The Glucose Sensitivity of Insulin Secretion-Lessons from In Vivo and In Vitro Studies in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Animal Disposition
2.3. Experiments
2.4. Assays
2.5. Data and Statistical Analysis
3. Results
3.1. Intravenous Glucose
3.2. Incubation of Islets
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Temple, R.C.; Carrington, C.A.; Luzio, S.D.; Owens, D.R.; Schneider, A.E.; Sobey, W.J.; Hales, C.N. Insulin deficiency in non-insulin-dependent diabetes. Lancet 1989, 1, 293–295. [Google Scholar] [CrossRef]
- Marshak, S.; Leibowitz, G.; Bertuzzi, F.; Socci, C.; Kaiser, N.; Gross, D.J.; Cerasi, E.; Melloul, D. Impaired ß-cell functions induced by chronic exposure of culture human pancreatic islets to high glucose. Diabetes 1999, 48, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Porte, D., Jr.; Kahn, S.E. ß-Cell dysfunction and failure in type 2 diabetes. Potential mechanisms. Diabetes 2001, 50, S160–S163. [Google Scholar] [CrossRef]
- Marchetti, P.; Dotta, F.; Lauro, D.; Purrello, F. An overview of pancreatic beta-cell defects in human type 2 diabetes: Implications for treatment. Regul. Pept. 2008, 146, 4–11. [Google Scholar] [CrossRef]
- Henquin, J.C. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 2000, 49, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Bratanova-Tochkova, T.; Cheng, H.; Daniel, S.; Gunawardana, S.; Liu, Y.J.; Mulvaney-Musa, J.; Schermerhorn, T.; Straub, S.G.; Yajima, H.; Sharp, G.W.G. Triggering and augmentation mechanisms, granule pools, and biphasic insulin secretion. Diabetes 2002, 51, S83–S90. [Google Scholar] [CrossRef]
- Matschinsky, F.M. Glucokinase as glucose sensor and metabolic signal generator in pancreatic β–cells and hepatocytes. Diabetes 1990, 39, 647–652. [Google Scholar] [CrossRef]
- Kalwat, M.A.; Cobb, M.H. Mechanisms of the amplifying pathway of insulin in the ß cell. Pharmacol. Ther. 2017, 179, 17–30. [Google Scholar] [CrossRef]
- Stozer, A.; Leitgeb, E.P.; Pohorec, V.; Dolensek, J.; Bombek, L.K.; Gosak, M.; Klemen, M.S. The role of cAMP in beta cell stimulus-secretion and intercellular coupling. Cells 2021, 10, 1658. [Google Scholar] [CrossRef]
- Freckmann, G.; Hagenlocher, S.; Baumstark, A.; Jendrike, N.; Gillen, R.C.; Rössner, K.; Haug, C. Continuous glucose profiles in healthy subjects under everyday life conditions and after different meals. J. Diabetes Sci. Technol. 2007, 1, 695–703. [Google Scholar] [CrossRef]
- Ahrén, B. Diurnal variation in circulating leptin is dependent on gender, food intake and circulating insulin in mice. Acta Physiol. Scand. 2000, 169, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Anello, M.; Gilon, P.; Henquin, J.C. Alterations of insulin secretion from mouse islets treated with sulphonylureas: Perturbations of Ca2+ regulation prevail over changes in insulin content. Brit. J. Pharmacol. 1999, 127, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Henquin, J.C.; Nenquin, M.; Stiernet, P.; Ahrén, B. In vivo and in vitro glucose-induced biphasic insulin secretion in the mouse: Pattern and role of cytoplasmic Ca2+ and amplification signals in ß-cells. Diabetes 2006, 55, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Alcazar, O.; Buchwald, P. Concentration-dependency and time profile of insulin secretion: Dynamic perifusion studies with human and murine islets. Front. Endocrinol. 2019, 10, 680. [Google Scholar] [CrossRef] [PubMed]
- Reimer, M.K.; Holst, J.J.; Ahrén, B. Long-term inhibition of dipeptidyl peptidase IV improves glucose tolerance and preserves islet function in mice. Eur. J. Endocrinol. 2002, 146, 717–727. [Google Scholar] [CrossRef]
- Pingitore, A.; Ruz-Maldonado, I.; Liu, B.; Huang, G.C.; Choudhary, P.; Persaud, S.J. Dynamic profiling of insulin secretion and ATP generation in isolated human and mouse islets reveals differential glucose sensitivity. Cell Physiol. Biochem. 2017, 44, 1352–1359. [Google Scholar] [CrossRef]
- Tura, A.; Pacini, G.; Yamada, Y.; Seino, Y.; Ahrén, B. Glucagon and insulin secretion, insulin clearance, and fasting glucose in GIP receptor and GLP-1 receptor knockout mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 316, R27–R37. [Google Scholar] [CrossRef]
- Ahrén, B.; Yamada, Y.; Seino, Y. The insulin response to oral glucose in GIP and GLP-1 receptor knockout mice: Review of the literature and stepwise glucose dose response studies in female mice. Front. Endocrinol. 2021, 12, 665537. [Google Scholar] [CrossRef]
- Ovlund, T.; Pacini, G.; Ahrén, B. Impact of incretin hormone receptors on insulin-independent glucose disposal in model experiments in mice. Front. Endocrinol. 2021, 12, 680153. [Google Scholar] [CrossRef]
- Pound, L.D.; Oeser, J.K.; O´Brien, P.; Wang, Y.; Faulman, C.J.; Dadi, P.K.; Jacobson, D.A.; Hutton, J.C.; McGuinness, O.P.; Shiota, M.; et al. G6PC2: A negative regulator of basal glucose-stimulated insulin secretion. Diabetes 2013, 62, 1547–1556. [Google Scholar] [CrossRef]
- Ritzel, R.A.; Veldhuis, J.D.; Butler, P.C. Glucose stimulates pulsatile insulin secretion from human pancreatic islets by increasing secretory burst mass: Dose-response relationships. J. Clin. Endocrinol. Metab. 2003, 88, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Persson, K.; Gingerich, R.L.; Nayak, S.; Wada, K.; Wada, E.; Ahrén, B. Reduced GLP-1 and insulin responses and glucose intolerance after gastric glucose in GRP receptor-deleted mice. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E956–E962. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ahrén, B. GIP secretion after oral macronutrient ingestion—The human literature revisited and a systematic study in model experiments in mice. J. Diabetes Investig. 2022; in press. [Google Scholar] [CrossRef]
- Glendinning, J.I.; Stano, S.; Holter, M.; Azenkot, T.; Goldman, O.; Margolskee, R.F.; Vasseli, J.R.; Sclafani, A. Sugar-induced cephalic-phase insulin release is mediated by a T1R2+T1r3-independent taste transduction pathway in mice. Am. J. Physiol. Regul. Integr. Comb. Physiol. 2015, 309, R552–R560. [Google Scholar] [CrossRef] [PubMed]
- Pacini, G.; Ahrén, B. Glucagon-like peptide-1 and glucose-dependent insulinotropic peptide: Effects alone and in combination on insulin secretion and glucose disappearance in mice. Physiol. Rep. 2017, 5, e13280. [Google Scholar] [CrossRef]
- Omar, B.; Ahlkvist, L.; Yamada, Y.; Seino, Y.; Ahrén, B. Incretin hormone receptors are required for normal beta cell development and function in female mice. Peptides 2016, 79, 58–65. [Google Scholar] [CrossRef]
- Ahrén, B. Glucagon-like peptide-1 and beta cell glucose sensitivity—A glucose ramp study in mice. Peptides 2021, 146, 170650. [Google Scholar] [CrossRef]
- Ahrén, B.; Pacini, G. Glucose effectiveness. Lessons from studies on insulin-independent glucose clearance in mice. J. Diabetes Investig. 2021, 12, 675–685. [Google Scholar] [CrossRef]
- Ahrén, B.; Pacini, G. A novel approach to assess insulin sensitivity reveals no increased insulin sensitivity in mice with a dominant-negative mutant hepatocyte nuclear factor-1α. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R131–R137. [Google Scholar] [CrossRef]
- Noonan, W.T.; Banks, R.O. Renal function and glucose transport in male and female mice with diet-induced type II diabetes mellitus. Proc. Soc. Exp. Biol. Med. 2000, 225, 221–230. [Google Scholar] [CrossRef]
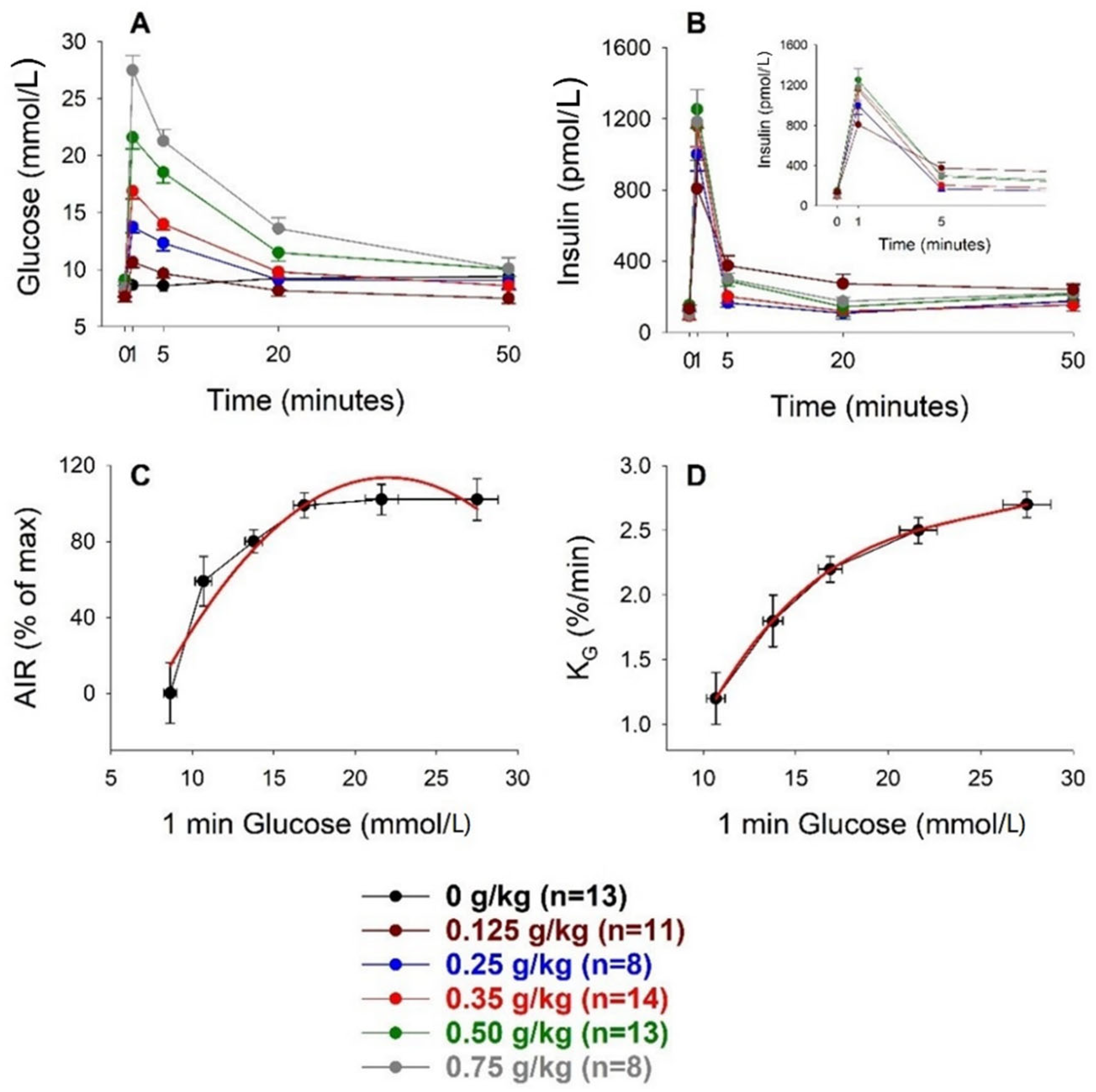
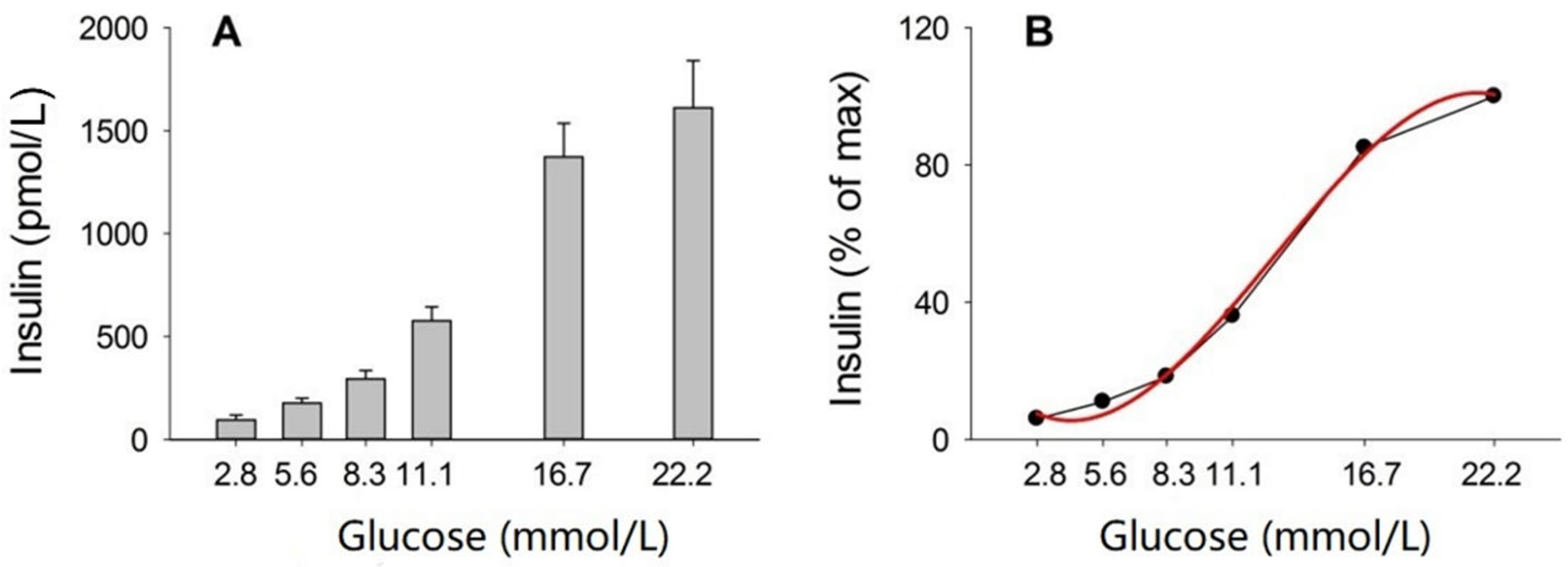
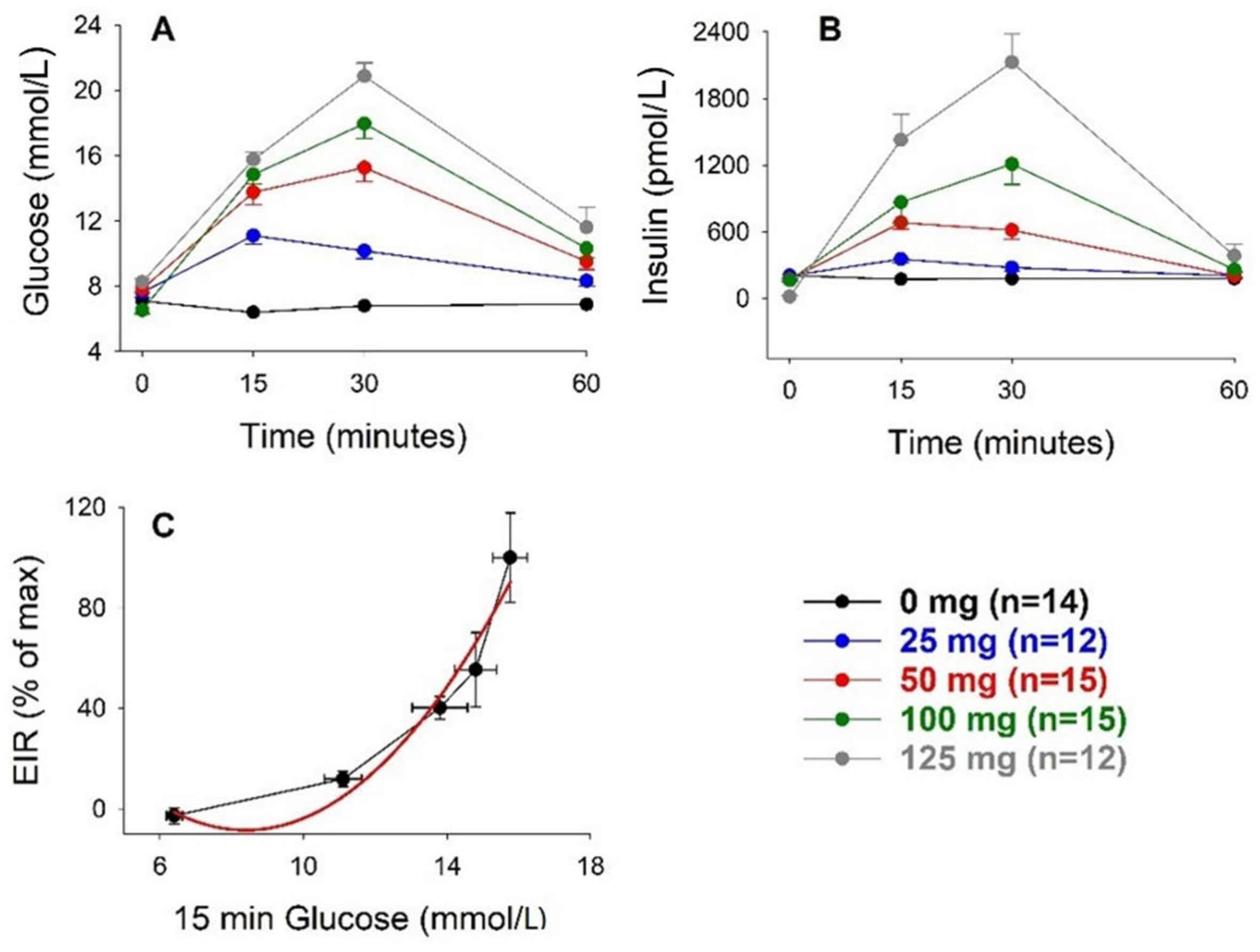
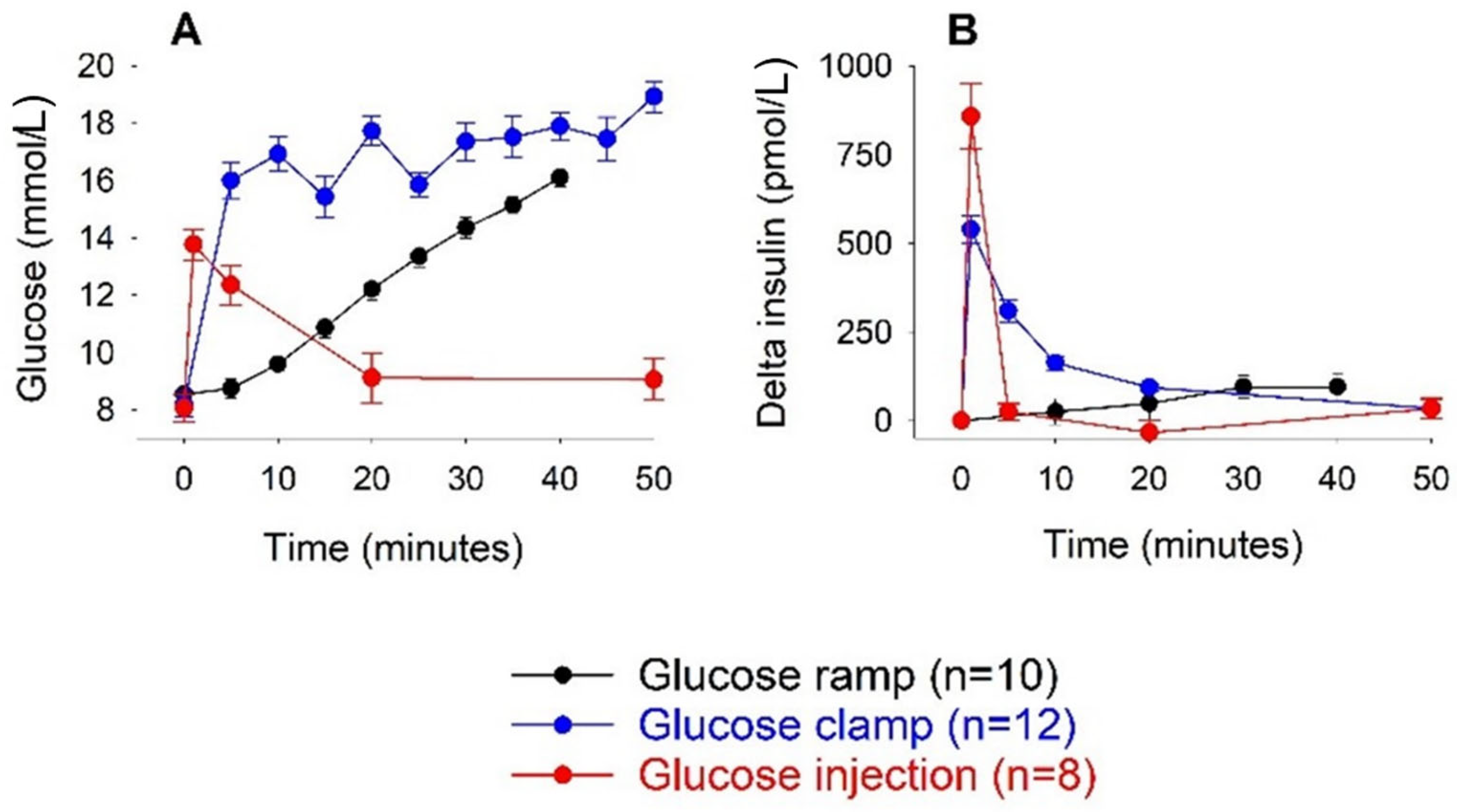

| Glucose Dose (g/kg) | n | Body Weight (g) | AIR (pmol/L) | AUCglucose (mmol/L min) | AUCinsulin (nmol/L min) | KG (%/min) |
|---|---|---|---|---|---|---|
| 0 | 13 | 21.7 ± 0.5 | −62 ± 8 | 18 ± 43 | −0.5 ± 1.1 | NR |
| 0.125 | 11 | 21.9 ± 0.6 | 326 ± 45 | 41 ± 16 | 4.3 ± 1.6 | 1.2 ± 0.2 |
| 0.25 | 8 | 21.7 ± 0.8 | 441 ± 50 | 99 ± 26 | 5.5 ± 1.8 | 1.8 ± 0.2 |
| 0.35 | 14 | 22.0 ± 0.8 | 588 ± 53 | 141 ± 14 | 8.4 ± 1.1 | 2.2 ± 0.1 |
| 0.50 | 13 | 22.1 ± 0.7 | 619 ± 64 | 200 ± 21 | 8.4 ± 2.2 | 2.5 ± 0.1 |
| 0.75 | 8 | 20.4 ± 0.2 | 616 ± 92 | 336 ± 29 | 11.0 ± 1.5 | 2.7 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahrén, B. The Glucose Sensitivity of Insulin Secretion-Lessons from In Vivo and In Vitro Studies in Mice. Biomolecules 2022, 12, 976. https://doi.org/10.3390/biom12070976
Ahrén B. The Glucose Sensitivity of Insulin Secretion-Lessons from In Vivo and In Vitro Studies in Mice. Biomolecules. 2022; 12(7):976. https://doi.org/10.3390/biom12070976
Chicago/Turabian StyleAhrén, Bo. 2022. "The Glucose Sensitivity of Insulin Secretion-Lessons from In Vivo and In Vitro Studies in Mice" Biomolecules 12, no. 7: 976. https://doi.org/10.3390/biom12070976
APA StyleAhrén, B. (2022). The Glucose Sensitivity of Insulin Secretion-Lessons from In Vivo and In Vitro Studies in Mice. Biomolecules, 12(7), 976. https://doi.org/10.3390/biom12070976





