Abstract
The dysfunction of pancreatic β-cells plays a central role in the onset and progression of type 2 diabetes mellitus (T2DM). Insulin secretory defects in β-cells are characterized by a selective impairment of glucose stimulation, and a reduction in glucose-induced ATP production, which is essential for insulin secretion. High glucose metabolism for insulin secretion generates reactive oxygen species (ROS) in mitochondria. In addition, the expression of antioxidant enzymes is very low in β-cells. Therefore, β-cells are easily exposed to oxidative stress. In islet studies using a nonobese T2DM animal model that exhibits selective impairment of glucose-induced insulin secretion (GSIS), quenching ROS generated by glucose stimulation and accumulated under glucose toxicity can improve impaired GSIS. Acute ROS generation and toxicity cause glucose metabolism disorders through different molecular mechanisms. Nuclear factor erythroid 2-related factor 2 (Nrf2), a transcription factor, is a master regulator of antioxidant defense and a potential therapeutic target in oxidative stress-related diseases, suggesting the possible involvement of Nrf2 in β-cell dysfunction caused by ROS. In this review, we describe the mechanisms of insulin secretory defects induced by oxidative stress in diabetic β-cells.
1. Introduction
Type 2 diabetes mellitus (T2DM), a metabolic disorder characterized by chronic hyperglycemia, is caused by relative insulin deficiency. An increase in insulin demand induced by insulin resistance in peripheral tissues, including the liver, muscle, and adipose tissue, exceeds the capacity for insulin secretion in pancreatic β-cells. The subsequent failure of pancreatic β-cells, which is attributed to the loss of their mass and functional deterioration, is essential for the onset and progression of T2DM [1,2,3]. Previous studies using specimens from autopsies or surgical operations have shown that β-cell mass is 30~41% lower in T2DM subjects than in normal, lean subjects (and 63% lower in obese T2DM subjects than in nondiabetic obese subjects) [4,5]. Furthermore, subsequent examination revealed that β-cell mass was 24 and 54% lower in subjects with <5 and >15 years of clinical diabetes, respectively, indicating that decreases in β-cell mass correspond to an increase in the duration of T2DM. These findings suggest that a small difference in β-cell mass observed within 5 years of onset is insufficient to cause T2DM, and that the decrease in mass with the increased duration of the disease can be a consequence of its progression [6]. Moreover, the possibility of overestimation of β-cell loss in T2DM has been reported [7]. Therefore, β-cell dysfunction likely plays a central role in disease progression. Pancreatic β-cells are metabolically active tissues, and ATP produced by glucose metabolism within the cells is essential for the exocytosis of insulin granules [8,9]. Previous studies using rodent and human islets have shown impaired glucose-stimulated insulin secretion (GSIS) and ATP production in T2DM [8]. A high level of glucose metabolism for insulin secretion generates reactive oxygen species (ROS) via the mitochondrial respiratory chain. Additionally, low levels of antioxidant enzymes in β-cells make them susceptible to oxidative stress-induced damage [10]. In this review, we discuss the role of ROS in glucose metabolism disorders that induce impaired insulin secretion in diabetic pancreatic β-cells.
2. Mechanism of Glucose-Stimulated Insulin Secretion in β-Cells
Glucose is the most important secretagogue for insulin, and the molecular mechanism of GSIS is shown in Figure 1. Glucose enters β-cells through glucose transporters (GLUT2 in rodents and GLUT1 in humans [11,12]) and is phosphorylated by glucokinase [13]. Glucose uptake is not rate-limiting because of the large capacity of transporters. Mutations in the human glucokinase gene cause the development of an autosomal dominant form of diabetes (maturity-onset diabetes of the young 2; MODY2) [14], therefore indicating the importance of glucokinase in the insulin secretory mechanism and as a glucose sensor in β-cells. Increases in glycolysis flux and the activity of the tricarboxylic acid (TCA) cycle lead to an increase in mitochondrial ATP production. The increased ATP/ADP ratio within the cytoplasm promotes the closure of ATP-sensitive K+ (KATP) channels that are composed of subunits of the potassium channel (Kir6.2) and sulfonylurea receptor 1 (SUR1) [15,16], followed by an attenuation of plasma membrane polarization. Membrane depolarization leads to the activation of voltage-dependent Ca2+ channels (VDCCs), and the influx of extracellular Ca2+. The resultant increase in intracellular Ca2+ levels triggers the exocytosis of insulin-containing secretory granules [8,9,17]. This pathway is critical for GSIS and is modulated by many factors, including hormones, neurotransmitters, and nutrients, which activate PKA and PKC via their specific G protein-coupled receptors (GPCRs) to act around the site of Ca2+ influx [18,19]. In addition to the classical mechanism (the triggering pathway), the glucose amplification pathway plays a role in GSIS [17]. The amplifying pathway was originally identified by pharmacological studies involving the clamping of the Ca2+ concentration within β-cells at a high level [20,21]. Under these conditions, an increase in glucose levels augments insulin secretion, even though glucose does not increase the Ca2+ concentration. These findings indicate that glucose produces a signal for insulin secretion other than increasing intracellular Ca2+. The metabolic amplifying pathway is physiologically and quantitatively important; however, the molecular mechanisms have not been completely understood [3,22], although it is known that ATP derived from glucose metabolism is involved [23,24,25].
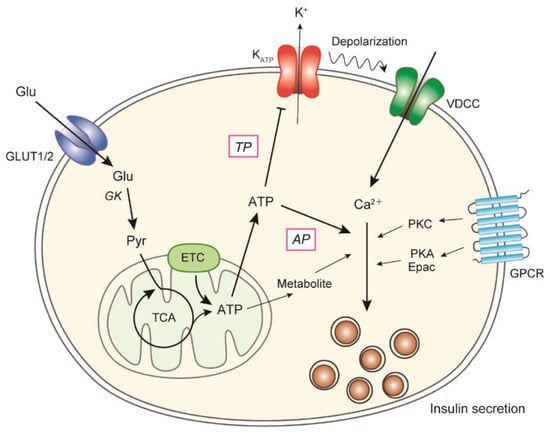
Figure 1.
Mechanism of glucose-induced insulin secretion in β-cells. Insulin secretion from β-cells is regulated by intracellular glucose metabolism, and glucose-induced ATP production in mitochondria plays an essential role. Increased ATP levels in β-cells lead to the closure of KATP channels, followed by membrane depolarization and the subsequent activation of VDCCs. An elevation of intracellular Ca2+ levels triggers the exocytosis of insulin granules. The triggering pathway is critical for GSIS and is modulated by the signals of various GPCRs. In addition, ATP and other metabolites amplify downstream of Ca2+ influx in the triggering pathway (the amplifying pathway). Glu, glucose; GK, glucokinase; Pyr, pyruvate; ETC, electron transport chain; TP, the triggering pathway; AP, the amplifying pathway.
The anaplerotic metabolism of glucose has been implicated in producing a source of coupling factors for GSIS [3]. The anaplerotic metabolism of pyruvate and other fuels leads to an increase in the levels of TCA cycle intermediates, followed by the export of mitochondrial intermediates to the cytosol and their subsequent engagement in cytosolic reactions. Recent studies have focused on the pyruvate–isocitrate cycle, in which the anaplerotic entry of pyruvate into the TCA cycle via pyruvate carboxylase (not mediated by acetyl-CoA) provides mitochondrial citrate and isocitrate [26,27]. Increased cytosolic isocitrate through the mitochondrial citrate/isocitrate carrier [28] engages with cytosolic NADP+-dependent isocitrate dehydrogenase 1 (IDH1) to generate 2-ketoglutarate and NADPH [29]. Cytosolic NADPH produced by the IDH1 reaction is linked to glutaredoxin-mediated activation of sentrin/SUMO-specific protease-1 (SENP1), a de-SUMOylase enzyme that activates the exocytosis of insulin granules [30].
The glycerol shunt operated by glycerol-3-phosphate phosphatase (G3PP) regulates β-cell metabolism and GSIS [31]. G3PP dephosphorylates glycerol-3-phosphate to form glycerol; glycerol-3-phosphate is produced from glucose via glycolysis and from lipolysis-derived glycerol by glycerol kinase. In islets from β-cell-specific G3PP deletion mice, ATP production and insulin secretion are enhanced at high glucose levels, suggesting that the glycerol shunt plays a role in preventing insulin hypersecretion in hyperglycemia [31].
Pyruvate kinase (PK) is an ATP/ADP generator that regulates the closure of KATP channels to stimulate insulin secretion [32]. PK converts ADP and phosphoenolpyruvate from glycolysis and mitochondrial anaplerosis into ATP and pyruvate. Glucose entry into β-cells lowers ADP through PK to levels sufficient to close KATP channels, although the flux of the TCA cycle is low. Following membrane depolarization, the level of ADP is restored, and the flux of the TCA cycle is increased, inducing mitochondrial oxidative phosphorylation-dependent ATP production. Therefore, glucose metabolism is spatially and temporally compartmentalized. Future studies focusing on all aspects of glucose metabolism, including unknown pathways other than the current consensus model, are warranted.
3. ROS Generation and Antioxidant Defense in β-Cells
ROS are a group of reactive molecules and free radicals derived from oxygen. The reduction of oxygen by a single electron generates a superoxide anion (O2−) that is the precursor of most forms of ROS. O2− is rapidly converted into hydrogen peroxide (H2O2) by superoxide dismutase (SOD). H2O2 is fully reduced to water by glutathione peroxidase (Gpx) or catalase. In the presence of high concentrations of transition metals, such as Cu2+ or Fe2+, H2O2 is reduced to hydroxyl radicals, one of the strongest oxidants in nature [33]. The mitochondrial electron transport chain is a major source of ROS because electron leakage occurs mainly in complexes I and III. O2− is one of the most abundant but short-lived ROS [34], and up to 4% of the total oxygen consumed is converted into O2− during oxidative phosphorylation [35]. In β-cells, a rapid increase in glycolytic flux is tightly coupled with the activation of mitochondrial oxidative phosphorylation [36], and almost 100% of the carbon in glucose is oxidized to CO2 [37]. Therefore, the high level of glucose metabolism in β-cells frequently generates ROS in mitochondria. Additionally, the expression of SOD and Gpx/catalase in β-cells is approximately 30 and 5% of the liver values, respectively [10]. Owing to the low expression of antioxidant enzymes, β-cells are susceptible to damage from oxidative stress. Since exposure of β-cells to high glucose induces mitochondrial ROS generation [38,39], the insulin secretory function of β-cells may be impaired.
An increase in intracellular Ca2+ induced by glucose metabolism activates protein kinase C and subsequently NADPH oxidase (NOX) [40]. The NOX family is a group of plasma or subcellular membrane-associated enzymes, which generate O2−. β-cells express NOX2 (prototypical NOX), which is composed of gp91phox (defining the NOX isoform) and p22phox, located in the plasma membrane, and cytosolic components of the NADPH complex (p47phox, p67phox, p40phox, and small G-protein Rac 1/2) [41,42]. NOX inhibition disrupts intracellular Ca2+ dynamics and impairs GSIS [43,44]. Therefore, NOX activation contributes to ROS generation during GSIS [42], and ROS can act as metabolic signaling molecules for GSIS [45,46]. In contrast, experiments using islets of Nox2 knockout mice showed that NOX2 generates O2− to reduce GSIS [47], and that NOX2 is involved in impaired insulin secretion by cytokines [48]. In islets of Zucker diabetic fatty rats, the expression of NOX2 components was higher than that in islets of lean control rats [49]. However, de Souza et al. indicated that NOX2 does not contribute to glucose-induced oxidative stress or alterations in β-cell function [50]. Therefore, the role of NOX in β-cell metabolism and function remains to be elucidated.
Peroxynitrite (ONOO−) is a reactive species generated by O2− and free radical nitric oxide (NO). It has been suggested that ONOO− contributes to β-cell damage in response to pro-inflammatory cytokines [51,52]. However, β-cells do not generate ONOO− by cytokine stimulation [53]. When β-cells are forced to generate ONOO− by providing exogenous O2− and NO, O2− scavenges NO to form ONOO−, resulting in the attenuation of NO-induced damage [53]. These findings suggest that NO is a toxic mediator of cytokines, and ONOO− generation protects β-cells from NO-induced damage.
Peroxiredoxins represent a family of antioxidant proteins, and six members of the peroxiredoxin family have been identified. Peroxiredoxin catalyzes the reduction of H2O2 to water, and oxidized peroxiredoxin is reduced by thioredoxin that, in turn, becomes oxidized. Oxidized thioredoxin is reduced by thioredoxin reductase using NADPH [54]. Peroxiredoxin and thioredoxin are important for redox signaling and are part of the first line of defense against H2O2. The expression of peroxiredoxins (1 and 2) in the cytoplasm of β-cells is upregulated by stressors, including H2O2, cytokines, streptozotocin, and alloxan [55]. Peroxiredoxin 3, a mitochondrial isoform, is also expressed in β-cells, and its expression is increased by cytokine stimulation [56]. β-cells also possess peroxiredoxin 4, an ER-specific isoform [57]. Overexpression of these peroxiredoxins reduces the damage of β-cells caused by several stressors [56,57,58]. In contrast, overexpression of thioredoxin in β-cells suppresses the progression of hyperglycemia in db/db mice, a diabetic model with obesity [59]. Interestingly, cytosolic thioredoxin 1 is secreted from β-cells by hypoxia and glucose stimulation, and exogenously added thioredoxin 1 improves impaired insulin secretion by hypoxia, suggesting paracrine regulation of β-cell function by thioredoxin [60]. Thioredoxin-interacting protein (TXNIP), identified as thioredoxin-binding protein-2, which is identical to vitamin D3 upregulated protein 1 [61], was found to be the most strongly upregulated gene in response to glucose in human islets [62]. TXNIP binds and inhibits thioredoxin, thereby inducing oxidative stress by modulating the cellular redox state [63]. TXNIP expression in β-cells is elevated in diabetic rodent models with and without obesity, and TXNIP deficiency protects β-cells from death [63]. Surprisingly, β-cells remove micromolar levels of H2O2 via the peroxiredoxin/thioredoxin antioxidant system [64]. Therefore, the peroxiredoxin/thioredoxin antioxidant system may compensate for inadequate antioxidant capacity owing to the low expression of antioxidant enzymes in β-cells.
4. Contribution of Acute ROS Generation by Glucose to Diabetic β-Cell Dysfunction
In T2DM, the characteristics of insulin secretory defects in β-cells include a selective impairment of glucose stimulation, while insulin secretion stimulated by arginine, a membrane-depolarizing agent, is preserved [8,65]. Our colleagues and other investigators have demonstrated the mechanism of impaired GSIS in T2DM animal models. The Goto-Kakizaki (GK) rat, an inbred polygenic model of nonobese T2DM, exhibits valuable characteristics that are functionally present in human diabetic patients [66,67,68]. Selective impairment of GSIS in GK rats has been demonstrated in vivo and in isolated pancreatic islets [69,70,71]. Studies on islet cells of GK rats have revealed that the responses to glucose are impaired, while the functions of KATP channels and VDCCs are maintained [72,73,74]. ATP production by glucose stimulation is lowered in the islets of GK rats [75], which parallels the impairment of glucose-induced ATP production in the islets of human patients with T2DM [76]. The glycerol phosphate shuttle is the metabolic site responsible for the impaired ATP production because of a low activity of mitochondrial glycerol phosphate dehydrogenase (mGPDH) [77,78,79]. However, mGPDH overexpression does not correct GSIS in the islets of GK rats [80]. Glucose utilization (the glycolytic rate) and glucose oxidation (the rate of the TCA cycle) have been reported to be decreased, unchanged, or even enhanced in the islets of GK rats [70,75,81], while these are decreased in islets of human T2DM patients [78,82]. These contradictory findings may be at least partly attributed to the differences between various GK rat colonies. Additionally, factors that impair glucose metabolism, rather than the failure of specific sites, may contribute to low ATP production.
We revealed that glucose-induced ROS generation is accelerated in the islets of GK rats and that ROS scavengers (α-tocopherol plus ascorbate) restore the GSIS and ATP production in the islets of GK rats to those of normal Wistar rats [83]. Interestingly, abnormalities in ROS generation, ATP production, and insulin secretion caused by glucose are also restored by Src inhibitors [83,84]. The Src family kinases (SFKs) are a family of nonreceptor tyrosine kinases associated with the cell membrane, and they play important roles in various signal transductions [85]. The c-Src proto-oncogene, the oldest and most studied member of the SFKs, plays major roles in the development, growth, progression, and metastasis of numerous cancers [86]. The pancreatic islets express most SFKs similarly in Wistar and GK rats (undetectable level of Fyn), except for c-Src [84]. The level of c-Src pY416, which indicates Src activation, has been found to be higher in the islets of GK rats than in those of Wistar rats, and the levels of c-Src and carboxyl terminal Src kinase, a negative regulator of SFKs [85], have been found to be lower in the islets of GK rats than in those of Wistar rats [84], indicating that c-Src is activated under diabetic conditions and is degraded by ubiquitination [87] (Figure 2). Although the mechanism of c-Src activation under diabetic conditions is unclear, ROS themselves may regulate c-Src activity [88]. c-Src has also been reported to mediate the transactivation of tyrosine kinase receptors by GPCRs [89,90,91]. A few hundred GPCRs expressed in human and rodent islets mediate the regulation of insulin secretion by various substrates including hormones [18,19]. Glucagon-like peptide (GLP)-1 is an incretin peptide released from the intestine in response to nutrient ingestion, and it augments GSIS [92,93]. GLP-1 binds to the GLP-1 receptor (GLP-1R), a member of the GPCRs, and induces the elevation of cAMP levels via adenylyl cyclase and the subsequent activation of PKA- and/or Epac-dependent pathways to increase the exocytosis of insulin granules [92,93,94]. A GLP-1R agonist suppresses the phosphorylation of c-Src pY416, i.e., c-Src activation, and ameliorates excess ROS generation by glucose (without affecting antioxidant enzyme activities), resulting in restored ATP production in the islets of GK rats [84]. c-Src inactivation by GLP-1R stimulation is Epac-dependent and not PKA-dependent. The regulation of downstream proteins by Src signaling is complex; typical PI3K/Akt signaling [85] is one of the downstream pathways of c-Src regulating ROS generation [84]. Therefore, GLP-1 signaling can acutely suppress excessive ROS generation via c-Src activation in diabetic β-cells, in addition to other beneficial long-term effects, including the induction of β-cell proliferation and enhanced resistance to apoptosis [92,93] (Figure 2). In the islets of Nox2 knockout mice, Nox2 deficiency decreases ROS generation and increases cAMP formation. The increase in cAMP reduces ROS generation, indicating a mutual relationship between cAMP and ROS levels [47]. Therefore, Nox2 may be partially involved in ROS generation in diabetic β-cells. GLP-1R agonists and dipeptidyl peptidase-4 inhibitors, which delay GLP-1 degradation, are widely used in T2DM treatment [95]. Several clinical trials have shown that patients receiving combination therapy of a GLP-1R agonist and sulfonylurea (SU), a KATP inhibitor, have a higher incidence of hypoglycemia than those treated with a GLP-1R agonist only [96,97]. KATP channel inhibition by SU has been shown to be attenuated under conditions of decreased intracellular ATP in β-cells because of a defect in signal transduction between SUR1 and Kir6.2 [98]. Therefore, recovery of ATP production by GLP-1 signaling may improve the attenuated KATP channel inhibition by SU under diabetic conditions, resulting in an excessive drop in blood glucose levels.
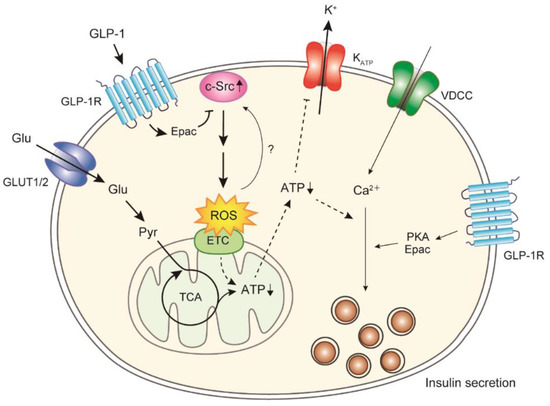
Figure 2.
Involvement of c-Src in glucose-induced ROS generation in diabetic β-cells. In diabetic β-cells, acute ROS generation induced by glucose is upregulated, which is attributed to c-Src activation. GLP-1 signaling can suppress c-Src activation and ROS generation, resulting in restored ATP production. Glu, glucose; Pyr, pyruvate; ETC, electron transport chain.
5. ROS Toxicity and the Change in Glucose Metabolism in Diabetic β-Cells
Oxidative stress is higher in the islets of both GK rats and human T2DM patients than in those of controls [4,99], and the elevation is dependent on the duration of chronic hyperglycemia [99]. Long-term exposure to high glucose causes impairment in insulin secretion and biosynthesis [100]. Prolonged oxidative stress activates stress-induced MAP kinases, such as JNK, suppressing the binding of transcriptional factors to the insulin promoter. Exposure of the islets of GK rats to cell-permeable antioxidant enzyme mimics (tempol plus ebselen) for 12 h leads to an ROS reduction, followed by an improvement in ATP production and insulin secretion [101]. Decreased glucose oxidation in GK rat islets is also elevated to levels observed in the islets of Wistar rats by treatment with antioxidant enzyme mimics. In addition, lactate production and the expression of lactate dehydrogenase A (LDHA), which converts pyruvate to lactate, are elevated in the islets of GK rats, and their elevations are reduced by treatment with antioxidant enzyme mimics. LDHA expression is upregulated in the islets of several T2DM model animals [102,103,104], and this LDHA overexpression is sufficient to disturb GSIS [105]. The expression of hypoxia-inducible factor-1α (HIF-1α), a potential upstream regulator of LDHA [106], is reduced by antioxidant treatment, and an HIF-1α inhibitor reduces lactate production and improves insulin secretion [101]. HIF-1 mediates adaptive responses to reduced O2 availability and regulates the balance between O2 supply and demand [106]. In properly oxygenated cells, HIF-1α is hydroxylated by prolyl hydroxylase domain (PHD) proteins using O2 and α-ketoglutarate, bound by von Hippel–Lindau (VHL) protein, ubiquitinylated, and degraded by the proteasome. In hypoxic cells, HIF-1α binds to HIF-1β, and the dimer induces the transcription of several genes, including pyruvate dehydrogenase (PDH) kinase 1 (PDK1), LDHA, and other genes of glycolytic enzymes. PDK1 phosphorylates and inactivates PDH, consequently inhibiting the conversion of pyruvate to acetyl-CoA for entry into the TCA cycle. Hypoxia occurs frequently in human cancers, which produce energy predominantly through accelerated glycolysis, resulting in lactate overproduction [106,107,108]. This metabolic phenomenon, known as aerobic glycolysis or the Warburg effect [109,110], can occur even under nonhypoxic conditions, indicating that HIF-1α is a central regulator of cancer metabolism. In addition, ROS inhibit PHD activity to stabilize HIF-1α under normoxia [111]. Therefore, ROS accumulation in diabetic β-cells may induce Warburg-like lactate production by HIF-1α as observed in cancer cells, resulting in the disruption of glucose sensing and insulin secretion [112] (Figure 3).
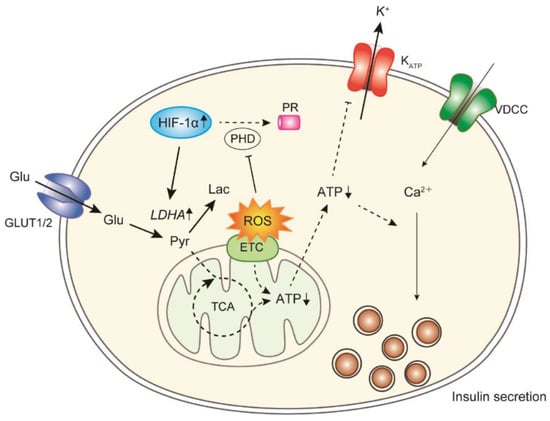
Figure 3.
HIF-1α regulates the change in glucose metabolism by ROS toxicity in diabetic β-cells. Under chronic oxidative stress, ROS inhibits PHD activity to activate HIF-1α. LDHA activation by HIF-1α causes excess lactate production from glucose. Glu, glucose; Pyr, pyruvate; Lac, lactate; ETC, electron transport chain; PR, proteasome.
Mice with a β-cell-specific deletion of VHL exhibit increased HIF-1α expression and impaired GSIS [113,114,115]. In contrast, mice with an HIF-1α deletion in β-cells do not exhibit obvious changes in glucose response, while HIF-1α overexpression in β-cells impairs GSIS [113,114]. However, Cheng et al. reported glucose intolerance in mice with a β-cell-specific deletion of HIF-1α, as well as impaired GSIS in their islets and in β-cells with an HIF-1α deletion by RNAi [116]. Moreover, high-fat diet-induced glucose intolerance in mice was improved by HIF-1α activation. The expression of HIF-1α mRNA is decreased in the islets of T2DM subjects compared to that in people with normal glucose tolerance [117]. However, HIF-1α expression in the islets of GK rats does not change compared with that in Wistar rats [101]. These discrepancies may be attributed to differences in the duration of T2DM and the levels of hypoxia and ROS. The expression of HIF-1β mRNA is decreased in islets of T2DM subjects, and mice with a β-cell-specific HIF-1β deletion exhibit abnormal glucose tolerance and impaired insulin secretion [117]. These findings indicate that HIF-1 is undoubtedly involved in diabetic β-cell dysfunction, and an elucidation of the role of HIF-1 in T2DM may be helpful in identifying HIF-1 as a therapeutic target.
6. A Master Regulator of Antioxidant Defense: Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2)
Nrf2, isolated in 1994 [118], is a transcription factor that regulates over 100 genes related to oxidative stress and cell survival [119,120]. Under nonstressed conditions, Nrf2 binds to its cytosolic repressor, Kelch-like ECH-associated protein 1 (Keap1) [121], which serves as an adaptor for the Cullin3-based ubiquitin E3 ligase complex and is engaged in proteasomal degradation [122]. Under oxidative stress, cysteine residues on Keap1 are modified, which leads to the disruption of Keap1–Keap1 homodimerization, Keap1–Cullin3 interaction, and Nrf2 translocation into the nucleus. Several cysteine residues within Keap1, including Cys151, are thought to be the sites for oxidation-induced modification of ROS sensors [120]. Nrf2 binds to the promoter region of the antioxidant response element (ARE) and initiates the transcription of various cytoprotective enzymes, including SOD and Gpx, resulting in the restoration of redox balance in response to oxidative stress (Figure 4). Nrf2 regulates the basal and inducible expression of various genes of antioxidant enzymes, antioxidants, xenobiotic-metabolizing enzymes, and enzymes of the pentose phosphate pathway/NADPH production [120]. Therefore, the Nrf2/Keap1 system may maintain GSIS under diabetic conditions through the regulation of antioxidant-related genes as well as genes involved in NADPH production, which help in amplifying insulin secretion [3]. In addition, Nrf2 affects mitochondrial membrane potential and ATP synthesis [123,124]. Therefore, Nrf2 may be a prominent regulator of glucose metabolism in diabetic β-cells.
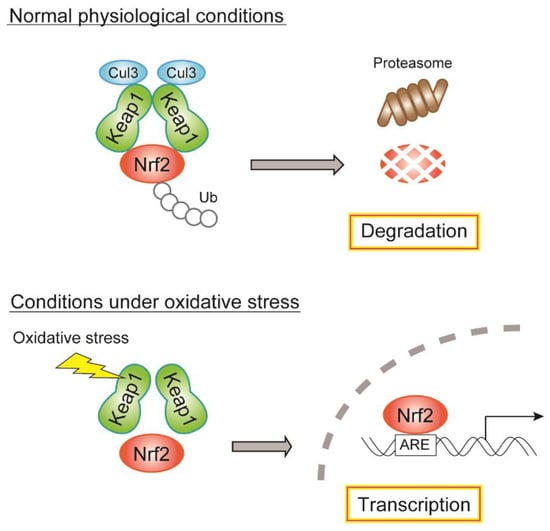
Figure 4.
Regulatory mechanism of gene expression by the Nrf2/Keap1 system. During normal physiological conditions, Nrf2 binds to Keap1 and is degraded through Cul3-mediated ubiquitination. During oxidative stress, Nrf2 is released from the binding of Keap1, translocated to the nucleus, and drives gene expression of antioxidant and NADPH-producing enzymes. Cul3, Cullin3; Ub, ubiquitin.
The mechanisms underlying alterations in the expression of Nrf2 and Keap1 in β-cells of T2DM patients are still unclear. The expression of Nrf2 mRNA in the islets is upregulated in streptozotocin-induced diabetic mice [125]. In contrast, exposure of β-cells to long-chain fatty acids (palmitate) for 48 h results in a decreased expression of Nrf2 mRNA (unpublished data). Since Nrf2 is translocated into the nucleus, further clarification regarding the change in Nrf2 levels in the nucleus under diabetic conditions is required. In a study using β-cell-specific Keap1 deletion mice, Nrf2 activation reversed the reduced β-cell mass, impaired GSIS, and glucose intolerance induced by NO stress [126]. It has been reported that dimethyl fumarate and oltipraz, Nrf2 activators, antagonize glucotoxicity-induced impairment of insulin secretion, whereas GSIS under normal conditions is reduced by both Nrf2 activators [127]. Elevation of Nrf2 levels following exposure to low levels of arsenite increases antioxidant capacity and reduces GSIS in normal β-cells [128], suggesting that overactivation of antioxidant capacity disturbs the physiological redox balance [127]. Therefore, unnecessary Nrf2 activation may not be beneficial for nonstressed β-cells. Accumulating evidence suggests that the Nrf2/Keap1 pathway is implicated in diabetic oxidative damage to several peripheral tissues, and the pharmacological activation of Nrf2 is expected to be a promising therapeutic approach for T2DM [119,129,130]. The bardoxolone family, including 2-cyano-3,12-dioxo-oleana-1,9(11)-dien-28-oic acid (CDDO) methyl ester (CDDO-Me) and CDDO imidazolide (CDDO-Im), derivatives of synthetic triterpenoids, reduces blood glucose levels and improves insulin resistance in high-fat diet-fed and db/db mice [131,132]. Several natural compounds, including sulforaphane, curcumin, resveratrol, and quercetin, have been identified as Nrf2 activators, which exhibit antioxidative potential in various tissues [119,129,130]. Accordingly, the Nrf2/Keap1 axis is a promising pharmacological target for protecting β-cells from oxidative damage under diabetic conditions.
7. Conclusions
Glucose metabolism is crucial for insulin secretion in β-cells, and a highly activated glucose metabolism causes ROS generation, which impairs insulin secretion. This contradictory mechanism might be associated with the dual effects of ROS. Although ROS have negative effects on cellular function, it has been proposed that they contribute to the stimulation of GSIS [133]. Measurements of redox changes at the whole-cell level are limited in clarifying the role of ROS. Recent developments of specific redox probes targeting cellular compartments of interest may allow the identification of the site of ROS generation and the site impaired by ROS at the subcellular level [133]. The mechanisms of the regulation of redox signaling in response to glucose and ROS toxicity caused by glucose exposure are complex. Therefore, evaluation of the process in subcellular compartments will be helpful in clarifying the role of ROS in detail, and future studies using advanced techniques may lead to the identification of new methods to attenuate oxidative stress for diabetic therapy.
Author Contributions
Conceptualization, E.M.; writing—original draft preparation, E.M.; writing—review and editing, E.M., S.F. and N.I.; visualization, E.M.; supervision, S.F. and N.I.; funding acquisition, E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the JSPS Grants-in-Aid for Scientific Research (21K08537).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Prentki, M.; Nolan, C.J. Islet β cell failure in type 2 diabetes. J. Clin. Investig. 2006, 116, 1802–1812. [Google Scholar] [CrossRef]
- Halban, P.A.; Polonsky, K.S.; Bowden, D.W.; Hawkins, M.A.; Ling, C.; Mather, K.J.; Powers, A.C.; Rhodes, C.J.; Sussel, L.; Weir, G.C. β-cell failure in type 2 diabetes: Postulated mechanisms and prospects for prevention and treatment. Diabetes Care 2014, 37, 1751–1758. [Google Scholar] [CrossRef]
- Campbell, J.E.; Newgard, C.B. Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat. Rev. Mol. Cell. Biol. 2021, 22, 142–158. [Google Scholar] [CrossRef]
- Sakuraba, H.; Mizukami, H.; Yagihashi, N.; Wada, R.; Hanyu, C.; Yagihashi, S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia 2002, 45, 85–96. [Google Scholar] [CrossRef]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. β-cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef]
- Rahier, J.; Guiot, Y.; Goebbels, R.M.; Sempoux, C.; Henquin, J.C. Pancreatic β-cell mass in European subjects with type 2 diabetes. Diabetes Obes. Metab. 2008, 10, 32–42. [Google Scholar] [CrossRef]
- Marselli, L.; Suleiman, M.; Masini, M.; Campani, D.; Bugliani, M.; Syed, F.; Martino, L.; Focosi, D.; Scatena, F.; Olimpico, F.; et al. Are we overestimating the loss of beta cells in type 2 diabetes? Diabetologia 2014, 57, 362–365. [Google Scholar] [CrossRef]
- Fujimoto, S.; Nabe, K.; Takehiro, M.; Shimodahira, M.; Kajikawa, M.; Takeda, T.; Mukai, E.; Inagaki, N.; Seino, Y. Impaired metabolism-secretion coupling in pancreatic β-cells: Role of determinants of mitochondrial ATP production. Diabetes Res. Clin. Pract. 2007, 77, S2–S10. [Google Scholar] [CrossRef]
- Rutter, G.A.; Pullen, T.J.; Hodson, D.J.; Martinez-Sanchez, A. Pancreatic β-cell identity, glucose sensing and the control of insulin secretion. Biochem. J. 2015, 466, 203–218. [Google Scholar] [CrossRef]
- Tiedge, M.; Lortz, S.; Drinkgern, J.; Lenzen, S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 1997, 46, 1733–1742. [Google Scholar] [CrossRef]
- McCulloch, L.J.; van de Bunt, M.; Braun, M.; Frayn, K.N.; Clark, A.; Gloyn, A.L. GLUT2 (SLC2A2) is not the principal glucose transporter in human pancreatic beta cells: Implications for understanding genetic association signals at this locus. Mol. Genet. Metab. 2011, 104, 648–653. [Google Scholar] [CrossRef]
- Thorens, B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia 2015, 58, 221–232. [Google Scholar] [CrossRef]
- Lenzen, S. A fresh view of glycolysis and glucokinase regulation: History and current status. J. Biol. Chem. 2014, 289, 12189–12194. [Google Scholar] [CrossRef]
- Matschinsky, F.M. Regulation of pancreatic β-cell glucokinase: From basics to therapeutics. Diabetes 2002, 51, S394–S404. [Google Scholar] [CrossRef]
- Inagaki, N.; Gonoi, T.; Clement IV, J.P.; Namba, N.; Inazawa, J.; Gonzalez, G.; Aguilar-Bryan, L.; Seino, S.; Bryan, J. Reconstitution of IKATP: An inward rectifier subunit plus the sulfonylurea receptor. Science 1995, 270, 1166–1170. [Google Scholar] [CrossRef]
- Aguilar-Bryan, L.; Nichols, C.G.; Wechsler, S.W.; Clement IV, J.P.; Boyd III, A.E.; González, G.; Herrera-Sosa, H.; Nguy, K.; Bryan, J.; Nelson, D.A. Cloning of the β cell high-affinity sulfonylurea receptor: A regulator of insulin secretion. Science 1995, 268, 423–426. [Google Scholar] [CrossRef]
- Henquin, J.C. Regulation of insulin secretion: A matter of phase control and amplitude modulation. Diabetologia 2009, 52, 739–751. [Google Scholar] [CrossRef]
- Winzell, M.S.; Ahrén, B. G-protein-coupled receptors and islet function-implications for treatment of type 2 diabetes. Pharmacol. Ther. 2007, 116, 437–448. [Google Scholar] [CrossRef]
- Thor, D. G protein-coupled receptors as regulators of pancreatic islet functionality. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119235. [Google Scholar] [CrossRef]
- Gembal, M.; Gilon, P.; Henquin, J.C. Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. J. Clin. Investig. 1992, 89, 1288–1295. [Google Scholar] [CrossRef]
- Sato, Y.; Aizawa, T.; Komatsu, M.; Okada, N.; Yamada, T. Dual functional role of membrane depolarization/Ca2+ influx in rat pancreatic B-cell. Diabetes 1992, 41, 438–443. [Google Scholar] [CrossRef]
- Prentki, M.; Matschinsky, F.M.; Murthy Madiraju, S.R. Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 2013, 18, 162–185. [Google Scholar] [CrossRef]
- Eliasson, L.; Renström, E.; Ding, W.G.; Proks, P.; Rorsman, P. Rapid ATP-dependent priming of secretory granules precedes Ca2+-induced exocytosis in mouse pancreatic B-cells. J. Physiol. 1997, 503, 399–412. [Google Scholar] [CrossRef]
- Takahashi, N.; Kadowaki, T.; Yazaki, Y.; Ellis-Davies, G.C.; Miyashita, Y.; Kasai, H. Post-priming actions of ATP on Ca2+-dependent exocytosis in pancreatic beta cells. Proc. Natl. Acad. Sci. USA 1999, 96, 760–765. [Google Scholar] [CrossRef]
- Fujimoto, S.; Mukai, E.; Hamamoto, Y.; Takeda, T.; Takehiro, M.; Yamada, Y.; Seino, Y. Prior exposure to high glucose augments depolarization-induced insulin release by mitigating the decline of ATP level in rat islets. Endocrinology 2002, 143, 213–221. [Google Scholar] [CrossRef][Green Version]
- Farfari, S.; Schulz, V.; Corkey, B.; Prentki, M. Glucose-regulated anaplerosis and cataplerosis in pancreatic β-cells: Possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes 2000, 49, 718–726. [Google Scholar] [CrossRef]
- Lu, D.; Mulder, H.; Zhao, P.; Burgess, S.C.; Jensen, M.V.; Kamzolova, S.; Newgard, C.B.; Sherry, A.D. 13C NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS). Proc. Natl. Acad. Sci. USA 2002, 99, 2708–2713. [Google Scholar] [CrossRef]
- Joseph, J.W.; Jensen, M.V.; Ilkayeva, O.; Palmieri, F.; Alárcon, C.; Rhodes, C.J.; Newgard, C.B. The mitochondrial citrate/isocitrate carrier plays a regulatory role in glucose-stimulated insulin secretion. J. Biol. Chem. 2006, 281, 35624–35632. [Google Scholar] [CrossRef]
- Ronnebaum, S.M.; Ilkayeva, O.; Burgess, S.C.; Joseph, J.W.; Lu, D.; Stevens, R.D.; Becker, T.C.; Sherry, A.D.; Newgard, C.B.; Jensen, M.V. A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretion. J. Biol. Chem. 2006, 281, 30593–30602. [Google Scholar] [CrossRef]
- Ferdaoussi, M.; Dai, X.; Jensen, M.V.; Wang, R.; Peterson, B.S.; Huang, C.; Ilkayeva, O.; Smith, N.; Miller, N.; Hajmrle, C.; et al. Isocitrate-to-SENP1 signaling amplifies insulin secretion and rescues dysfunctional β cells. J. Clin. Investig. 2015, 125, 3847–3860. [Google Scholar] [CrossRef]
- Al-Mass, A.; Poursharifi, P.; Peyot, M.L.; Lussier, R.; Levens, E.J.; Guida, J.; Mugabo, Y.; Possik, E.; Ahmad, R.; Al-Mulla, F.; et al. Glycerol-3-phosphate phosphatase operates a glycerol shunt in pancreatic β-cells that controls insulin secretion and metabolic stress. Mol. Metab. 2022, 60, 101471. [Google Scholar] [CrossRef]
- Lewandowski, S.L.; Cardone, R.L.; Foster, H.R.; Ho, T.; Potapenko, E.; Poudel, C.; VanDeusen, H.R.; Sdao, S.M.; Alves, T.C.; Zhao, X.; et al. Pyruvate kinase controls signal strength in the insulin secretory pathway. Cell Metab. 2020, 32, 736–750.e5. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Gray, J.P.; Heart, E.; Bhattacharya, S. Usurping the mitochondrial supremacy: Extramitochondrial sources of reactive oxygen intermediates and their role in beta cell metabolism and insulin secretion. Toxicol. Mech. Methods 2010, 20, 167–174. [Google Scholar] [CrossRef]
- Cadenas, E.; Davies, K.J.A. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- Sekine, N.; Cirulli, V.; Regazzi, R.; Brown, L.J.; Gine, E.; Tamarit-Rodriguez, J.; Girotti, M.; Marie, S.; MacDonald, M.J.; Wollheim, C.B.; et al. Low lactate dehydrogenase and high mitochondrial glycerol phosphate dehydrogenase in pancreatic β-cells. Potential role in nutrient sensing. J. Biol. Chem. 1994, 269, 4895–4902. [Google Scholar] [CrossRef]
- Schuit, F.; De Vos, A.; Farfari, S.; Moens, K.; Pipeleers, D.; Brun, T.; Prentk, M. Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in β cells. J. Biol. Chem. 1997, 272, 18572–18579. [Google Scholar] [CrossRef]
- Bindokas, V.P.; Kuznetsov, A.; Sreenan, S.; Polonsky, K.S.; Roe, M.W.; Philipson, L.H. Visualizing superoxide production in normal and diabetic rat islets of Langerhans. J. Biol. Chem. 2003, 278, 9796–9801. [Google Scholar] [CrossRef]
- Sakai, K.; Matsumoto, K.; Nishikawa, T.; Suefuji, M.; Nakamaru, K.; Hirashima, Y.; Kawashima, J.; Shirotani, T.; Ichinose, K.; Brownlee, M.; et al. Mitochondrial reactive oxygen species reduce insulin secretion by pancreatic β-cells. Biochem. Biophys. Res. Commun. 2003, 300, 216–222. [Google Scholar] [CrossRef]
- Morgan, D.; Oliveira-Emilio, H.R.; Keane, D.; Hirata, A.E.; Santos da Rocha, M.; Bordin, S.; Curi, R.; Newsholme, P.; Carpinelli, A.R. Glucose, palmitate and pro-inflammatory cytokines modulate production and activity of a phagocyte-like NADPH oxidase in rat pancreatic islets and a clonal beta cell line. Diabetologia 2007, 50, 359–369. [Google Scholar] [CrossRef]
- Oliveira, H.R.; Verlengia, R.; Carvalho, C.R.O.; Britto, L.R.G.; Curi, R.; Carpinelli, A.R. Pancreatic β-cells express phagocyte-like NAD(P)H oxidase. Diabetes 2003, 52, 1457–1463. [Google Scholar] [CrossRef]
- Newsholme, P.; Morgan, D.; Rebelato, E.; Oliveira-Emilio, H.C.; Procopio, J.; Curi, R.; Carpinelli, A. Insights into the critical role of NADPH oxidase(s) in the normal and dysregulated pancreatic beta cell. Diabetologia 2009, 52, 2489–2498. [Google Scholar] [CrossRef]
- Imoto, H.; Sasaki, N.; Iwase, M.; Nakamura, U.; Oku, M.; Sonoki, K.; Uchizono, Y.; Iida, M. Impaired insulin secretion by diphenyleneiodium associated with perturbation of cytosolic Ca2+ dynamics in pancreatic β-cells. Endocrinology 2008, 149, 5391–5400. [Google Scholar] [CrossRef]
- Morgan, D.; Rebelato, E.; Abdulkader, F.; Graciano, M.F.R.; Oliveira-Emilio, H.R.; Hirata, A.E.; Rocha, M.S.; Bordin, S.; Curi, R.; Carpinelli, A.R. Association of NAD(P)H oxidase with glucose-induced insulin secretion by pancreatic β-cells. Endocrinology 2009, 150, 2197–2201. [Google Scholar] [CrossRef]
- Pi, J.; Bai, Y.; Zhang, Q.; Wong, V.; Floering, L.M.; Daniel, K.; Reece, J.M.; Deeney, J.T.; Andersen, M.E.; Corkey, B.E.; et al. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes 2007, 56, 1783–1791. [Google Scholar] [CrossRef]
- Leloup, C.; Tourrel-Cuzin, C.; Magnan, C.; Karaca, M.; Castel, J.; Carneiro, L.; Colombani, A.L.; Ktorza, A.; Casteilla, L.; Pénicaud, L. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes 2009, 58, 673–681. [Google Scholar] [CrossRef]
- Li, N.; Li, B.; Brun, T.; Deffert-Delbouille, C.; Mahiout, Z.; Daali, Y.; Ma, X.J.; Krause, K.H.; Maechler, P. NADPH oxidase NOX2 defines a new antagonistic role for reactive oxygen species and cAMP/PKA in the regulation of insulin secretion. Diabetes 2012, 61, 2842–2850. [Google Scholar] [CrossRef]
- Xiang, F.L.; Lu, X.; Strutt, B.; Hill, D.J.; Feng, Q. NOX2 deficiency protects against streptozotocin-induced β-cell destruction and development of diabetes in mice. Diabetes 2010, 59, 2603–2611. [Google Scholar] [CrossRef]
- Syed, I.; Kyathanahalli, C.N.; Jayaram, B.; Govind, S.; Rhodes, C.J.; Kowluru, R.A.; Kowluru, A. Increased phagocyte-like NADPH oxidase and ROS generation in type 2 diabetic ZDF rat and human islets: Role of Rac1-JNK1/2 signaling pathway in mitochondrial dysregulation in the diabetic islet. Diabetes 2011, 60, 2843–2852. [Google Scholar] [CrossRef]
- de Souza, A.H.; Santos, L.R.B.; Roma, L.P.; Bensellam, M.; Carpinelli, A.R.; Jonas, J.C. NADPH oxidase-2 does not contribute to β-cell glucotoxicity in cultured pancreatic islets from C57BL/6J mice. Mol. Cell Endocrinol. 2017, 439, 354–362. [Google Scholar] [CrossRef]
- Suarez-Pinzon, W.L.; Szabó, C.; Rabinovitch, A. Development of autoimmune diabetes in NOD mice is associated with the formation of peroxynitrite in pancreatic islet β-cells. Diabetes 1997, 46, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Lakey, J.R.; Suarez-Pinzon, W.L.; Strynadka, K.; Korbutt, G.S.; Rajotte, R.V.; Mabley, J.G.; Szabó, C.; Rabinovitch, A. Peroxynitrite is a mediator of cytokine-induced destruction of human pancreatic islet β cells. Lab. Investig. 2001, 81, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Broniowska, K.A.; Mathews, C.E.; Corbett, J.A. Do β-cells generate peroxynitrite in response to cytokine treatment? J. Biol. Chem. 2013, 288, 36567–36578. [Google Scholar] [CrossRef] [PubMed]
- Stancill, J.S.; Corbett, J.A. The role of thioredoxin/peroxiredoxin in the β-cell defense against oxidative damage. Front. Endocrinol. 2021, 12, 718235. [Google Scholar] [CrossRef] [PubMed]
- Bast, A.; Wolf, G.; Oberbäumer, I.; Walther, R. Oxidative and nitrosative stress induces peroxiredoxins in pancreatic beta cells. Diabetologia 2002, 45, 867–876. [Google Scholar] [CrossRef]
- Wolf, G.; Aumann, N.; Michalska, M.; Bast, A.; Sonnemann, J.; Beck, J.F.; Lendeckel, U.; Newsholme, P.; Walther, R. Peroxiredoxin III protects pancreatic β cells from apoptosis. J. Endocrinol. 2010, 207, 163–175. [Google Scholar] [CrossRef]
- Mehmeti, I.; Lortz, S.; Elsner, M.; Lenzen, S. Peroxiredoxin 4 improves insulin biosynthesis and glucose-induced insulin secretion in insulin-secreting INS-1E cells. J. Biol. Chem. 2014, 289, 26904–26913. [Google Scholar] [CrossRef]
- Stancill, J.S.; Happ, J.T.; Broniowska, K.A.; Hogg, N.; Corbett, J.A. Peroxiredoxin 1 plays a primary role in protecting pancreatic β-cells from hydrogen peroxide and peroxynitrite. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R1004–R1013. [Google Scholar] [CrossRef]
- Yamamoto, M.; Yamato, E.; Toyoda, S.; Tashiro, F.; Ikegami, H.; Yodoi, J.; Miyazaki, J. Transgenic expression of antioxidant protein thioredoxin in pancreatic β cells prevents progression of type 2 diabetes mellitus. Antioxid. Redox Signal. 2008, 10, 43–49. [Google Scholar] [CrossRef]
- Hanschmann, E.M.; Petry, S.F.; Eitner, S.; Maresch, C.C.; Lingwal, N.; Lillig, C.H.; Linn, T. Paracrine regulation and improvement of β-cell function by thioredoxin. Redox Biol. 2020, 34, 101570. [Google Scholar] [CrossRef]
- Nishiyama, A.; Matsui, M.; Iwata, S.; Hirota, K.; Masutani, H.; Nakamura, H.; Takagi, Y.; Sono, H.; Gon, Y.; Yodoi, J. Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J. Biol. Chem. 1999, 274, 21645–21650. [Google Scholar] [CrossRef] [PubMed]
- Shalev, A.; Pise-Masison, C.A.; Radonovich, M.; Hoffmann, S.C.; Hirshberg, B.; Brady, J.N.; Harlan, D.M. Oligonucleotide microarray analysis of intact human pancreatic islets: Identification of glucose-responsive genes and a highly regulated TGFβ signaling pathway. Endocrinology 2002, 143, 3695–3698. [Google Scholar] [CrossRef] [PubMed]
- Shalev, A. Minireview: Thioredoxin-interacting protein: Regulation and function in the pancreatic β-cell. Mol. Endocrinol. 2014, 28, 1211–1220. [Google Scholar] [CrossRef]
- Stancill, J.S.; Broniowska, K.A.; Oleson, B.J.; Naatz, A.; Corbett, J.A. Pancreatic β-cells detoxify H2O2 through the peroxiredoxin/thioredoxin antioxidant system. J. Biol. Chem. 2019, 294, 4843–4853. [Google Scholar] [CrossRef] [PubMed]
- Leahy, J.L.; Bonner-Weir, S.; Weir, G.C. β-cell dysfunction induced by chronic hyperglycemia. Current ideas on mechanism of impaired glucose-induced insulin secretion. Diabetes Care 1992, 15, 442–455. [Google Scholar] [CrossRef]
- Östenson, C.G.; Efendic, S. Islet gene expression and function in type 2 diabetes; studies in the Goto-Kakizaki rat and humans. Diabetes Obes. Metab. 2007, 9, 180–186. [Google Scholar] [CrossRef]
- Portha, B.; Giroix, M.H.; Tourrel-Cuzin, C.; Le-Stunff, H.; Movassat, J. The GK rat: A prototype for the study of non-overweight type 2 diabetes. Methods Mol. Biol. 2012, 933, 125–159. [Google Scholar] [CrossRef]
- Akash, M.S.; Rehman, K.; Chen, S. Goto-Kakizaki rats: Its suitability as non-obese diabetic animal model for spontaneous type 2 diabetes mellitus. Curr. Diabetes Rev. 2013, 9, 387–396. [Google Scholar] [CrossRef]
- Portha, B.; Serradas, P.; Bailbé, D.; Suzuki, K.; Goto, Y.; Giroix, M.H. β-cell insensitivity to glucose in the GK rat, a spontaneous nonobese model for type II diabetes. Diabetes 1991, 40, 486–491. [Google Scholar] [CrossRef]
- Östenson, C.G.; Khan, A.; Abdel-Halim, S.M.; Guenifi, A.; Suzuki, K.; Goto, Y.; Efendic, S. Abnormal insulin secretion and glucose metabolism in pancreatic islets from the spontaneously diabetic GK rat. Diabetologia 1993, 36, 3–8. [Google Scholar] [CrossRef]
- Hughes, S.J.; Suzuki, K.; Goto, Y. The role of islet secretory function in the development of diabetes in the GK Wistar rat. Diabetologia 1994, 37, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Tsuura, Y.; Ishida, H.; Okamoto, Y.; Kato, S.; Sakamoto, K.; Horie, M.; Ikeda, H.; Okada, Y.; Seino, Y. Glucose sensitivity of ATP-sensitive K+ channels is impaired in β-cells of the GK rat. A new genetic model of NIDDM. Diabetes 1993, 42, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Ishida, H.; Tsuura, Y.; Yasuda, K.; Kato, S.; Matsubara, H.; Nishimura, M.; Mizuno, N.; Ikeda, H.; Seino, Y. Hyperresponse in calcium-induced insulin release from electrically permeabilized pancreatic islets of diabetics GK rats and its defective augmentation by glucose. Diabetologia 1995, 38, 772–778. [Google Scholar] [CrossRef]
- Kato, S.; Ishida, H.; Tsuura, Y.; Tsuji, K.; Nishimura, M.; Horie, M.; Taminato, T.; Ikehara, S.; Odaka, H.; Ikeda, I.; et al. Alterations in basal and glucose-stimulated voltage-dependent Ca2+ channel activities in pancreatic β cells of non-insulin-dependent diabetes mellitus GK rats. J. Clin. Investig. 1996, 97, 2417–2425. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.J.; Faehling, M.; Thorneley, C.W.; Proks, P.; Ashcroft, F.M.; Smith, P.A. Electrophysiological and metabolic characterization of single β-cells and islets from diabetic GK rats. Diabetes 1998, 47, 73–81. [Google Scholar] [CrossRef]
- Anello, M.; Lupi, R.; Spampinato, D.; Piro, S.; Masini, M.; Boggi, U.; Del Prato, S.; Rabuazzo, A.M.; Purrello, F.; Marchetti, P. Functional and morphological alterations of mitochondria in pancreatic beta cells from type 2 diabetic patients. Diabetologia 2005, 48, 282–289. [Google Scholar] [CrossRef]
- Östenson, C.G.; Abdel-Halim, S.M.; Rasschaert, J.; Malaisse-Lagae, F.; Meuris, S.; Sener, A.; Efendic, S.; Malaisse, W.J. Deficient activity of FAD-linked glycerophosphate dehydrogenase in islets of GK rats. Diabetologia 1993, 36, 722–726. [Google Scholar] [CrossRef]
- Fernandez-Alvarez, J.; Conget, I.; Rasschaert, J.; Sener, A.; Gomis, R.; Malaisse, W.J. Enzymatic, metabolic and secretory patterns in human islets of type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1994, 37, 177–181. [Google Scholar] [CrossRef]
- MacDonald, M.J.; Efendic, S.; Östenson, C.G. Normalization by insulin treatment of low mitochondrial glycerol phosphate dehydrogenase and pyruvate carboxylase in pancreatic islets of the GK rat. Diabetes 1996, 45, 886–890. [Google Scholar] [CrossRef]
- Ueda, K.; Tanizawa, Y.; Ishihara, H.; Kizuki, N.; Ohta, Y.; Matsutani, A.; Oka, Y. Overexpression of mitochondrial FAD-linked glycerol-3-phosphate dehydrogenase does not correct glucose-stimulated insulin secretion from diabetic GK rat pancreatic islets. Diabetologia 1998, 41, 649–653. [Google Scholar] [CrossRef]
- Ling, Z.C.; Efendic, S.; Wibom, R.; Abdel-Halim, S.M.; Ostenson, C.G.; Landau, B.R.; Khan, A. Glucose metabolism in Goto-Kakizaki rat islets. Endocrinology 1998, 139, 2670–2675. [Google Scholar] [CrossRef] [PubMed]
- Del Guerra, S.; Lupi, R.; Marselli, L.; Masini, M.; Bugliani, M.; Sbrana, S.; Torri, S.; Pollera, M.; Boggi, U.; Mosca, F.; et al. Functional and molecular defects of pancreatic islets in human type 2 diabetes. Diabetes 2005, 54, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Kominato, R.; Fujimoto, S.; Mukai, E.; Nakamura, Y.; Nabe, K.; Shimodahira, M.; Nishi, Y.; Funakoshi, S.; Seino, Y.; Inagaki, N. Src activation generates reactive oxygen species and impairs metabolism–secretion coupling in diabetic Goto–Kakizaki and ouabain-treated rat pancreatic islets. Diabetologia 2008, 51, 1226–1235. [Google Scholar] [CrossRef]
- Mukai, E.; Fujimoto, S.; Sato, H.; Oneyama, C.; Kominato, R.; Sato, Y.; Sasaki, M.; Nishi, Y.; Okada, M.; Inagaki, N. Exendin-4 suppresses SRC activation and reactive oxygen species production in diabetic Goto-Kakizaki rat islets in an Epac-dependent manner. Diabetes 2011, 60, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Ingley, E. Src family kinases: Regulation of their activities, levels and identification of new pathways. Biochim. Biophys. Acta 2008, 1784, 56–65. [Google Scholar] [CrossRef]
- Jha, V.; Macchia, M.; Tuccinardi, T.; Poli, G. Three-dimensional interactions analysis of the anticancer target c-Src kinase with its inhibitors. Cancers 2020, 12, 2327. [Google Scholar] [CrossRef]
- Harris, K.F.; Shoji, I.; Cooper, E.M.; Kumar, S.; Oda, H.; Howley, P.M. Ubiquitin-mediated degradation of active Src tyrosine kinase. Proc. Natl. Acad. Sci. USA 1999, 96, 13738–13743. [Google Scholar] [CrossRef]
- MacKay, C.E.; Knock, G.A. Control of vascular smooth muscle function by Src-family kinases and reactive oxygen species in health and disease. J. Physiol. 2015, 593, 3815–3828. [Google Scholar] [CrossRef]
- Cattaneo, F.; Guerra, G.; Parisi, M.; De Marinis, M.; Tafuri, D.; Cinelli, M.; Ammendola, R. Cell-surface receptors transactivation mediated by G protein-coupled receptors. Int. J. Mol. Sci. 2014, 15, 19700–19728. [Google Scholar] [CrossRef]
- Rozengurt, E. Mitogenic signaling pathways induced by G protein-coupled receptors. J. Cell. Physiol. 2007, 213, 589–602. [Google Scholar] [CrossRef]
- Buteau, J.; Foisy, S.; Joly, E.; Prentki, M. Glucagon-like peptide 1 induces pancreatic β-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes 2003, 52, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. The biology of incretin hormones. Cell Metab. 2006, 3, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef] [PubMed]
- Seino, S.; Takahashi, H.; Fujimoto, W.; Shibasaki, T. Roles of cAMP signalling in insulin granule exocytosis. Diabetes Obes. Metab. 2009, 11, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J.; Nauck, M.A. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006, 368, 1696–1705. [Google Scholar] [CrossRef]
- Buse, J.B.; Rosenstock, J.; Sesti, G.; Schmidt, W.E.; Montanya, E.; Brett, J.H.; Zychma, M.; Blonde, L.; LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: A 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009, 374, 39–47. [Google Scholar] [CrossRef]
- Kaku, K.; Rasmussen, M.F.; Clauson, P.; Seino, Y. Improved glycaemic control with minimal hypoglycaemia and no weight change with the once-daily human glucagon-like peptide-1 analogue liraglutide as add-on to sulphonylurea in Japanese patients with type 2 diabetes. Diabetes Obes. Metab. 2010, 12, 341–347. [Google Scholar] [CrossRef]
- Mukai, E.; Ishida, H.; Kato, S.; Tsuura, Y.; Fujimoto, S.; Ishida-Takahashi, A.; Horie, M.; Tsuda, K.; Seino, Y. Metabolic inhibition impairs ATP-sensitive K+ channel block by sulfonylurea in pancreatic β-cells. Am. J. Physiol. 1998, 274, E38–E44. [Google Scholar] [CrossRef]
- Ihara, Y.; Toyokuni, S.; Uchida, K.; Odaka, H.; Tanaka, T.; Ikeda, H.; Hiai, H.; Seino, Y.; Yamada, Y. Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats, a model of type 2 diabetes. Diabetes 1999, 48, 927–932. [Google Scholar] [CrossRef]
- Boland, B.B.; Rhodes, C.J.; Grimsby, J.S. The dynamic plasticity of insulin production in β-cells. Mol. Metab. 2017, 6, 958–973. [Google Scholar] [CrossRef]
- Sasaki, M.; Fujimoto, S.; Sato, Y.; Nishi, Y.; Mukai, E.; Yamano, G.; Sato, H.; Tahara, Y.; Ogura, K.; Nagashima, K.; et al. Reduction of reactive oxygen species ameliorates metabolism-secretion coupling in islets of diabetic GK rats by suppressing lactate overproduction. Diabetes 2013, 62, 1996–2003. [Google Scholar] [CrossRef] [PubMed]
- Kjørholt, C.; Akerfeldt, M.C.; Biden, T.J.; Laybutt, D.R. Chronic hyperglycemia, independent of plasma lipid levels, is sufficient for the loss of β-cell differentiation and secretory function in the db/db mouse model of diabetes. Diabetes 2005, 54, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Homo-Delarche, F.; Calderari, S.; Irminger, J.C.; Gangnerau, M.N.; Coulaud, J.; Rickenbach, K.; Dolz, M.; Halban, P.; Portha, B.; Serradas, P. Islet inflammation and fibrosis in a spontaneous model of type 2 diabetes, the GK rat. Diabetes 2006, 55, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Meshinchi, S.; Dias-Leme, C.; Raffin, D.; Johnson, J.D.; Treutelaar, M.K.; Burant, C.F. Islet microvasculature in islet hyperplasia and failure in a model of type 2 diabetes. Diabetes 2006, 55, 2965–2973. [Google Scholar] [CrossRef]
- Ainscow, E.K.; Zhao, C.; Rutter, G.A. Acute overexpression of lactate dehydrogenase-A perturbs β-cell mitochondrial metabolism and insulin secretion. Diabetes 2000, 49, 1149–1155. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J. Clin. Investig. 2013, 123, 3664–3671. [Google Scholar] [CrossRef]
- Courtnay, R.; Ngo, D.C.; Malik, N.; Ververis, K.; Tortorella, S.M.; Karagiannis, T.C. Cancer metabolism and the Warburg effect: The role of HIF-1 and PI3K. Mol. Biol. Rep. 2015, 42, 841–851. [Google Scholar] [CrossRef]
- Nagao, A.; Kobayashi, M.; Koyasu, S.; Chow, C.C.T.; Harada, H. HIF-1-dependent reprogramming of glucose metabolic pathway of cancer cells and its therapeutic significance. Int. J. Mol. Sci. 2019, 20, 238. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef]
- Movafagh, S.; Crook, S.; Vo, K. Regulation of hypoxia-inducible factor-1a by reactive oxygen species: New developments in an old debate. J. Cell. Biochem. 2015, 116, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Cantley, J.; Biden, T.J. Sweet and sour β-cells: ROS and Hif1α induce Warburg-like lactate production during type 2 diabetes. Diabetes 2013, 62, 1823–1825. [Google Scholar] [CrossRef] [PubMed]
- Zehetner, J.; Danzer, C.; Collins, S.; Eckhardt, K.; Gerber, P.A.; Ballschmieter, P.; Galvanovskis, J.; Shimomura, K.; Ashcroft, F.M.; Thorens, B.; et al. PVHL is a regulator of glucose metabolism and insulin secretion in pancreatic β cells. Genes Dev. 2008, 22, 3135–3146. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.; Cano, D.A.; Hebrok, M. A role for Von-Hippel Lindau protein in pancreatic β-cell function. Diabetes 2009, 58, 433–441. [Google Scholar] [CrossRef]
- Cantley, J.; Selman, C.; Shukla, D.; Abramov, A.Y.; Forstreuter, F.; Esteban, M.A.; Claret, M.; Lingard, S.J.; Clements, M.; Harten, S.K.; et al. Deletion of the von Hippel-Lindau gene in pancreatic β cells impairs glucose homeostasis in mice. J. Clin. Investig. 2009, 119, 125–135. [Google Scholar] [CrossRef]
- Cheng, K.; Ho, K.; Stokes, R.; Scott, C.; Lau, S.M.; Hawthorne, W.J.; O’Connell, P.J.; Loudovaris, T.; Kay, T.W.; Kulkarni, R.N.; et al. Hypoxia-inducible factor-1α regulates β cell function in mouse and human islets. J. Clin. Investig. 2010, 20, 2171–2183. [Google Scholar] [CrossRef]
- Gunton, J.E.; Kulkarni, R.N.; Yim, S.; Okada, T.; Hawthorne, W.J.; Tseng, Y.H.; Roberson, R.S.; Ricordi, C.; O’Connell, P.J.; Gonzalez, F.J.; et al. Loss of ARNT/HIF1β mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell 2005, 122, 337–349. [Google Scholar] [CrossRef]
- Moi, P.; Chan, K.; Asunis, I.; Cao, A.; Kan, Y.W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. USA 1994, 91, 9926–9930. [Google Scholar] [CrossRef]
- David, J.A.; Rifkin, W.J.; Rabbani, P.S.; Ceradini, D.J. The Nrf2/Keap1/ARE pathway and oxidative stress as a therapeutic target in type II diabetes mellitus. J. Diabetes Res. 2017, 2017, 4826724. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J. Biol. Chem. 2003, 278, 21592–21600. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Abramov, A.Y. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 2015, 88, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Katz, L.S.; Schulz, A.M.; Kim, M.; Honig, L.B.; Li, L.; Davenport, B.; Homann, D.; Garcia-Ocaña, A.; Herman, M.A.; et al. Activation of Nrf2 is required for normal and ChREBPα-augmented glucose-stimulated β-cell proliferation. Diabetes 2018, 67, 1561–1575. [Google Scholar] [CrossRef]
- Li, W.; Wu, W.; Song, H.; Wang, F.; Li, H.; Chen, L.; Lai, Y.; Janicki, J.S.; Ward, K.W.; Meyer, C.J.; et al. Targeting Nrf2 by dihydro-CDDO-trifluoroethyl amide enhances autophagic clearance and viability of β-cells in a setting of oxidative stress. FEBS Lett. 2014, 588, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Yagishita, Y.; Fukutomi, T.; Sugawara, A.; Kawamura, H.; Takahashi, T.; Pi, J.; Uruno, A.; Yamamoto, M. Nrf2 protects pancreatic β-cells from oxidative and nitrosative stress in diabetic model mice. Diabetes 2014, 63, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Schultheis, J.; Beckmann, D.; Mulac, D.; Müller, L.; Esselen, M.; Düfer, M. Nrf2 activation protects mouse beta cells from glucolipotoxicity by restoring mitochondrial function and physiological redox balance. Oxid. Med. Cell. Longev. 2019, 2019, 7518510. [Google Scholar] [CrossRef]
- Fu, J.; Woods, C.G.; Yehuda-Shnaidman, E.; Zhang, Q.; Wong, V.; Collins, S.; Sun, G.; Andersen, M.E.; Pi, J. Low-level arsenic impairs glucose-stimulated insulin secretion in pancreatic beta cells: Involvement of cellular adaptive response to oxidative stress. Environ. Health Perspect. 2010, 118, 864–870. [Google Scholar] [CrossRef]
- Subba, R.; Ahmad, M.H.; Ghosh, B.; Mondal, A.C. Targeting NRF2 in type 2 diabetes mellitus and depression: Efficacy of natural and synthetic compounds. Eur. J. Pharmacol. 2022, 925, 174993. [Google Scholar] [CrossRef]
- Robledinos-Antón, N.; Fernández-Ginés, R.; Manda, G.; Cuadrado, A. Activators and inhibitors of NRF2: A review of their potential for clinical development. Oxid. Med. Cell. Longev. 2019, 2019, 9372182. [Google Scholar] [CrossRef]
- Saha, P.K.; Reddy, V.T.; Konopleva, M.; Andreeff, M.; Chan, L. The triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic-acid methyl ester has potent anti-diabetic effects in diet-induced diabetic mice and Leprdb/db mice. J. Biol. Chem. 2010, 285, 40581–40592. [Google Scholar] [CrossRef] [PubMed]
- Uruno, A.; Furusawa, Y.; Yagishita, Y.; Fukutomi, T.; Muramatsu, H.; Negishi, T.; Sugawara, A.; Kensler, T.W.; Yamamoto, M. The Keap1-Nrf2 system prevents onset of diabetes mellitus. Mol. Cell. Biol. 2013, 33, 2996–3010. [Google Scholar] [CrossRef] [PubMed]
- Roma, L.P.; Jonas, J.C. Nutrient metabolism, subcellular redox state, and oxidative stress in pancreatic islets and β-cells. J. Mol. Biol. 2020, 432, 1461–1493. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).