Characterization and Investigation of Novel Benzodioxol Derivatives as Antidiabetic Agents: An In Vitro and In Vivo Study in an Animal Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.2.1. General Procedure for the Synthesis of Benzodioxol-Carboxamide (Ia-IIe)
2.2.2. MicroED Sample Preparation, Data Collection, and Refinement Procedure
2.2.3. MicroED Method
2.3. α-Amylase Inhibitory Evaluation
2.4. In Vivo Evaluation of the Antidiabetic Effect
2.4.1. Animals
2.4.2. Induction of Experimental Diabetes
2.4.3. Drug Administration and Measurement of Blood Glucose Levels
2.5. Cell Culture and MTS Assay
3. Results
3.1. Chemistry
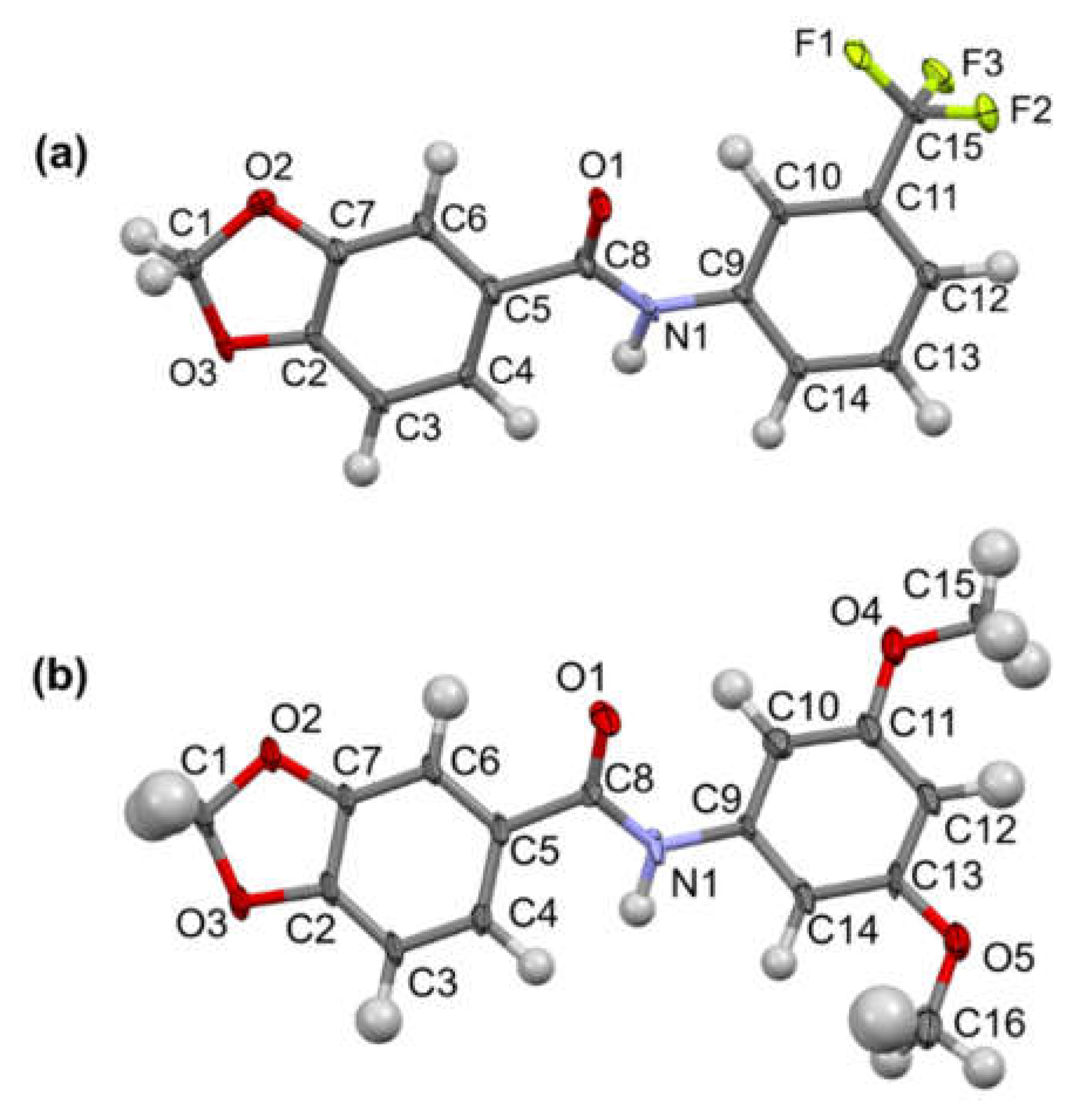
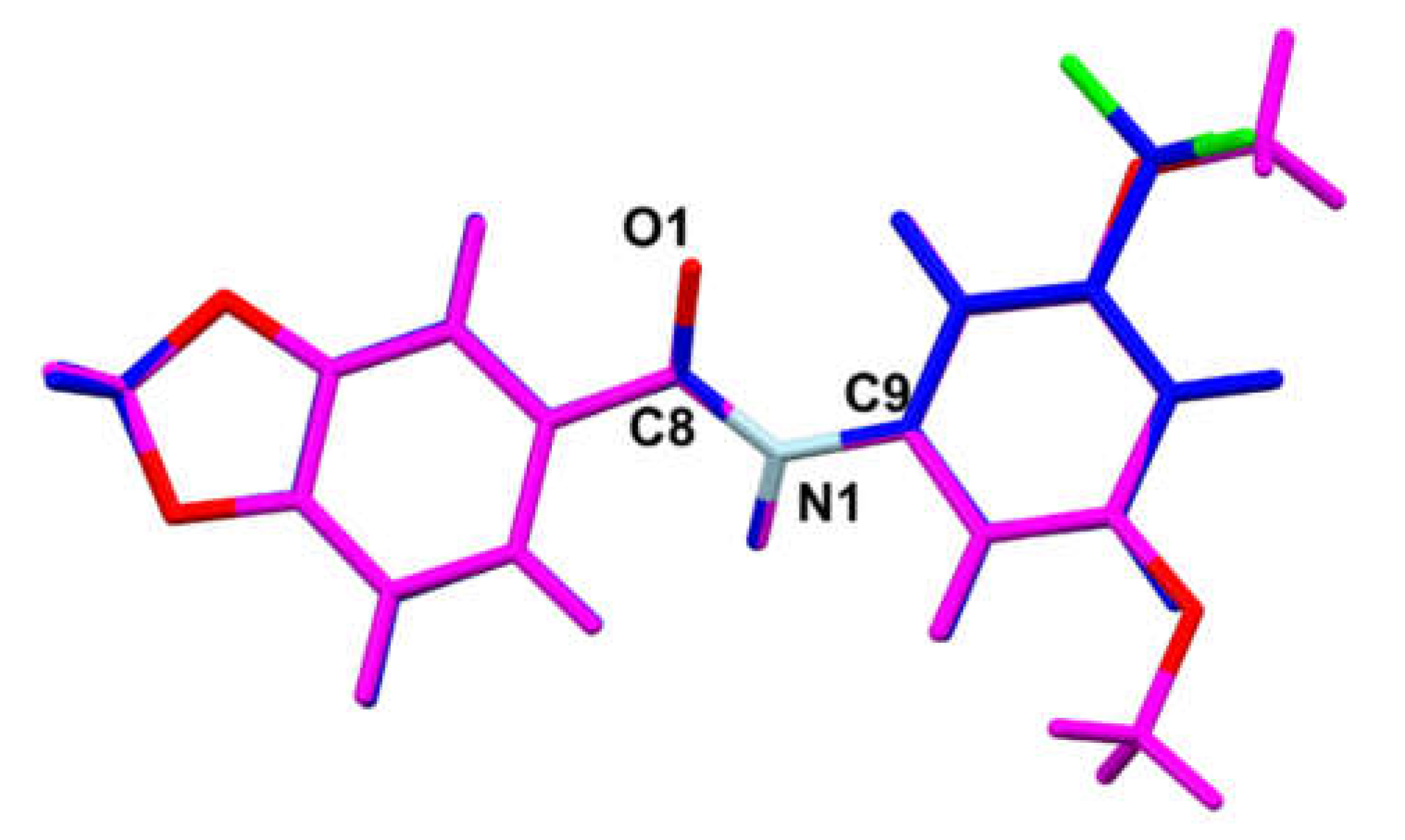
3.2. MicroED Characterization
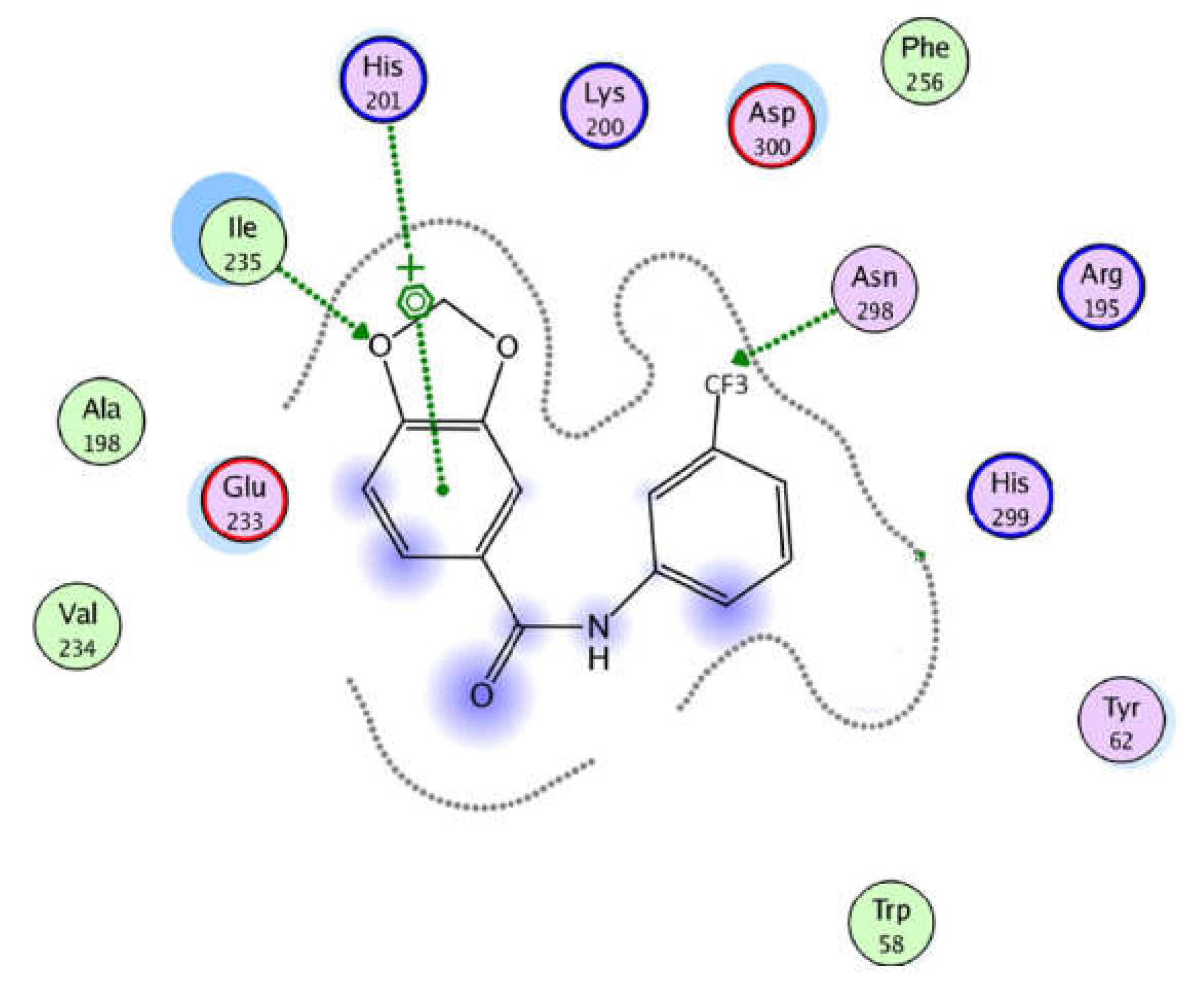
3.3. In Vitro α-Amylase Inhibitory Effects
3.4. In Vivo Hypoglycemic Effect of Compound IIc on Mice with STZ-Induced Diabetes
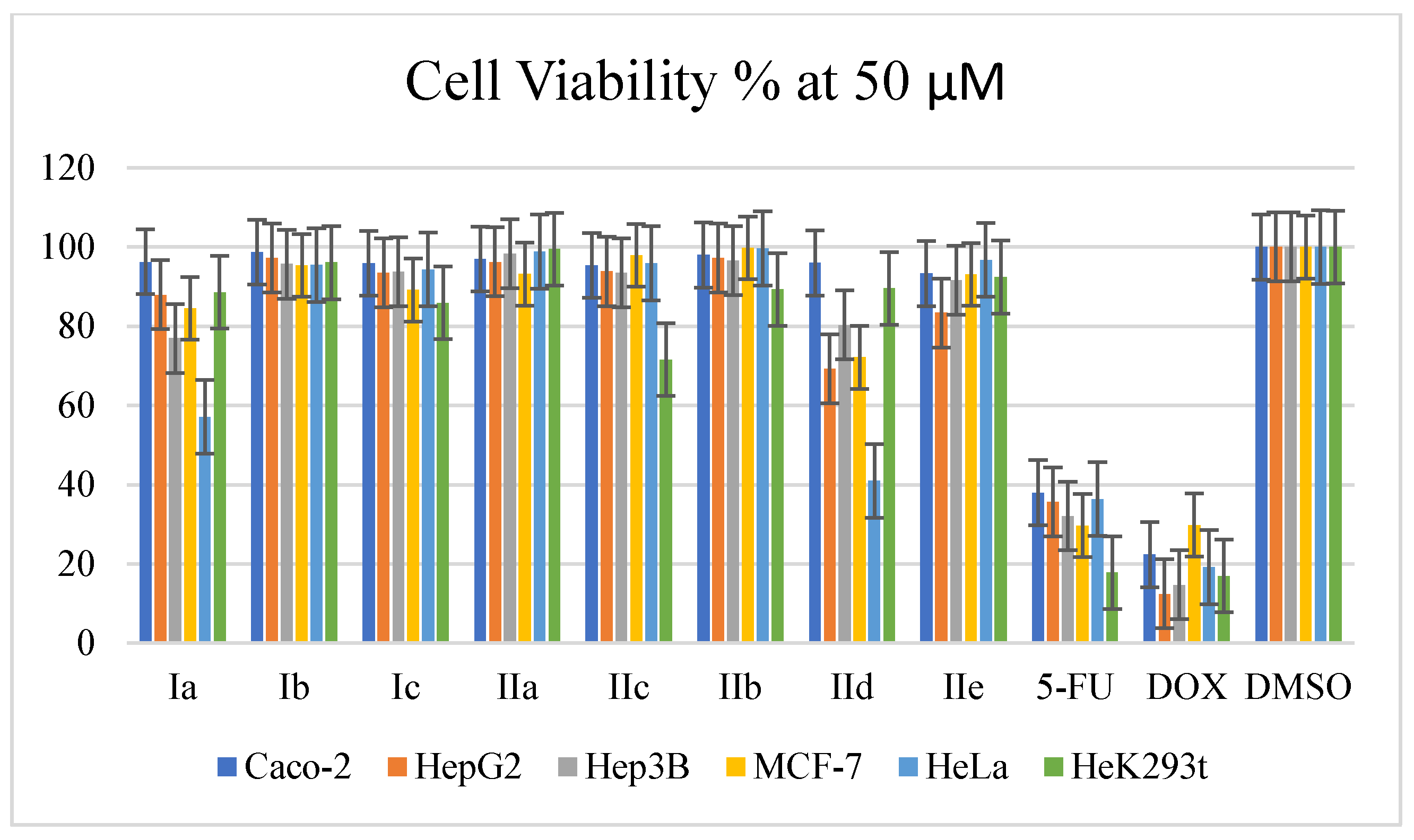
3.5. Cytotoxicity Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Association, A.D. Economic costs of diabetes in the US in 2007. Diabetes Care 2008, 31, 596–615. [Google Scholar] [CrossRef] [PubMed]
- Zaccardi, F.; Webb, D.R.; Yates, T.; Davies, M.J. Pathophysiology of type 1 and type 2 diabetes mellitus: A 90-year perspective. Postgrad. Med. J. 2016, 92, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.S.; Ande, C.; Moremen, K.W.; Crich, D. Influence of side chain conformation on the activity of glycosidase inhibitors. Angew. Chem. 2023, 135, e202217809. [Google Scholar] [CrossRef]
- Compain, P.; Martin, O.R. Iminosugars: From Synthesis to Therapeutic Applications; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Rajasekaran, P.; Ande, C.; Vankar, Y.D. Synthesis of (5, 6 & 6, 6)-oxa-oxa annulated sugars as glycosidase inhibitors from 2-formyl galactal using iodocyclization as a key step. Arkivoc 2022, 2022, 5–23. [Google Scholar]
- Compain, P.; Martin, O.R. Iminosugars: Past, present and future. In Iminosugars: From Synthesis to Therapeutic Applications; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 1–6. [Google Scholar]
- Sadiq, A.; Mahnashi, M.H.; Rashid, U.; Jan, M.S.; Alshahrani, M.A.; Huneif, M.A. 3-(((1 S, 3 S)-3-((R)-Hydroxy (4-(trifluoromethyl) phenyl) methyl)-4-oxocyclohexyl) methyl) pentane-2, 4-dione: Design and Synthesis of New Stereopure Multi-Target Antidiabetic Agent. Molecules 2022, 27, 3265. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, A.; Azam, U.; Mehreen, S.; Naseer, M.M. Synthetic α-glucosidase inhibitors as promising anti-diabetic agents: Recent developments and future challenges. Eur. J. Med. Chem. 2023, 249, 115119. [Google Scholar] [CrossRef] [PubMed]
- Hill, R. The Chemistry of Life: Eight Lectures on the History of Biochemistry; CUP Archive: Cambridge, UK, 1970. [Google Scholar]
- Stenesh, J. Biochemistry 2; Elsevier: New York, NY, USA, 1998; p. 83. [Google Scholar]
- Flefel, E.M.; El-Sofany, W.I.; Al-Harbi, R.A.; El-Shahat, M. Development of a novel series of anticancer and antidiabetic: Spirothiazolidines analogs. Molecules 2019, 24, 2511. [Google Scholar] [CrossRef] [PubMed]
- Balu, P.; Jas, J.S.; Govindaraj, M. Design and evaluation of chalconeimine derivatives as α-amylase inhibitors. Bioinformation 2019, 15, 523. [Google Scholar] [CrossRef]
- Renehan, A.G.; Zwahlen, M.; Minder, C.; T O’Dwyer, S.; Shalet, S.M.; Egger, M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: Systematic review and meta-regression analysis. Lancet 2004, 363, 1346–1353. [Google Scholar] [CrossRef]
- Frasca, F.; Pandini, G.; Sciacca, L.; Pezzino, V.; Squatrito, S.; Belfiore, A.; Vigneri, R. The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch. Physiol. Biochem. 2008, 114, 23–37. [Google Scholar] [CrossRef]
- Clayton, P.E.; Banerjee, I.; Murray, P.G.; Renehan, A.G. Growth hormone, the insulin-like growth factor axis, insulin and cancer risk. Nat. Rev. Endocrinol. 2011, 7, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.R.; Nagrale, S.N.; Patil, M.V.; Chavan, S.S. Novel 3,4-methylenedioxybenzene scaffold incorporated 1,3,5-trisubstituted-2-pyrazolines: Synthesis, characterization and evaluation for chemotherapeutic activity. Indian. J. Pharm. Sci. 2015, 77, 24. [Google Scholar] [CrossRef] [PubMed]
- Rollas, S.; Küçükgüzel, S.G. Biological Activities of Hydrazone Derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.C.; Swanson, A.B.; Phillips, D.H.; Fletcher, T.L.; Liem, A.; Miller, J.A. Structure-Activity Studies of the Carcinogenicities in the Mouse and Rat of Some Naturally Occurring and Synthetic Alkenylbenzene Derivatives Related to Safrole and Estragol. Cancer Res. 1983, 43, 1124–1134. [Google Scholar] [PubMed]
- Lima, P.C.; Lima, L.M.; Silva, K.C.M.; Léda, P.H.O.; Miranda, A.L.P.; Fraga, C.A.M.; Barreiro, E.J. Synthesis and analgesic activity of novel N-acylarylhydrazones and isosters, derived from natural safrole. Eur. J. Med. Chem. 2000, 35, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Khayyat, S.A. Photosynthesis of dimeric cinnamaldehyde, eugenol, and safrole as antimicrobial agents. J. Saudi. Chem. Soc. 2013, 17, 61–65. [Google Scholar] [CrossRef]
- Espahbodinia, M.; Ettari, R.; Wen, W.; Wu, A.; Shen, Y.C.; Niu, L.; Grasso, S.; Zappala, M. Development of novel N-3-bromoisoxazolin-5-yl substituted 2,3-benzodiazepines as noncompetitive AMPAR antagonists. Bioorg. Med. Chem. 2017, 25, 3631–3637. [Google Scholar] [CrossRef]
- da Silva, L.M.M.G.; de Oliveira, J.F.; Silva, W.L.; da Silva, A.L.; de Almeida Junior, A.S.A.; dos Santos, V.H.B.; Alves, L.C.; dos Santos, F.A.B.; Costa, V.M.A.; de Lima Aires, A. New 1, 3-benzodioxole derivatives: Synthesis, evaluation of in vitro schistosomicidal activity and ultrastructural analysis. Chem. Biol. Interact. 2018, 283, 20–29. [Google Scholar] [CrossRef]
- Qneibi, M.; Hawash, M.; Bdir, S.; Nacak Baytas, S. Targeting the kinetics mechanism of AMPA receptor inhibition by 2-oxo-3H-benzoxazole derivatives. Bioorganic Chem. 2022, 129, 106163. [Google Scholar] [CrossRef]
- Qin, X.; Ren, L.; Yang, X.; Bai, F.; Wang, L.; Geng, P.; Bai, G.; Shen, Y. Structures of human pancreatic α-amylase in complex with acarviostatins: Implications for drug design against type II diabetes. J. Struct. Biol. 2011, 174, 196–202. [Google Scholar] [CrossRef]
- Williams, L.K.; Li, C.; Withers, S.G.; Brayer, G.D. Order and disorder: Differential structural impacts of myricetin and ethyl caffeate on human amylase, an antidiabetic target. J. Med. Chem. 2012, 55, 10177–10186. [Google Scholar] [CrossRef]
- Kawamura-Konishi, Y.; Watanabe, N.; Saito, M.; Nakajima, N.; Sakaki, T.; Katayama, T.; Enomoto, T. Isolation of a new phlorotannin, a potent inhibitor of carbohydrate-hydrolyzing enzymes, from the brown alga Sargassum patens. J. Agric. Food Chem. 2012, 60, 5565–5570. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.S.; Nandre, K.P.; Ghosh, S.; Rao, V.J.; Chopade, B.A.; Sridhar, B.; Bhosale, S.V.; Bhosale, S.V. Synthesis, crystal structure and antidiabetic activity of substituted (E)-3-(Benzo[d]thiazol-2-ylamino) phenylprop-2-en-1-one. Eur. J. Med. Chem. 2013, 59, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Hawash, M.; Jaradat, N.; Shekfeh, S.; Abualhasan, M.; Eid, A.M.; Issa, L. Molecular docking, chemo-informatic properties, alpha-amylase, and lipase inhibition studies of benzodioxol derivatives. BMC Chem. 2021, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.M.; Hawash, M.; Amer, J.; Jarrar, A.; Qadri, S.; Alnimer, I.; Sharaf, A.; Zalmoot, R.; Hammoudie, O.; Hameedi, S. Synthesis and biological evaluation of novel isoxazole-amide analogues as anticancer and antioxidant agents. BioMed Res. Int. 2021, 2021, 6633297. [Google Scholar] [CrossRef]
- Kabsch, W. xds. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Kabsch, W. Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 133–144. [Google Scholar] [CrossRef]
- Sebbar, N.; Ellouz, M.; Essassi, E.; Ouzidan, Y.; Mague, J. Crystal structure of 4-benzyl-2H-benzo[b][1,4]thiazin-3(4H)-one. Acta Crystallogr. Sect. E Crystallogr. Commun. 2015, 71, o999. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Chodkiewicz, M.L.; Migacz, S.; Rudnicki, W.; Makal, A.; Kalinowski, J.A.; Moriarty, N.W.; Grosse-Kunstleve, R.W.; Afonine, P.V.; Adams, P.D.; Dominiak, P.M. DiSCaMB: A software library for aspherical atom model X-ray scattering factor calculations with CPUs and GPUs. J. Appl. Crystallogr. 2018, 51, 193–199. [Google Scholar] [CrossRef]
- Jha, K.K.; Gruza, B.; Sypko, A.; Kumar, P.; Chodkiewicz, M.L.; Dominiak, P.M. Multipolar Atom Types from Theory and Statistical Clustering (MATTS) Data Bank: Restructurization and Extension of UBDB. J. Chem. Inf. Model. 2022, 62, 3752–3765. [Google Scholar] [CrossRef] [PubMed]
- Kleemiss, F.; Dolomanov, O.V.; Bodensteiner, M.; Peyerimhoff, N.; Midgley, L.; Bourhis, L.J.; Genoni, A.; Malaspina, L.A.; Jayatilaka, D.; Spencer, J.L. Accurate crystal structures and chemical properties from NoSpherA2. Chem. Sci. 2021, 12, 1675–1692. [Google Scholar] [CrossRef]
- Gruza, B.; Chodkiewicz, M.L.; Krzeszczakowska, J.; Dominiak, P.M. Refinement of organic crystal structures with multipolar electron scattering factors. Acta Crystallogr. Sect. A Found. Adv. 2020, 76, 92–109. [Google Scholar] [CrossRef] [PubMed]
- Wickramaratne, M.N.; Punchihewa, J.; Wickramaratne, D. In-vitro alpha amylase inhibitory activity of the leaf extracts of Adenanthera pavonina. BMC Complement. Altern. Med. 2016, 16, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Hawash, M.; Jaradat, N.; Elaraj, J.; Hamdan, A.; Lebdeh, S.A.; Halawa, T. Evaluation of the hypoglycemic effect of seven wild folkloric edible plants from Palestine. J. Complement. Integr. Med. 2019, 17, 201900032. [Google Scholar] [CrossRef]
- Sharma, B.; Salunke, R.; Balomajumder, C.; Daniel, S.; Roy, P. Anti-diabetic potential of alkaloid rich fraction from Capparis decidua on diabetic mice. J. Ethnopharmacol. 2010, 127, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.; Brown, P. Parenteral injections. Curr. Protoc. Immunol. 2006, 73, 10. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, N.; Al-Maharik, N. Fingerprinting, Antimicrobial, Antioxidant, Anticancer, Cyclooxygenase and Metabolic Enzymes Inhibitory Characteristic Evaluations of Stachys viticina Boiss. Essential Oil. Molecules 2019, 24, 3880. [Google Scholar] [CrossRef]
- Hawash, M.; Jaradat, N.; Abualhasan, M.; Şüküroğlu, M.K.; Qaoud, M.T.; Kahraman, D.C.; Daraghmeh, H.; Maslamani, L.; Sawafta, M.; Ratrout, A.; et al. Design, synthesis, molecular docking studies and biological evaluation of thiazole carboxamide derivatives as COX inhibitors. BMC Chem. 2023, 17, 11. [Google Scholar] [CrossRef]
- Xie, D.; Hu, X.; Ren, X.; Yang, Z. Synthesis and Bioactivities of Novel Piperonylic Acid Derivatives Containing a Sulfonic Acid Ester Moiety. Front. Chem. 2022, 10, 913003. [Google Scholar] [CrossRef]
- Li, S.-C.; Jhang, W.-F.; Liou, T.-J.; Yang, D.-Y. Photochemical synthesis of indazolo [3, 2-b] quinazolines and their redox-switching properties. Dyes Pigments 2015, 114, 259–266. [Google Scholar] [CrossRef]
- Samulitis, B.; Goda, T.; Lee, S.; Koldovský, O. Inhibitory mechanism of acarbose and 1-deoxynojirimycin derivatives on carbohydrases in rat small intestine. Drugs Exp. Clin. Res. 1987, 13, 517–524. [Google Scholar]
- Williams, L.K.; Zhang, X.; Caner, S.; Tysoe, C.; Nguyen, N.T.; Wicki, J.; Williams, D.E.; Coleman, J.; McNeill, J.H.; Yuen, V. The amylase inhibitor montbretin A reveals a new glycosidase inhibition motif. Nat. Chem. Biol. 2015, 11, 691–696. [Google Scholar] [CrossRef]
- Gilles, C.; Astier, J.P.; Marchis-Mouren, G.; Cambillau, C.; Payan, F. Crystal structure of pig pancreatic α-amylase isoenzyme II, in complex with the carbohydrate inhibitor acarbose. Eur. J. Biochem. 1996, 238, 561–569. [Google Scholar] [CrossRef]
- Hasegawa, K.; Kubota, M.; Matsuura, Y. Roles of catalytic residues in α-amylases as evidenced by the structures of the product-complexed mutants of a maltotetraose-forming amylase. Protein Eng. 1999, 12, 819–824. [Google Scholar] [CrossRef] [PubMed]


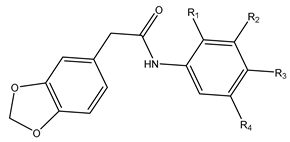 St. I | 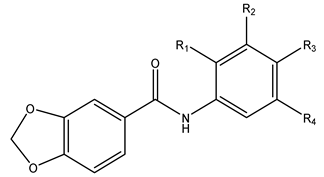 St. II | ||||||
|---|---|---|---|---|---|---|---|
| Compound | St. | R1 | R2 | R3 | R4 | IC50 µM | |
| α-Amylase | Hek293t | ||||||
| Ia | I | O-CH3 | H | Cl | O-CH3 | 16.77 ± 1.55 | >300 |
| Ib | I | H | H | H | H | 8.44 ± 1.01 | 269.23 ± 1.73 |
| Ic | I | O-CH3 | H | H | O-CH3 | 16.40 ± 1.58 | 206.64 ± 2.59 |
| IIa | II | H | H | H | H | 0.85 ± 0.18 | >300 |
| IIb | II | H | O-CH3 | O-CH3 | O-CH3 | 15.30 ± 1.07 | >300 |
| IIc | II | H | CF3 | H | H | 0.68 ± 0.25 | 155.87 ± 2.98 |
| IId | II | H | H | 2-OCH3Ph | H | 5.76 ± 1.92 | >300 |
| IIe | II | H | O-CH3 | H | O-CH3 | 14.97 ± 3.52 [21] | >300 |
| Acarbose | 1.55 ± 0.85 | - | |||||
| DOX | - | 0.038 ± 0.02 | |||||
| IC50 µM | ||||||
|---|---|---|---|---|---|---|
| Caco-2 | HepG2 | Hep3B | MCF-7 | HeLa | LX-2 | |
| Ia | >300 | 169.44 ± 2.16 | 196.52 ± 1.99 | 123.58 ± 2.73 | 43.65 ± 2.97 | 70.62 ± 2.45 |
| Ib | >300 | >300 | >300 | >300 | >300 | 280.61 ± 2.33 |
| Ic | >300 | >300 | >300 | >300 | >300 | 208.87 ± 2.20 |
| IIa | >300 | >300 | >300 | >300 | >300 | >300 |
| IIb | >300 | >300 | >300 | >300 | >300 | 96.60 ± 2.70 |
| IIc | >300 | >300 | >300 | >300 | >300 | >300 |
| IId | >300 | 55.33 ± 2.72 | 65.16 ± 2.53 | 55.7592 ± 2.51 | 26.59 ± 2.41 | 31.43 ± 2.71 |
| IIe | >300 | >300 | >300 | >300 | >300 | 183.43 ± 2.00 |
| 5-FU | 4.75 ± 1.71 | 4.07 ± 1.42 | 5.11 ± 1.67 | 6.51 ± 1.82 | 7.79 ± 1.60 | 0.178 ± 0.11 |
| DOX | 0.024 ± 0.001 | <0.01 | 0.025 ± 0.005 | 0.053 ± 0.009 | <0.01 | 0.21 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawash, M.; Al-Smadi, D.; Kumar, A.; Olech, B.; Dominiak, P.M.; Jaradat, N.; Antari, S.; Mohammed, S.; Nasasrh, A.; Abualhasan, M.; et al. Characterization and Investigation of Novel Benzodioxol Derivatives as Antidiabetic Agents: An In Vitro and In Vivo Study in an Animal Model. Biomolecules 2023, 13, 1486. https://doi.org/10.3390/biom13101486
Hawash M, Al-Smadi D, Kumar A, Olech B, Dominiak PM, Jaradat N, Antari S, Mohammed S, Nasasrh A, Abualhasan M, et al. Characterization and Investigation of Novel Benzodioxol Derivatives as Antidiabetic Agents: An In Vitro and In Vivo Study in an Animal Model. Biomolecules. 2023; 13(10):1486. https://doi.org/10.3390/biom13101486
Chicago/Turabian StyleHawash, Mohammed, Derar Al-Smadi, Anil Kumar, Barbara Olech, Paulina Maria Dominiak, Nidal Jaradat, Sarah Antari, Sarah Mohammed, Ala’a Nasasrh, Murad Abualhasan, and et al. 2023. "Characterization and Investigation of Novel Benzodioxol Derivatives as Antidiabetic Agents: An In Vitro and In Vivo Study in an Animal Model" Biomolecules 13, no. 10: 1486. https://doi.org/10.3390/biom13101486
APA StyleHawash, M., Al-Smadi, D., Kumar, A., Olech, B., Dominiak, P. M., Jaradat, N., Antari, S., Mohammed, S., Nasasrh, A., Abualhasan, M., Musa, A., Suboh, S., Çapan, İ., Qneibi, M., & Natsheh, H. (2023). Characterization and Investigation of Novel Benzodioxol Derivatives as Antidiabetic Agents: An In Vitro and In Vivo Study in an Animal Model. Biomolecules, 13(10), 1486. https://doi.org/10.3390/biom13101486







