Outcomes and Adverse Effects of Voretigene Neparvovec Treatment for Biallelic RPE65-Mediated Inherited Retinal Dystrophies in a Cohort of Patients from a Single Center
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Procedures
2.3. Best-Corrected Visual Acuity
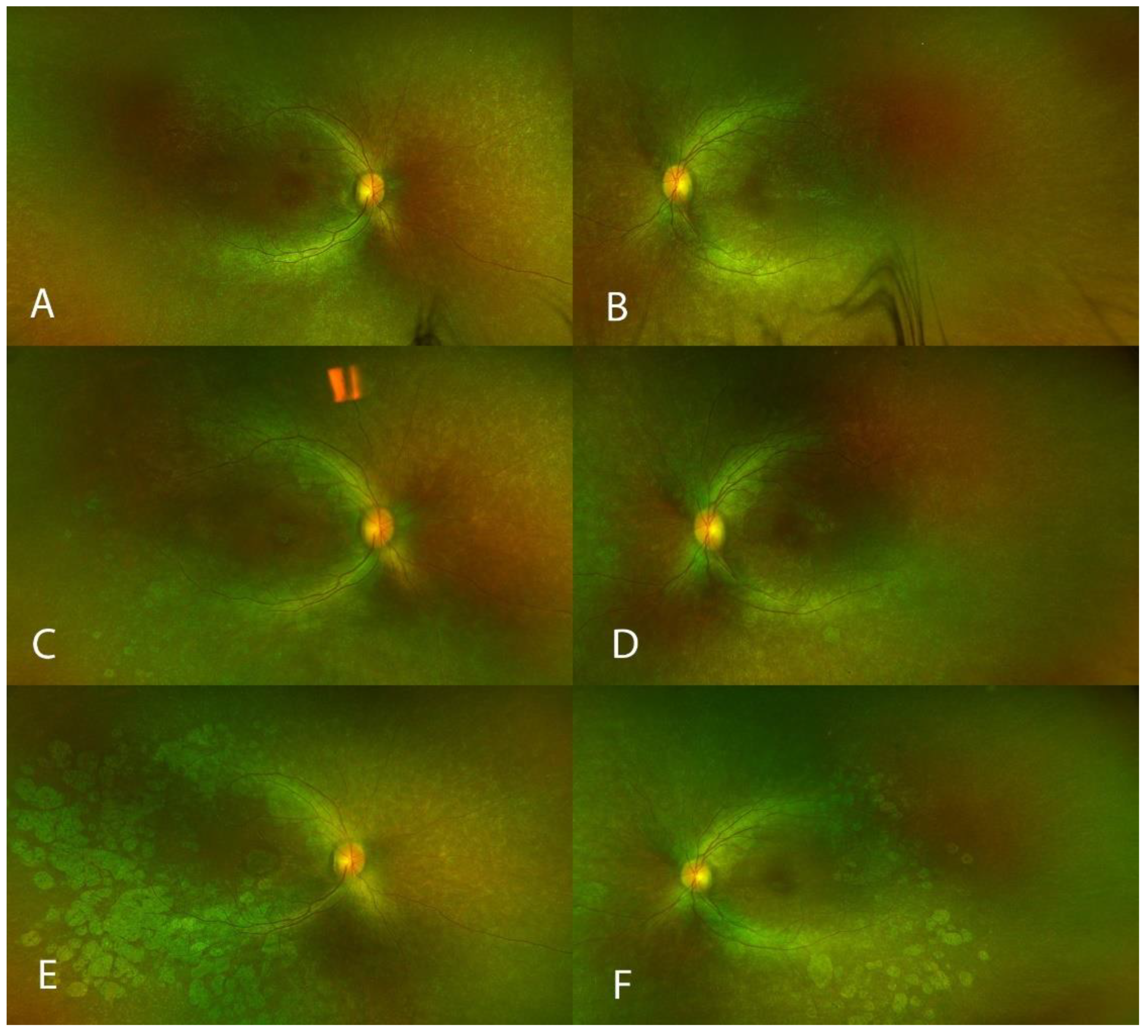
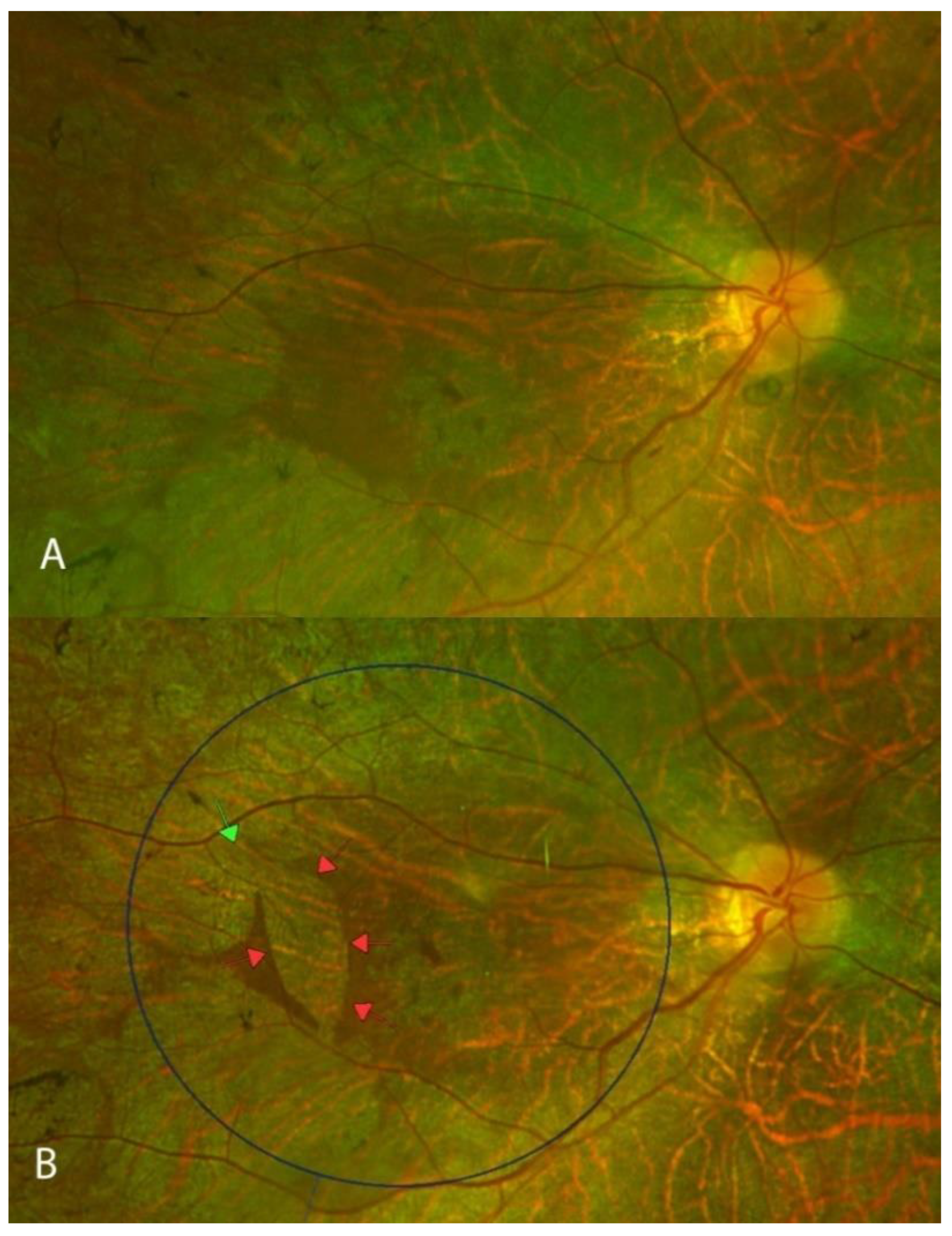
2.4. Multimodal Imaging
2.5. Full-Field Stimulus Testing
2.6. Statistical Analysis
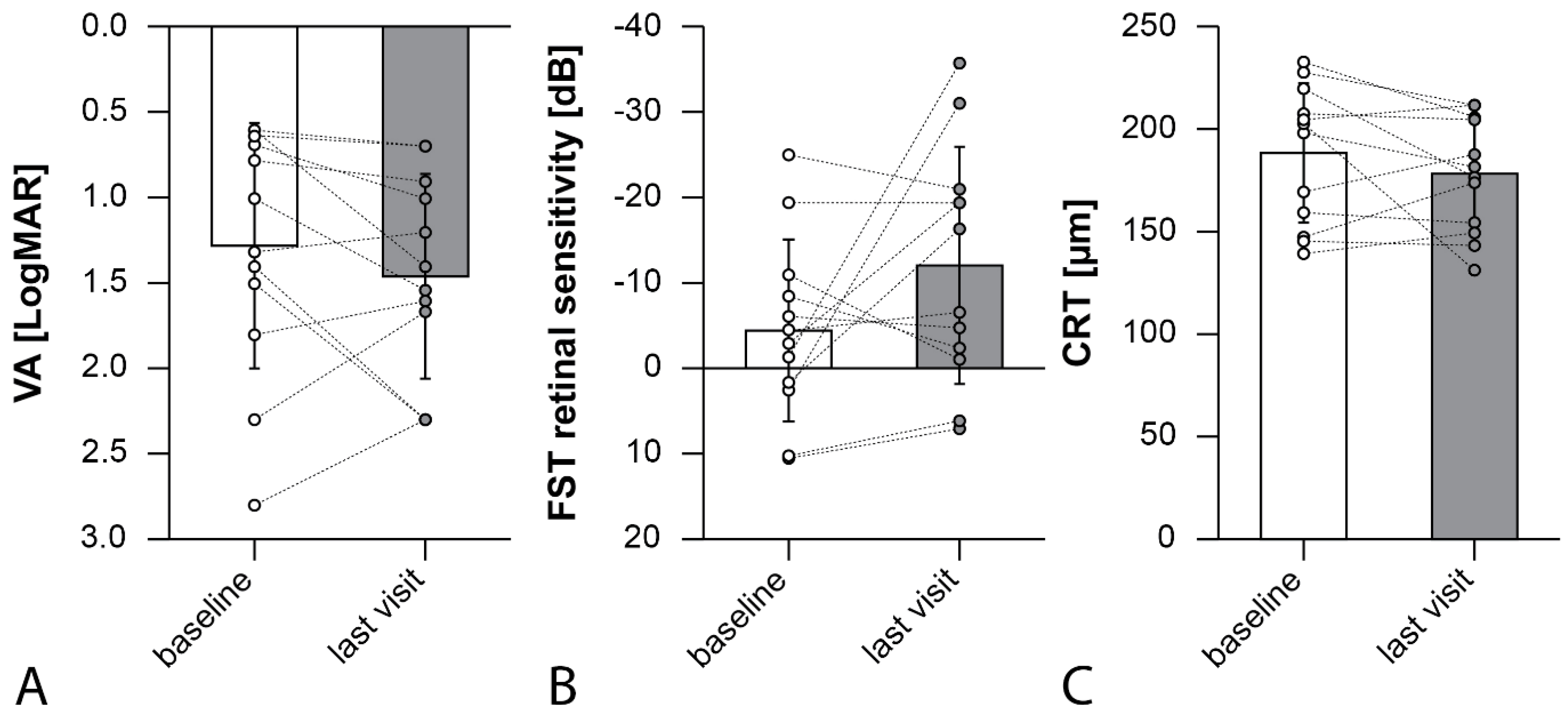
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heath Jeffery, R.C.; Mukhtar, S.A.; McAllister, I.L.; Morgan, W.H.; Mackey, D.A.; Chen, F.K. Inherited retinal diseases are the most common cause of blindness in the working-age population in Australia. Ophthalmic Genet. 2021, 42, 431–439. [Google Scholar] [CrossRef]
- García Bohórquez, B.; Aller, E.; Rodríguez Muñoz, A.; Jaijo, T.; García García, G.; Millán, J.M. Updating the Genetic Landscape of Inherited Retinal Dystrophies. Front. Cell Dev. Biol. 2021, 9, 645600. [Google Scholar] [CrossRef] [PubMed]
- Sallum, J.M.F.; Kaur, V.P.; Shaikh, J.; Banhazi, J.; Spera, C.; Aouadj, C.; Viriato, D.; Fischer, M.D. Epidemiology of Mutations in the 65-kDa Retinal Pigment Epithelium (RPE65) Gene-Mediated Inherited Retinal Dystrophies: A Systematic Literature Review. Adv. Ther. 2022, 39, 1179–1198. [Google Scholar] [CrossRef]
- Maguire, A.M.; Russell, S.; Wellman, J.A.; Chung, D.C.; Yu, Z.F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; Marshall, K.A.; et al. Efficacy, Safety, and Durability of Voretigene Neparvovec-rzyl in RPE65 Mutation-Associated Inherited Retinal Dystrophy: Results of Phase 1 and 3 Trials. Ophthalmology 2019, 126, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet 2017, 390, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Maguire, A.M.; Russell, S.; Chung, D.C.; Yu, Z.F.; Tillman, A.; Drack, A.V.; Simonelli, F.; Leroy, B.P.; Reape, K.Z.; High, K.A.; et al. Durability of Voretigene Neparvovec for Biallelic RPE65-Mediated Inherited Retinal Disease: Phase 3 Results at 3 and 4 Years. Ophthalmology 2021, 128, 1460–1468. [Google Scholar] [CrossRef]
- Ku, C.A.; Igelman, A.D.; Huang, S.J.; Vasconcelos, H.; da Palma, M.M.; Bailey, S.T.; Lauer, A.K.; Weleber, R.G.; Yang, P.; Pennesi, M.E. Improved Rod Sensitivity as Assessed by Two-Color Dark-Adapted Perimetry in Patients With RPE65-Related Retinopathy Treated With Voretigene Neparvovec-rzyl. Transl. Vis. Sci. Technol. 2023, 12, 17. [Google Scholar] [CrossRef]
- Testa, F.; Melillo, P.; Di Iorio, V.; Iovino, C.; Farinaro, F.; Karali, M.; Banfi, S.; Rossi, S.; Della Corte, M.; Simonelli, F. Visual function and retinal changes after voretigene neparvovec treatment in children with biallelic RPE65-related inherited retinal dystrophy. Sci. Rep. 2022, 12, 17637. [Google Scholar] [CrossRef] [PubMed]
- Stingl, K.; Kempf, M.; Bartz-Schmidt, K.U.; Dimopoulos, S.; Reichel, F.; Jung, R.; Kelbsch, C.; Kohl, S.; Kortüm, F.C.; Nasser, F.; et al. Spatial and temporal resolution of the photoreceptors rescue dynamics after treatment with voretigene neparvovec. Br. J. Ophthalmol. 2022, 106, 831–838. [Google Scholar] [CrossRef]
- Reichel, F.F.; Seitz, I.; Wozar, F.; Dimopoulos, S.; Jung, R.; Kempf, M.; Kohl, S.; Kortüm, F.C.; Ott, S.; Pohl, L.; et al. Development of retinal atrophy after subretinal gene therapy with voretigene neparvovec. Br. J. Ophthalmol. 2022, 107, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, M.J.; Priglinger, C.S.; Rudolph, G.; Hufendiek, K.; Framme, C.; Jägle, H.; Salchow, D.J.; Anschütz, A.; Michalakis, S.; Priglinger, S.G. Gene Therapy with Voretigene Neparvovec Improves Vision and Partially Restores Electrophysiological Function in Pre-School Children with Leber Congenital Amaurosis. Biomedicines 2022, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Testa, F.; Melillo, P.; Della Corte, M.; Di Iorio, V.; Brunetti-Pierri, R.; Citro, A.; Ferrara, M.; Karali, M.; Annibale, R.; Banfi, S.; et al. Voretigene Neparvovec Gene Therapy in Clinical Practice: Treatment of the First Two Italian Pediatric Patients. Transl. Vis. Sci. Technol. 2021, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Kortüm, F.C.; Kempf, M.; Jung, R.; Kohl, S.; Ott, S.; Kortuem, C.; Sting, K.; Stingl, K. Short term morphological rescue of the fovea after gene therapy with voretigene neparvovec. Acta Ophthalmol. 2022, 100, e807–e812. [Google Scholar] [CrossRef]
- Levi, S.R.; Oh, J.K.; de Carvalho, J.R.L., Jr.; Mahajan, V.B.; Tsang, S.H.; Sparrow, J.R. Quantitative Autofluorescence Following Gene Therapy With Voretigene Neparvovec. JAMA Ophthalmol. 2020, 138, 919–921. [Google Scholar] [CrossRef] [PubMed]
- von Krusenstiern, L.; Liu, J.; Liao, E.; Gow, J.A.; Chen, G.; Ong, T.; Lotery, A.J.; Jalil, A.; Lam, B.L.; MacLaren, R.E. Changes in Retinal Sensitivity Associated With Cotoretigene Toliparvovec in X-Linked Retinitis Pigmentosa With RPGR Gene Variations. JAMA Ophthalmol. 2023, 141, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Ducloyer, J.B.; Le Meur, G.; Lebranchu, P.; Billaud, F.; Weber, M. Macular Fold Complicating a Subretinal Injection of Voretigene Neparvovec. Ophthalmology. Retin. 2020, 4, 456–458. [Google Scholar] [CrossRef]
- Price, K.W.; Pennesi, M.E.; Lauer, A.K.; Bailey, S.T. Iatrogenic choroidal neovascularization associated with subretinal gene therapy surgery. Am. J. Ophthalmol. Case Rep. 2022, 27, 101677. [Google Scholar] [CrossRef]
- Gange, W.S.; Sisk, R.A.; Besirli, C.G.; Lee, T.C.; Havunjian, M.; Schwartz, H.; Borchert, M.; Sengillo, J.D.; Mendoza, C.; Berrocal, A.M.; et al. Perifoveal Chorioretinal Atrophy after Subretinal Voretigene Neparvovec-rzyl for RPE65-Mediated Leber Congenital Amaurosis. Ophthalmology. Retin. 2022, 6, 58–64. [Google Scholar] [CrossRef]
- Lopez, J.; Borchert, M.; Lee, T.C.; Nagiel, A. Subretinal deposits in young patients treated with voretigene neparvovec-rzyl for RPE65-mediated retinal dystrophy. Br. J. Ophthalmol. 2023, 107, 299–301. [Google Scholar] [CrossRef]
- Blonde, L.; Khunti, K.; Harris, S.B.; Meizinger, C.; Skolnik, N.S. Interpretation and Impact of Real-World Clinical Data for the Practicing Clinician. Adv. Ther. 2018, 35, 1763–1774. [Google Scholar] [CrossRef]
- Fischer, M.D.; Maier, R.; Suhner, A.; Stiehl, D.; Fasser, C.; Leroy, B.P. PERCEIVE study report: Real-world safety and effectiveness of voretigene neparvovec. Investig. Ophthalmol. Vis. Sci. 2022, 63, 451. [Google Scholar]
- Kaiser, P.K. Prospective evaluation of visual acuity assessment: A comparison of snellen versus ETDRS charts in clinical practice (An AOS Thesis). Trans. Am. Ophthalmol. Soc. 2009, 107, 311–324. [Google Scholar]
- Bailey, I.L.; Jackson, A.J.; Minto, H.; Greer, R.B.; Chu, M.A. The Berkeley Rudimentary Vision Test. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2012, 89, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Testa, F.; Murro, V.; Signorini, S.; Colombo, L.; Iarossi, G.; Parmeggiani, F.; Falsini, B.; Salvetti, A.P.; Brunetti-Pierri, R.; Aprile, G.; et al. RPE65-Associated Retinopathies in the Italian Population: A Longitudinal Natural History Study. Investig. Ophthalmol. Vis. Sci. 2022, 63, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, C.; Tzekov, R.T.; Zhu, Y.; Li, W. The effect of human gene therapy for RPE65-associated Leber’s congenital amaurosis on visual function: A systematic review and meta-analysis. Orphanet J. Rare Dis. 2020, 15, 49. [Google Scholar] [CrossRef]
- Maguire, A.M.; High, K.A.; Auricchio, A.; Wright, J.F.; Pierce, E.A.; Testa, F.; Mingozzi, F.; Bennicelli, J.L.; Ying, G.S.; Rossi, S.; et al. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: A phase 1 dose-escalation trial. Lancet 2009, 374, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Dell’Osso, L.F. The diagnosis and treatment of infantile nystagmus syndrome (INS). Sci. World J. 2006, 6, 1385–1397. [Google Scholar] [CrossRef]
- Roman, A.J.; Cideciyan, A.V.; Wu, V.; Garafalo, A.V.; Jacobson, S.G. Full-field stimulus testing: Role in the clinic and as an outcome measure in clinical trials of severe childhood retinal disease. Prog. Retin. Eye Res. 2022, 87, 101000. [Google Scholar] [CrossRef]
- Deng, C.; Zhao, P.Y.; Branham, K.; Schlegel, D.; Fahim, A.T.; Jayasundera, T.K.; Khan, N.; Besirli, C.G. Real-world outcomes of voretigene neparvovec treatment in pediatric patients with RPE65-associated Leber congenital amaurosis. Graefe’s Arch. Clin. Exp. Ophthalmol. = Albrecht Von Graefes Arch. Fur Klin. Und Exp. Ophthalmol. 2022, 260, 1543–1550. [Google Scholar] [CrossRef]
- Leroy, B.P.; Fischer, M.D.; Flannery, J.G.; MacLaren, R.E.; Dalkara, D.; Scholl, H.P.N.; Chung, D.C.; Spera, C.; Viriato, D.; Banhazi, J. Gene therapy for inherited retinal disease: Long-term durability of effect. Ophthalmic Res. 2022, 66, 179–196. [Google Scholar] [CrossRef]
- Jolly, J.K.; Menghini, M.; Johal, P.A.; Buckley, T.M.W.; Bridge, H.; Maclaren, R.E. Inner retinal thickening affects microperimetry thresholds in the presence of photoreceptor thinning in patients with RPGR retinitis pigmentosa. Br. J. Ophthalmol. 2022, 106, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Berlin, J.A.; Glasser, S.C.; Ellenberg, S.S. Adverse event detection in drug development: Recommendations and obligations beyond phase 3. Am. J. Public Health 2008, 98, 1366–1371. [Google Scholar] [CrossRef] [PubMed]
- Pillai, G.S.; Varkey, R.; Unnikrishnan, U.G.; Radhakrishnan, N. Incidence and risk factors for intraocular pressure rise after transconjunctival vitrectomy. Indian J. Ophthalmol. 2020, 68, 812–817. [Google Scholar] [CrossRef]
- Sihota, R.; Konkal, V.L.; Dada, T.; Agarwal, H.C.; Singh, R. Prospective, long-term evaluation of steroid-induced glaucoma. Eye 2008, 22, 26–30. [Google Scholar] [CrossRef] [PubMed]

| Age at Surgery, Sex, Ethnicity | Clinical Diagnosis | RPE65 Mutation(s) | Gene Mutation Classification | Functional Changes |
|---|---|---|---|---|
| P1, 18 years, Male, Black British | LCA2 | Compound Heterozygous RPE65 C.271C > T; RPE65 C.1102T > C | Missense; Missense | p.(Arg91Trp); p.(Tyr368His) |
| P2, 49 years, Male, White | RP20 | Compound Heterozygous RPE65 C.11 + 5G > A; RPE65 C.1543C > T | Intronic; Missense | Disruption of normal splicing; p.(Arg515Trp) |
| P3, 19 years, female, Arabic | LCA2 | Homozygous RPE65 C.271C > T | Missense | p.(Arg91Trp) |
| P4, 40 years, Male, Pakistani | RP20 | Homozygous RPE65 C.179T > C | Missense | p.(Leu60Pro) |
| P5, 48 years, Male, Pakistani | RP20 | Homozygous RPE65 C.179T > C | Missense | p.(Leu60Pro) |
| P6, 44 years, Male, White | RP20 | Homozygous RPE65 C.560G > A | Missense | p.(Gly187Glu) |
| Prior to Gene Therapy | At the Last Follow-up | ||||||
|---|---|---|---|---|---|---|---|
| BCVA logMAR | FST (White) dB | CRT μm | BCVA logMAR | FST (White) dB | CRT μm | Follow-up Months | |
| P1, 18 years, right eye | 0.64 | −2.9 | 227 | 0.69 | −19.4 | 211 | 12 |
| P1, 18 years, left eye * | 0.78 | 1.9 | 219 | 0.9 | −16.25 | 176 | 12 |
| P2, 49 years, right eye * | 1.8 | −6 | 147 | 1.6 | −4.7 | 174 | 12 |
| P2, 49 years, left eye | 2.8 | −4.5 | 145 | 2.3 | −6.5 | 143 | 12 |
| P3, 19 years, right eye * | 1.32 | −1.3 | 139 | 1.2 | −35.53 | 149 | 12 |
| P3, 19 years, left eye | 0.6 | 2.53 | 159 | 1.4 | −30.95 | 154 | 12 |
| P4, 40 years, right eye | 0.6 | −10.9 | 202 | 0.69 | −1.2 | 131 | 6 |
| P4, 40 years, left eye * | 0.69 | −8.4 | 207 | 1 | −2.35 | 205 | 6 |
| P5, 48 years, right eye * | 1.5 | 10.35 | 198 | 2.3 | 6.15 | 181 | 6 |
| P5, 48 years, left eye | 1.4 | 10.5 | 169 | 2.3 | 7.06 | 187 | 6 |
| P6, 44 years, right eye * | 2.3 | −19.3 | 232 | 1.66 | −19.3 | 206 | 1 |
| P6, 44 years, left eye | 1 | −24.9 | 204 | 1.54 | −20.9 | 211 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiraly, P.; Cottriall, C.L.; Taylor, L.J.; Jolly, J.K.; Cehajic-Kapetanovic, J.; Yusuf, I.H.; Martinez-Fernandez de la Camara, C.; Shanks, M.; Downes, S.M.; MacLaren, R.E.; et al. Outcomes and Adverse Effects of Voretigene Neparvovec Treatment for Biallelic RPE65-Mediated Inherited Retinal Dystrophies in a Cohort of Patients from a Single Center. Biomolecules 2023, 13, 1484. https://doi.org/10.3390/biom13101484
Kiraly P, Cottriall CL, Taylor LJ, Jolly JK, Cehajic-Kapetanovic J, Yusuf IH, Martinez-Fernandez de la Camara C, Shanks M, Downes SM, MacLaren RE, et al. Outcomes and Adverse Effects of Voretigene Neparvovec Treatment for Biallelic RPE65-Mediated Inherited Retinal Dystrophies in a Cohort of Patients from a Single Center. Biomolecules. 2023; 13(10):1484. https://doi.org/10.3390/biom13101484
Chicago/Turabian StyleKiraly, Peter, Charles L. Cottriall, Laura J. Taylor, Jasleen K. Jolly, Jasmina Cehajic-Kapetanovic, Imran H. Yusuf, Cristina Martinez-Fernandez de la Camara, Morag Shanks, Susan M. Downes, Robert E. MacLaren, and et al. 2023. "Outcomes and Adverse Effects of Voretigene Neparvovec Treatment for Biallelic RPE65-Mediated Inherited Retinal Dystrophies in a Cohort of Patients from a Single Center" Biomolecules 13, no. 10: 1484. https://doi.org/10.3390/biom13101484
APA StyleKiraly, P., Cottriall, C. L., Taylor, L. J., Jolly, J. K., Cehajic-Kapetanovic, J., Yusuf, I. H., Martinez-Fernandez de la Camara, C., Shanks, M., Downes, S. M., MacLaren, R. E., & Fischer, M. D. (2023). Outcomes and Adverse Effects of Voretigene Neparvovec Treatment for Biallelic RPE65-Mediated Inherited Retinal Dystrophies in a Cohort of Patients from a Single Center. Biomolecules, 13(10), 1484. https://doi.org/10.3390/biom13101484





