Liquid–Liquid Phase Separation Prediction of Proteins in Salt Solution by Deep Neural Network
Abstract
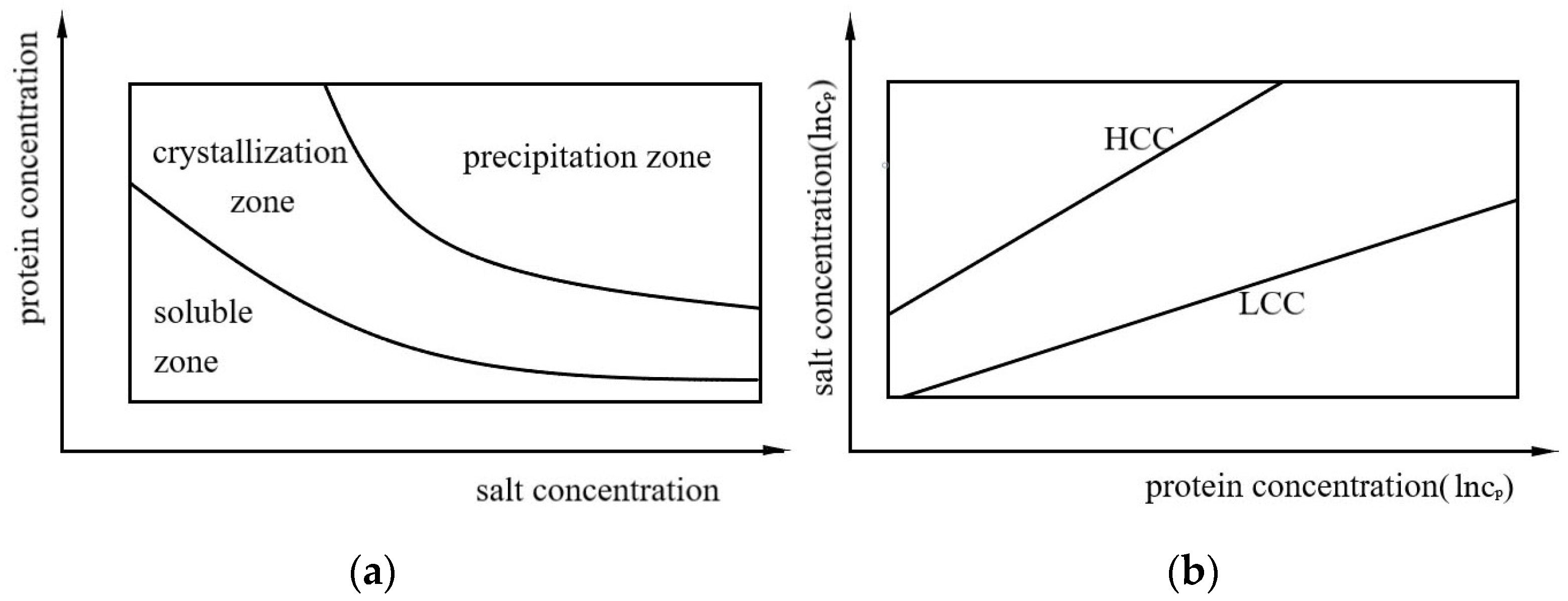
1. Introduction
2. Materials and Methods
2.1. Description of Neural Networks
2.2. Analysis of Data
2.3. Experimental Materials
3. Results and Discussion
3.1. Prediction by ANNs
3.2. Accuracy and Loss Functions
3.3. Experimental Verification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zhang, F.J.; Roth, R.; Wolf, M.; Roosen-Runge, F.; Skoda, M.W.A.; Jacobs, R.M.J.; Stzucki, M.; Schreiber, F. Charge-controlled metastable liquid-liquid phase separation in protein solutions as a universal pathway towards crystallization. Soft Matter 2012, 8, 1313–1316. [Google Scholar] [CrossRef]
- Alberti, S.; Gladfelter, A.; Mittag, T. Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell 2019, 176, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Krainer, G.; Welsh, T.J.; Joseph, J.A.; Espinosa, J.R.; Wittmann, S.; de Csillery, E.; Sridhar, A.; Toprakcioglu, Z.; Gudiskyte, G.; Czekalska, M.A.; et al. Reentrant liquid condensate phase of proteins is stabilized by hydrophobic and non-ionic interactions. Nat. Commun. 2021, 12, 1085. [Google Scholar] [CrossRef] [PubMed]
- Babinchak, W.M.; Dumm, B.K.; Venus, S.; Boyko, S.; Putnam, A.A.; Jankowsky, E.; Surewicz, W.K. Small molecules as potent biphasic modulators of protein liquid-liquid phase separation. Nat. Commun. 2020, 11, 5574. [Google Scholar] [CrossRef]
- Fisher, R.S.; Elbaum-Garfinkle, S. Tunable multiphase dynamics of arginine and lysine liquid condensates. Nat. Commun. 2020, 11, 4628. [Google Scholar] [CrossRef]
- Vekilov, P.G. Nucleation. Cryst. Growth Des. 2010, 10, 5007–5019. [Google Scholar] [CrossRef]
- Watanabe, E.O.; Popova, E.; Miranda, E.A.; Maurer, G.; Filho, P.d.A.P. Phase equilibria for salt-induced lysozyme precipitation: Effect of salt type and temperature. Fluid Phase Equilibria 2009, 281, 32–39. [Google Scholar] [CrossRef]
- Wolf, M.; Roosen-Runge, F.; Zhang, F.; Roth, R.; Skoda, M.W.A.; Jacobs, R.M.J.; Sztucki, M.; Schreiber, F. Effective interactions in protein–salt solutions approaching liquid–liquid phase separation. J. Mol. Liq. 2014, 200, 20–27. [Google Scholar] [CrossRef]
- Zhang, F.J.; Roosen-Runge, F.; Sauter, A.; Wolf, M.; Jacobs, R.M.J.; Schreiber, F. Reentrant condensation, liquid-liquid phase separation and crystallization in protein solutions induced by multivalent metal ions. Pure Appl. Chem. 2014, 86, 191–202. [Google Scholar] [CrossRef]
- Asherie, N. Protein crystallization and phase diagrams. Methods 2004, 34, 266–272. [Google Scholar] [CrossRef]
- Cacioppo, E.; Pusey, M.L. The solubility of the tetragonal form of hen egg white lysozyme from pH 4.0 to 5.4. J. Cryst. Growth 1991, 114, 286–292. [Google Scholar] [CrossRef]
- Judge, R.A.; Jacobs, R.S.; Frazier, T.; Snell, E.H.; Pusey, M.L. The Effect of Temperature and Solution pH on the Nucleation of Tetragonal Lysozyme Crystals. Biophys. J. 1999, 77, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- ten Wolde, P.R.; Frenkel, D. Enhancement of protein crystal nucleation by critical density fluctuations. Science 1997, 277, 1975–1978. [Google Scholar] [CrossRef]
- Broide, M.L.; Tominc, T.M.; Saxowsky, M.D. Using phase transitions to investigate the effect of salts on protein interactions. Phys. Rev. E 1996, 53, 6325–6335. [Google Scholar] [CrossRef] [PubMed]
- Grigsby, J.J.; Blanch, H.W.; Prausnitz, J.M. Cloud-point temperatures for lysozyme in electrolyte solutions: Effect of salt type, salt concentration and pH. Biophys. Chem. 2001, 91, 231–243. [Google Scholar] [CrossRef]
- Chang, B.H.; Bae, Y.C. Lysozyme−Lysozyme and Lysozyme−Salt Interactions in the Aqueous Saline Solution: A New Square-Well Potential. Biomacromolecules 2003, 4, 1713–1718. [Google Scholar] [CrossRef]
- Heijna, M.C.R.; Van Enckevort, W.J.P.; Vlieg, E. Crystal growth in a three-phase system: Diffusion and liquid-liquid phase separation in lysozyme crystal growth. Phys. Rev. E 2007, 76, 011604. [Google Scholar] [CrossRef]
- Huang, J.; Li, S.B.; Zhang, X.H.; Huang, G. Neural network model for structure factor of polymer systems. J. Chem. Phys. 2020, 153, 124902. [Google Scholar] [CrossRef]
- Saar, K.L.; Morgunov, A.S.; Qi, R.Z.; Arter, W.E.; Krainer, G.; Lee, A.A.; Knowles, T.P.J. Learning the molecular grammar of protein condensates from sequence determinants and embeddings. Proc. Natl. Acad. Sci. USA 2021, 118, e2019053118. [Google Scholar] [CrossRef]
- Zhang, X.P.; Zhang, S.J.; He, X.Z. Prediction of solubility of lysozyme in lysozyme-NaCl-H2O system with artificial neural network. J. Cryst. Growth 2004, 264, 409–416. [Google Scholar] [CrossRef]
- Huang, G.B.; Zhu, Q.Y.; Siew, C.K. Extreme learning machine: Theory and applications. Neurocomputing 2006, 70, 489–501. [Google Scholar] [CrossRef]
- Veesler, S.; Revalor, E.; Bottini, O.; Hoff, C. Crystallization in the Presence of a Liquid−Liquid Phase Separation. Org. Process Res. Dev. 2006, 10, 841–845. [Google Scholar] [CrossRef]
- Anwar, S.M.; Majid, M.; Qayyum, A.; Awais, M.; Alnowami, M.; Khan, M.K. Medical Image Analysis using Convolutional Neural Networks: A Review. J. Med. Syst. 2018, 42, 1–13. [Google Scholar] [CrossRef]
- Kingma, D.P.; Ba, J. Adam: A method for stochastic optimization. arXiv 2014, arXiv:1412.6980. [Google Scholar]
- Forsythe, E.L.; Judge, R.A.; Pusey, M.L. Tetragonal Chicken Egg White Lysozyme Solubility in Sodium Chloride Solutions. J. Chem. Eng. Data 1999, 44, 637–640. [Google Scholar] [CrossRef]
- Muschol, M.; Rosenberger, F. Liquid–liquid phase separation in supersaturated lysozyme solutions and associated precipitate formation/crystallization. J. Chem. Phys. 1997, 107, 1953–1962. [Google Scholar] [CrossRef]
- Darcy, P.A.; Wiencek, J.M. Identifying nucleation temperatures for lysozyme via differential scanning calorimetry. J. Cryst. Growth 1999, 196, 243–249. [Google Scholar] [CrossRef]
- Petsev, D.N.; Wu, X.; Galkin, O.; Vekilov, P.G. Thermodynamic Functions of Concentrated Protein Solutions from Phase Equilibria. J. Phys. Chem. B 2003, 107, 3921–3926. [Google Scholar] [CrossRef]
- Lu, J.; Carpenter, K.; Li, R.-J.; Wang, X.-J.; Ching, C.-B. Cloud-point temperature and liquid–liquid phase separation of supersaturated lysozyme solution. Biophys. Chem. 2004, 109, 105–112. [Google Scholar] [CrossRef]
- Wentzel, N.; Gunton, J.D. Liquid−Liquid Coexistence Surface for Lysozyme: Role of Salt Type and Salt Concentration. J. Phys. Chem. B 2007, 111, 1478–1481. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Wang, X.; Ching, C.B. Toward Further Understanding of Lysozyme Crystallization: Phase Diagram, Protein−Protein Interaction, Nucleation Kinetics, and Growth Kinetics. Cryst. Growth Des. 2010, 10, 548–558. [Google Scholar] [CrossRef]
- Taratuta, V.G.; Holschbach, A.; Thurston, G.M.; Blankschtein, D.; Benedek, G.B. Liquid-liquid phase separation of aqueous lysozyme solutions: Effects of pH and salt identity. J. Phys. Chem. 1990, 94, 2140–2144. [Google Scholar] [CrossRef]
- Liao, Z.J. Regulation of Dodecyl Dimethylamine Oxide on Liquid-Liquid Separation of Bovine Serum Albumin. Master’s Thesis, Wenzhou University, Wenzhou, China, 2022. [Google Scholar]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, S.; Wang, Y.; Yang, G. Liquid–Liquid Phase Separation Prediction of Proteins in Salt Solution by Deep Neural Network. Biomolecules 2023, 13, 42. https://doi.org/10.3390/biom13010042
Wei S, Wang Y, Yang G. Liquid–Liquid Phase Separation Prediction of Proteins in Salt Solution by Deep Neural Network. Biomolecules. 2023; 13(1):42. https://doi.org/10.3390/biom13010042
Chicago/Turabian StyleWei, Suwen, Yanwei Wang, and Guangcan Yang. 2023. "Liquid–Liquid Phase Separation Prediction of Proteins in Salt Solution by Deep Neural Network" Biomolecules 13, no. 1: 42. https://doi.org/10.3390/biom13010042
APA StyleWei, S., Wang, Y., & Yang, G. (2023). Liquid–Liquid Phase Separation Prediction of Proteins in Salt Solution by Deep Neural Network. Biomolecules, 13(1), 42. https://doi.org/10.3390/biom13010042




