N-Acetylcysteine, an ROS Inhibitor, Alleviates the Pathophysiology of Hyperthyroidism-Induced Cardiomyopathy via the ROS/Ca2+ Pathway
Abstract
:1. Introduction
2. Results
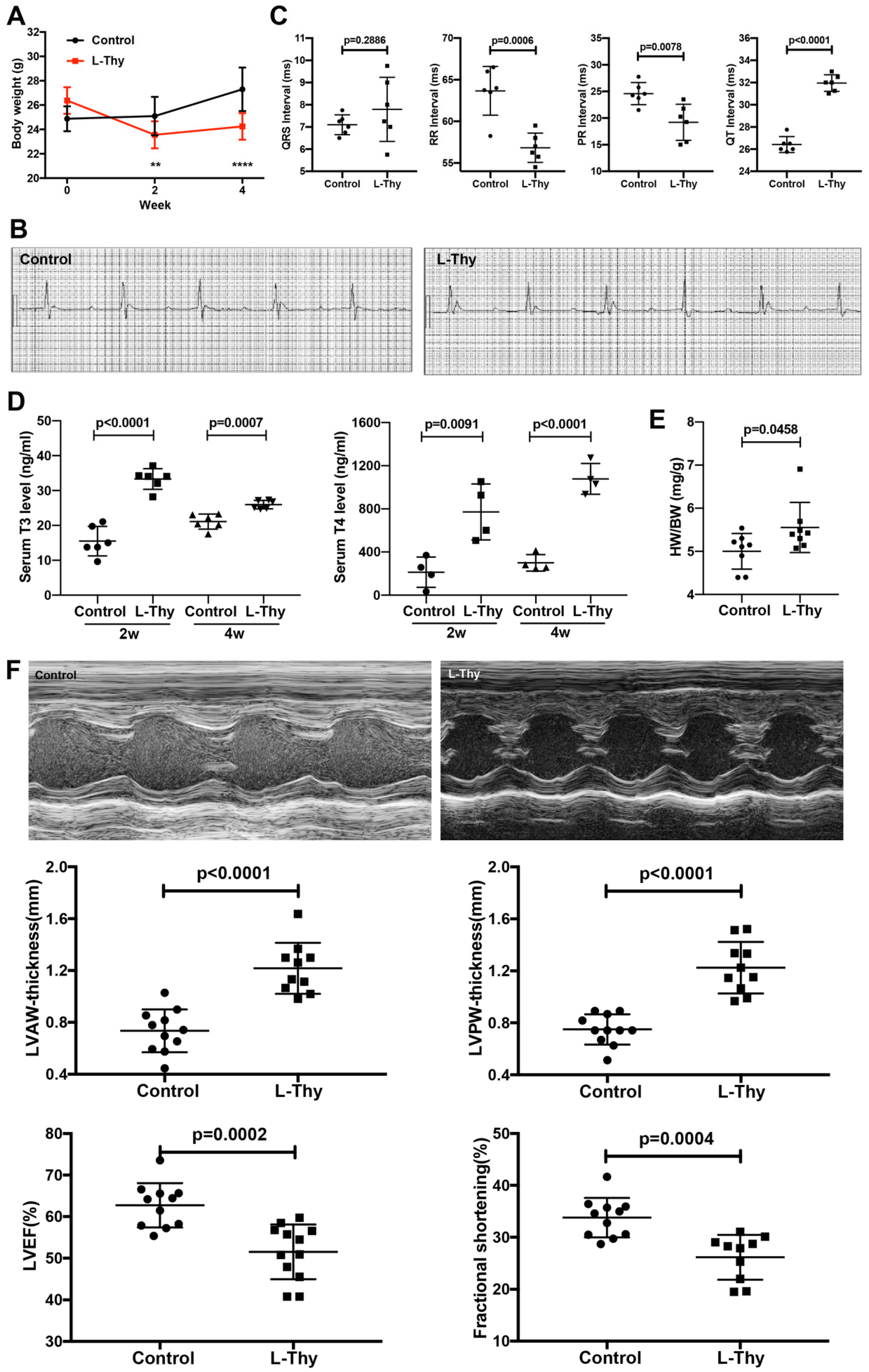
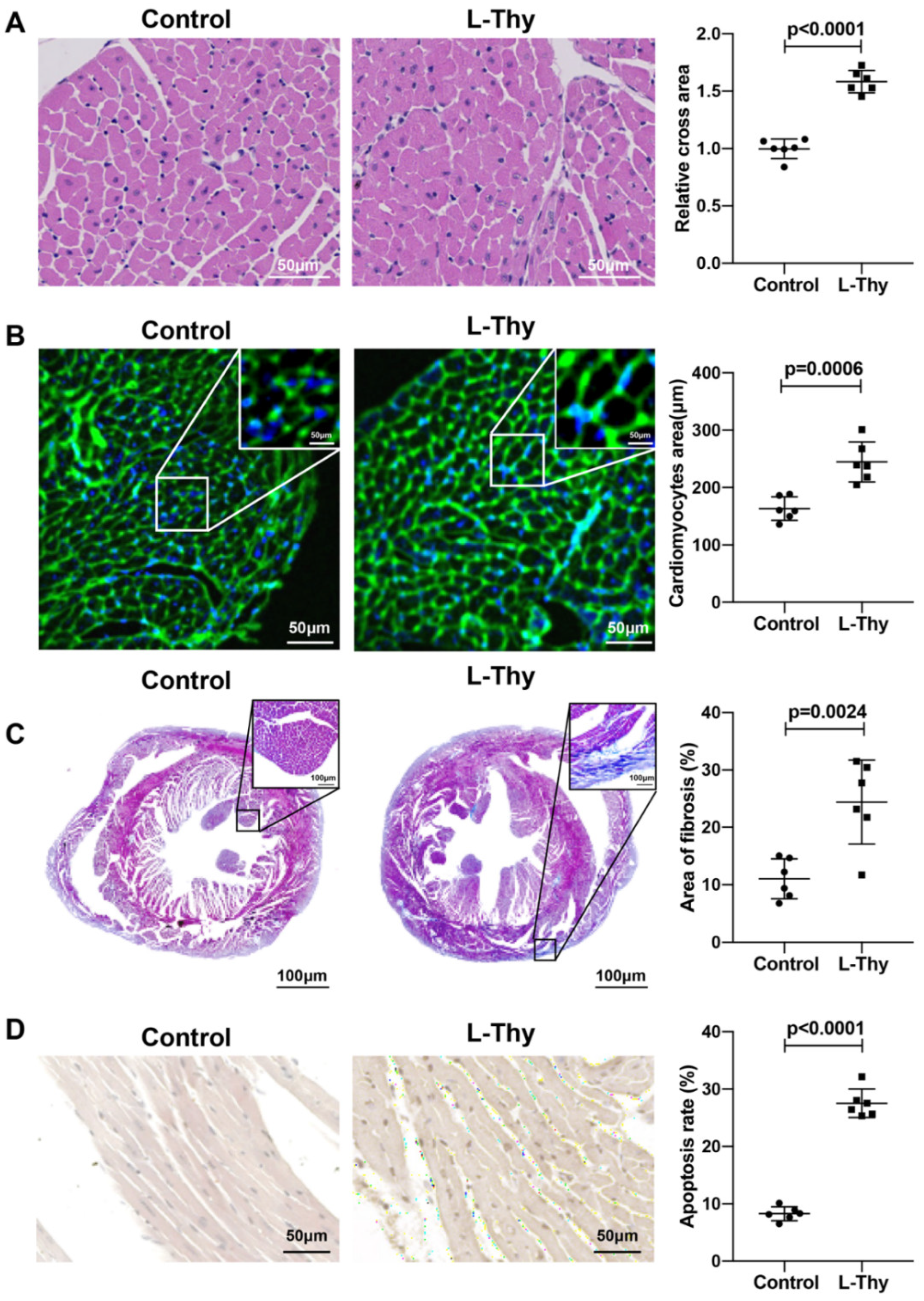
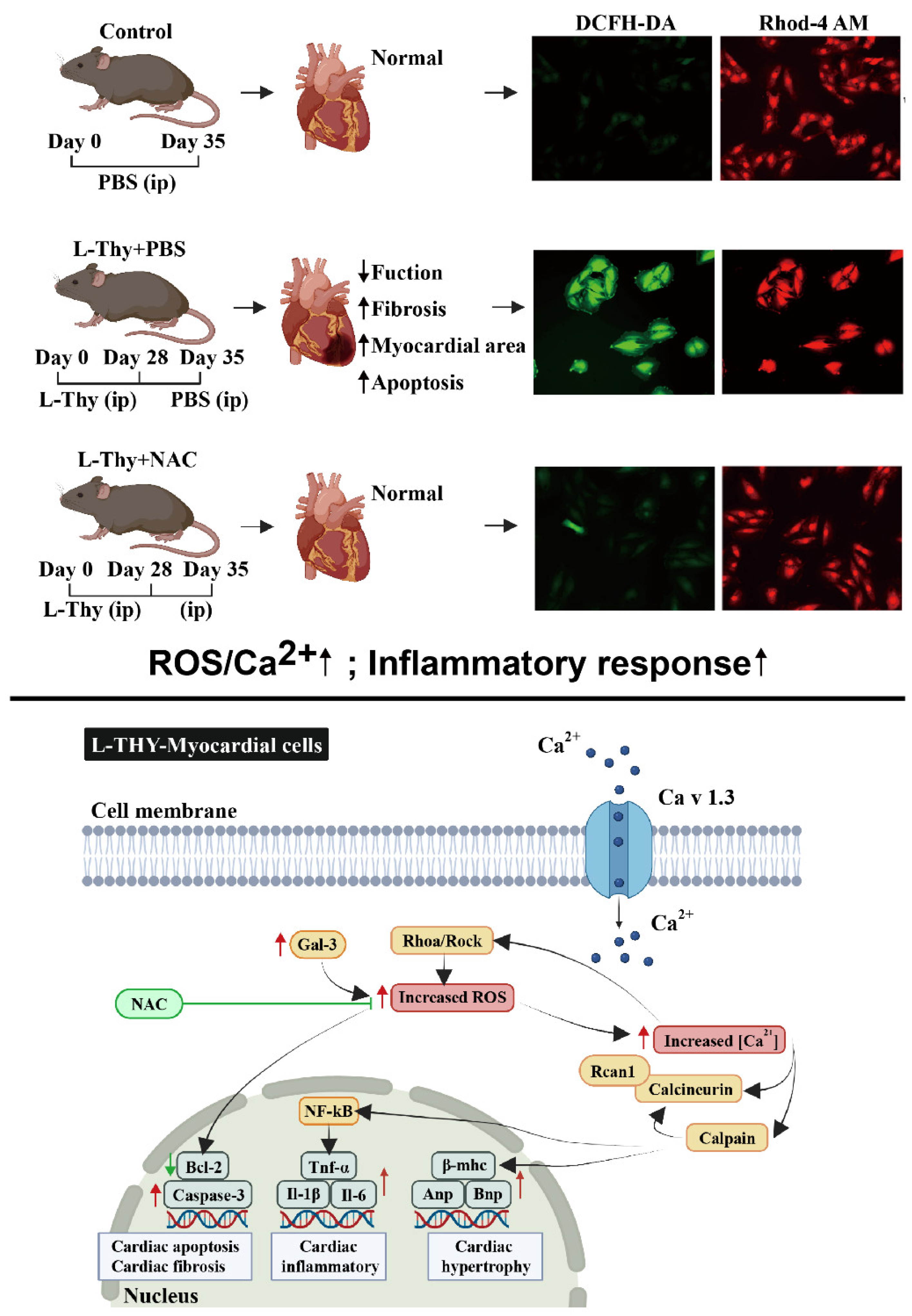
2.1. High L-Thy Induced the Occurrence of Cardiac Structural and Functional Changes in Mice
2.2. Increased ROS and Inflammatory Gene Expression Was Higher in the Hyperthyroidism-Induced Cardiomyopathy Group
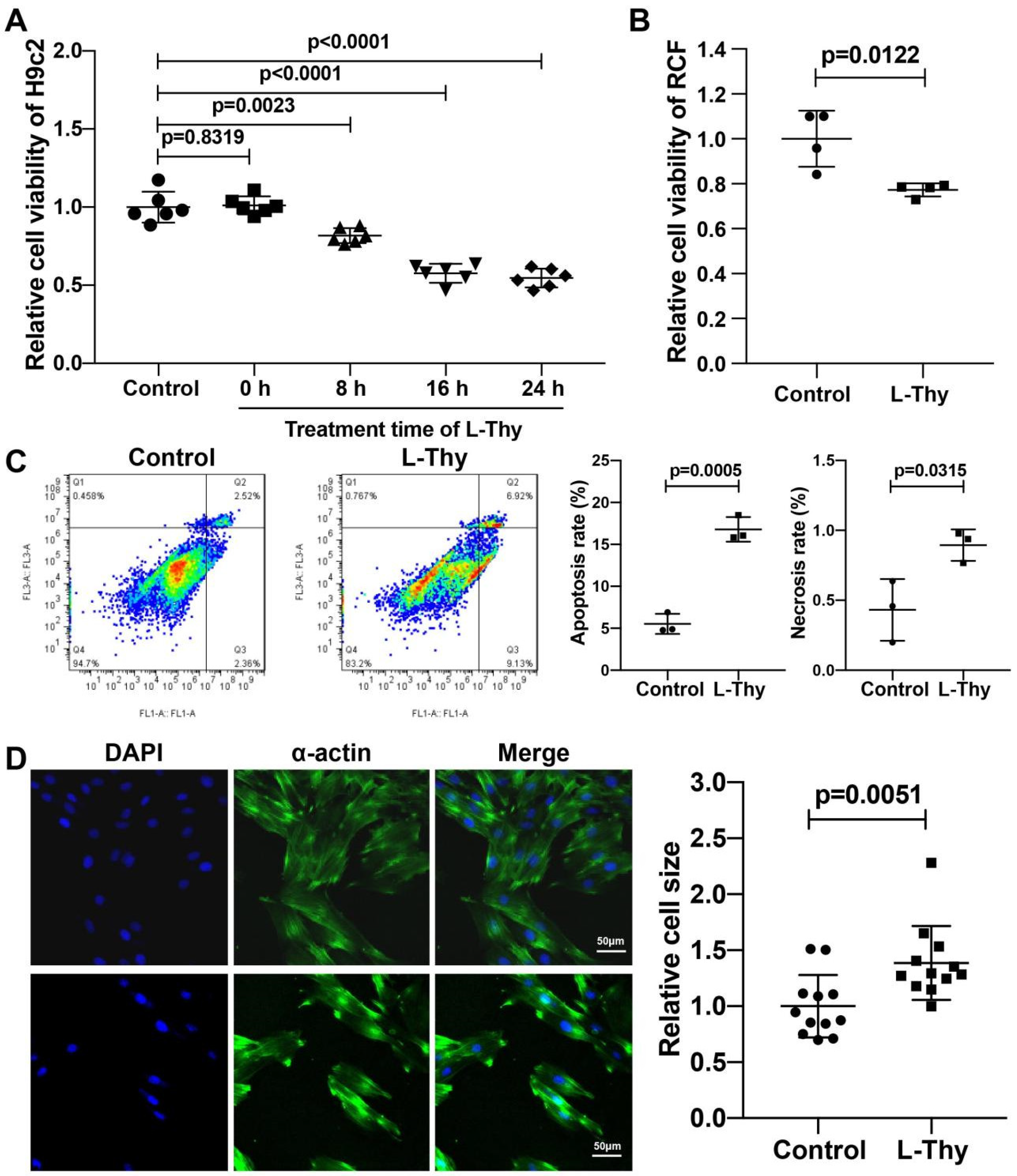
2.3. Hyperthyroidism-Induced Cardiomyopathy Cell Model for Mechanistic Investigation
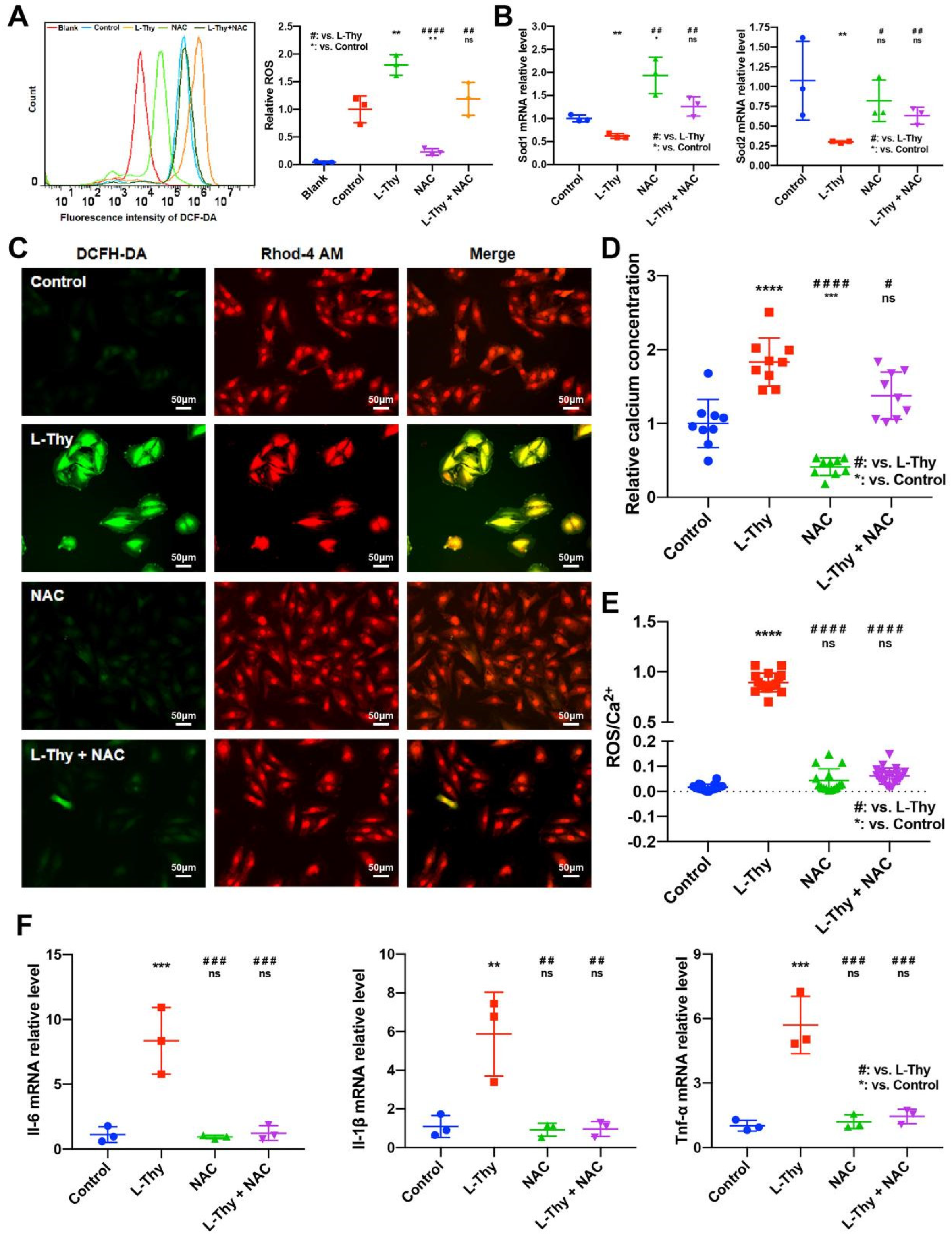
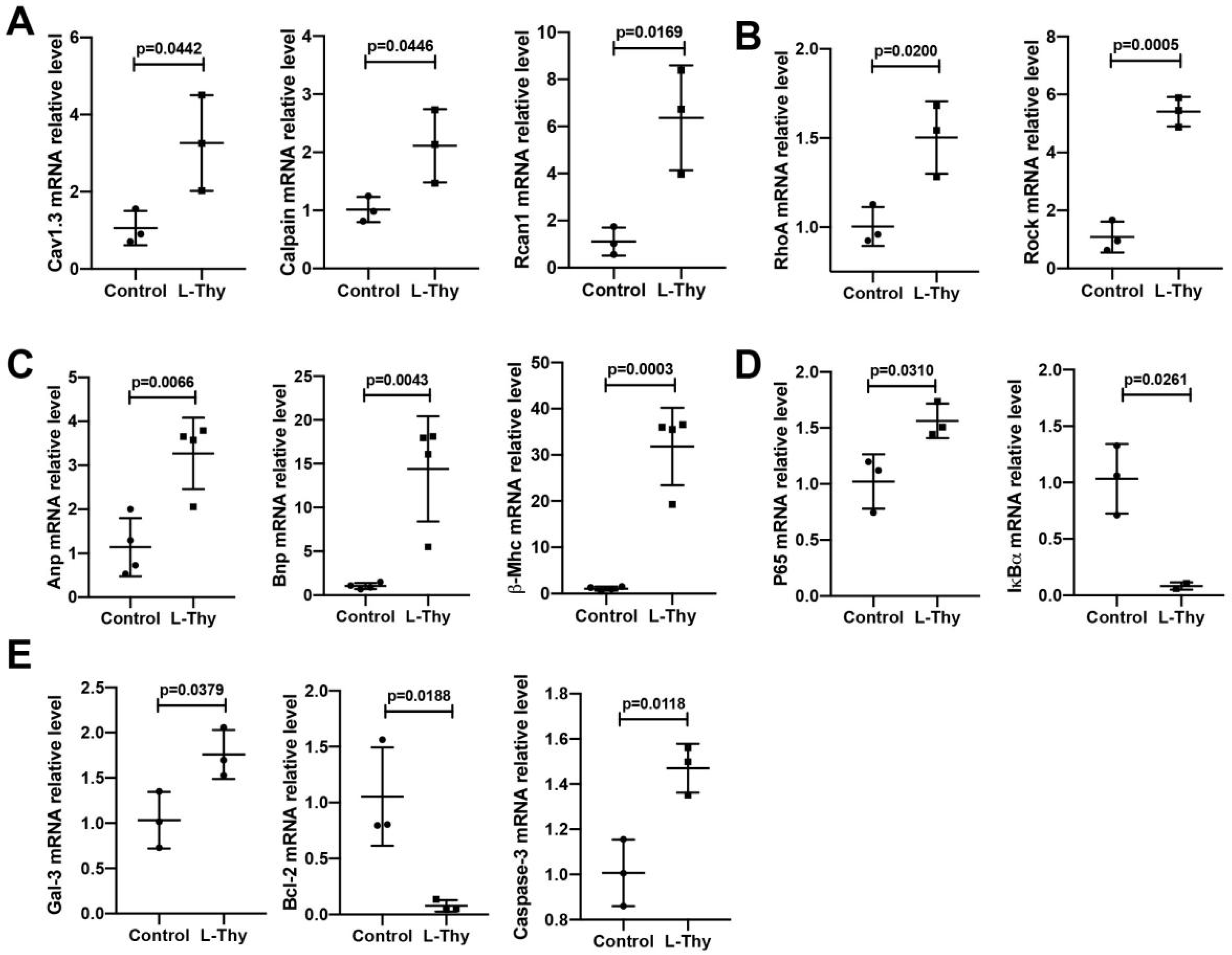
2.4. ROS/Ca2+ Pathway and Inflammatory Gene Expression in a Hyperthyroidism-Induced Cardiomyopathy Cell Model, Consistent with the Performance of the Animal Model
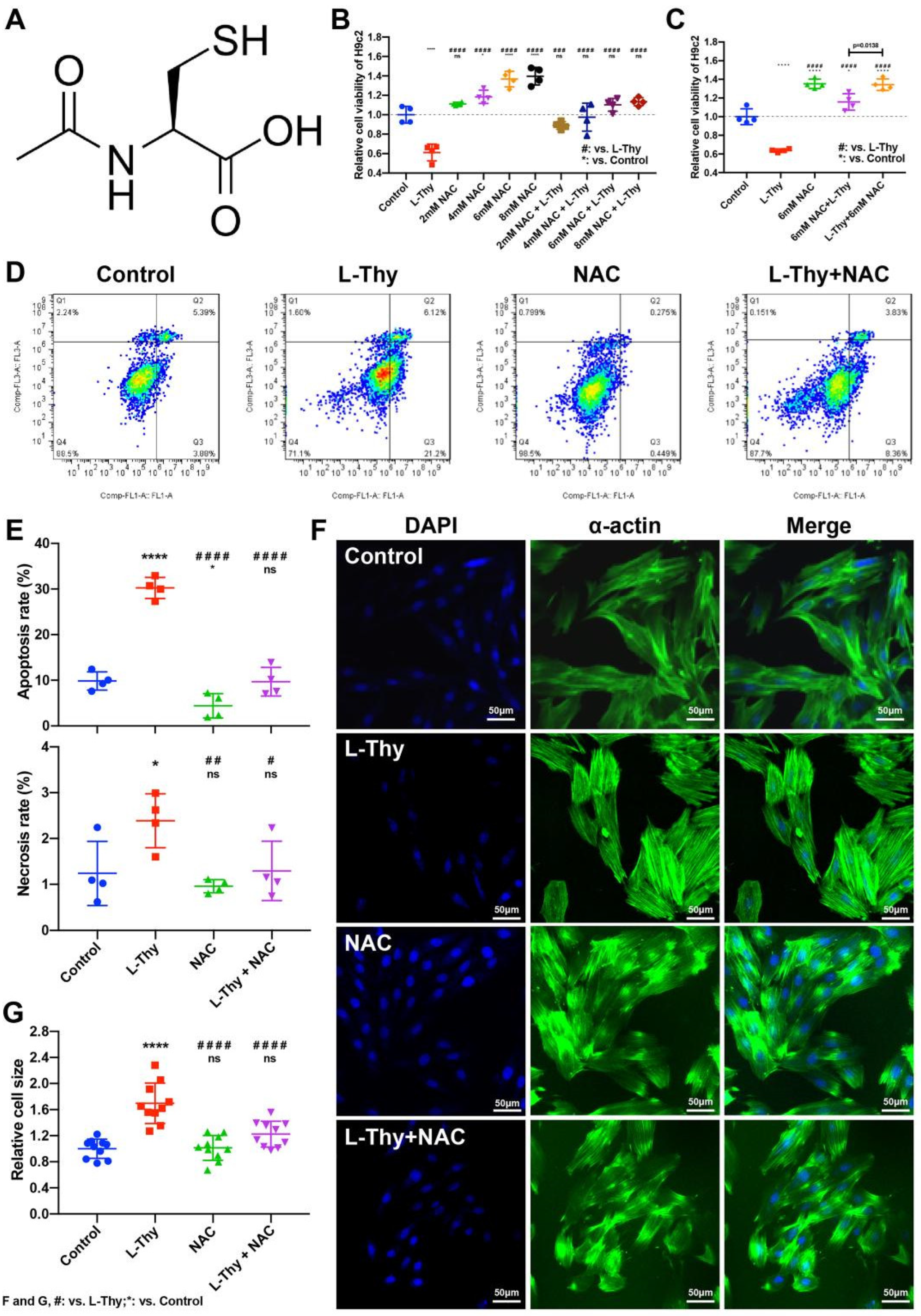
2.5. NAC Could Relieve L-Thy-Induced Myocardial Hypertrophy and the Cardiac ROS/Ca2+ Pathway
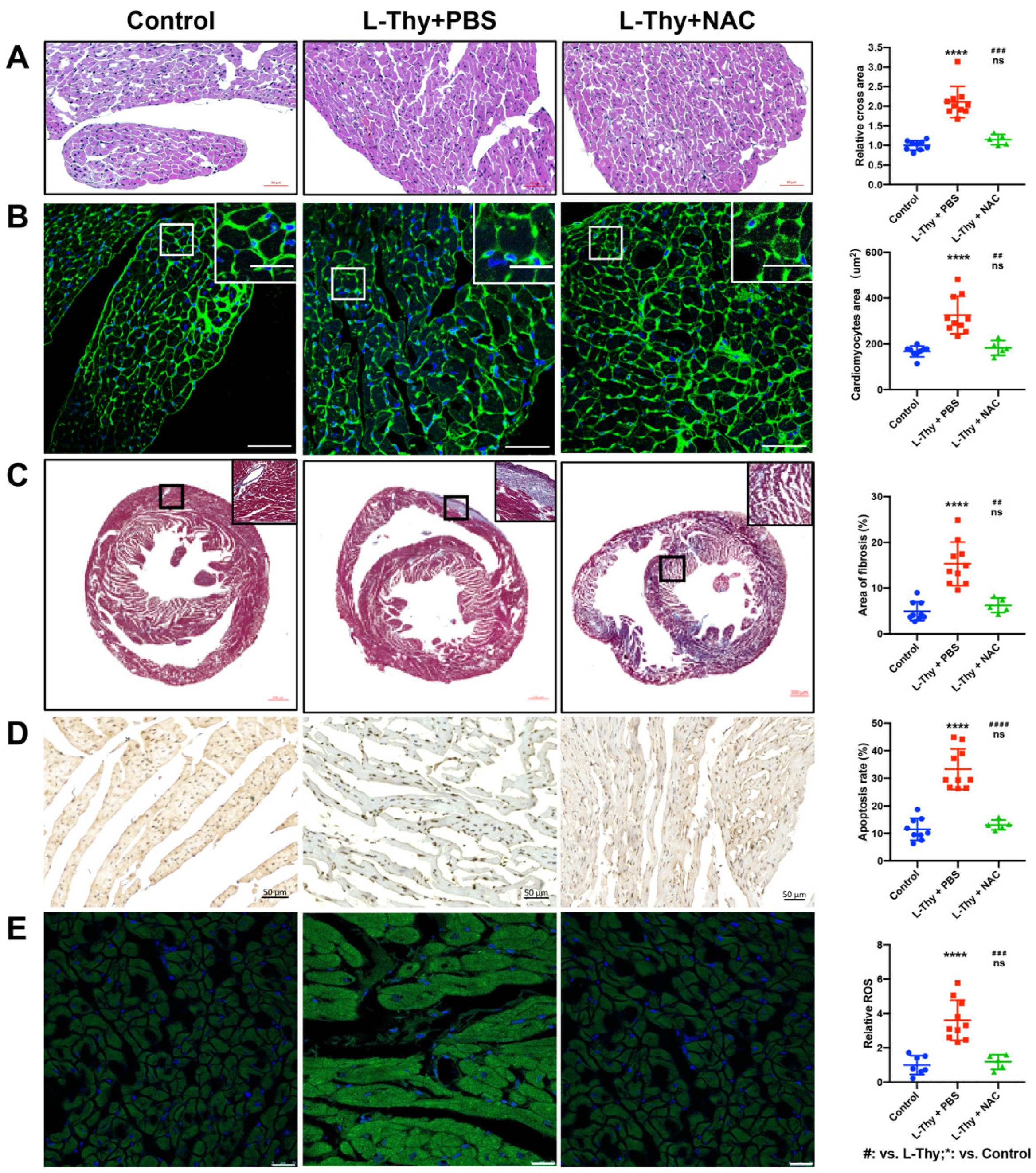
2.6. NAC Attenuated the Phenotype of Hyperthyroidism-Induced Cardiomyopathy In Vivo
3. Discussion
4. Conclusions
5. Methods
5.1. Cell Culture
5.2. Hyperthyroid Cardiomyopathy Model Induction
5.3. Experimental Grouping
5.4. Haematoxylin-Eosin (HE) and Masson Staining
5.5. TUNEL Assay
5.6. Cell Counting Kit-8 (CCK-8) Assay
5.7. WGA, FITC-Phalloidin, DCFH-DA, and Rhod-4 Staining
5.8. Flow Cytometry
5.9. Quantitative Real Time-Polymerase Chain Reaction (qRT-PCR)
5.10. Western Blot (WB)
5.11. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamakawa, H.; Kato, T.S.; Noh, J.Y.; Yuasa, S.; Kawamura, A.; Fukuda, K.; Aizawa, Y. Thyroid Hormone Plays an Important Role in Cardiac Function: From Bench to Bedside. Front. Physiol. 2021, 12, 606931. [Google Scholar] [CrossRef] [PubMed]
- Danzi, S.; Klein, I. Thyroid disease and the cardiovascular system. Endocrinol. Metab. Clin. N. Am. 2014, 43, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Elnakish, M.T.; Ahmed, A.A.; Mohler, P.J.; Janssen, P.M. Role of Oxidative Stress in Thyroid Hormone-Induced Cardiomyocyte Hypertrophy and Associated Cardiac Dysfunction: An Undisclosed Story. Oxid. Med. Cell. Longev. 2015, 2015, 854265. [Google Scholar] [CrossRef] [PubMed]
- Klein, I.; Danzi, S. Thyroid disease and the heart. Circulation 2007, 116, 1725–1735. [Google Scholar] [CrossRef]
- Khan, R.; Sikanderkhel, S.; Gui, J.; Adeniyi, A.R.; O’Dell, K.; Erickson, M.; Malpartida, J.; Mufti, Z.; Khan, T.; Mufti, H.; et al. Thyroid and Cardiovascular Disease: A Focused Review on the Impact of Hyperthyroidism in Heart Failure. Cardiol. Res. 2020, 11, 68–75. [Google Scholar] [CrossRef]
- Klein, I.; Ojamaa, K. Thyroid hormone and the cardiovascular system. N. Engl. J. Med. 2001, 344, 501–509. [Google Scholar] [CrossRef]
- Cooper, D.S.; Biondi, B. Subclinical thyroid disease. Lancet 2012, 379, 1142–1154. [Google Scholar] [CrossRef]
- Madreiter-Sokolowski, C.T.; Thomas, C.; Ristow, M. Interrelation between ROS and Ca2+ in aging and age-related diseases. Redox Biol. 2020, 36, 101678. [Google Scholar] [CrossRef]
- Bertero, E.; Maack, C. Calcium Signaling and Reactive Oxygen Species in Mitochondria. Circ. Res. 2018, 122, 1460–1478. [Google Scholar] [CrossRef]
- Li, S.; Ma, Y.; Ye, S.; Tang, S.; Liang, N.; Liang, Y.; Xiao, F. Polystyrene microplastics trigger hepatocyte apoptosis and abnormal glycolytic flux via ROS-driven calcium overload. J. Hazard. Mater. 2021, 417, 126025. [Google Scholar] [CrossRef]
- Rushworth, G.F.; Megson, I.L. Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 2014, 141, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Wang, F.T.; Hsuuw, Y.D.; Huang, F.J.; Chan, W.H. Non-embryotoxic dosage of alternariol aggravates ochratoxin A-triggered deleterious effects on embryonic development through ROS-dependent apoptotic processes. Toxicol. Res. 2021, 10, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.X.; Zhang, C.J.; Li, Y.Z.; Sun, Y.M. Effect of beta-casomorphin-7 on myocardial hypertrophy in hyperthyroidism-induced cardiomyopathy. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6380–6389. [Google Scholar] [PubMed]
- Liu, M.; Li, Z.; Liang, B.; Li, L.; Liu, S.; Tan, W.; Long, J.; Tang, F.; Chu, C.; Yang, J. Hydrogen sulfide ameliorates rat myocardial fibrosis induced by thyroxine through PI3K/AKT signaling pathway. Endocr. J. 2018, 65, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.K.; Guo, J.L.; Xu, S.X.; Han, Y.W.; Lee, S.C.; Zhao, J.Z. Hexavalent chromium induces centrosome amplification through ROS-ATF6-PLK4 pathway in colon cancer cells. Cell Biol. Int. 2022, 46, 1128–1136. [Google Scholar] [CrossRef]
- Torrano, A.A.; Blechinger, J.; Osseforth, C.; Argyo, C.; Reller, A.; Bein, T.; Michaelis, J.; Brauchle, C. A fast analysis method to quantify nanoparticle uptake on a single cell level. Nanomedicine 2013, 8, 1815–1828. [Google Scholar] [CrossRef]
- Sirenko, O.; Hancock, M.K.; Hesley, J.; Hong, D.; Cohen, A.; Gentry, J.; Carlson, C.B.; Mann, D.A. Phenotypic Characterization of Toxic Compound Effects on Liver Spheroids Derived from iPSC Using Confocal Imaging and Three-Dimensional Image Analysis. Assay Drug Dev. Technol. 2016, 14, 381–394. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, S.A.; Li, W.; Ma, Y.; Shen, J.; Wang, Y.; Shen, Y.; Chen, J.; Ji, Y.; Xie, Y.; et al. Piezo1-Mediated Mechanotransduction Promotes Cardiac Hypertrophy by Impairing Calcium Homeostasis to Activate Calpain/Calcineurin Signaling. Hypertension 2021, 78, 647–660. [Google Scholar] [CrossRef]
- Kochman, J.; Jakubczyk, K.; Bargiel, P.; Janda-Milczarek, K. The Influence of Oxidative Stress on Thyroid Diseases. Antioxidants 2021, 10, 1442. [Google Scholar] [CrossRef]
- Razvi, S.; Jabbar, A.; Pingitore, A.; Danzi, S.; Biondi, B.; Klein, I.; Peeters, R.; Zaman, A.; Iervasi, G. Thyroid Hormones and Cardiovascular Function and Diseases. J. Am. Coll. Cardiol. 2018, 71, 1781–1796. [Google Scholar] [CrossRef]
- Xun, Y.; Zhou, P.; Yang, Y.; Li, C.; Zhang, J.; Hu, H.; Qin, B.; Zhang, Z.; Wang, Q.; Lu, Y.; et al. Role of Nox4 in High Calcium-Induced Renal Oxidative Stress Damage and Crystal Deposition. Antioxid. Redox Signal. 2022, 36, 15–38. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.Y.; Dong, Y.; Huang, X.J.; Murtaza, G.; Huang, Y.J.; Sarfaraz Iqbal, M. Anshen Buxin Liuwei Pill, a Mongolian Medicinal Formula, Could Protect H2O2-Induced H9c2 Myocardial Cell Injury by Suppressing Apoptosis, Calcium Channel Activation, and Oxidative Stress. Evid. Based Complement. Alternat. Med. 2022, 2022, 5023654. [Google Scholar] [CrossRef] [PubMed]
- Uthman, L.; Li, X.; Baartscheer, A.; Schumacher, C.A.; Baumgart, P.; Hermanides, J.; Preckel, B.; Hollmann, M.W.; Coronel, R.; Zuurbier, C.J.; et al. Empagliflozin reduces oxidative stress through inhibition of the novel inflammation/NHE/[Na+]c/ROS-pathway in human endothelial cells. Biomed. Pharmacother. 2022, 146, 112515. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, D.; Ward, J.P.T.; Yarova, P.L.; Janssen, L.J.; Lee, T.H.; Ying, S.; Corrigan, C.J. Topical therapy with negative allosteric modulators of the calcium-sensing receptor (calcilytics) for the management of asthma: The beginning of a new era? Eur. Respir. J. 2022, 60, 2102103. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.Q.; Bai, S.Z.; Sun, F.Q.; Wu, R.; Wei, C.; Wen, X.; Xi, Y.X.; Hao, J.H.; Zaid, A.; Li, H.Z. SKF38393 prevents high glucose (HG)-induced endothelial dysfunction by inhibiting the effects of HG on cystathionine gamma-lyase/hydrogen sulfide activity and via a RhoA/ROCK1 pathway. Front. Biosci. 2022, 27, 49. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Kim, N.K.; Seo, S.R. Cynanchi atrati and Its Phenolic Constituent Sinapic Acid Target Regulator of Calcineurin 1 (RCAN1) to Control Skin Inflammation. Antioxidants 2022, 11, 205. [Google Scholar] [CrossRef] [PubMed]
- Frey, N.; McKinsey, T.A.; Olson, E.N. Decoding calcium signals involved in cardiac growth and function. Nat. Med. 2000, 6, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Wettschureck, N.; Rutten, H.; Zywietz, A.; Gehring, D.; Wilkie, T.M.; Chen, J.; Chien, K.R.; Offermanns, S. Absence of pressure overload induced myocardial hypertrophy after conditional inactivation of Galphaq/Galpha11 in cardiomyocytes. Nat. Med. 2001, 7, 1236–1240. [Google Scholar] [CrossRef]
- Ren, Z.; Liang, W.; Sheng, J.; Xun, C.; Xu, T.; Cao, R.; Sheng, W. Gal-3 is a potential biomarker for spinal cord injury and Gal-3 deficiency attenuates neuroinflammation through ROS/TXNIP/NLRP3 signaling pathway. Biosci. Rep. 2019, 39, BSR20192368. [Google Scholar] [CrossRef]
- Barman, S.A.; Bordan, Z.; Batori, R.; Haigh, S.; Fulton, D.J.R. Galectin-3 Promotes ROS, Inflammation, and Vascular Fibrosis in Pulmonary Arterial Hypertension. Adv. Exp. Med. Biol. 2021, 1303, 13–32. [Google Scholar]
- Li, Y.; Yang, L.; Hou, Y.; Zhang, Z.; Chen, M.; Wang, M.; Liu, J.; Wang, J.; Zhao, Z.; Xie, C.; et al. Polydopamine-mediated graphene oxide and nanohydroxyapatite-incorporated conductive scaffold with an immunomodulatory ability accelerates periodontal bone regeneration in diabetes. Bioact. Mater. 2022, 18, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Shi, Y.; Dai, Y.; Wang, F.; Chen, X.; Li, X. Baicalin clears inflammation by enhancing macrophage efferocytosis via inhibition of RhoA/ROCK signaling pathway and regulating macrophage polarization. Int. Immunopharmacol. 2022, 105, 108532. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.J.; Hyon, J.Y.; Lee, H.W.; Han, E.H.; Kim, Y.; Cha, Y.S.; Ha, S.K. Glycolaldehyde, an Advanced Glycation End Products Precursor, Induces Apoptosis via ROS-Mediated Mitochondrial Dysfunction in Renal Mesangial Cells. Antioxidants 2022, 11, 934. [Google Scholar] [CrossRef]
- Wu, H.Y.; Yang, F.L.; Li, L.H.; Rao, Y.K.; Ju, T.C.; Wong, W.T.; Hsieh, C.Y.; Pivkin, M.V.; Hua, K.F.; Wu, S.H. Ergosterol peroxide from marine fungus Phoma sp. induces ROS-dependent apoptosis and autophagy in human lung adenocarcinoma cells. Sci. Rep. 2018, 8, 17956. [Google Scholar] [CrossRef] [PubMed]
- Clozel, J.P.; Danchin, N.; Genton, P.; Thomas, J.L.; Cherrier, F. Effects of propranolol and of verapamil on heart rate and blood pressure in hyperthyroidism. Clin. Pharmacol. Ther. 1984, 36, 64–69. [Google Scholar] [CrossRef]
- Subahi, A.; Ibrahim, W.; Abugroun, A. Diltiazem-Associated Cardiogenic Shock in Thyrotoxic Crisis. Am. J. Ther. 2018, 25, e666–e669. [Google Scholar] [CrossRef] [PubMed]
- Brandt, F.; Green, A.; Hegedus, L.; Brix, T.H. A critical review and meta-analysis of the association between overt hyperthyroidism and mortality. Eur. J. Endocrinol. 2011, 165, 491–497. [Google Scholar] [CrossRef] [Green Version]



| Primer | Forward/Reverse Primer (5′-3′) |
|---|---|
| H9c2-Il-6-F | CCAGCCAGTTGCCTTCTTG |
| H9c2-Il-6-R | CAATCAGAATTGCCATTGCAC |
| H9c2-Il-1β-F | ACAGTGCATCATCGCTGTTC |
| H9c2-Il-1β-R | GAAGAATCTATACCTGTCCT |
| H9c2-Tnf-α-F | CTTTTCCATCTTCTTCTTTG |
| H9c2-Tnf-α-R | TGCTTGTTCCTCAGCCTCTT |
| H9c2-Sod1-F | TGGGCTACAGGCTTGTCACT |
| H9c2-Sod1-R | AACCAGTTGTGGTGTCAGGA |
| H9c2-Sod2-F | CTCCTGAGAGTGAGATCACA |
| H9c2-Sod2-R | TTCTGGACAAACCTGAGCCCTAA |
| H9c2-β-actin-F | GAACCTTGGACTCCCACAGACAC |
| H9c2-β-actin-R | CCCTAAGGCCAACCGTGAAAAG |
| H9c2-Cav1.3-F | TTGGTACGGACGGCTCTCA- |
| H9c2-Cav1.3-R | CCCCACGGTTACCTCATCAT |
| H9c2-Calpain-F | CAAAGTGGACCCCTATGAACG |
| H9c2-Calpain-R | TAAGGGCGTCAGGTGTAAGGT |
| H9c2-Rcan1-F | CTTCAGCAACCCCCTGTC |
| H9c2-Rcan1-R | ACTGGGGTAGCGTCTTCT |
| H9c2-RhoA-F | TCGGAATGATGAGCACACAA |
| H9c2-RhoA-R | GCTTCACAAGATGAGGCAC |
| H9c2-Rock-F | GTGATGGCTATTATGGACG |
| H9c2-Rock-R | AGGAAGGCACAAATGAGAT |
| H9c2-Anp-F | GATCTGCCCTCTTGAAAAGC |
| H9c2-Anp-R | CCAGGAGGGTATTCACCAC |
| H9c2-Bnp-F | CACGATGCAGAAGCTGCTGG |
| H9c2-Bnp-R | ACAACCTCTGCCCGTCACA |
| H9c2-β-Mhc-F | GACAGGAAGAACCTACTGCG |
| H9c2-β-Mhc-R | CTCCAGGTCTCAGGGCTTCAC |
| H9c2-p65-F | AGAGCAACGATTCCACCAA |
| H9c2-p65-R | GCAGTCTTTTCCCACCAGC |
| H9c2-IκBα-F | CACTCCATCCTGAAGGCTACCAA |
| H9c2-IκBα-R | AAGGGCAGTCCGGCCATTA |
| H9c2-Gal-3-F | AGCCCAACGCAAACAGTATC |
| H9c2-Gal-3-R | GGCTTCAACCAGGACCTGTA |
| H9c2-Bcl-2-F | GCCTTCTTTGAGTTCGGTG |
| H9c2-Bcl-2-R | GAAATCAAACAGAGGTCGC |
| H9c2-Caspase-3-F | GGTATTGAGACAGACAGTGG |
| H9c2-Caspase-3-R | CATGGGATCTGTTTCTTTGC |
| Mice-Il-6-F | TACGTACATGGCTGGGGTGT |
| Mice-Il-6-R | TAGTCCTTCCTACCCCAATTTCC |
| Mice-Il-1β-F | TTGGTCCTTAGCCACTCCTTC |
| Mice-Il-1β-R | AGGCAAACCGTGAAAAGATG |
| Mice-Tnf-α-F | ATCTTTTGGGGTCCGTCAACT |
| Mice-Tnf-α-R | CCTCACACTCAGATCATCTTCT |
| Mice-Sod1-F | GCTACGACGTGGGCTACAG |
| Mice-Sod1-R | GAGACCTGGGCAATGTGACT |
| Mice-Sod2-F | AACTCAGGTCGCTCTTCAGC |
| Mice-Sod2-R | TTGTTTCTCATGGACCACCA |
| Mice-β-actin-F | GCTTGATAGCCTCCAGCAAC |
| Mice-β-actin-R | AGGCAAACCGTGAAAAGATG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, M.; Hua, X.; Mo, H.; Sun, Z.; Xu, B.; Chen, X.; Xu, M.; Xu, X.; Song, J. N-Acetylcysteine, an ROS Inhibitor, Alleviates the Pathophysiology of Hyperthyroidism-Induced Cardiomyopathy via the ROS/Ca2+ Pathway. Biomolecules 2022, 12, 1195. https://doi.org/10.3390/biom12091195
Bao M, Hua X, Mo H, Sun Z, Xu B, Chen X, Xu M, Xu X, Song J. N-Acetylcysteine, an ROS Inhibitor, Alleviates the Pathophysiology of Hyperthyroidism-Induced Cardiomyopathy via the ROS/Ca2+ Pathway. Biomolecules. 2022; 12(9):1195. https://doi.org/10.3390/biom12091195
Chicago/Turabian StyleBao, Mengni, Xiumeng Hua, Han Mo, Zhe Sun, Bo Xu, Xiao Chen, Mengda Xu, Xinjie Xu, and Jiangping Song. 2022. "N-Acetylcysteine, an ROS Inhibitor, Alleviates the Pathophysiology of Hyperthyroidism-Induced Cardiomyopathy via the ROS/Ca2+ Pathway" Biomolecules 12, no. 9: 1195. https://doi.org/10.3390/biom12091195
APA StyleBao, M., Hua, X., Mo, H., Sun, Z., Xu, B., Chen, X., Xu, M., Xu, X., & Song, J. (2022). N-Acetylcysteine, an ROS Inhibitor, Alleviates the Pathophysiology of Hyperthyroidism-Induced Cardiomyopathy via the ROS/Ca2+ Pathway. Biomolecules, 12(9), 1195. https://doi.org/10.3390/biom12091195






