Group I Metabotropic Glutamate Receptors Modulate Motility and Enteric Neural Activity in the Mouse Colon
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
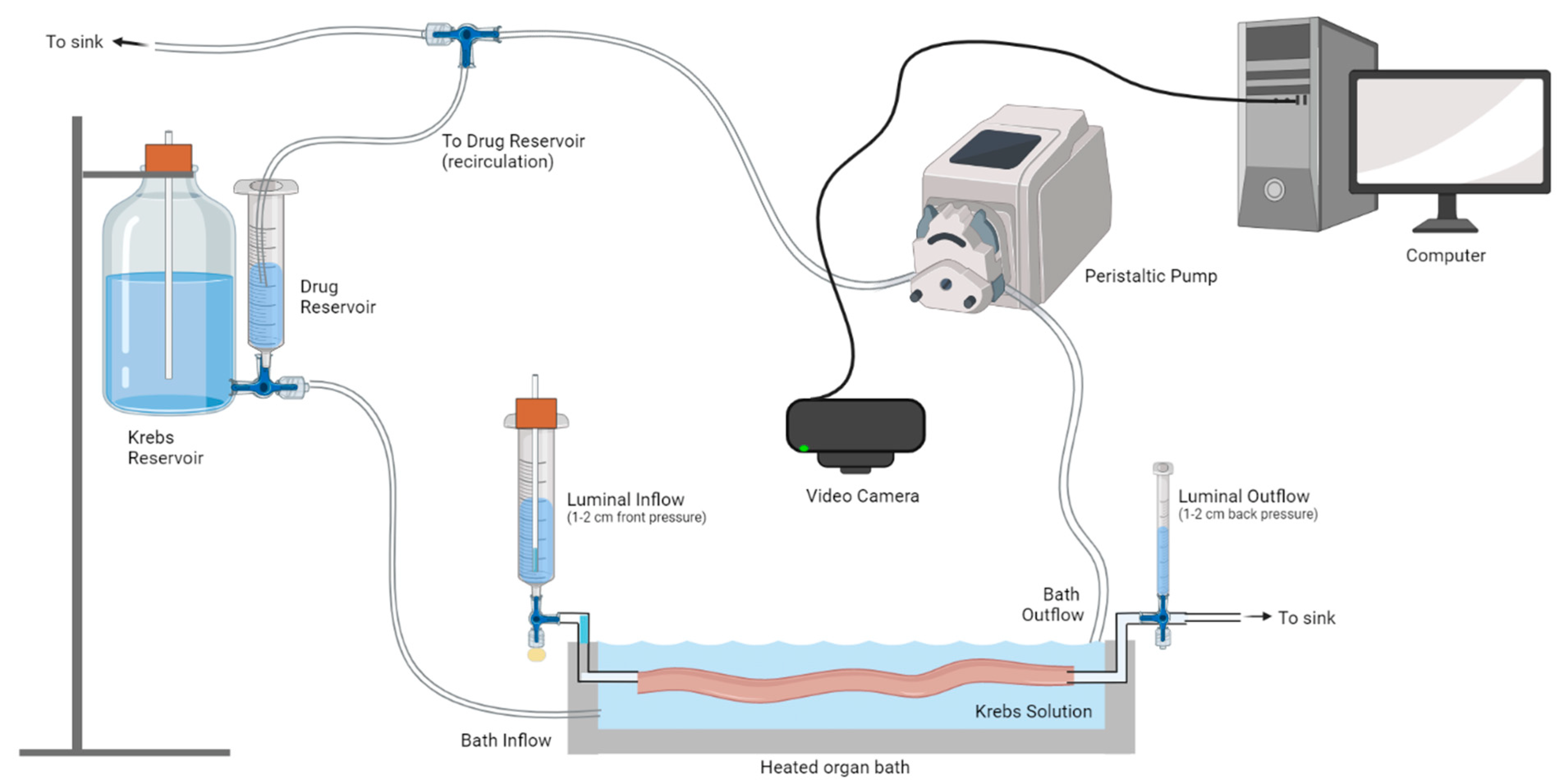
2.2. Calcium Imaging
2.3. Video Imaging of Ex Vivo Colonic Motility
2.4. Pharmacological Agents
2.5. RNAscope In Situ Hybridization
2.6. Immunofluorescence Staining
2.7. Imaging and Analysis
2.8. Data Analysis
3. Results
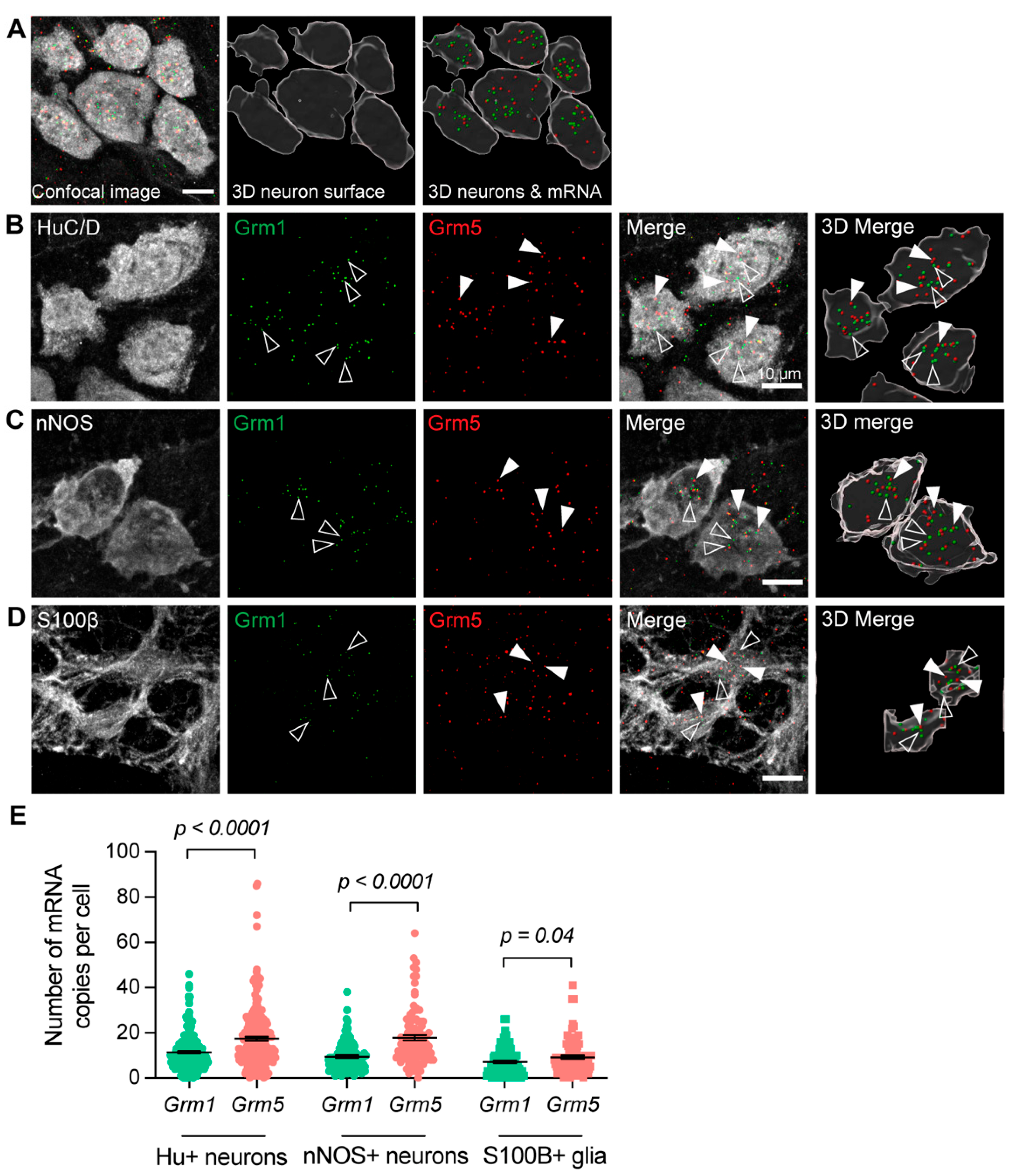
3.1. Group-I mGlu Receptors Are Widely Expressed in the Myenteric Plexus
3.2. Group-I mGlu Receptors and Myenteric Neuron Activity

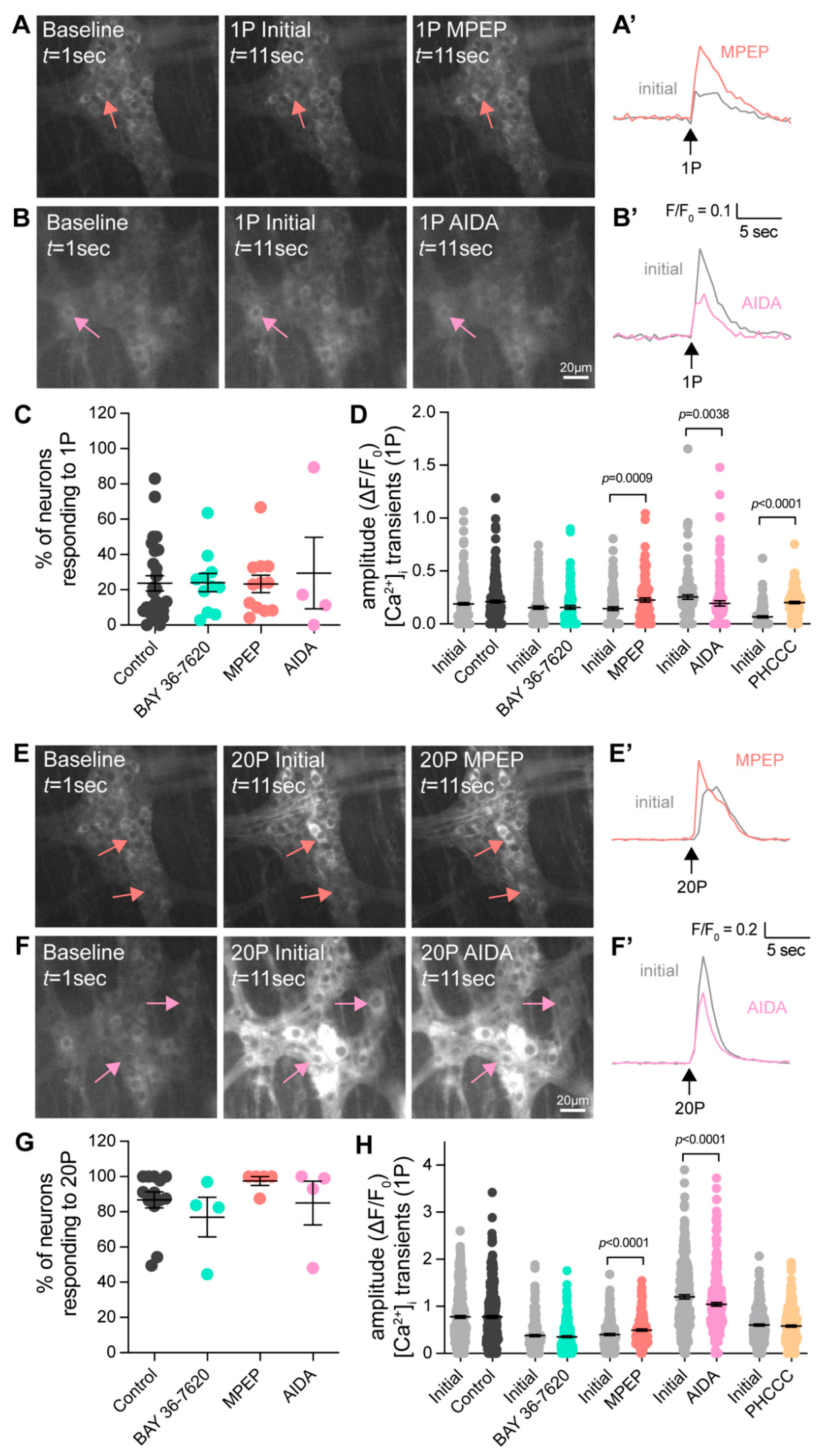
3.3. Group-I mGlu Receptors Are Involved in Initiation of Colonic Motility

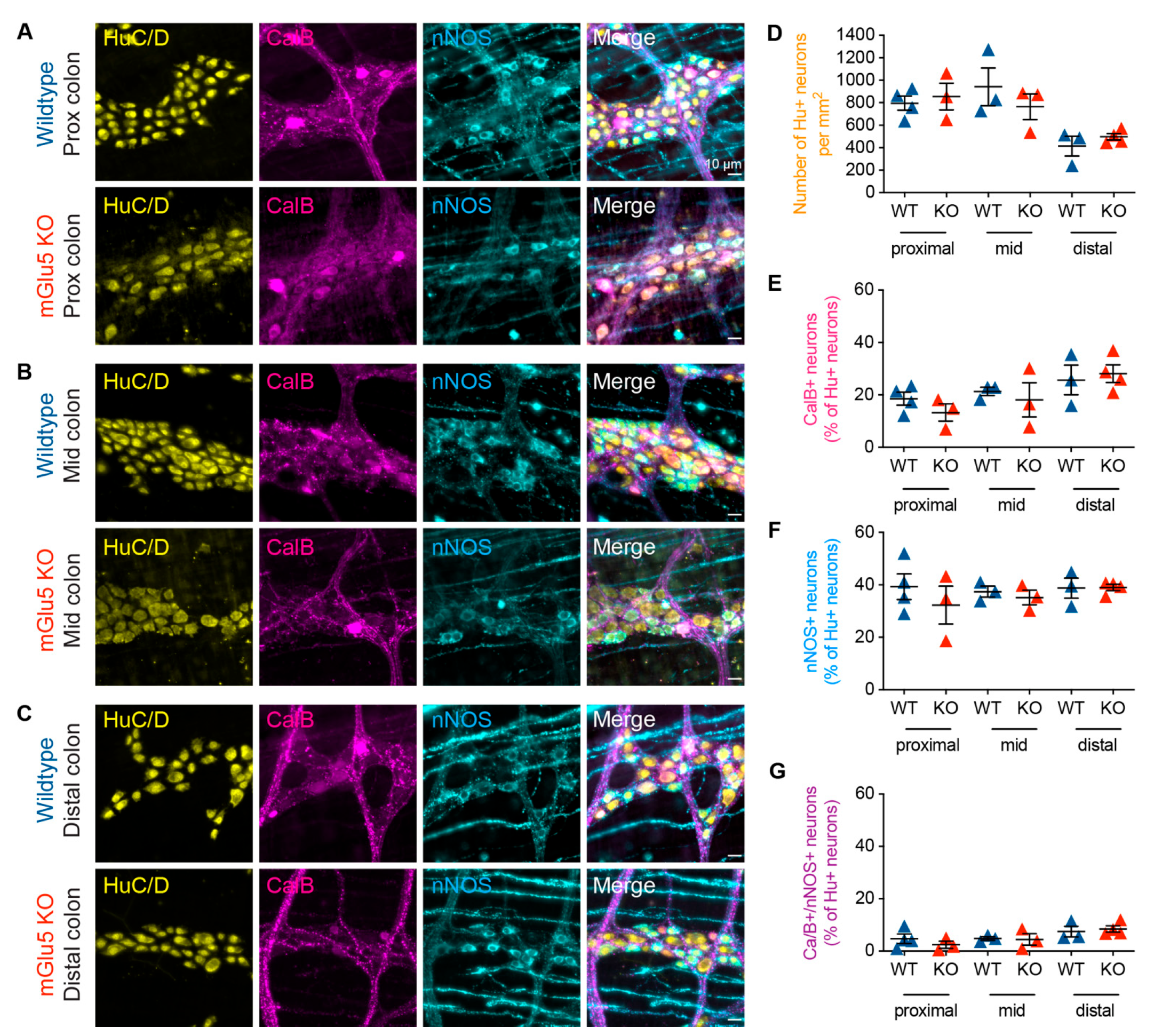
3.4. mGlu5-KO Mice Show No Change in Myenteric Neurochemistry
4. Discussion
4.1. The Role of Group-I mGlu Receptors on Synaptic Transmission and Colonic Motility
4.2. Group-I mGlu Receptors in Enteric Glia
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, S.A.; Raiten, D.J. Safety of Amino Acids Used as Dietary Supplements; Federation of American Societies for Experimental Biology: Bethesda, MD, USA, 1992. [Google Scholar]
- Beyreuther, K.; Biesalski, H.K.; Fernstrom, J.D.; Grimm, P.; Hammes, W.P.; Heinemann, U.; Kempski, O.; Stehle, P.; Steinhart, H.; Walker, R. Consensus meeting: Monosodium glutamate—An update. Eur. J. Clin. Nutr. 2007, 61, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef] [PubMed]
- Reeds, P.J.; Burrin, D.G.; Stoll, B.; Jahoor, F. Intestinal glutamate metabolism. J. Nutr. 2000, 130, 978s–982s. [Google Scholar] [CrossRef] [PubMed]
- Tomé, D. The Roles of Dietary Glutamate in the Intestine. Ann. Nutr. Metab. 2018, 73 (Suppl. 5), 15–20. [Google Scholar] [CrossRef] [PubMed]
- Pin, J.P.; Duvoisin, R. The metabotropic glutamate receptors: Structure and functions. Neuropharmacology 1995, 34, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Mahato, P.K.; Ramsakha, N.; Ojha, P.; Gulia, R.; Sharma, R.; Bhattacharyya, S. Group I Metabotropic Glutamate Receptors (mGluRs): Ins and Outs. Adv. Exp. Med. Biol. 2018, 1112, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Baude, A.; Nusser, Z.; Roberts, J.D.; Mulvihill, E.; McIlhinney, R.A.; Somogyi, P. The metabotropic glutamate receptor (mGluR1 alpha) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron 1993, 11, 771–787. [Google Scholar] [CrossRef]
- Shigemoto, R.; Nomura, S.; Ohishi, H.; Sugihara, H.; Nakanishi, S.; Mizuno, N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci. Lett. 1993, 163, 53–57. [Google Scholar] [CrossRef]
- Kim, C.H.; Lee, J.; Lee, J.Y.; Roche, K.W. Metabotropic glutamate receptors: Phosphorylation and receptor signaling. J. Neurosci. Res. 2008, 86, 1–10. [Google Scholar] [CrossRef]
- Conn, P.J.; Pin, J.P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 205–237. [Google Scholar] [CrossRef]
- Lüscher, C.; Huber, K.M. Group 1 mGluR-dependent synaptic long-term depression: Mechanisms and implications for circuitry and disease. Neuron 2010, 65, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Anwyl, R. Metabotropic glutamate receptor-dependent long-term potentiation. Neuropharmacology 2009, 56, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef]
- Spencer, N.J.; Hu, H. Enteric nervous system: Sensory transduction, neural circuits and gastrointestinal motility. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Nasser, Y.; Keenan, C.M.; Ma, A.C.; McCafferty, D.M.; Sharkey, K.A. Expression of a functional metabotropic glutamate receptor 5 on enteric glia is altered in states of inflammation. Glia 2007, 55, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Burns, G.A.; Stephens, K.E.; Benson, J.A. Expression of mRNA for the N-methyl-D-aspartate (NMDAR1) receptor by the enteric neurons of the rat. Neurosci. Lett. 1994, 170, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.H. Function of GABAergic and glutamatergic neurons in the stomach. J. Biomed. Sci. 2005, 12, 255–266. [Google Scholar] [CrossRef]
- Liu, M.T.; Rothstein, J.D.; Gershon, M.D.; Kirchgessner, A.L. Glutamatergic enteric neurons. J. Neurosci. 1997, 17, 4764–4784. [Google Scholar] [CrossRef]
- Seifi, M.; Swinny, J.D. Immunolocalization of AMPA receptor subunits within the enteric nervous system of the mouse colon and the effect of their activation on spontaneous colonic contractions. Neurogastroenterol. Motil. 2016, 28, 705–720. [Google Scholar] [CrossRef]
- Galligan, J.J. Ligand-gated ion channels in the enteric nervous system. Neurogastroenterol. Motil. 2002, 14, 611–623. [Google Scholar] [CrossRef]
- Hu, H.Z.; Ren, J.; Liu, S.; Gao, C.; Xia, Y.; Wood, J.D. Functional group I metabotropic glutamate receptors in submucous plexus of guinea-pig ileum. Br. J. Pharmacol. 1999, 128, 1631–1635. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Kirchgessner, A.L. Agonist- and reflex-evoked internalization of metabotropic glutamate receptor 5 in enteric neurons. J. Neurosci. 2000, 20, 3200–3205. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Hu, H.Z.; Liu, S.; Xia, Y.; Wood, J.D. Glutamate receptors in the enteric nervous system: Ionotropic or metabotropic? Neurogastroenterol. Motil. 2000, 12, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, M.; Hill-Yardin, E.L.; Bornstein, J.C.; Foong, J.P.P. Endogenous Glutamate Excites Myenteric Calbindin Neurons by Activating Group I Metabotropic Glutamate Receptors in the Mouse Colon. Front. Neurosci. 2019, 13, 426. [Google Scholar] [CrossRef] [PubMed]
- Danielian, P.S.; Muccino, D.; Rowitch, D.H.; Michael, S.K.; McMahon, A.P. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr. Biol. 1998, 8, 1323–1326. [Google Scholar] [CrossRef]
- Chen, T.-W.; Wardill, T.J.; Sun, Y.; Pulver, S.R.; Renninger, S.L.; Baohan, A.; Schreiter, E.R.; Kerr, R.A.; Orger, M.B.; Jayaraman, V.; et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 2013, 499, 295–300. [Google Scholar] [CrossRef]
- Li, Z.; Hao, M.M.; Van den Haute, C.; Baekelandt, V.; Boesmans, W.; Vanden Berghe, P. Regional complexity in enteric neuron wiring reflects diversity of motility patterns in the mouse large intestine. eLife 2019, 8, e42914. [Google Scholar] [CrossRef]
- Swaminathan, M.; Hill-Yardin, E.; Ellis, M.; Zygorodimos, M.; Johnston, L.A.; Gwynne, R.M.; Bornstein, J.C. Video Imaging and Spatiotemporal Maps to Analyze Gastrointestinal Motility in Mice. J. Vis. Exp. 2016, 108, 53828. [Google Scholar] [CrossRef]
- Neal, K.B.; Parry, L.J.; Bornstein, J.C. Strain-specific genetics, anatomy and function of enteric neural serotonergic pathways in inbred mice. J. Physiol. 2009, 587, 567–586. [Google Scholar] [CrossRef] [PubMed]
- Herath, M.; Cho, E.; Franks, A.E.; Bornstein, J.C.; Hill-Yardin, E.L. Quantitative analysis of neuroligin-3 expression in the enteric nervous system of the Neuroligin-3 R451C mouse model of autism. bioRxiv 2022. [Google Scholar] [CrossRef]
- Leembruggen, A.J.L.; Balasuriya, G.K.; Zhang, J.; Schokman, S.; Swiderski, K.; Bornstein, J.C.; Nithianantharajah, J.; Hill-Yardin, E.L. Colonic dilation and altered ex vivo gastrointestinal motility in the neuroligin-3 knockout mouse. Autism Res. Off. J. Int. Soc. Autism Res. 2019, 13, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.D.; Thacker, M.; Castelucci, P.; Bagyanszki, M.; Epstein, M.; Furness, J.B. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell Tissue Res. 2008, 334, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Ferraguti, F.; Crepaldi, L.; Nicoletti, F. Metabotropic glutamate 1 receptor: Current concepts and perspectives. Pharmacol. Rev. 2008, 60, 536–581. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Boesmans, W.; Kazwiny, Y.; Hao, M.M.; Vanden Berghe, P. Simultaneous whole-cell patch-clamp and calcium imaging on myenteric neurons. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 323, G341–G347. [Google Scholar] [CrossRef] [PubMed]
- Foong, J.P.; Bornstein, J.C. mGluR(1) Receptors Contribute to Non-Purinergic Slow Excitatory Transmission to Submucosal VIP Neurons of Guinea-Pig Ileum. Front. Neurosci. 2009, 3, 46. [Google Scholar] [CrossRef]
- Niswender, C.M.; Conn, P.J. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef]
- Coutinho, V.; Knöpfel, T. Metabotropic glutamate receptors: Electrical and chemical signaling properties. Neuroscientist 2002, 8, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Valenti, O.; Conn, P.J.; Marino, M.J. Distinct physiological roles of the Gq-coupled metabotropic glutamate receptors Co-expressed in the same neuronal populations. J. Cell. Physiol. 2002, 191, 125–137. [Google Scholar] [CrossRef]
- Fiorillo, C.D.; Williams, J.T. Glutamate mediates an inhibitory postsynaptic potential in dopamine neurons. Nature 1998, 394, 78–82. [Google Scholar] [CrossRef]
- Tack, J.F.; Wood, J.D. Electrical behaviour of myenteric neurones in the gastric antrum of the guinea-pig. J. Physiol. 1992, 447, 49–66. [Google Scholar] [CrossRef]
- Deng, H.; Gerencser, A.A.; Jasper, H. Signal integration by Ca(2+) regulates intestinal stem-cell activity. Nature 2015, 528, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Murthy, K.S.; Grider, J.R. Expression patterns of L-amino acid receptors in the murine STC-1 enteroendocrine cell line. Cell Tissue Res. 2019, 378, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Mannaioni, G.; Marino, M.J.; Valenti, O.; Traynelis, S.F.; Conn, P.J. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J. Neurosci. 2001, 21, 5925–5934. [Google Scholar] [CrossRef] [PubMed]
- Maj, M.; Bruno, V.; Dragic, Z.; Yamamoto, R.; Battaglia, G.; Inderbitzin, W.; Stoehr, N.; Stein, T.; Gasparini, F.; Vranesic, I.; et al. (−)-PHCCC, a positive allosteric modulator of mGluR4: Characterization, mechanism of action, and neuroprotection. Neuropharmacology 2003, 45, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Nunez-Salces, M.; Li, H.; Feinle-Bisset, C.; Young, R.L.; Page, A.J. Nutrient-sensing components of the mouse stomach and the gastric ghrelin cell. Neurogastroenterol. Motil. 2020, 32, e13944. [Google Scholar] [CrossRef] [PubMed]
- Julio-Pieper, M.; Flor, P.J.; Dinan, T.G.; Cryan, J.F. Exciting times beyond the brain: Metabotropic glutamate receptors in peripheral and non-neural tissues. Pharmacol. Rev. 2011, 63, 35–58. [Google Scholar] [CrossRef] [PubMed]
- Panaccione, I.; King, R.; Molinaro, G.; Riozzi, B.; Battaglia, G.; Nicoletti, F.; Bashir, Z.I. Constitutively active group I mGlu receptors and PKMzeta regulate synaptic transmission in developing perirhinal cortex. Neuropharmacology 2013, 66, 143–150. [Google Scholar] [CrossRef]
- Spencer, N.J. Constitutively Active 5-HT Receptors: An Explanation of How 5-HT Antagonists Inhibit Gut Motility in Species Where 5-HT is Not an Enteric Neurotransmitter? Front. Cell. Neurosci. 2015, 9, 487. [Google Scholar] [CrossRef]
- Boesmans, W.; Cirillo, C.; Van den Abbeel, V.; Van den Haute, C.; Depoortere, I.; Tack, J.; Vanden Berghe, P. Neurotransmitters involved in fast excitatory neurotransmission directly activate enteric glial cells. Neurogastroenterol. Motil. 2013, 25, e151–e160. [Google Scholar] [CrossRef]
- Fung, C.; Boesmans, W.; Cirillo, C.; Foong, J.P.P.; Bornstein, J.C.; Vanden Berghe, P. VPAC Receptor Subtypes Tune Purinergic Neuron-to-Glia Communication in the Murine Submucosal Plexus. Front. Cell. Neurosci. 2017, 11, 118. [Google Scholar] [CrossRef]
- Kim, S.K.; Nabekura, J.; Koizumi, S. Astrocyte-mediated synapse remodeling in the pathological brain. Glia 2017, 65, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Lalo, U.; Koh, W.; Lee, C.J.; Pankratov, Y. The tripartite glutamatergic synapse. Neuropharmacology 2021, 199, 108758. [Google Scholar] [CrossRef] [PubMed]
- Cornell-Bell, A.H.; Finkbeiner, S.M.; Cooper, M.S.; Smith, S.J. Glutamate induces calcium waves in cultured astrocytes: Long-range glial signaling. Science 1990, 247, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Glaum, S.R.; Holzwarth, J.A.; Miller, R.J. Glutamate receptors activate Ca2+ mobilization and Ca2+ influx into astrocytes. Proc. Natl. Acad. Sci. USA 1990, 87, 3454–3458. [Google Scholar] [CrossRef]
- Broadhead, M.J.; Bayguinov, P.O.; Okamoto, T.; Heredia, D.J.; Smith, T.K. Ca2+ transients in myenteric glial cells during the colonic migrating motor complex in the isolated murine large intestine. J. Physiol. 2012, 590, 335–350. [Google Scholar] [CrossRef]
- McClain, J.L.; Fried, D.E.; Gulbransen, B.D. Agonist-evoked Ca(2+) signaling in enteric glia drives neural programs that regulate intestinal motility in mice. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 631–645. [Google Scholar] [CrossRef]
- McClain, J.; Grubišić, V.; Fried, D.; Gomez-Suarez, R.A.; Leinninger, G.M.; Sévigny, J.; Parpura, V.; Gulbransen, B.D. Ca2+ responses in enteric glia are mediated by connexin-43 hemichannels and modulate colonic transit in mice. Gastroenterology 2014, 146, 497–507.e1. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leembruggen, A.J.L.; Lu, Y.; Wang, H.; Uzungil, V.; Renoir, T.; Hannan, A.J.; Stamp, L.A.; Hao, M.M.; Bornstein, J.C. Group I Metabotropic Glutamate Receptors Modulate Motility and Enteric Neural Activity in the Mouse Colon. Biomolecules 2023, 13, 139. https://doi.org/10.3390/biom13010139
Leembruggen AJL, Lu Y, Wang H, Uzungil V, Renoir T, Hannan AJ, Stamp LA, Hao MM, Bornstein JC. Group I Metabotropic Glutamate Receptors Modulate Motility and Enteric Neural Activity in the Mouse Colon. Biomolecules. 2023; 13(1):139. https://doi.org/10.3390/biom13010139
Chicago/Turabian StyleLeembruggen, Anita J. L., Yuqing Lu, Haozhe Wang, Volkan Uzungil, Thibault Renoir, Anthony J. Hannan, Lincon A. Stamp, Marlene M. Hao, and Joel C. Bornstein. 2023. "Group I Metabotropic Glutamate Receptors Modulate Motility and Enteric Neural Activity in the Mouse Colon" Biomolecules 13, no. 1: 139. https://doi.org/10.3390/biom13010139
APA StyleLeembruggen, A. J. L., Lu, Y., Wang, H., Uzungil, V., Renoir, T., Hannan, A. J., Stamp, L. A., Hao, M. M., & Bornstein, J. C. (2023). Group I Metabotropic Glutamate Receptors Modulate Motility and Enteric Neural Activity in the Mouse Colon. Biomolecules, 13(1), 139. https://doi.org/10.3390/biom13010139





