Parental Programming of Offspring Health: The Intricate Interplay between Diet, Environment, Reproduction and Development
Abstract
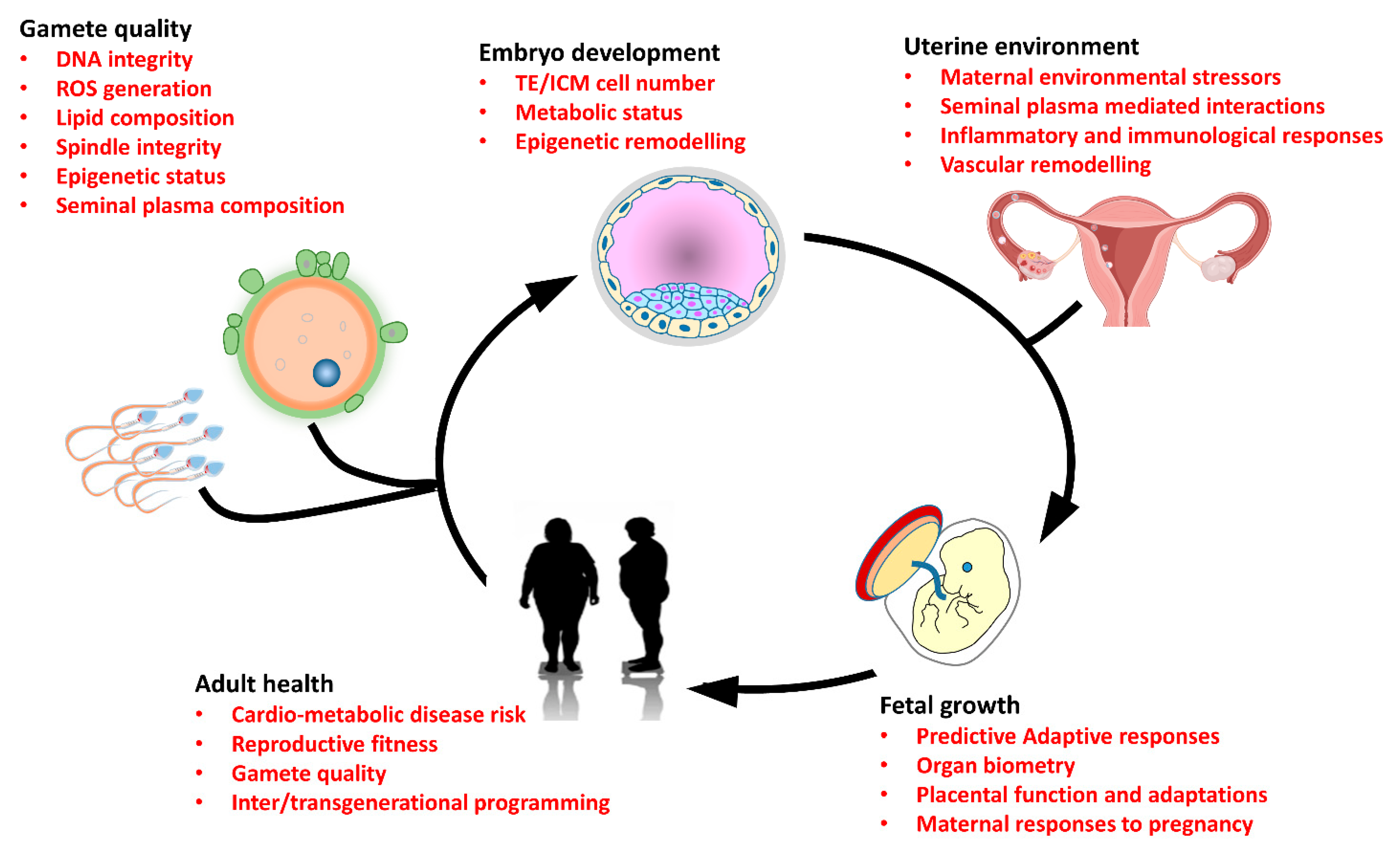
1. Introduction
2. Parental Diet and Obesity
3. Parental Age
4. Parental Exposure to Environmental Pollutants
5. Mechanisms Underlying Parental Programming
5.1. Parental Epigenetic Status
5.2. Embryo Directed Mechanisms of Parental Programming
5.3. Parental Influences on the Uterine Environment
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Hanson, M.A.; Gluckman, P.D. Early developmental conditioning of later health and disease: Physiology or pathophysiology? Physiol. Rev. 2014, 94, 1027–1076. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef] [PubMed]
- West-Eberhard, M.J. Developmental plasticity and the origin of species differences. Proc. Natl. Acad. Sci. USA 2005, 102 (Suppl. S1), 6543–6549. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Thornburg, K.L. The obstetric origins of health for a lifetime. Clin. Obstet. Gynecol. 2013, 56, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.T.; Dasinger, J.H.; Intapad, S. Fetal programming and cardiovascular pathology. Compr. Physiol. 2015, 5, 997–1025. [Google Scholar] [CrossRef]
- Nielsen, J.H.; Haase, T.N.; Jaksch, C.; Nalla, A.; Sostrup, B.; Nalla, A.A.; Larsen, L.; Rasmussen, M.; Dalgaard, L.T.; Gaarn, L.W.; et al. Impact of fetal and neonatal environment on beta cell function and development of diabetes. Acta Obstet. Et Gynecol. Scand. 2014, 93, 1109–1122. [Google Scholar] [CrossRef]
- Argeri, R.; Thomazini, F.; Lichtenecker, D.C.K.; Thieme, K.; do Carmo Franco, M.; Gomes, G.N. Programmed Adult Kidney Disease: Importance of Fetal Environment. Front. Physiol. 2020, 11, 586290. [Google Scholar] [CrossRef]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of lifetime health around the time of conception: Causes and consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef]
- Roseboom, T.J. Developmental plasticity and its relevance to assisted human reproduction. Hum. Reprod. 2018, 33, 546–552. [Google Scholar] [CrossRef]
- Robker, R.L.; Akison, L.K.; Bennett, B.D.; Thrupp, P.N.; Chura, L.R.; Russell, D.L.; Lane, M.; Norman, R.J. Obese women exhibit differences in ovarian metabolites, hormones, and gene expression compared with moderate-weight women. J. Clin. Endocrinol. Metab. 2009, 94, 1533–1540. [Google Scholar] [CrossRef]
- Turner, N.; Robker, R.L. Developmental programming of obesity and insulin resistance: Does mitochondrial dysfunction in oocytes play a role? Mol. Hum. Reprod. 2015, 21, 23–30. [Google Scholar] [CrossRef]
- Velazquez, M.A.; Fleming, T.P.; Watkins, A.J. Periconceptional environment and the developmental origins of disease. J. Endocrinol. 2019, 242, T33–T49. [Google Scholar] [CrossRef]
- Padmanabhan, V.; Cardoso, R.C.; Puttabyatappa, M. Developmental Programming, a Pathway to Disease. Endocrinology 2016, 157, 1328–1340. [Google Scholar] [CrossRef]
- Watkins, A.J.; Rubini, E.; Hosier, E.D.; Morgan, H.L. Paternal programming of offspring health. Early Hum. Dev. 2020, 150, 105185. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, J.; Rassoulzadegan, M.; Tuorto, F.; Chen, Q. Sperm RNA code programmes the metabolic health of offspring. Nat. Rev. Endocrinol. 2019, 15, 489–498. [Google Scholar] [CrossRef]
- Eurenius, E.; Lindkvist, M.; Sundqvist, M.; Ivarsson, A.; Mogren, I. Maternal and paternal self-rated health and BMI in relation to lifestyle in early pregnancy: The Salut Programme in Sweden. Scand. J. Public Health 2011, 39, 730–741. [Google Scholar] [CrossRef]
- Zhu, L.; Marjani, S.L.; Jiang, Z. The Epigenetics of Gametes and Early Embryos and Potential Long-Range Consequences in Livestock Species-Filling in the Picture with Epigenomic Analyses. Front. Genet. 2021, 12, 557934. [Google Scholar] [CrossRef]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef]
- Segal, T.R.; Giudice, L.C. Before the beginning: Environmental exposures and reproductive and obstetrical outcomes. Fertil. Steril. 2019, 112, 613–621. [Google Scholar] [CrossRef]
- Aggerholm, A.S.; Thulstrup, A.M.; Toft, G.; Ramlau-Hansen, C.H.; Bonde, J.P. Is overweight a risk factor for reduced semen quality and altered serum sex hormone profile? Fertil. Steril. 2008, 90, 619–626. [Google Scholar] [CrossRef]
- Teerds, K.J.; de Rooij, D.G.; Keijer, J. Functional relationship between obesity and male reproduction: From humans to animal models. Hum. Reprod. Update 2011, 17, 667–683. [Google Scholar] [CrossRef]
- Raad, G.; Azouri, J.; Rizk, K.; Zeidan, N.S.; Azouri, J.; Grandjean, V.; Hazzouri, M. Adverse effects of paternal obesity on the motile spermatozoa quality. PLoS ONE 2019, 14, e0211837. [Google Scholar] [CrossRef]
- Tunc, O.; Bakos, H.W.; Tremellen, K. Impact of body mass index on seminal oxidative stress. Andrologia 2011, 43, 121–128. [Google Scholar] [CrossRef]
- Montjean, D.; Zini, A.; Ravel, C.; Belloc, S.; Dalleac, A.; Copin, H.; Boyer, P.; McElreavey, K.; Benkhalifa, M. Sperm global DNA methylation level: Association with semen parameters and genome integrity. Andrology 2015, 3, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Tunc, O.; Tremellen, K. Oxidative DNA damage impairs global sperm DNA methylation in infertile men. J. Assist. Reprod. Genet. 2009, 26, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Binder, N.K.; Sheedy, J.R.; Hannan, N.J.; Gardner, D.K. Male obesity is associated with changed spermatozoa Cox4i1 mRNA level and altered seminal vesicle fluid composition in a mouse model. Mol. Hum. Reprod. 2015, 21, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Ramaraju, G.A.; Teppala, S.; Prathigudupu, K.; Kalagara, M.; Thota, S.; Kota, M.; Cheemakurthi, R. Association between obesity and sperm quality. Andrologia 2018, 50, e12888. [Google Scholar] [CrossRef]
- Igosheva, N.; Abramov, A.Y.; Poston, L.; Eckert, J.J.; Fleming, T.P.; Duchen, M.R.; McConnell, J. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS ONE 2010, 5, e10074. [Google Scholar] [CrossRef]
- Wu, L.L.; Dunning, K.R.; Yang, X.; Russell, D.L.; Lane, M.; Norman, R.J.; Robker, R.L. High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology 2010, 151, 5438–5445. [Google Scholar] [CrossRef]
- Reynolds, K.A.; Boudoures, A.L.; Chi, M.M.; Wang, Q.; Moley, K.H. Adverse effects of obesity and/or high-fat diet on oocyte quality and metabolism are not reversible with resumption of regular diet in mice. Reprod. Fertil. Dev. 2015, 27, 716–724. [Google Scholar] [CrossRef]
- Loy, S.L.; Chan, D.W.K.; Ku, C.W.; Cheung, Y.B.; Godfrey, K.M.; Tan, K.M.L.; Chong, Y.S.; Shek, L.P.; Tan, K.H.; Chan, S.Y.; et al. Metabolic health status and fecundability in a Singapore preconception cohort study. Am. J. Obs. Gynecol 2022, 226, 714.e711–714.e716. [Google Scholar] [CrossRef]
- Leary, C.; Leese, H.J.; Sturmey, R.G. Human embryos from overweight and obese women display phenotypic and metabolic abnormalities. Hum. Reprod. 2015, 30, 122–132. [Google Scholar] [CrossRef]
- Andersen, C.Y.; Ezcurra, D. Human steroidogenesis: Implications for controlled ovarian stimulation with exogenous gonadotropins. Reprod. Biol. Endocrinol. 2014, 12. [Google Scholar] [CrossRef]
- Silvestris, E.; de Pergola, G.; Rosania, R.; Loverro, G. Obesity as disruptor of the female fertility. Reprod. Biol. Endocrinol. 2018, 16, 22. [Google Scholar] [CrossRef]
- Fullston, T.; Mitchell, M.; Wakefield, S.; Lane, M. Mitochondrial inhibition during preimplantation embryogenesis shifts the transcriptional profile of fetal mouse brain. Reprod. Fertil Dev. 2011, 23, 691–701. [Google Scholar] [CrossRef]
- Luzzo, K.M.; Wang, Q.; Purcell, S.H.; Chi, M.; Jimenez, P.T.; Grindler, N.; Schedl, T.; Moley, K.H. High fat diet induced developmental defects in the mouse: Oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS ONE 2012, 7, e49217. [Google Scholar] [CrossRef]
- McIntyre, H.D.; Kapur, A.; Divakar, H.; Hod, M. Gestational Diabetes Mellitus-Innovative Approach to Prediction, Diagnosis, Management, and Prevention of Future NCD-Mother and Offspring. Front. Endocrinol. 2020, 11, 614533. [Google Scholar] [CrossRef]
- Group, H.S.C.R.; Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef]
- Powe, C.E. Early Pregnancy Biochemical Predictors of Gestational Diabetes Mellitus. Curr. Diab Rep. 2017, 17, 12. [Google Scholar] [CrossRef]
- Sevilla-Domingo, M.; Olivo-Ramirez, C.G.; Huerta-Padilla, V.M.; Gomez-Diaz, R.A.; Gonzalez-Carranza, E.; Acevedo-Rodriguez, G.E.; Hernandez-Zuniga, V.E.; Gonzalez, A.L.V.; Mateos-Sanchez, L.; Mondragon-Gonzalez, R.; et al. Downregulation of SLC16A11 is Present in Offspring of Mothers with Gestational Diabetes. Arch. Med. Res. 2022, 53, 516–523. [Google Scholar] [CrossRef]
- Zhou, T.; Huang, L.; Wang, M.; Chen, D.; Chen, Z.; Jiang, S.W. A Critical Review of Proteomic Studies in Gestational Diabetes Mellitus. J. Diabetes Res. 2020, 2020, 6450352. [Google Scholar] [CrossRef]
- Herrera-Van Oostdam, A.S.; Salgado-Bustamante, M.; Lopez, J.A.; Herrera-Van Oostdam, D.A.; Lopez-Hernandez, Y. Placental exosomes viewed from an ‘omics’ perspective: Implications for gestational diabetes biomarkers identification. Biomark. Med. 2019, 13, 675–684. [Google Scholar] [CrossRef]
- Nasiri-Amiri, F.; Sepidarkish, M.; Shirvani, M.A.; Habibipour, P.; Tabari, N.S.M. The effect of exercise on the prevention of gestational diabetes in obese and overweight pregnant women: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2019, 11, 72. [Google Scholar] [CrossRef]
- Keats, E.C.; Haider, B.A.; Tam, E.; Bhutta, Z.A. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019, 3, CD004905. [Google Scholar] [CrossRef]
- Petry, C.J.; Ong, K.K.; Hughes, I.A.; Dunger, D.B. Multiple Micronutrient Supplementation during Pregnancy and Increased Birth Weight and Skinfold Thicknesses in the Offspring: The Cambridge Baby Growth Study. Nutrients 2020, 12, 3466. [Google Scholar] [CrossRef]
- Fisher, A.L.; Nemeth, E. Iron homeostasis during pregnancy. Am. J. Clin. Nutr. 2017, 106, 1567S–1574S. [Google Scholar] [CrossRef]
- Georgieff, M.K. Iron deficiency in pregnancy. Am. J. Obstet. Gynecol. 2020, 223, 516–524. [Google Scholar] [CrossRef]
- Kataria, Y.; Wu, Y.; Horskjaer, P.H.; Mandrup-Poulsen, T.; Ellervik, C. Iron Status and Gestational Diabetes-A Meta-Analysis. Nutrients 2018, 10, 621. [Google Scholar] [CrossRef]
- Zhang, C.; Rawal, S. Dietary iron intake, iron status, and gestational diabetes. Am. J. Clin. Nutr. 2017, 106, 1672S–1680S. [Google Scholar] [CrossRef]
- Lao, T.T.; Tam, K.F.; Chan, L.Y. Third trimester iron status and pregnancy outcome in non-anaemic women; pregnancy unfavourably affected by maternal iron excess. Hum. Reprod. 2000, 15, 1843–1848. [Google Scholar] [CrossRef]
- Rayman, M.P.; Barlis, J.; Evans, R.W.; Redman, C.W.; King, L.J. Abnormal iron parameters in the pregnancy syndrome preeclampsia. Am. J. Obstet. Gynecol. 2002, 187, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.J.; Gibson, R.A.; Crowther, C.A.; Baghurst, P.; Makrides, M. Effect of iron supplementation during pregnancy on the intelligence quotient and behavior of children at 4 y of age: Long-term follow-up of a randomized controlled trial. Am. J. Clin. Nutr. 2006, 83, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Finger, B.J.; Harvey, A.J.; Green, M.P.; Gardner, D.K. Combined parental obesity negatively impacts preimplantation mouse embryo development, kinetics, morphology and metabolism. Hum. Reprod. 2015, 30, 2084–2096. [Google Scholar] [CrossRef] [PubMed]
- Binder, N.K.; Hannan, N.J.; Gardner, D.K. Paternal diet-induced obesity retards early mouse embryo development, mitochondrial activity and pregnancy health. PLoS ONE 2012, 7, e52304. [Google Scholar] [CrossRef]
- KŠiŇanovÁ, M.; KovaŘÍkovÁ, V.; ŠefČÍkovÁ, Z.; ŠpirkovÁ, A.; ČikoŠ, Š.; Pisko, J.; Fabian, D. Different response of embryos originating from control and obese mice to insulin in vitro. J. Reprod. Dev. 2021, 67, 25–34. [Google Scholar] [CrossRef]
- McPherson, N.O.; Bell, V.G.; Zander-Fox, D.L.; Fullston, T.; Wu, L.L.; Robker, R.L.; Lane, M. When two obese parents are worse than one! Impacts on embryo and fetal development. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E568–E581. [Google Scholar] [CrossRef]
- Stothard, K.J.; Tennant, P.W.; Bell, R.; Rankin, J. Maternal overweight and obesity and the risk of congenital anomalies: A systematic review and meta-analysis. JAMA 2009, 301, 636–650. [Google Scholar] [CrossRef]
- Zalbahar, N.; Najman, J.; McIntyre, H.D.; Mamun, A. Parental pre-pregnancy obesity and the risk of offspring weight and body mass index change from childhood to adulthood. Clin. Obes. 2017, 7, 206–215. [Google Scholar] [CrossRef]
- Vickers, M.H. Developmental programming and transgenerational transmission of obesity. Ann. Nutr. Metab. 2014, 64 (Suppl. S1), 26–34. [Google Scholar] [CrossRef]
- Fuemmeler, B.F.; Lovelady, C.A.; Zucker, N.L.; Østbye, T. Parental obesity moderates the relationship between childhood appetitive traits and weight. Obesity 2013, 21, 815–823. [Google Scholar] [CrossRef]
- Ornellas, F.; Souza-Mello, V.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Programming of obesity and comorbidities in the progeny: Lessons from a model of diet-induced obese parents. PLoS ONE 2015, 10, e0124737. [Google Scholar] [CrossRef]
- Linabery, A.M.; Nahhas, R.W.; Johnson, W.; Choh, A.C.; Towne, B.; Odegaard, A.O.; Czerwinski, S.A.; Demerath, E.W. Stronger influence of maternal than paternal obesity on infant and early childhood body mass index: The Fels Longitudinal Study. Pediatr. Obes. 2013, 8, 159–169. [Google Scholar] [CrossRef]
- Bellieni, C. The Best Age for Pregnancy and Undue Pressures. J. Fam. Reprod. Health 2016, 10, 104–107. [Google Scholar]
- Levenson, D.; Romero, R.; Garcia-Flores, V.; Miller, D.; Xu, Y.; Sahi, A.; Hassan, S.S.; Gomez-Lopez, N. The effects of advanced maternal age on T-cell subsets at the maternal-fetal interface prior to term labor and in the offspring: A mouse study. Clin. Exp. Immunol. 2020, 201, 58–75. [Google Scholar] [CrossRef]
- Sampino, S.; Stankiewicz, A.M.; Zacchini, F.; Goscik, J.; Szostak, A.; Swiergiel, A.H.; Drago, G.; Modlinski, J.A.; Ptak, G.E. Pregnancy at Advanced Maternal Age Affects Behavior and Hippocampal Gene Expression in Mouse Offspring. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1465–1473. [Google Scholar] [CrossRef]
- Halvaei, I.; Litzky, J.; Esfandiari, N. Advanced paternal age: Effects on sperm parameters, assisted reproduction outcomes and offspring health. Reprod. Biol. Endocrinol. 2020, 18, 110. [Google Scholar] [CrossRef]
- Reichenberg, A.; Gross, R.; Weiser, M.; Bresnahan, M.; Silverman, J.; Harlap, S.; Rabinowitz, J.; Shulman, C.; Malaspina, D.; Lubin, G.; et al. Advancing paternal age and autism. Arch. Gen. Psychiatry 2006, 63, 1026–1032. [Google Scholar] [CrossRef]
- Idring, S.; Magnusson, C.; Lundberg, M.; Ek, M.; Rai, D.; Svensson, A.C.; Dalman, C.; Karlsson, H.; Lee, B.K. Parental age and the risk of autism spectrum disorders: Findings from a Swedish population-based cohort. Int. J. Epidemiol. 2014, 43, 107–115. [Google Scholar] [CrossRef]
- Mutsaerts, M.A.; Groen, H.; Huiting, H.G.; Kuchenbecker, W.K.; Sauer, P.J.; Land, J.A.; Stolk, R.P.; Hoek, A. The influence of maternal and paternal factors on time to pregnancy—A Dutch population-based birth-cohort study: The GECKO Drenthe study. Hum. Reprod. 2012, 27, 583–593. [Google Scholar] [CrossRef]
- Sandin, S.; Schendel, D.; Magnusson, P.; Hultman, C.; Surén, P.; Susser, E.; Grønborg, T.; Gissler, M.; Gunnes, N.; Gross, R.; et al. Autism risk associated with parental age and with increasing difference in age between the parents. Mol. Psychiatry 2016, 21, 693–700. [Google Scholar] [CrossRef]
- Meeker, J.D. Exposure to environmental endocrine disruptors and child development. Arch. Pediatr. Adolesc. Med. 2012, 166, E1–E7. [Google Scholar] [CrossRef]
- Cariati, F.; D’Uonno, N.; Borrillo, F.; Iervolino, S.; Galdiero, G.; Tomaiuolo, R. Bisphenol a: An emerging threat to male fertility. Reprod. Biol. Endocrinol. 2019, 17, 6. [Google Scholar] [CrossRef]
- Wisniewski, P.; Romano, R.M.; Kizys, M.M.; Oliveira, K.C.; Kasamatsu, T.; Giannocco, G.; Chiamolera, M.I.; Dias-da-Silva, M.R.; Romano, M.A. Adult exposure to bisphenol A (BPA) in Wistar rats reduces sperm quality with disruption of the hypothalamic-pituitary-testicular axis. Toxicology 2015, 329, 1–9. [Google Scholar] [CrossRef]
- Li, D.K.; Zhou, Z.; Miao, M.; He, Y.; Wang, J.; Ferber, J.; Herrinton, L.J.; Gao, E.; Yuan, W. Urine bisphenol-A (BPA) level in relation to semen quality. Fertil. Steril. 2011, 95. [Google Scholar] [CrossRef]
- You, L.; Wang, Y.X.; Zeng, Q.; Li, M.; Huang, Y.H.; Hu, Y.; Cao, W.C.; Liu, A.L.; Lu, W.Q. Semen phthalate metabolites, spermatozoa apoptosis, and DNA damage: A cross-sectional study in China. Environ. Sci. Technol. 2015, 49, 3805–3812. [Google Scholar] [CrossRef]
- Dodge, L.E.; Williams, P.L.; Williams, M.A.; Missmer, S.A.; Souter, I.; Calafat, A.M.; Hauser, R.; Team, E.S. Associations between paternal urinary phthalate metabolite concentrations and reproductive outcomes among couples seeking fertility treatment. Reprod. Toxicol. 2015, 58, 184–193. [Google Scholar] [CrossRef]
- Huang, X.F.; Li, Y.; Gu, Y.H.; Liu, M.; Xu, Y.; Yuan, Y.; Sun, F.; Zhang, H.Q.; Shi, H.J. The effects of Di-(2-ethylhexyl)-phthalate exposure on fertilization and embryonic development in vitro and testicular genomic mutation in vivo. PLoS ONE 2012, 7, e50465. [Google Scholar] [CrossRef]
- Wu, H.; Ashcraft, L.; Whitcomb, B.W.; Rahil, T.; Tougias, E.; Sites, C.K.; Pilsner, J.R. Parental contributions to early embryo development: Influences of urinary phthalate and phthalate alternatives among couples undergoing IVF treatment. Hum. Reprod. 2017, 32, 65–75. [Google Scholar] [CrossRef]
- Carran, M.; Shaw, I.C. New Zealand Malayan war veterans’ exposure to dibutylphthalate is associated with an increased incidence of cryptorchidism, hypospadias and breast cancer in their children. N. Z. Med. J. 2012, 125, 52–63. [Google Scholar]
- Messerlian, C.; Braun, J.M.; Minguez-Alarcon, L.; Williams, P.L.; Ford, J.B.; Mustieles, V.; Calafat, A.M.; Souter, I.; Toth, T.; Hauser, R.; et al. Paternal and maternal urinary phthalate metabolite concentrations and birth weight of singletons conceived by subfertile couples. Environ. Int. 2017, 107, 55–64. [Google Scholar] [CrossRef]
- Robledo, C.A.; Yeung, E.; Mendola, P.; Sundaram, R.; Maisog, J.; Sweeney, A.M.; Barr, D.B.; Louis, G.M. Preconception maternal and paternal exposure to persistent organic pollutants and birth size: The LIFE study. Env. Health Perspect. 2015, 123, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Souter, I.; Smith, K.W.; Dimitriadis, I.; Ehrlich, S.; Williams, P.L.; Calafat, A.M.; Hauser, R. The association of bisphenol-A urinary concentrations with antral follicle counts and other measures of ovarian reserve in women undergoing infertility treatments. Reprod. Toxicol. 2013, 42, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.; Favetta, L.A.; King, W.A. Bisphenol A Exposure during Oocyte Maturation in vitro Results in Spindle Abnormalities and Chromosome Misalignment in Bos taurus. Cytogenet. Genome Res. 2015, 145, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.; Mahboubi, K.; MacLusky, N.; King, W.A.; Favetta, L.A. BPA exposure during in vitro oocyte maturation results in dose-dependent alterations to embryo development rates, apoptosis rate, sex ratio and gene expression. Reprod. Toxicol. 2016, 59, 128–138. [Google Scholar] [CrossRef]
- Ehrlich, S.; Williams, P.L.; Missmer, S.A.; Flaws, J.A.; Berry, K.F.; Calafat, A.M.; Ye, X.; Petrozza, J.C.; Wright, D.; Hauser, R. Urinary bisphenol A concentrations and implantation failure among women undergoing in vitro fertilization. Env. Health Perspect. 2012, 120, 978–983. [Google Scholar] [CrossRef]
- Minguez-Alarcon, L.; Gaskins, A.J.; Chiu, Y.H.; Williams, P.L.; Ehrlich, S.; Chavarro, J.E.; Petrozza, J.C.; Ford, J.B.; Calafat, A.M.; Hauser, R.; et al. Urinary bisphenol A concentrations and association with in vitro fertilization outcomes among women from a fertility clinic. Hum. Reprod. 2015, 30, 2120–2128. [Google Scholar] [CrossRef]
- Wright, D.L.; Afeiche, M.C.; Ehrlich, S.; Smith, K.; Williams, P.L.; Chavarro, J.E.; Batsis, M.; Toth, T.L.; Hauser, R. Hair mercury concentrations and in vitro fertilization (IVF) outcomes among women from a fertility clinic. Reprod. Toxicol. 2015, 51, 125–132. [Google Scholar] [CrossRef]
- Hauser, R.; Gaskins, A.J.; Souter, I.; Smith, K.W.; Dodge, L.E.; Ehrlich, S.; Meeker, J.D.; Calafat, A.M.; Williams, P.L.; Team, E.S. Urinary Phthalate Metabolite Concentrations and Reproductive Outcomes among Women Undergoing in Vitro Fertilization: Results from the EARTH Study. Env. Health Perspect. 2016, 124, 831–839. [Google Scholar] [CrossRef]
- Takai, Y.; Tsutsumi, O.; Ikezuki, Y.; Kamei, Y.; Osuga, Y.; Yano, T.; Taketan, Y. Preimplantation exposure to bisphenol A advances postnatal development. Reprod. Toxicol. 2001, 15, 71–74. [Google Scholar] [CrossRef]
- Xiao, S.; Diao, H.; Smith, M.A.; Song, X.; Ye, X. Preimplantation exposure to bisphenol A (BPA) affects embryo transport, preimplantation embryo development, and uterine receptivity in mice. Reprod. Toxicol. 2011, 32, 434–441. [Google Scholar] [CrossRef]
- Susiarjo, M.; Sasson, I.; Mesaros, C.; Bartolomei, M.S. Bisphenol a exposure disrupts genomic imprinting in the mouse. PLoS Genet. 2013, 9, e1003401. [Google Scholar] [CrossRef]
- Guerrero-Bosagna, C.; Covert, T.R.; Haque, M.M.; Settles, M.; Nilsson, E.E.; Anway, M.D.; Skinner, M.K. Epigenetic transgenerational inheritance of vinclozolin induced mouse adult onset disease and associated sperm epigenome biomarkers. Reprod. Toxicol. 2012, 34, 694–707. [Google Scholar] [CrossRef]
- Mustieles, V.; Minguez-Alarcon, L.; Christou, G.; Ford, J.B.; Dimitriadis, I.; Hauser, R.; Souter, I.; Messerlian, C.; Environment Reproductive Health Study Team. Placental weight in relation to maternal and paternal preconception and prenatal urinary phthalate metabolite concentrations among subfertile couples. Env. Res. 2019, 169, 272–279. [Google Scholar] [CrossRef]
- Mustieles, V.; Zhang, Y.; Yland, J.; Braun, J.M.; Williams, P.L.; Wylie, B.J.; Attaman, J.A.; Ford, J.B.; Azevedo, A.; Calafat, A.M.; et al. Maternal and paternal preconception exposure to phenols and preterm birth. Environ. Int. 2020, 137, 105523. [Google Scholar] [CrossRef]
- Mustieles, V.; Williams, P.L.; Fernandez, M.F.; Minguez-Alarcon, L.; Ford, J.B.; Calafat, A.M.; Hauser, R.; Messerlian, C.; Environment Reproductive Health Study Team. Maternal and paternal preconception exposure to bisphenols and size at birth. Hum. Reprod. 2018, 33, 1528–1537. [Google Scholar] [CrossRef]
- Marcho, C.; Oluwayiose, O.A.; Pilsner, J.R. The preconception environment and sperm epigenetics. Andrology 2020, 8, 924–942. [Google Scholar] [CrossRef]
- Bohacek, J.; Mansuy, I.M. Molecular insights into transgenerational non-genetic inheritance of acquired behaviours. Nat. Rev. Genet. 2015, 16, 641–652. [Google Scholar] [CrossRef]
- Han, L.; Ren, C.; Li, L.; Li, X.; Ge, J.; Wang, H.; Miao, Y.L.; Guo, X.; Moley, K.H.; Shu, W.; et al. Publisher Correction: Embryonic defects induced by maternal obesity in mice derive from Stella insufficiency in oocytes. Nat. Genet. 2018, 50, 768. [Google Scholar] [CrossRef]
- Kessler, N.J.; Waterland, R.A.; Prentice, A.M.; Silver, M.J. Establishment of environmentally sensitive DNA methylation states in the very early human embryo. Sci. Adv. 2018, 4, eaat2624. [Google Scholar] [CrossRef]
- Jenkins, T.G.; Aston, K.I.; James, E.R.; Carrell, D.T. Sperm epigenetics in the study of male fertility, offspring health, and potential clinical applications. Syst. Biol. Reprod. Med. 2017, 63, 69–76. [Google Scholar] [CrossRef]
- Watkins, A.J.; Sinclair, K.D. Paternal low protein diet affects adult offspring cardiovascular and metabolic function in mice. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1444–H1452. [Google Scholar] [CrossRef]
- Watkins, A.J.; Dias, I.; Tsuro, H.; Allen, D.; Emes, R.D.; Moreton, J.; Wilson, R.; Ingram, R.J.M.; Sinclair, K.D. Paternal diet programs offspring health through sperm- and seminal plasma-specific pathways in mice. Proc. Natl. Acad. Sci. USA 2018, 115, 10064–10069. [Google Scholar] [CrossRef]
- Yoshizaki, K.; Kimura, R.; Kobayashi, H.; Oki, S.; Kikkawa, T.; Mai, L.L.; Koike, K.; Mochizuki, K.; Inada, H.; Matsui, Y.; et al. Paternal age affects offspring via an epigenetic mechanism involving REST/NRSF. Embo. Rep. 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Milekic, M.H.; Xin, Y.; O’Donnell, A.; Kumar, K.K.; Bradley-Moore, M.; Malaspina, D.; Moore, H.; Brunner, D.; Ge, Y.; Edwards, J.; et al. Age-related sperm DNA methylation changes are transmitted to offspring and associated with abnormal behavior and dysregulated gene expression. Mol. Psychiatry 2015, 20, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Ost, A.; Lempradl, A.; Casas, E.; Weigert, M.; Tiko, T.; Deniz, M.; Pantano, L.; Boenisch, U.; Itskov, P.M.; Stoeckius, M.; et al. Paternal diet defines offspring chromatin state and intergenerational obesity. Cell 2014, 159, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Sun, F.; Conine, C.C.; Reichholf, B.; Kukreja, S.; Herzog, V.A.; Ameres, S.L.; Rando, O.J. Small RNAs Are Trafficked from the Epididymis to Developing Mammalian Sperm. Dev. Cell 2018, 46, 481–494.e486. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yan, M.; Cao, Z.; Li, X.; Zhang, Y.; Shi, J.; Feng, G.H.; Peng, H.; Zhang, X.; Qian, J.; et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 2016, 351, 397–400. [Google Scholar] [CrossRef]
- Nätt, D.; Kugelberg, U.; Casas, E.; Nedstrand, E.; Zalavary, S.; Henriksson, P.; Nijm, C.; Jäderquist, J.; Sandborg, J.; Flinke, E.; et al. Human sperm displays rapid responses to diet. PLoS Biol. 2019, 17, e3000559. [Google Scholar] [CrossRef]
- Donkin, I.; Versteyhe, S.; Ingerslev, L.R.; Qian, K.; Mechta, M.; Nordkap, L.; Mortensen, B.; Appel, E.V.; Jorgensen, N.; Kristiansen, V.B.; et al. Obesity and Bariatric Surgery Drive Epigenetic Variation of Spermatozoa in Humans. Cell Metab. 2016, 23, 369–378. [Google Scholar] [CrossRef]
- Denham, J.; O’Brien, B.J.; Harvey, J.T.; Charchar, F.J. Genome-wide sperm DNA methylation changes after 3 months of exercise training in humans. Epigenomics 2015, 7, 717–731. [Google Scholar] [CrossRef]
- Palmer, N.O.; Bakos, H.W.; Owens, J.A.; Setchell, B.P.; Lane, M. Diet and exercise in an obese mouse fed a high-fat diet improve metabolic health and reverse perturbed sperm function. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E768–780. [Google Scholar] [CrossRef]
- Cropley, J.E.; Eaton, S.A.; Aiken, A.; Young, P.E.; Giannoulatou, E.; Ho, J.W.K.; Buckland, M.E.; Keam, S.P.; Hutvagner, G.; Humphreys, D.T.; et al. Male-lineage transmission of an acquired metabolic phenotype induced by grand-paternal obesity. Mol. Metab. 2016, 5, 699–708. [Google Scholar] [CrossRef]
- Short, A.K.; Yeshurun, S.; Powell, R.; Perreau, V.M.; Fox, A.; Kim, J.H.; Pang, T.Y.; Hannan, A.J. Exercise alters mouse sperm small noncoding RNAs and induces a transgenerational modification of male offspring conditioned fear and anxiety. Transl. Psychiatry 2017, 7, e1114. [Google Scholar] [CrossRef]
- Stanford, K.I.; Rasmussen, M.; Baer, L.A.; Lehnig, A.C.; Rowland, L.A.; White, J.D.; So, K.; De Sousa-Coelho, A.L.; Hirshman, M.F.; Patti, M.E.; et al. Paternal Exercise Improves Glucose Metabolism in Adult Offspring. Diabetes 2018, 67, 2530–2540. [Google Scholar] [CrossRef]
- Laker, R.C.; Lillard, T.S.; Okutsu, M.; Zhang, M.; Hoehn, K.L.; Connelly, J.J.; Yan, Z. Exercise prevents maternal high-fat diet-induced hypermethylation of the Pgc-1α gene and age-dependent metabolic dysfunction in the offspring. Diabetes 2014, 63, 1605–1611. [Google Scholar] [CrossRef]
- Jawaid, A.; Jehle, K.L.; Mansuy, I.M. Impact of Parental Exposure on Offspring Health in Humans. Trends Genet. 2021, 37, 373–388. [Google Scholar] [CrossRef]
- Kusuyama, J.; Alves-Wagner, A.B.; Makarewicz, N.S.; Goodyear, L.J. Effects of maternal and paternal exercise on offspring metabolism. Nat. Metab. 2020, 2, 858–872. [Google Scholar] [CrossRef]
- Skinner, M.K.; Manikkam, M.; Guerrero-Bosagna, C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol. Metab. 2010, 21, 214–222. [Google Scholar] [CrossRef]
- Zacchini, F.; Sampino, S.; Ziętek, M.; Chan, A. Delayed parenthood and its influence on offspring health: What have we learned from the mouse model†. Biol. Reprod. 2022, 106, 58–65. [Google Scholar] [CrossRef]
- Denomme, M.M.; Parks, J.C.; McCallie, B.R.; McCubbin, N.I.; Schoolcraft, W.B.; Katz-Jaffe, M.G. Advanced paternal age directly impacts mouse embryonic placental imprinting. PLoS ONE 2020, 15, e0229904. [Google Scholar] [CrossRef]
- Stankiewicz, A.M.; Goscik, J.; Wieczorek, M.; Ptak, G.E.; Modlinski, J.A.; Sampino, S. Developmental sequelae of advanced paternal age on fetal brain and placenta in mice. Mech. Dev. 2017, 145, S24–S25. [Google Scholar] [CrossRef]
- Hann, M.; Roberts, S.A.; D’Souza, S.W.; Clayton, P.; Macklon, N.; Brison, D.R. The growth of assisted reproductive treatment-conceived children from birth to 5 years: A national cohort study. BMC Med. 2018, 16, 224. [Google Scholar] [CrossRef]
- Ramos-Ibeas, P.; Heras, S.; Gómez-Redondo, I.; Planells, B.; Fernández-González, R.; Pericuesta, E.; Laguna-Barraza, R.; Pérez-Cerezales, S.; Gutiérrez-Adán, A. Embryo responses to stress induced by assisted reproductive technologies. Mol. Reprod. Dev. 2019, 86, 1292–1306. [Google Scholar] [CrossRef]
- Kleijkers, S.H.; Mantikou, E.; Slappendel, E.; Consten, D.; van Echten-Arends, J.; Wetzels, A.M.; van Wely, M.; Smits, L.J.; van Montfoort, A.P.; Repping, S.; et al. Influence of embryo culture medium (G5 and HTF) on pregnancy and perinatal outcome after IVF: A multicenter RCT. Hum. Reprod. 2016, 31, 2219–2230. [Google Scholar] [CrossRef]
- Gkourogianni, A.; Kosteria, I.; Telonis, A.G.; Margeli, A.; Mantzou, E.; Konsta, M.; Loutradis, D.; Mastorakos, G.; Papassotiriou, I.; Klapa, M.I.; et al. Plasma metabolomic profiling suggests early indications for predisposition to latent insulin resistance in children conceived by ICSI. PLoS ONE 2014, 9, e94001. [Google Scholar] [CrossRef]
- Watkins, A.J.; Platt, D.; Papenbrock, T.; Wilkins, A.; Eckert, J.J.; Kwong, W.Y.; Osmond, C.; Hanson, M.; Fleming, T.P. Mouse embryo culture induces changes in postnatal phenotype including raised systolic blood pressure. Proc. Natl. Acad. Sci. USA 2007, 104, 5449–5454. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, U.; Rimoldi, S.F.; Rexhaj, E.; Stuber, T.; Duplain, H.; Garcin, S.; de Marchi, S.F.; Nicod, P.; Germond, M.; Allemann, Y.; et al. Response to letters regarding article, “Systemic and pulmonary vascular dysfunction in children conceived by assisted reproductive technologies”. Circulation 2013, 127, e478. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, S.L.; Shi, X.Y.; Zheng, H.Y.; Wu, F.R.; Luo, C. Aberrant DNA methylation of imprinted H19 gene in human preimplantation embryos. Fertil. Steril. 2010, 94. [Google Scholar] [CrossRef] [PubMed]
- Turan, N.; Katari, S.; Gerson, L.F.; Chalian, R.; Foster, M.W.; Gaughan, J.P.; Coutifaris, C.; Sapienza, C. Inter- and intra-individual variation in allele-specific DNA methylation and gene expression in children conceived using assisted reproductive technology. PLoS Genet. 2010, 6, e1001033. [Google Scholar] [CrossRef]
- Michalakis, K.; Mintziori, G.; Kaprara, A.; Tarlatzis, B.C.; Goulis, D.G. The complex interaction between obesity, metabolic syndrome and reproductive axis: A narrative review. Metabolism 2013, 62, 457–478. [Google Scholar] [CrossRef]
- Bray, G.A. Medical consequences of obesity. J. Clin. Endocrinol. Metab. 2004, 89, 2583–2589. [Google Scholar] [CrossRef]
- Batra, V.; Dagar, K.; Nayak, S.; Kumaresan, A.; Kumar, R.; Datta, T.K. A Higher Abundance of O-Linked Glycans Confers a Selective Advantage to High Fertile Buffalo Spermatozoa for Immune-Evasion from Neutrophils. Front. Immunol. 2020, 11, 1928. [Google Scholar] [CrossRef]
- Sharkey, D.J.; Tremellen, K.P.; Jasper, M.J.; Gemzell-Danielsson, K.; Robertson, S.A. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J. Immunol. 2012, 188, 2445–2454. [Google Scholar] [CrossRef]
- Smyth, S.P.; Nixon, B.; Anderson, A.L.; Murray, H.C.; Martin, J.H.; MacDougall, L.A.; Robertson, S.A.; Skerrett-Byrne, D.A.; Schjenken, J.E. Elucidation of the protein composition of mouse seminal vesicle fluid. Proteomics 2022, 22, e2100227. [Google Scholar] [CrossRef]
- Schuberth, H.J.; Taylor, U.; Zerbe, H.; Waberski, D.; Hunter, R.; Rath, D. Immunological responses to semen in the female genital tract. Theriogenology 2008, 70, 1174–1181. [Google Scholar] [CrossRef]
- Aluvihare, V.R.; Kallikourdis, M.; Betz, A.G. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 2004, 5, 266–271. [Google Scholar] [CrossRef]
- Lan, R.; Xin, M.; Hao, Z.; You, S.; Xu, Y.; Wu, J.; Dang, L.; Zhang, X.; Sun, S. Biological Functions and Large-Scale Profiling of Protein Glycosylation in Human Semen. J. Proteome Res. 2020, 19, 3877–3889. [Google Scholar] [CrossRef]
- Bromfield, J.J.; Schjenken, J.E.; Chin, P.Y.; Care, A.S.; Jasper, M.J.; Robertson, S.A. Maternal tract factors contribute to paternal seminal fluid impact on metabolic phenotype in offspring. Proc. Natl. Acad. Sci. USA 2014, 111, 2200–2205. [Google Scholar] [CrossRef]
- Juyena, N.S.; Stelletta, C. Seminal plasma: An essential attribute to spermatozoa. J. Androl. 2012, 33, 536–551. [Google Scholar] [CrossRef]
- Samanta, L.; Parida, R.; Dias, T.R.; Agarwal, A. The enigmatic seminal plasma: A proteomics insight from ejaculation to fertilization. Reprod. Biol. Endocrinol. 2018, 16, 41. [Google Scholar] [CrossRef]
- Schjenken, J.E.; Moldenhauer, L.M.; Sharkey, D.J.; Chan, H.Y.; Chin, P.Y.; Fullston, T.; McPherson, N.O.; Robertson, S.A. High-fat Diet Alters Male Seminal Plasma Composition to Impair Female Immune Adaptation for Pregnancy in Mice. Endocrinology 2021, 162, bqab123. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Gluckman, P.D.; Hanson, M.A. Developmental origins of metabolic disease: Life course and intergenerational perspectives. Trends Endocrinol. Metab. 2010, 21, 199–205. [Google Scholar] [CrossRef]
- Robertson, S.A. Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res. 2005, 322, 43–52. [Google Scholar] [CrossRef]
- Tremellen, K.P.; Seamark, R.F.; Robertson, S.A. Seminal transforming growth factor beta1 stimulates granulocyte-macrophage colony-stimulating factor production and inflammatory cell recruitment in the murine uterus. Biol. Reprod. 1998, 58, 1217–1225. [Google Scholar] [CrossRef]
- Schumacher, A.; Sharkey, D.J.; Robertson, S.A.; Zenclussen, A.C. Immune Cells at the Fetomaternal Interface: How the Microenvironment Modulates Immune Cells to Foster Fetal Development. J. Immunol. 2018, 201, 325–334. [Google Scholar] [CrossRef]
- Chan, J.C.; Morgan, C.P.; Adrian Leu, N.; Shetty, A.; Cisse, Y.M.; Nugent, B.M.; Morrison, K.E.; Jasarevic, E.; Huang, W.; Kanyuch, N.; et al. Reproductive tract extracellular vesicles are sufficient to transmit intergenerational stress and program neurodevelopment. Nat. Commun. 2020, 11, 1499. [Google Scholar] [CrossRef]
- Rando, O.J. Daddy issues: Paternal effects on phenotype. Cell 2012, 151, 702–708. [Google Scholar] [CrossRef]
- Hardikar, A.A.; Satoor, S.N.; Karandikar, M.S.; Joglekar, M.V.; Puranik, A.S.; Wong, W.; Kumar, S.; Limaye, A.; Bhat, D.S.; Januszewski, A.S.; et al. Multigenerational Undernutrition Increases Susceptibility to Obesity and Diabetes that Is Not Reversed after Dietary Recuperation. Cell Metab. 2015, 22, 312–319. [Google Scholar] [CrossRef]
| Factor | Singular/Combined | Major Outcomes/Findings | Species | References |
|---|---|---|---|---|
| Parental obesity | Paternal alone | Minimal impacts on seminal quality. Some studies indicate links between the Kisspeptin signaling pathway and obesity-induced male infertility Obesity may disturb early embryonic cell cycles kinetics, seminal/spermatozoal biomolecular composition, diminish reproductive performance and increase sperm oxidative stress | Human/Rodents | [20,21,22,23,24,25,26,27] |
| Maternal alone | Maternal obesity been associated with high rates of meiotic spindle abnormalities, defective mitochondria, increased levels of reactive oxygen species, dyslipidemia, perturbed follicular fluid composition and oocyte quality | Humans/Mice | [28,29,30,31,32,33,34,35,36] | |
| Combined parental | Combined parental obesity leads to impaired post-fertilization development and offspring health, e.g., increased risk of birth defects, elevated weight gain in postnatal life and metabolic disorders in adulthood | Humans/Mice | [53,54,55,56,57,58,59,60,61,62] | |
| Advanced parental age | Paternal alone | Perturbed growth and development, dysregulated immune cell profiles, increased risk of autism spectrum disorders and impaired neurocognition | Humans/Mice | [66,67,68] |
| Maternal alone | Programming effects similar to those observed in advanced paternal age studies | Mice | [64,65] | |
| Combined parental | Reduced fecundity and compromised neuro-development | Humans | [69,70] | |
| Parental Exposure to Environmental Pollutants | Paternal alone | EDCs disrupt the hypothalamic-pituitary-gonadal axis, thus compromising sperm/semen quality, conception potential and embryonic development | Humans/Rodents | [72,73,74,75,76,77,79] |
| Maternal alone | EDCs disrupt the hypothalamic-pituitary-gonadal axis, affecting oocyte’s structural and functional integrity and thus compromising fertilization potential | Humans/Mice/Cattle | [82,83,84,85,86,87,88,89,90,91,92] | |
| Combined parental | Adverse pregnancy outcomes, e.g., altered morphometry of fetus/embryo, impaired growth | Humans | [78,80,81,93,94,95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batra, V.; Norman, E.; Morgan, H.L.; Watkins, A.J. Parental Programming of Offspring Health: The Intricate Interplay between Diet, Environment, Reproduction and Development. Biomolecules 2022, 12, 1289. https://doi.org/10.3390/biom12091289
Batra V, Norman E, Morgan HL, Watkins AJ. Parental Programming of Offspring Health: The Intricate Interplay between Diet, Environment, Reproduction and Development. Biomolecules. 2022; 12(9):1289. https://doi.org/10.3390/biom12091289
Chicago/Turabian StyleBatra, Vipul, Emily Norman, Hannah L. Morgan, and Adam J. Watkins. 2022. "Parental Programming of Offspring Health: The Intricate Interplay between Diet, Environment, Reproduction and Development" Biomolecules 12, no. 9: 1289. https://doi.org/10.3390/biom12091289
APA StyleBatra, V., Norman, E., Morgan, H. L., & Watkins, A. J. (2022). Parental Programming of Offspring Health: The Intricate Interplay between Diet, Environment, Reproduction and Development. Biomolecules, 12(9), 1289. https://doi.org/10.3390/biom12091289







