Head-to-Head Comparison of Different Blood Collecting Tubes for Quantification of Alzheimer’s Disease Biomarkers in Plasma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Blood Collection
2.3. Quantification of AD Biomarkers
2.4. Statistical Analyses
3. Results
3.1. Demographic and Clinical Characteristics
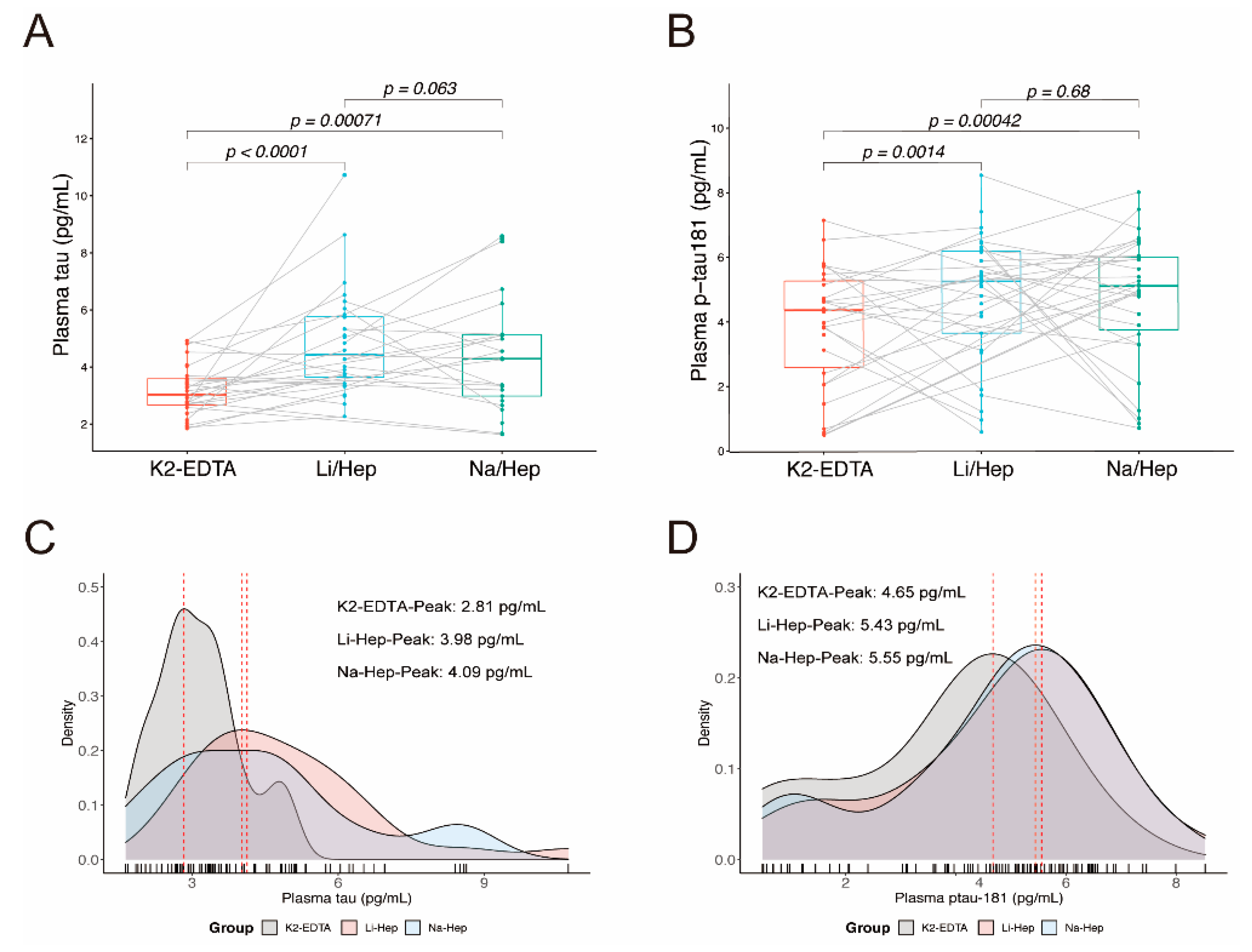
3.2. Blood Collection Methods Affect the Results of Plasma Tau
3.3. Blood Collection Methods Do Not Affect the Results of Plasma Aβ
3.4. Blood Collection Methods Does Not Affect the Difference in Biomarkers between Sexes
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hansson, O. Biomarkers for neurodegenerative diseases. Nat. Med. 2021, 27, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Teunissen, C.E.; Zetterberg, H.; Allue, J.A.; Sarasa, L.; Eichenlaub, U.; Bittner, T.; Ovod, V.; Verberk, I.M.W.; Toba, K.; et al. Head-to-Head Comparison of 8 Plasma Amyloid-beta 42/40 Assays in Alzheimer Disease. JAMA Neurol. 2021, 78, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Mattsson, N.; Palmqvist, S.; Smith, R.; Beach, T.G.; Serrano, G.E.; Chai, X.; Proctor, N.K.; Eichenlaub, U.; Zetterberg, H.; et al. Plasma P-tau181 in Alzheimer’s disease: Relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat. Med. 2020, 26, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, E.H.; La Joie, R.; Wolf, A.; Strom, A.; Wang, P.; Iaccarino, L.; Bourakova, V.; Cobigo, Y.; Heuer, H.; Spina, S.; et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat. Med. 2020, 26, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Mielke, M.M.; Hagen, C.E.; Xu, J.; Chai, X.; Vemuri, P.; Lowe, V.J.; Airey, D.C.; Knopman, D.S.; Roberts, R.O.; Machulda, M.M.; et al. Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement 2018, 14, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Lantero Rodriguez, J.; Karikari, T.K.; Suarez-Calvet, M.; Troakes, C.; King, A.; Emersic, A.; Aarsland, D.; Hye, A.; Zetterberg, H.; Blennow, K.; et al. Plasma p-tau181 accurately predicts Alzheimer’s disease pathology at least 8 years prior to post-mortem and improves the clinical characterisation of cognitive decline. Acta Neuropathol. 2020, 140, 267–278. [Google Scholar] [CrossRef]
- Meng, J.; Lei, P. Plasma pTau181 as a biomarker for Alzheimer’s disease. MedComm 2020, 1, 74–76. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, S.; Jiang, L.; Wang, L.; Li, T.; Lei, P. Ultrasensitive assays for detection of plasma tau and phosphorylated tau 181 in Alzheimer’s disease: A systematic review and meta-analysis. Transl. Neurodegener. 2021, 10, 10. [Google Scholar] [CrossRef]
- Meng, Q.H.; Krahn, J. Lithium heparinised blood-collection tubes give falsely low albumin results with an automated bromcresol green method in haemodialysis patients. Clin. Chem. Lab. Med. 2008, 46, 396–400. [Google Scholar] [CrossRef]
- Cerón, J.J.; Martínez-Subiela, S.; Hennemann, C.; Tecles, F. The effects of different anticoagulants on routine canine plasma biochemistry. Vet. J. 2004, 167, 294–301. [Google Scholar] [CrossRef]
- Lan, H.; Du, W.; Mo, Z.; Huang, H. The Influence of Blood Collection Tubes on Measurement of Cardiac Biomarkers. Clin. Lab. 2016, 62, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Smets, E.M.; Dijkstra-Lagemaat, J.E.; Blankenstein, M.A. Influence of blood collection in plastic vs. glass evacuated serum-separator tubes on hormone and tumour marker levels. Clin. Chem. Lab. Med. 2004, 42, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Banfi, G.; Salvagno, G.L.; Lippi, G. The role of ethylenediamine tetraacetic acid (EDTA) as in vitro anticoagulant for diagnostic purposes. Clin. Chem. Lab. Med. 2007, 45, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Abrahams, J.P.; Skinner, R.; Petitou, M.; Pike, R.N.; Carrell, R.W. The anticoagulant activation of antithrombin by heparin. Proc Natl Acad Sci USA 1997, 94, 14683–14688. [Google Scholar] [CrossRef] [PubMed]
- Jafri, L.; Khan, A.H.; Azeem, S. Ionized calcium measurement in serum and plasma by ion selective electrodes: Comparison of measured and calculated parameters. Indian J. Clin. Biochem. 2014, 29, 327–332. [Google Scholar] [CrossRef]
- Tami, C.; Puig, M.; Reepmeyer, J.C.; Ye, H.; D’Avignon, D.A.; Buhse, L.; Verthelyi, D. Inhibition of Taq polymerase as a method for screening heparin for oversulfated contaminants. Biomaterials 2008, 29, 4808–4814. [Google Scholar] [CrossRef]
- Park, J.C.; Han, S.H.; Yi, D.; Byun, M.S.; Lee, J.H.; Jang, S.; Ko, K.; Jeon, S.Y.; Lee, Y.S.; Kim, Y.K.; et al. Plasma tau/amyloid-beta1-42 ratio predicts brain tau deposition and neurodegeneration in Alzheimer’s disease. Brain 2019, 142, 771–786. [Google Scholar] [CrossRef]
- Bergman, L.; Zetterberg, H.; Kaihola, H.; Hagberg, H.; Blennow, K.; Akerud, H. Blood-based cerebral biomarkers in preeclampsia: Plasma concentrations of NfL, tau, S100B and NSE during pregnancy in women who later develop preeclampsia—A nested case control study. PLoS ONE 2018, 13, e0196025. [Google Scholar] [CrossRef]
- Pase, M.P.; Beiser, A.S.; Himali, J.J.; Satizabal, C.L.; Aparicio, H.J.; DeCarli, C.; Chene, G.; Dufouil, C.; Seshadri, S. Assessment of Plasma Total Tau Level as a Predictive Biomarker for Dementia and Related Endophenotypes. JAMA Neurol. 2019, 76, 598–606. [Google Scholar] [CrossRef]
- Kovacs, G.G.; Andreasson, U.; Liman, V.; Regelsberger, G.; Lutz, M.I.; Danics, K.; Keller, E.; Zetterberg, H.; Blennow, K. Plasma and cerebrospinal fluid tau and neurofilament concentrations in rapidly progressive neurological syndromes: A neuropathology-based cohort. Eur. J. Neurol. 2017, 24, e1326–e1377. [Google Scholar] [CrossRef] [Green Version]
- Alosco, M.L.; Tripodis, Y.; Jarnagin, J.; Baugh, C.M.; Martin, B.; Chaisson, C.E.; Estochen, N.; Song, L.; Cantu, R.C.; Jeromin, A.; et al. Repetitive head impact exposure and later-life plasma total tau in former National Football League players. Alzheimers Dement 2017, 7, 33–40. [Google Scholar] [CrossRef]
- Rissin, D.M.; Kan, C.W.; Campbell, T.G.; Howes, S.C.; Fournier, D.R.; Song, L.; Piech, T.; Patel, P.P.; Chang, L.; Rivnak, A.J.; et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol. 2010, 28, 595–599. [Google Scholar] [CrossRef]
- Verberk, I.M.W.; Misdorp, E.O.; Koelewijn, J.; Ball, A.J.; Blennow, K.; Dage, J.L.; Fandos, N.; Hansson, O.; Hirtz, C.; Janelidze, S.; et al. Characterization of pre-analytical sample handling effects on a panel of Alzheimer’s disease-related blood-based biomarkers: Results from the Standardization of Alzheimer’s Blood Biomarkers (SABB) working group. Alzheimers Dement 2022, 18, 1484–1497. [Google Scholar] [CrossRef] [PubMed]
- Lippa, S.M.; Yeh, P.H.; Gill, J.; French, L.M.; Brickell, T.A.; Lange, R.T. Plasma Tau and Amyloid Are Not Reliably Related to Injury Characteristics, Neuropsychological Performance, or White Matter Integrity in Service Members with a History of Traumatic Brain Injury. J. Neurotrauma 2019, 36, 2190–2199. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.L.; Tuo, Q.Z.; Lei, P. An Introduction to Ultrasensitive Assays for Plasma Tau Detection. J. Alzheimers Dis. 2021, 80, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Rojas, C.; Cabezas-Opazo, F.; Deaton, C.A.; Vergara, E.H.; Johnson, G.V.W.; Quintanilla, R.A. It’s all about tau. Prog. Neurobiol. 2019, 175, 54–76. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Jakes, R.; Spillantini, M.G.; Hasegawa, M.; Smith, M.J.; Crowther, R.A. Assembly of microtubule-associated protein tau into Alzheimer-like filaments induced by sulphated glycosaminoglycans. Nature 1996, 383, 550–553. [Google Scholar] [CrossRef]
- Chirita, C.N.; Kuret, J. Evidence for an Intermediate in Tau Filament Formation. Biochemistry 2004, 43, 1704–1714. [Google Scholar] [CrossRef]
- Pérez, M.; Valpuesta, J.M.; Medina, M.; Garcini, E.M.D.; Avila, J. Polymerization of Tau into filaments in the presence of heparin: The minimal sequence required for Tau-Tau interaction. J. Neurochem. 1996, 67, 1183–1190. [Google Scholar] [CrossRef]
- Zhu, H.L.; Fernandez, C.; Fan, J.-B.; Shewmaker, F.; Chen, J.; Minton, A.P.; Liang, Y. Quantitative Characterization of Heparin Binding to Tau Protein: IMPLICATION FOR INDUCER-MEDIATED TAU FILAMENT FORMATION. J. Biol. Chem. 2010, 285, 3592–3599. [Google Scholar] [CrossRef] [Green Version]
- Kuret, J.; Chirita, C.N.; Congdon, E.E.; Kannanayakal, T.; Li, G.; Necula, M.; Yin, H.; Zhong, Q. Pathways of tau fibrillization. BBA—Mol. Basis Dis. 2005, 1739, 167–178. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.; Karikari, T.K.; Poole, T.; Ashton, N.J.; Lantero Rodriguez, J.; Khatun, A.; Swift, I.; Heslegrave, A.J.; Abel, E.; Chung, E.; et al. Plasma phospho-tau181 in presymptomatic and symptomatic familial Alzheimer’s disease: A longitudinal cohort study. Mol. Psychiatry 2021, 26, 5967–5976. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Values |
|---|---|
| Maximum, n | 34 |
| Age, years | 28.71 (7.55) |
| Male, n (%) | 11 (32.40) |
| Education, years | 18.38 (1.72) |
| K2-EDTA-tau, pg/mL | 3.15 (0.86) |
| Li-Hep-tau, pg/mL | 4.87 (1.85) |
| Na-Hep-tau, pg/mL | 4.41 (2.02) |
| K2-EDTA-p-tau181, pg/mL | 3.95 (1.86) |
| Li-Hep-p-tau181, pg/mL | 4.84 (1.95) |
| Na-Hep-p-tau181, pg/mL | 4.78 (1.97) |
| K2-EDTA-Aβ40, pg/mL | 193.20 (35.65) |
| Li-Hep-Aβ40, pg/mL | 203.20 (47.94) |
| Na-Hep-Aβ40, pg/mL | 197.10 (46.04) |
| K2-EDTA- Aβ42, pg/mL | 13.92 (2.66) |
| Li-Hep-Aβ42, pg/mL | 14.41 (3.43) |
| Na-Hep-Aβ42, pg/mL | 13.87 (3.28) |
| K2-EDTA-Aβ42/40 ratio, pg/mL | 0.073 (0.014) |
| Li-Hep-Aβ42/40 ratio, pg/mL | 0.073 (0.015) |
| Na-Hep-Aβ42/40 ratio, pg/mL | 0.073 (0.017) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Ding, X.; Wang, W.; Yang, X.; Li, T.; Lei, P. Head-to-Head Comparison of Different Blood Collecting Tubes for Quantification of Alzheimer’s Disease Biomarkers in Plasma. Biomolecules 2022, 12, 1194. https://doi.org/10.3390/biom12091194
Jiang L, Ding X, Wang W, Yang X, Li T, Lei P. Head-to-Head Comparison of Different Blood Collecting Tubes for Quantification of Alzheimer’s Disease Biomarkers in Plasma. Biomolecules. 2022; 12(9):1194. https://doi.org/10.3390/biom12091194
Chicago/Turabian StyleJiang, Lijun, Xulong Ding, Wenxiao Wang, Xiaobin Yang, Tao Li, and Peng Lei. 2022. "Head-to-Head Comparison of Different Blood Collecting Tubes for Quantification of Alzheimer’s Disease Biomarkers in Plasma" Biomolecules 12, no. 9: 1194. https://doi.org/10.3390/biom12091194
APA StyleJiang, L., Ding, X., Wang, W., Yang, X., Li, T., & Lei, P. (2022). Head-to-Head Comparison of Different Blood Collecting Tubes for Quantification of Alzheimer’s Disease Biomarkers in Plasma. Biomolecules, 12(9), 1194. https://doi.org/10.3390/biom12091194






