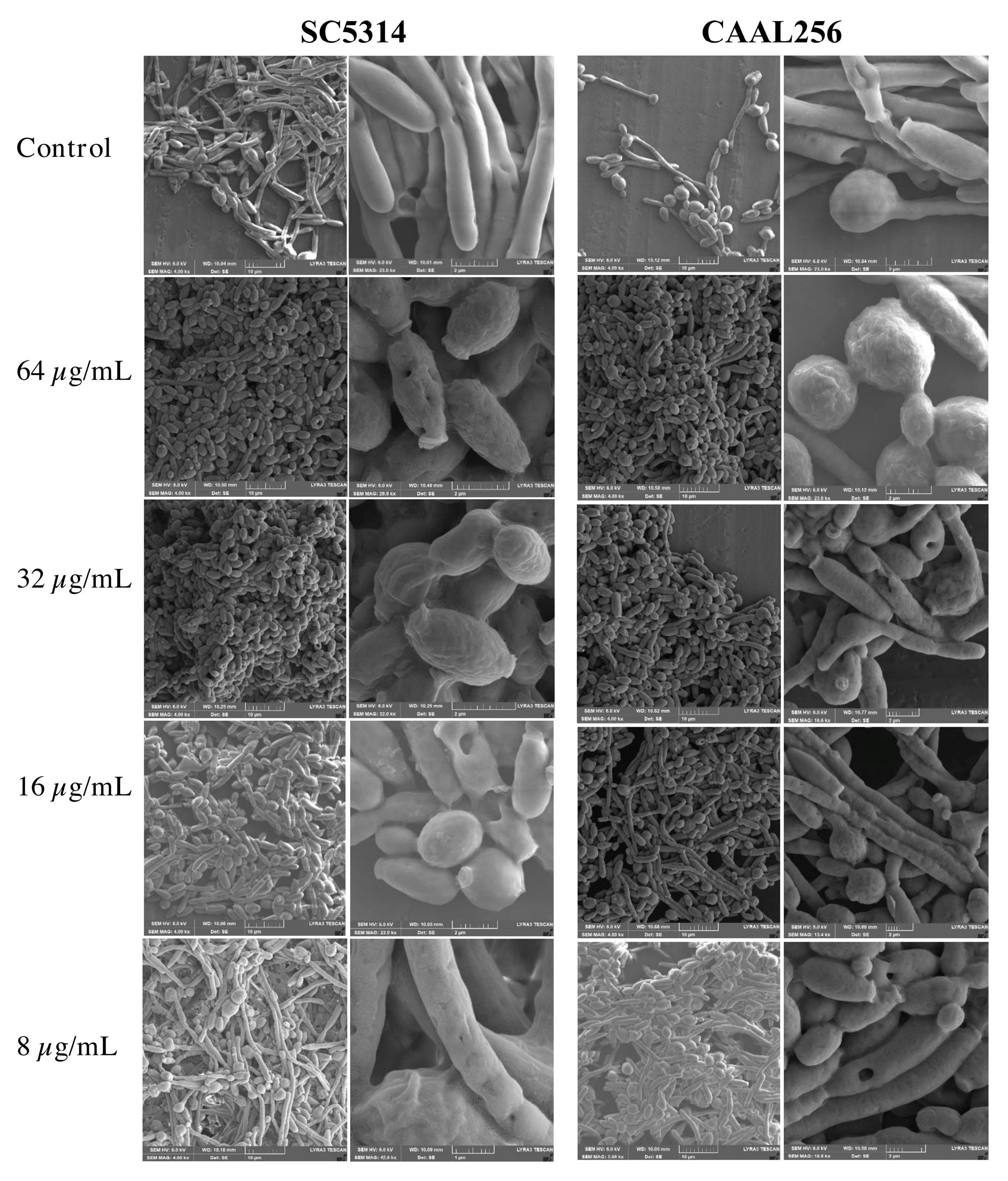
2.9. Scanning Electron Microscopy (SEM)
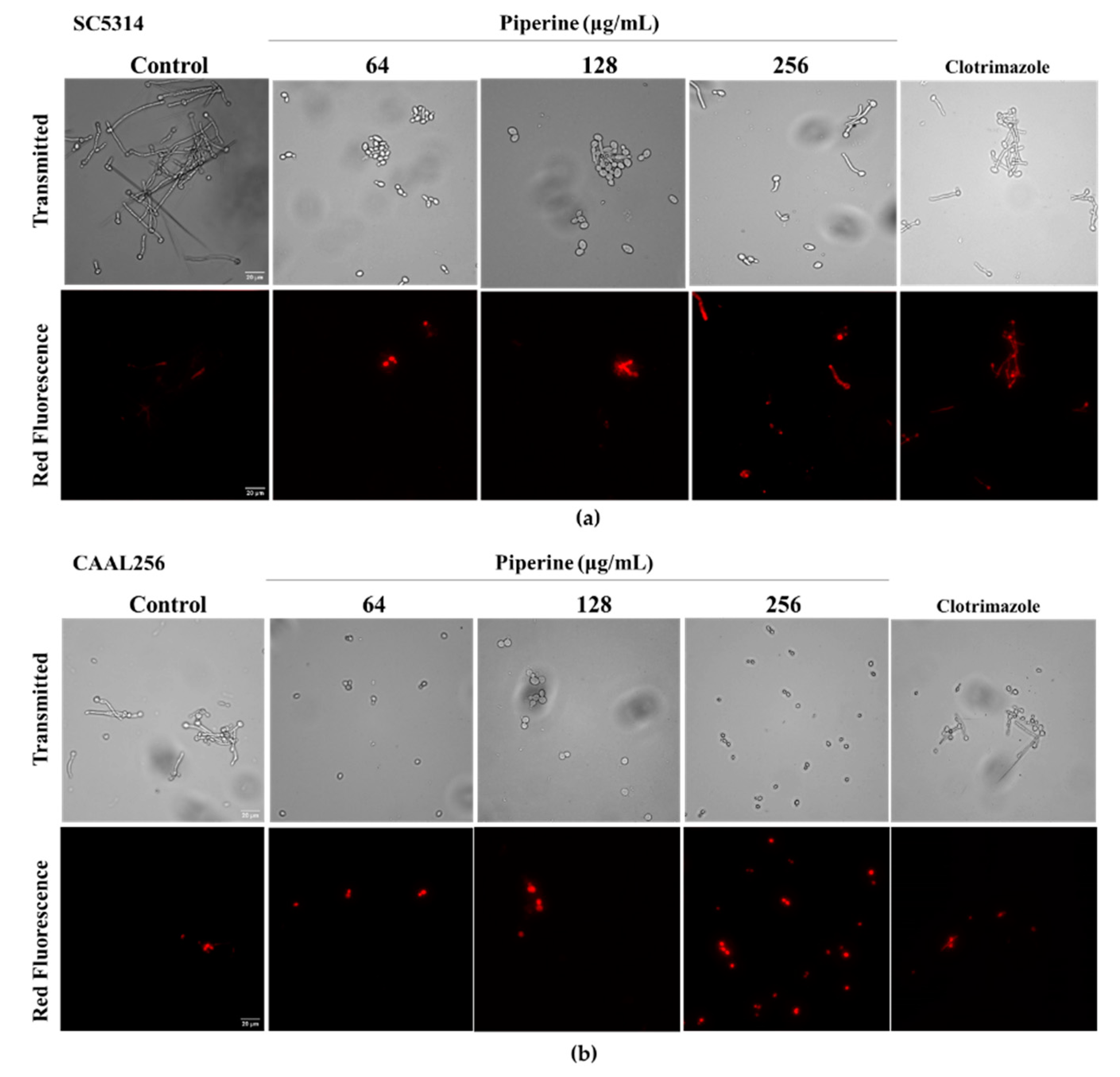
2.10. Membrane Permeabilization Assay
2.11. Measurement of Mitochondrial Membrane Potential
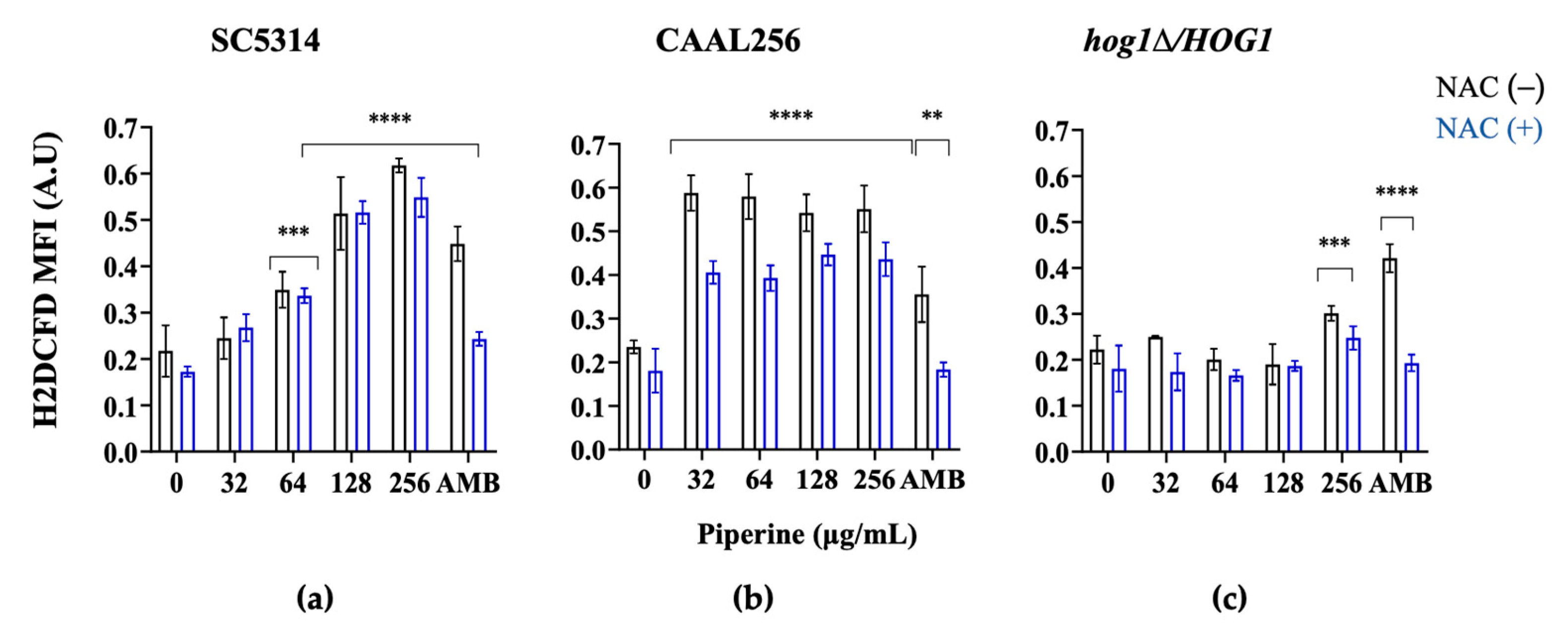
2.12. ROS Detection
2.13. Checkerboard Assay
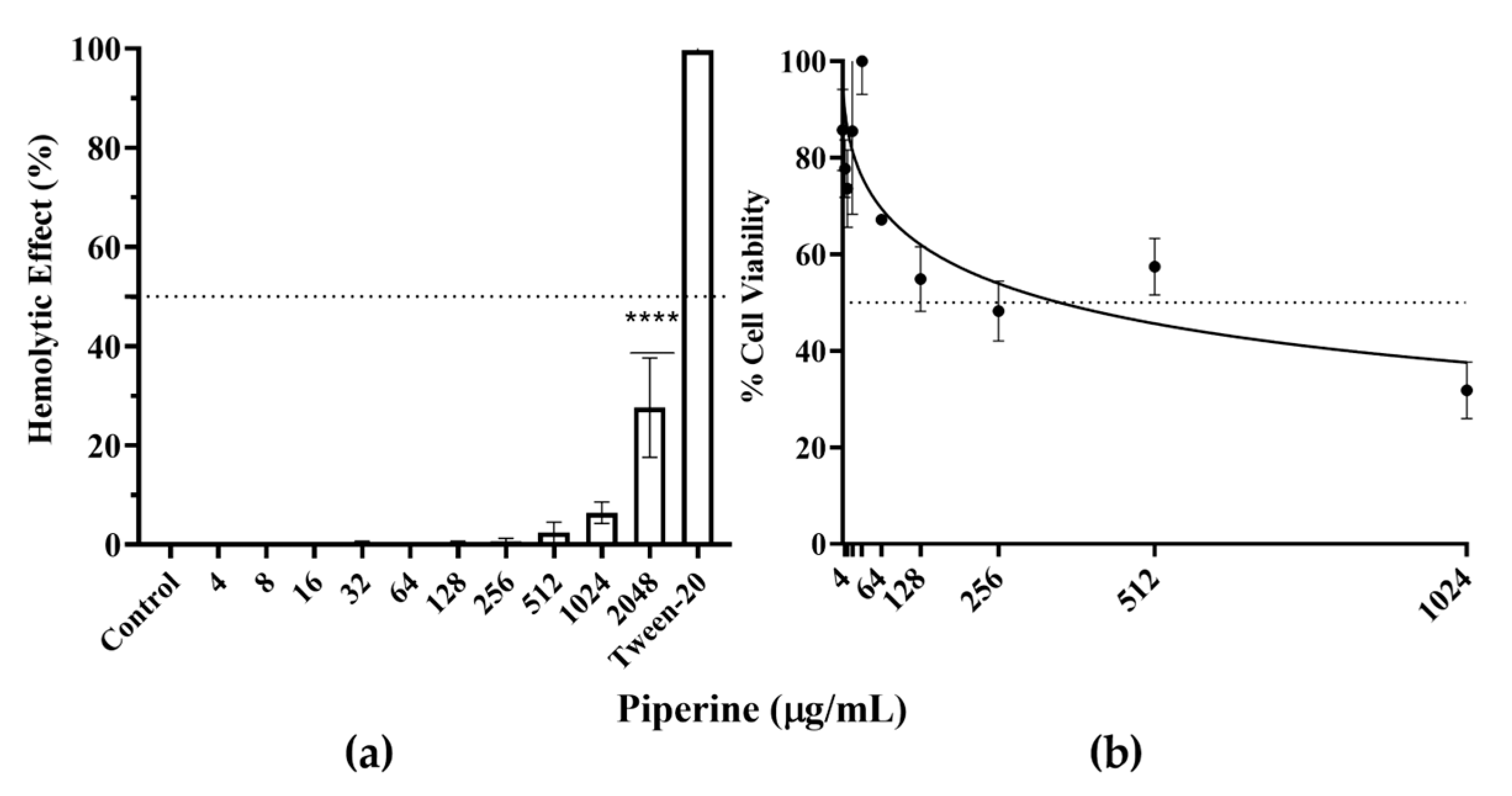
2.14. Hemolytic Activity Assay
2.15. Cell Viability Assay
2.16. Statistical Analysis
3. Results and Discussion
3.1. Structural Elucidation of Isolated Piperine
3.2. Piperine Inhibit C. albicans Growth
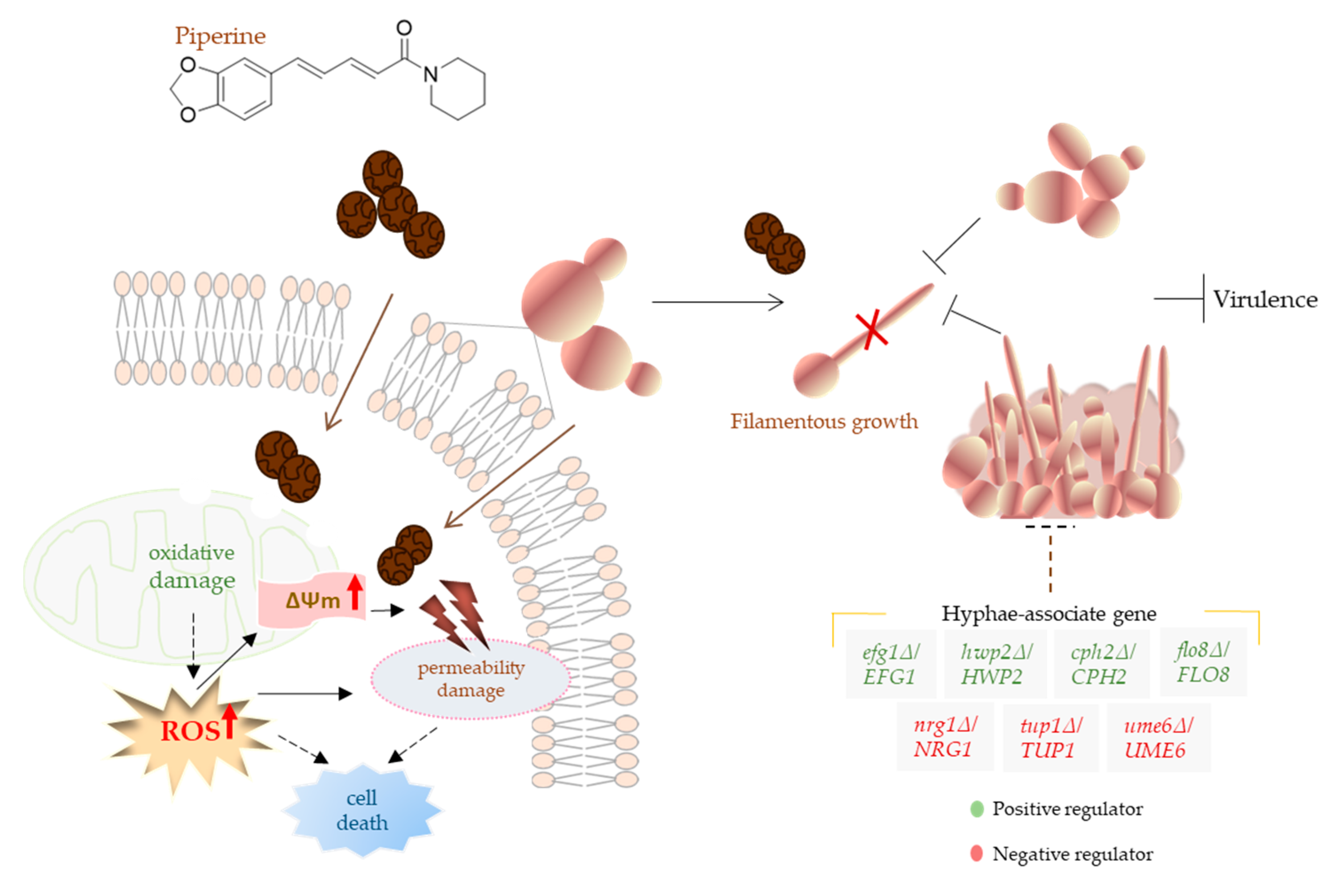
3.3. Piperine Inhibits Biofilm Formation and Hyphal Morphogenesis of C. albicans
3.4. Piperine Affects Membrane Permeability of C. albicans
3.5. Piperine Increases Intracellular Concentration of ROS and Causes Disruption of Mitochondrial Membrane Potential
3.6. Piperine Interact on the Growth of C. albicans with Fluconazole and NAC
3.7. Piperine Shows No Appreciable Toxicity against Human Red Blood Cells and Fibroblast Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nobile, C.J.; Johnson, A.D. Candida albicans Biofilms and Human Disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.; Sherry, L.; Nile, C.J.; Sherriff, A.; Johnson, E.M.; Hanson, M.F.; Williams, C.; Munro, C.A.; Jones, B.J.; Ramage, G. Biofilm Formation Is a Risk Factor for Mortality in Patients with Candida albicans Bloodstream Infection—Scotland, 2012–2013. Clin. Microbiol. Infect. 2016, 22, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Lockhart, S.R.; Berkow, E.L.; Calandra, T. Changes in the Epidemiological Landscape of Invasive Candidiasis. J. Antimicrob. Chemother. 2018, 73, i4–i13. [Google Scholar] [CrossRef] [PubMed]
- Perfect, J.R. The Antifungal Pipeline: A Reality Check. Nat. Rev. Drug Discov. 2017, 16, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Phuna, Z.X.; Yu, J.K.E.; Tee, J.Y.; Chuah, S.Q.; Tan, N.W.H.; Vijayabalan, S.; Abdul Manap, A.S.; Sisinthy, S.P.; Madhavan, P. In Vitro Evaluation of Nanoemulsions of Curcumin, Piperine, and Tualang Honey as Antifungal Agents for Candida Species. J. Appl. Biotechnol. Rep. 2020, 7, 189–197. [Google Scholar]
- Gow, N.A.R.; Brown, A.J.P.; Odds, F.C. Fungal Morphogenesis and Host Invasion. Curr. Opin. Microbiol. 2002, 5, 366–371. [Google Scholar] [CrossRef]
- Vediyappan, G.; Dumontet, V.; Pelissier, F.; d’Enfert, C. Gymnemic Acids Inhibit Hyphal Growth and Virulence in Candida albicans. PLoS ONE 2013, 8, e74189. [Google Scholar] [CrossRef]
- Ramage, G.; Saville, S.P.; Thomas, D.P.; Lopez-Ribot, J.L. Candida Biofilms: An Update. Eukaryot. Cell 2005, 4, 633–638. [Google Scholar] [CrossRef]
- Tsang, P.W.K.; Bandara, H.M.H.N.; Fong, W.P. Purpurin Suppresses Candida albicans Biofilm Formation and Hyphal Development. PLoS ONE 2012, 7, e50866. [Google Scholar] [CrossRef]
- Tsui, C.; Kong, E.F.; Jabra-Rizk, M.A. Pathogenesis of Candida albicans Biofilm. Pathog. Dis. 2016, 74, ftw018. [Google Scholar] [CrossRef]
- Lopes, G.; Pinto, E.; Andrade, P.B.; Valentao, P. Antifungal Activity of Phlorotannins against Dermatophytes and Yeasts: Approaches to the Mechanism of Action and Influence on Candida albicans Virulence Factor. PLoS ONE 2013, 8, e72203. [Google Scholar] [CrossRef] [PubMed]
- Shlezinger, N.; Goldfinger, N.; Sharon, A. Apoptotic-like Programed Cell Death in Fungi: The Benefits in Filamentous Species. Front. Oncol. 2012, 2, 97. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, D.G. Novel Approaches for Efficient Antifungal Drug Action. J. Microbiol. Biotechnol. 2018, 28, 1771–1781. [Google Scholar] [CrossRef]
- Zida, A.; Bamba, S.; Yacouba, A.; Ouedraogo-Traore, R.; Guiguemdé, R.T. Anti-Candida albicans Natural Products, Sources of New Antifungal Drugs: A Review. J. Mycol. Med. 2017, 27, 1–19. [Google Scholar] [CrossRef]
- Bravo-Chaucanes, C.P.; Vargas-casanova, Y.; Chitiva-chitiva, L.C.; Ceballos-garzon, A.; Modesti-costa, G.; Parra-giraldo, C.M. Evaluation of Anti- Candida Potential of Piper nigrum Extract in Inhibiting Growth, Yeast-Hyphal Transition, Virulent Enzymes, and Biofilm Formation. J. Fungi 2022, 8, 784. [Google Scholar] [CrossRef]
- Wu, S.; Pang, Y.; He, Y.; Zhang, X.; Peng, L.; Guo, J.; Zeng, J. A Comprehensive Review of Natural Products against Atopic Dermatitis: Flavonoids, Alkaloids, Terpenes, Glycosides and Other Compounds. Biomed. Pharmacother. 2021, 140, 111741. [Google Scholar] [CrossRef]
- Stojanović-Radić, Z.; Pejčić, M.; Dimitrijević, M.; Aleksić, A.; V Anil Kumar, N.; Salehi, B.; C Cho, W.; Sharifi-Rad, J. Piperine-a Major Principle of Black Pepper: A Review of Its Bioactivity and Studies. Appl. Sci. 2019, 9, 4270. [Google Scholar] [CrossRef]
- Aldaly, Z.T.K. Antimicrobial Activity of Piperine Purified from Piper nigrum. J. Basrah Res. 2010, 36, 54–61. [Google Scholar]
- Hikal, D.M. Antibacterial Activity of Piperine and Black Pepper Oil. Biosci. Biotechnol. Res. Asia 2018, 15, 877. [Google Scholar] [CrossRef]
- Maitra, J. Synergistic Effect of Piperine, Extracted from Piper nigrum, with Ciprofloxacin on Escherichia coli, Bacillus subtilis. Pharm. Sin. 2017, 8, 29–34. [Google Scholar]
- Priya, A.; Pandian, S.K. Piperine Impedes Biofilm Formation and Hyphal Morphogenesis of Candida albicans. Front. Microbiol. 2020, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Thakre, A.; Jadhav, V.; Kazi, R.; Shelar, A.; Patil, R.; Kharat, K.; Zore, G.; Karuppayil, S.M. Oxidative Stress Induced by Piperine Leads to Apoptosis in Candida albicans. Med. Mycol. 2021, 59, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Chanda, S.; Rakholiya, K. Combination Therapy: Synergism between Natural Plant Extracts and Antibiotics against Infectious Diseases. Microbiol. Book Series 2011, 1, 520–529. [Google Scholar]
- Kanaki, N.; Dave, M.; Padh, H.; Rajani, M. A Rapid Method for Isolation of Piperine from the Fruits of Piper nigrum Linn. J. Nat. Med. 2008, 62, 281–283. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, J.B.; Zhang, J.; Li, X.J.; Shen, M.H. One Step Purification of Piperine Directly from Piper nigrum L. by High Performance Centrifugal Partition Chromatography. Sep. Sci. Technol. 2009, 44, 1884–1893. [Google Scholar] [CrossRef]
- Epstein, W.W.; Netz, D.F.; Seidel, J.L. Isolation of Piperine from Black Pepper. J. Chem. Educ. 1993, 70, 598. [Google Scholar] [CrossRef]
- Saha, K.C.; Seal, H.P.; Noor, M.A. Isolation and Characterization of Piperine from the Fruits of Black Pepper (Piper nigrum). J. Bangladesh Agric. Univ. 2013, 11, 11–16. [Google Scholar] [CrossRef]
- Ceballos-Garzon, A.; Wintaco-Martínez, L.M.; Velez, N.; Hernandez-Padilla, C.; De la Hoz, A.; Valderrama-Beltrán, S.L.; Alvarez-Moreno, C.A.; Le Pape, P.; Ramírez, J.D.; Parra-Giraldo, C.M. Persistence of Clonal Azole-Resistant Isolates of Candida albicans from a Patient with Chronic Mucocutaneous Candidiasis in Colombia. J. Glob. Infect. Dis. 2020, 12, 16. [Google Scholar]
- Xu, D.; Jiang, B.; Ketela, T.; Lemieux, S.; Veillette, K.; Martel, N.; Davison, J.; Sillaots, S.; Trosok, S.; Bachewich, C. Genome-Wide Fitness Test and Mechanism-of-Action Studies of Inhibitory Compounds in Candida albicans. PLoS Pathog. 2007, 3, e92. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. CLSI Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi: Approved Standard-Second Edition.CLSI Document. CLSI document M27-A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard—Second Edition Serving the World’s Medical Science Community through Voluntary Consensus; NCCLS: Wayne, PA, USA, 2017; Volume 22, ISBN 1562384694. [Google Scholar]
- Alexander, B.D.; Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Suscetibility Testing of Yeasts; Document M27-A4; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2012. [Google Scholar]
- Pfaller, M.A.; Diekema, D. Progress in Antifungal Susceptibility Testing of Candida Spp. by Use of Clinical and Laboratory Standards Institute Broth Microdilution Methods, 2010 to 2012. J. Clin. Microbiol. 2012, 50, 2846–2856. [Google Scholar] [CrossRef]
- Bagiu, R.V.; Vlaicu, B.; Butnariu, M. Chemical Composition and in Vitro Antifungal Activity Screening of the Allium ursinum L. (Liliaceae). Int. J. Mol. Sci. 2012, 13, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Kim, J.-G.; Kim, K.-Y. Anti-Candida Potential of Sclareol in Inhibiting Growth, Biofilm Formation, and Yeast–Hyphal Transition. J. Fungi 2023, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Sheehan, D.J.; Rex, J.H. Determination of Fungicidal Activities against Yeasts and Molds: Lessons Learned from Bactericidal Testing and the Need for Standardization. Clin. Microbiol. Rev. 2004, 17, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, Y.G.; Choi, P.; Ham, J.; Park, J.G.; Lee, J. Antibiofilm and Antivirulence Activities of 6-Gingerol and 6-Shogaol against Candida albicans Due to Hyphal Inhibition. Front. Cell. Infect. Microbiol. 2018, 8, 299. [Google Scholar] [CrossRef] [PubMed]
- Iadnut, A.; Mamoon, K.; Thammasit, P.; Pawichai, S.; Tima, S.; Preechasuth, K.; Kaewkod, T.; Tragoolpua, Y.; Tragoolpua, K. In Vitro Antifungal and Antivirulence Activities of Biologically Synthesized Ethanolic Extract of Propolis-Loaded PLGA Nanoparticles against Candida albicans. Evid.-Based Complement. Altern. Med. 2019, 2019, 3715481. [Google Scholar] [CrossRef]
- Chaves, G.M.; da Silva, W.P. Superoxide Dismutases and Glutaredoxins Have a Distinct Role in the Response of Candida albicans to Oxidative Stress Generated by the Chemical Compounds Menadione and Diamide. Mem. Inst. Oswaldo Cruz. 2012, 107, 998–1005. [Google Scholar] [CrossRef]
- Roscetto, E.; Contursi, P.; Vollaro, A.; Fusco, S.; Notomista, E.; Catania, M.R. Antifungal and Anti-Biofilm Activity of the First Cryptic Antimicrobial Peptide from an Archaeal Protein against Candida Spp. Clinical Isolates. Sci. Rep. 2018, 8, 17570. [Google Scholar] [CrossRef]
- Mishra, B.; Leishangthem, G.D.; Gill, K.; Singh, A.K.; Das, S.; Singh, K.; Xess, I.; Dinda, A.; Kapil, A.; Patro, I.K. A Novel Antimicrobial Peptide Derived from Modified N-Terminal Domain of Bovine Lactoferrin: Design, Synthesis, Activity against Multidrug-Resistant Bacteria and Candida. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 677–686. [Google Scholar] [CrossRef]
- Friedman, P.L.; Ellisman, M.H. Enhanced Visualization of Peripheral Nerve and Sensory Receptors in the Scanning Electron Microscope Using Cryofracture and Osmium-Thiocarbohydrazide-Osmium Impregnation. J. Neurocytol. 1981, 10, 111–131. [Google Scholar] [CrossRef]
- Brana, C.; Benham, C.; Sundstrom, L. A Method for Characterising Cell Death in Vitro by Combining Propidium Iodide Staining with Immunohistochemistry. Brain Res. Protoc. 2002, 10, 109–114. [Google Scholar] [CrossRef]
- Chang, C.-K.; Kao, M.-C.; Lan, C.-Y. Antimicrobial Activity of the Peptide LfcinB15 against Candida albicans. J. Fungi 2021, 7, 519. [Google Scholar] [CrossRef] [PubMed]
- Endo, E.H.; Cortez, D.A.G.; Ueda-Nakamura, T.; Nakamura, C.V.; Dias Filho, B.P. Potent Antifungal Activity of Extracts and Pure Compound Isolated from Pomegranate Peels and Synergism with Fluconazole against Candida albicans. Res. Microbiol. 2010, 161, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Solarte, V.A.; Rosas, J.E.; Rivera, Z.J.; Arango-Rodríguez, M.L.; García, J.E.; Vernot, J.P. A Tetrameric Peptide Derived from Bovine Lactoferricin Exhibits Specific Cytotoxic Effects against Oral Squamous-Cell Carcinoma Cell Lines. Biom. Res. Int. 2015, 2015, 20497–20504. [Google Scholar] [CrossRef] [PubMed]
- Barragán-Cárdenas, A.; Urrea-Pelayo, M.; Niño-Ramírez, V.A.; Umaña-Pérez, A.; Vernot, J.P.; Parra-Giraldo, C.M.; Fierro-Medina, R.; Rivera-Monroy, Z.; García-Castañeda, J. Selective Cytotoxic Effect against the MDA-MB-468 Breast Cancer Cell Line of the Antibacterial Palindromic Peptide Derived from Bovine Lactoferricin. RSC Adv. 2020, 10, 17593–17601. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, A.M.; Castro, R.H.A.; Cordeiro, R.P.; Peixoto Sobrinho, T.J.S.; Castro, V.; Amorim, E.L.C.; Xavier, H.S.; Pisciottano, M.N.C. In Vitro Evaluation of Antioxidant, Antimicrobial and Toxicity Properties of Extracts of Schinopsis Brasiliensis Engl.(Anacardiaceae). Afr. J. Pharm. Pharmacol. 2011, 5, 1724–1731. [Google Scholar] [CrossRef]
- Berkow, E.L.; Lockhart, S.R. Fluconazole Resistance in Candida Species: A Current Perspective. Infect. Drug Resist. 2017, 10, 237–245. [Google Scholar] [CrossRef]
- Mgbeahuruike, E.E.; Holm, Y.; Vuorela, H.; Amandikwa, C.; Fyhrquist, P. An Ethnobotanical Survey and Antifungal Activity of Piper Guineense Used for the Treatment of Fungal Infections in West-African Traditional Medicine. J. Ethnopharmacol. 2019, 229, 157–166. [Google Scholar] [CrossRef]
- Lee, J.-G.; Kim, Y.-J. Comparison Study of Validation Parameters and Measurement Uncertainty of Rapid Analytical Methods for Piperine in Black Pepper by Ultraviolet Spectroscopy and High-Performance Liquid Chromatography. Food Sci. Biotechnol. 2022, 31, 1133–1143. [Google Scholar] [CrossRef]
- World Health Organization. Quality Assurance of Pharmaceuticals: A Compendium of Guidelines and Related Materials. Good Manufacturing Practices and Inspection; World Health Organization: Geneva, Switzerland, 2007; Volume 2, ISBN 9241547081. [Google Scholar]
- Laboratory, G.A. CPC 250: Mass-Directed Purification of Piperine from Piper nigrum Technical Note 208; Gilson, Inc.: Middleton, WI, USA, 2017; pp. 2–4. [Google Scholar]
- Rahman Khan, Z.; Moni, F.; Sharmin, S.; Al-Mansur, M.A.; Gafur, A.; Rahman, O.; Afroz, F. Isolation of Bulk Amount of Piperine as Active Pharmaceutical Ingredient (API) from Black Pepper and White Pepper (Piper nigrum L.). Pharmacol. Pharm. 2017, 8, 253–262. [Google Scholar] [CrossRef]
- Turner, S.A.; Butler, G. The Candida Pathogenic Species Complex. Cold Spring Harb Perspect. Med. 2014, 4, a019778. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of Invasive Candidiasis: A Persistent Public Health Problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, G.; Navazio, A.S.; Giuliani, A.; Giordano, A.; Proli, E.M.; Antonelli, G.; Raponi, G. Candida Blood Stream Infections Observed between 2011 and 2016 in a Large Italian University Hospital: A Time-Based Retrospective Analysis on Epidemiology, Biofilm Production, Antifungal Agents Consumption and Drug-Susceptibility. PLoS ONE 2019, 14, e0224678. [Google Scholar] [CrossRef] [PubMed]
- Trindade, E.O.; Souza, H.D.S.; Brandão, M.C.R.; Neto, H.D.; Lima, E.O.; Lira, B.F.; de Athayde-Filho, P.F.; Barbosa-Filho, J.M. New Diesters Derived from Piperine: In Silico Study and Evaluation of Their Antimicrobial Potential. J. Braz. Chem. Soc. 2020, 31, 1668–1678. [Google Scholar] [CrossRef]
- Shariati, A.; Didehdar, M.; Razavi, S.; Heidary, M.; Soroush, F.; Chegini, Z. Natural Compounds: A Hopeful Promise as an Antibiofilm Agent against Candida Species. Front. Pharmacol. 2022, 13, 917787. [Google Scholar] [CrossRef]
- Atriwal, T.; Azeem, K.; Husain, F.M.; Hussain, A.; Khan, M.N.; Alajmi, M.F.; Abid, M. Mechanistic Understanding of Candida albicans Biofilm Formation and Approaches for Its Inhibition. Front. Microbiol. 2021, 12, 638609. [Google Scholar] [CrossRef]
- Priya, A.; Pandian, S.K. Biofilm and Hyphal Inhibitory Synergistic Effects of Phytoactives Piperine and Cinnamaldehyde against Candida albicans. Med. Mycol. 2022, 60, myac039. [Google Scholar] [CrossRef]
- Talapko, J.; Juzbašić, M.; Matijević, T.; Pustijanac, E.; Bekić, S.; Kotris, I.; Škrlec, I. Candida albicans—The Virulence Factors and Clinical Manifestations of Infection. J. Fungi 2021, 7, 79. [Google Scholar] [CrossRef]
- Priya, A.; Nivetha, S.; Pandian, S.K. Synergistic Interaction of Piperine and Thymol on Attenuation of the Biofilm Formation, Hyphal Morphogenesis and Phenotypic Switching in Candida albicans. Front. Cell. Infect. Microbiol. 2022, 11, 1417. [Google Scholar] [CrossRef]
- Lindsay, A.K.; Morales, D.K.; Liu, Z.; Grahl, N.; Zhang, A.; Willger, S.D.; Myers, L.C.; Hogan, D.A. Analysis of Candida albicans Mutants Defective in the Cdk8 Module of Mediator Reveal Links between Metabolism and Biofilm Formation. PLoS Genet. 2014, 10, e1004567. [Google Scholar] [CrossRef]
- Braun, B.R.; Kadosh, D.; Johnson, A.D. NRG1, a Repressor of Filamentous Growth in C. Albicans, Is down-Regulated during Filament Induction. EMBO J. 2001, 20, 4753–4761. [Google Scholar] [CrossRef]
- Banerjee, M.; Thompson, D.S.; Lazzell, A.; Carlisle, P.L.; Pierce, C.; Monteagudo, C.; Lopez-Ribot, J.L.; Kadosh, D. UME6, a Novel Filament-Specific Regulator of Candida albicans Hyphal Extension and Virulence. Mol. Biol. Cell 2008, 19, 1354–1365. [Google Scholar] [PubMed]
- Carlisle, P.L.; Banerjee, M.; Lazzell, A.; Monteagudo, C.; López-Ribot, J.L.; Kadosh, D. Expression Levels of a Filament-Specific Transcriptional Regulator Are Sufficient to Determine Candida albicans Morphology and Virulence. Proc. Natl. Acad. Sci. USA 2009, 106, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Lane, S.; Raniga, P.P.; Lu, Y.; Zhou, Z.; Ramon, K.; Chen, J.; Liu, H. The Flo8 Transcription Factor Is Essential for Hyphal Development and Virulence in Candida albicans. Mol. Biol. Cell 2006, 17, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Bain, J.M.; Alonso, M.F.; Childers, D.S.; Walls, C.A.; Mackenzie, K.; Pradhan, A.; Lewis, L.E.; Louw, J.; Avelar, G.M.; Larcombe, D.E. Immune Cells Fold and Damage Fungal Hyphae. Proc. Natl. Acad. Sci. USA 2021, 118, e2020484118. [Google Scholar] [CrossRef]
- Kornitzer, D. Regulation of Candida albicans Hyphal Morphogenesis by Endogenous Signals. J. Fungi 2019, 5, 21. [Google Scholar]
- Villa, S.; Hamideh, M.; Weinstock, A.; Qasim, M.N.; Hazbun, T.R.; Sellam, A.; Hernday, A.D.; Thangamani, S. Transcriptional Control of Hyphal Morphogenesis in Candida albicans. FEMS Yeast Res. 2020, 20, foaa005. [Google Scholar] [CrossRef]
- Wakade, R.S.; Huang, M.; Mitchell, A.P.; Wellington, M.; Krysan, D.J. Intravital Imaging of Candida albicans Identifies Differential In Vitro and In Vivo Filamentation Phenotypes for Transcription Factor Deletion Mutants. mSphere 2021, 6, e0043621. [Google Scholar] [CrossRef]
- Lane, S.; Zhou, S.; Pan, T.; Dai, Q.; Liu, H. The Basic Helix-Loop-Helix Transcription Factor Cph2 Regulates Hyphal Development in Candida albicans Partly via TEC1. Mol. Cell. Biol. 2001, 21, 6418–6428. [Google Scholar]
- Hayek, P.; Dib, L.; Yazbeck, P.; Beyrouthy, B.; Khalaf, R.A. Characterization of Hwp2, a Candida albicans Putative GPI-Anchored Cell Wall Protein Necessary for Invasive Growth. Microbiol. Res. 2010, 165, 250–258. [Google Scholar]
- Sohn, K.; Urban, C.; Brunner, H.; Rupp, S. EFG1 Is a Major Regulator of Cell Wall Dynamics in Candida albicans as Revealed by DNA Microarrays. Mol. Microbiol. 2003, 47, 89–102. [Google Scholar] [CrossRef]
- Srivastava, V.; Wani, M.Y.; Al-Bogami, A.S.; Ahmad, A. Piperidine Based 1,2,3-Triazolylacetamide Derivatives Induce Cell Cycle Arrest and Apoptotic Cell Death in Candida Auris. J. Adv. Res. 2021, 29, 121–135. [Google Scholar] [PubMed]
- Tripathi, A.K.; Ray, A.K.; Mishra, S.K. Molecular and Pharmacological Aspects of Piperine as a Potential Molecule for Disease Prevention and Management: Evidence from Clinical Trials. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 16. [Google Scholar]
- Parimi, U.; Deepti, K.; Durvasula, V. Synthesis, Anticancer and Antibacterial Activities of Piperine Analogs. Med. Chem. Res. 2013, 22, 5466–5471. [Google Scholar] [CrossRef]
- Setiawati, S.; Nuryastuti, T.; Ngatidjan, N.; Mustofa, M.; Jumina, J.; Fitriastuti, D. In Vitro Antifungal Activity of (1)-N-2-Methoxybenzyl-1, 10-Phenanthrolinium Bromide against Candida albicans and Its Effects on Membrane Integrity. Mycobiology 2017, 45, 25–30. [Google Scholar]
- Haller, I. Mode of Action of Clotrimazole: Implications for Therapy. Am. J. Obstet. Gynecol. 1985, 152, 939–944. [Google Scholar] [PubMed]
- Davey, H.M.; Hexley, P. Red but Not Dead? Membranes of Stressed Saccharomyces Cerevisiae Are Permeable to Propidium Iodide. Environ. Microbiol. 2011, 13, 163–171. [Google Scholar] [PubMed]
- Khajuria, A.; Thusu, N.; Zutshi, U. Piperine Modulates Permeability Characteristics of Intestine by Inducing Alterations in Membrane Dynamics: Influence on Brush Border Membrane Fluidity, Ultrastructure and Enzyme Kinetics. Phytomedicine 2002, 9, 224–231. [Google Scholar]
- Akins, R.A. An Update on Antifungal Targets and Mechanisms of Resistance in Candida albicans. Med. Mycol. 2005, 43, 285–318. [Google Scholar]
- Zhitkovich, A. N-Acetylcysteine: Antioxidant, Aldehyde Scavenger, and More. Chem. Res. Toxicol. 2019, 32, 1318–1319. [Google Scholar]
- Alonso-Monge, R.; Navarro-García, F.; Román, E.; Negredo, A.I.; Eisman, B.; Nombela, C.; Pla, J. The Hog1 Mitogen-Activated Protein Kinase Is Essential in the Oxidative Stress Response and Chlamydospore Formation in Candida albicans. Eukaryot. Cell 2003, 2, 351–361. [Google Scholar]
- Enjalbert, B.; Smith, D.A.; Cornell, M.J.; Alam, I.; Nicholls, S.; Brown, A.J.P.; Quinn, J. Role of the Hog1 Stress-Activated Protein Kinase in the Global Transcriptional Response to Stress in the Fungal Pathogen Candida albicans. Mol. Biol. Cell 2006, 17, 1018–1032. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.A.; Nicholls, S.; Morgan, B.A.; Brown, A.J.P.; Quinn, J. A Conserved Stress-Activated Protein Kinase Regulates a Core Stress Response in the Human Pathogen Candida albicans. Mol. Biol. Cell 2004, 15, 4179–4190. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [PubMed]
- Murphy, M.P. Mitochondrial Dysfunction Indirectly Elevates ROS Production by the Endoplasmic Reticulum. Cell Metab. 2013, 18, 145–146. [Google Scholar] [CrossRef]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative Stress: The Mitochondria-Dependent and Mitochondria-Independent Pathways of Apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar]
- Skrzypek, M.S.; Binkley, J.; Binkley, G.; Miyasato, S.R.; Simison, M.; Sherlock, G. The Candida Genome Database (CGD): Incorporation of Assembly 22, Systematic Identifiers and Visualization of High Throughput Sequencing Data. Nucleic Acids Res. 2016, 45, gkw924. [Google Scholar]
- Gil-Bona, A.; Parra-Giraldo, C.M.; Hernáez, M.L.; Reales-Calderon, J.A.; Solis, N.V.; Filler, S.G.; Monteoliva, L.; Gil, C. Candida albicans Cell Shaving Uncovers New Proteins Involved in Cell Wall Integrity, Yeast to Hypha Transition, Stress Response and Host–Pathogen Interaction. J. Proteom. 2015, 127, 340–351. [Google Scholar] [CrossRef]
- Kamat, J.P.; Devasagayam, T.P.A.; Priyadarsini, K.I.; Mohan, H. Reactive Oxygen Species Mediated Membrane Damage Induced by Fullerene Derivatives and Its Possible Biological Implications. Toxicology 2000, 155, 55–61. [Google Scholar] [CrossRef]
- Li, Y.; Shan, M.; Zhu, Y.; Yao, H.; Li, H.; Gu, B.; Zhu, Z. Kalopanaxsaponin A Induces Reactive Oxygen Species Mediated Mitochondrial Dysfunction and Cell Membrane Destruction in Candida albicans. PLoS ONE 2020, 15, e0243066. [Google Scholar] [CrossRef]
- Kobayashi, D.; Kondo, K.; Uehara, N.; Otokozawa, S.; Tsuji, N.; Yagihashi, A.; Watanabe, N. Endogenous Reactive Oxygen Species Is an Important Mediator of Miconazole Antifungal Effect. Antimicrob. Agents Chemother. 2002, 46, 3113–3117. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Q.; Ma, K.; Shi, P.; Liu, W.; Huang, Z. Fluconazole Inhibits Cellular Ergosterol Synthesis to Confer Synergism with Berberine against Yeast Cells. J. Glob. Antimicrob. Resist. 2018, 13, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, H.Y.; Back, S.Y.; Han, H.-K. Piperine-Mediated Drug Interactions and Formulation Strategy for Piperine: Recent Advances and Future Perspectives. Expert Opin. Drug Metab. Toxicol. 2018, 14, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, A.M.; Manuel-Y-Keenoy, B.; De Backer, W.A. Antioxidant and Anti-Inflammatory Efficacy of NAC in the Treatment of COPD: Discordant in Vitro and in Vivo Dose-Effects: A Review. Pulm. Pharmacol. Ther. 2007, 20, 9–22. [Google Scholar] [CrossRef]
- Yang, J.; Wu, L.-J.; Tashino, S.-I.; Onodera, S.; Ikejima, T. Reactive Oxygen Species and Nitric Oxide Regulate Mitochondria-Dependent Apoptosis and Autophagy in Evodiamine-Treated Human Cervix Carcinoma HeLa Cells. Free Radic. Res. 2008, 42, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.S.; Lim, J.H.; Oh, W.K.; Park, J.T.; Park, S.C.; Cho, K.A. Piperine: An Anticancer and Senostatic Drug. Front. Biosci.-Landmark 2022, 27, 137. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.P.; de Sousa, S.O.; Gusson-Zanetoni, J.P.; de Melo Moreira Silva, L.L.; Frigieri, B.M.; Henrique, T.; Tajara, E.H.; Oliani, S.M.; Rodrigues-Lisoni, F.C. Piperine Reduces Neoplastic Progression in Cervical Cancer Cells by Downregulating the Cyclooxygenase 2 Pathway. Pharmaceuticals 2023, 16, 103. [Google Scholar] [CrossRef]
- Dhanasekaran, S.; Pushparaj Selvadoss, P.; Sundar Manoharan, S.; Jeyabaslan, S.; Devi Rajeswari, V. Revealing Anti-Fungal Potential of Plant-Derived Bioactive Therapeutics in Targeting Secreted Aspartyl Proteinase (SAP) of Candida albicans: A Molecular Dynamics Approach. J. Biomol. Struct. Dyn. 2023, 6, 1–15. [Google Scholar] [CrossRef]




| Candida spp. | Piperine (μg/mL) | ||
|---|---|---|---|
| MIC80 (24 h) | MIC80 (48 h) | MFC (24 h) | |
| C. albicans (SC5314) (ATCC) a | 1024 | 2048 | >2048 |
| C. albicans (CAAL256) b | 512 | 512 | 2048 |
| C. parapsilosis (ATCC 22019) a | 1024 | 2048 | 2048 |
| C. tropicalis (ATCC 1369) a | 512 | 1024 | 2048 |
| C. krusei (ATCC 6258) b | 2048 | >2048 | >2048 |
| Candida spp. | MIC of FLC | MIC of Piperine | ||||
| Alone | With piperine | Alone | With FLC | FICI | Outcome | |
| SC5314 | 1 | 0.25 | 2048 | 256 | 0.375 | synergy |
| CAAL256 | 32 | 16 | 1024 | 32 | 0.531 | additive |
| MIC of NAC | MIC of Piperine | |||||
| Alone | With piperine | Alone | With FLC | FICI | Outcome | |
| SC5314 | 25 | 25 | 2048 | 64 | 1.031 | indifference |
| CAAL256 | 50 | 50 | 1024 | 64 | 1.031 | indifference |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bravo-Chaucanés, C.P.; Chitiva, L.C.; Vargas-Casanova, Y.; Diaz-Santoyo, V.; Hernández, A.X.; Costa, G.M.; Parra-Giraldo, C.M. Exploring the Potential Mechanism of Action of Piperine against Candida albicans and Targeting Its Virulence Factors. Biomolecules 2023, 13, 1729. https://doi.org/10.3390/biom13121729
Bravo-Chaucanés CP, Chitiva LC, Vargas-Casanova Y, Diaz-Santoyo V, Hernández AX, Costa GM, Parra-Giraldo CM. Exploring the Potential Mechanism of Action of Piperine against Candida albicans and Targeting Its Virulence Factors. Biomolecules. 2023; 13(12):1729. https://doi.org/10.3390/biom13121729
Chicago/Turabian StyleBravo-Chaucanés, Claudia Patricia, Luis Carlos Chitiva, Yerly Vargas-Casanova, Valentina Diaz-Santoyo, Andrea Ximena Hernández, Geison M. Costa, and Claudia Marcela Parra-Giraldo. 2023. "Exploring the Potential Mechanism of Action of Piperine against Candida albicans and Targeting Its Virulence Factors" Biomolecules 13, no. 12: 1729. https://doi.org/10.3390/biom13121729
APA StyleBravo-Chaucanés, C. P., Chitiva, L. C., Vargas-Casanova, Y., Diaz-Santoyo, V., Hernández, A. X., Costa, G. M., & Parra-Giraldo, C. M. (2023). Exploring the Potential Mechanism of Action of Piperine against Candida albicans and Targeting Its Virulence Factors. Biomolecules, 13(12), 1729. https://doi.org/10.3390/biom13121729











