The Patent Ductus Arteriosus in Extremely Preterm Neonates Is More than a Hemodynamic Challenge: New Molecular Insights
Abstract
1. Introduction
2. Methods
2.1. Study Cohort
2.2. Echocardiography and Clinical Information
2.3. Plasma Samples
2.4. Preparation of Samples for Proteomics
2.5. LC-MS/MS Analysis
2.6. Proteomic Data Analysis
2.7. Multiplexed Luminex Analyses
2.8. Statistical and Bioinformatics Analyses
2.9. Periostin Enzyme-Linked Immunosorbent Assay
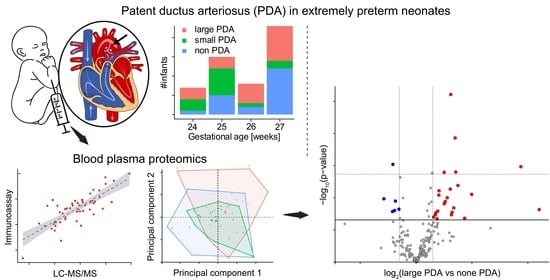
3. Results
3.1. Study Cohort
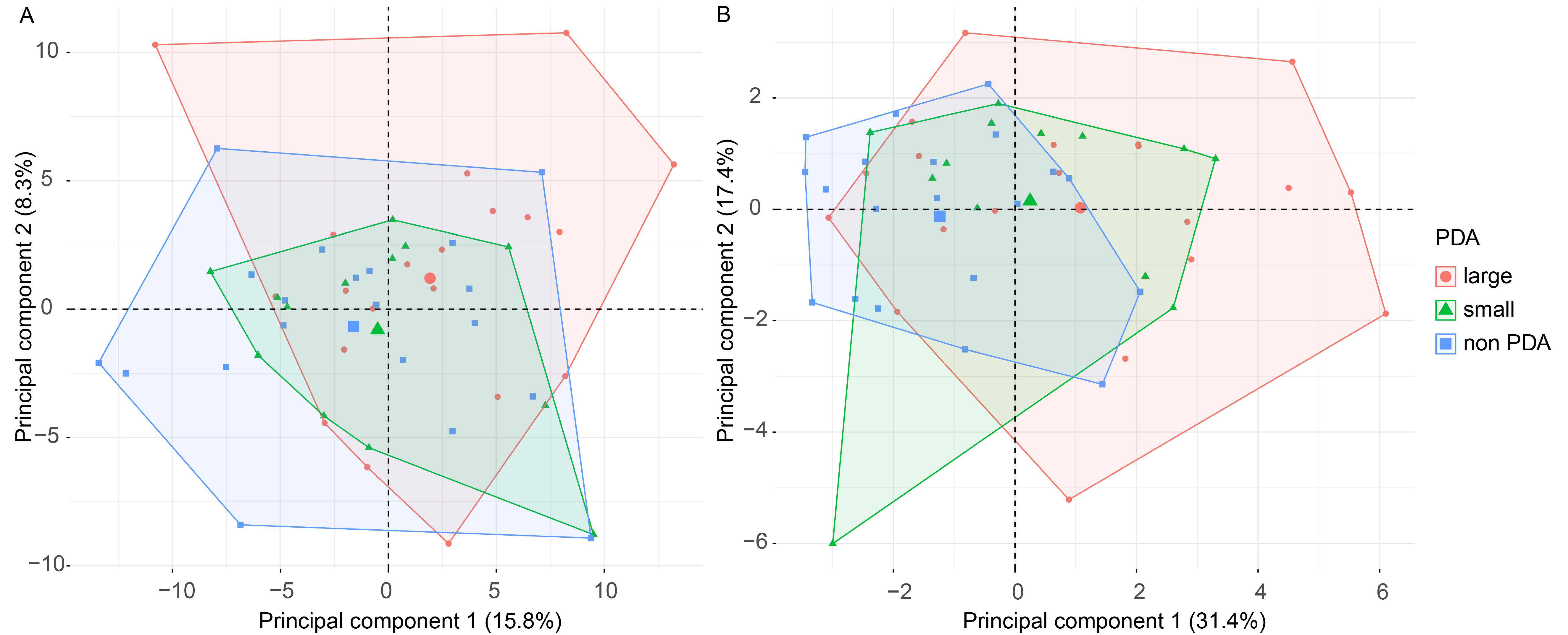
3.2. Plasma Proteomics
3.3. Identifying Proteins Differentiating PDA from No PDA Samples
3.4. Immunoassay Validation of Periostin (POSTN) Levels
3.5. PDA and Increased Levels of NT-proBNP Levels
4. Discussion
4.1. Short Presentation of Main Results
4.2. Activation of the RAAS System in Neonates with PDA
4.3. Ang II and IL-10 Stimulated Increase in SPP1 in Neonates with PDA
4.4. Which Cytokines Are Associated with Large PDA?
4.5. Balancing Pro- and Anti-Inflammation in the Preterm Neonates
4.6. Complement System
4.7. Is Periostin a Marker of Pressure Overload or of Pulmonary Remodeling?
4.8. Do the Levels of sCD163 Indicate Inflammation Involving Macrophages System?
4.9. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Bennet, L.; Dhillon, S.; Lear, C.A.; van den Heuij, L.; King, V.; Dean, J.M.; Wassink, G.; Davidson, J.O.; Gunn, A.J. Chronic inflammation and impaired development of the preterm brain. J. Reprod. Immunol. 2018, 125, 45–55. [Google Scholar] [CrossRef] [PubMed]
- D’Angio, C.T.; Ambalavanan, N.; Carlo, W.A.; McDonald, S.A.; Skogstrand, K.; Hougaard, D.M.; Shankaran, S.; Goldberg, R.N.; Ehrenkranz, R.A.; Tyson, J.E.; et al. Blood Cytokine Profiles Associated with Distinct Patterns of Bronchopulmonary Dysplasia among Extremely Low Birth Weight Infants. J. Pediatr. 2016, 174, 45–51.e5. [Google Scholar] [CrossRef] [PubMed]
- Humberg, A.; Fortmann, I.; Siller, B.; Kopp, M.V.; Herting, E.; Gopel, W.; Hartel, C.; German Neonatal Network, German Center for Lung Research; Priming Immunity at the beginning of life (PRIMAL) Consortium. Preterm birth and sustained inflammation: Consequences for the neonate. Semin. Immunopathol. 2020, 42, 451–468. [Google Scholar] [CrossRef]
- Sabic, D.; Koenig, J.M. A perfect storm: Fetal inflammation and the developing immune system. Pediatr. Res. 2020, 87, 319–326. [Google Scholar] [CrossRef]
- Stoll, B.J.; Gordon, T.; Korones, S.B.; Shankaran, S.; Tyson, J.E.; Bauer, C.R.; Fanaroff, A.A.; Lemons, J.A.; Donovan, E.F.; Oh, W.; et al. Early-onset sepsis in very low birth weight neonates: A report from the National Institute of Child Health and Human Development Neonatal Research Network. J. Pediatr. 1996, 129, 72–80. [Google Scholar] [CrossRef]
- Reiss, J.D.; Peterson, L.S.; Nesamoney, S.N.; Chang, A.L.; Pasca, A.M.; Marić, I.; Shaw, G.M.; Gaudilliere, B.; Wong, R.J.; Sylvester, K.G.; et al. Perinatal infection, inflammation, preterm birth, and brain injury: A review with proposals for future investigations. Exp. Neurol. 2022, 351, 113988. [Google Scholar] [CrossRef] [PubMed]
- Aslam, B.; Basit, M.; Nisar, M.A.; Khurshid, M.; Rasool, M.H. Proteomics: Technologies and Their Applications. J. Chromatogr. Sci. 2017, 55, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Mann, M. Is proteomics the new genomics? Cell 2007, 130, 395–398. [Google Scholar] [CrossRef]
- Palstrom, N.B.; Matthiesen, R.; Rasmussen, L.M.; Beck, H.C. Recent Developments in Clinical Plasma Proteomics-Applied to Cardiovascular Research. Biomedicines 2022, 10, 162. [Google Scholar] [CrossRef] [PubMed]
- Sellmer, A.; Bjerre, J.V.; Schmidt, M.R.; McNamara, P.J.; Hjortdal, V.E.; Host, B.; Bech, B.H.; Henriksen, T.B. Morbidity and mortality in preterm neonates with patent ductus arteriosus on day 3. Arch. Dis. Child.-Fetal Neonatal Ed. 2013, 98, F505–F510. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.W.; Geva, T.; Shirali, G.S.; Frommelt, P.C.; Humes, R.A.; Brook, M.M.; Pignatelli, R.H.; Rychik, J. Guidelines and standards for performance of a pediatric echocardiogram: A report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2006, 19, 1413–1430. [Google Scholar] [CrossRef] [PubMed]
- Papile, L.A.; Burstein, J.; Burstein, R.; Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1500 gm. J. Pediatr. 1978, 92, 529–534. [Google Scholar] [CrossRef]
- Sellmer, A.; Hjortdal, V.E.; Bjerre, J.V.; Schmidt, M.R.; Bech, B.H.; Henriksen, T.B. Cardiovascular biomarkers in the evaluation of patent ductus arteriosus in very preterm neonates: A cohort study. Early Hum. Dev. 2020, 149, 105142. [Google Scholar] [CrossRef] [PubMed]
- Edhager, A.V.; Povlsen, J.A.; Løfgren, B.; Bøtker, H.E.; Palmfeldt, J. Proteomics of the Rat Myocardium during Development of Type 2 Diabetes Mellitus Reveals Progressive Alterations in Major Metabolic Pathways. J. Proteome Res. 2018, 17, 2521–2532. [Google Scholar] [CrossRef]
- Deeb, S.J.; D’Souza, R.C.; Cox, J.; Schmidt-Supprian, M.; Mann, M. Super-SILAC allows classification of diffuse large B-cell lymphoma subtypes by their protein expression profiles. Mol. Cell Proteom. 2012, 11, 77–89. [Google Scholar] [CrossRef]
- Bennike, T.B.; Fatou, B.; Angelidou, A.; Diray-Arce, J.; Falsafi, R.; Ford, R.; Gill, E.E.; van Haren, S.D.; Idoko, O.T.; Lee, A.H.; et al. Preparing for Life: Plasma Proteome Changes and Immune System Development During the First Week of Human Life. Front. Immunol. 2020, 11, 578505. [Google Scholar] [CrossRef]
- Douglas Bates, M.M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Yoav, B.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. Royal Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 5 July 2022).
- R Studio Team. RStudio: Integrated Development Environment for R; R Studio Team: Boston, MA, USA, 2015. [Google Scholar]
- Wickham, H.; François, R.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation, R Package Version 1.0.7. 2021. Available online: https://dplyr.tidyverse.org/ (accessed on 5 July 2022).
- Rohart, F.; Gautier, B.; Singh, A.; KA, L.C. mixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Comput Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots, R Package Version 0.4.0. 2020. Available online: https://rpkgs.datanovia.com/ggpubr/ (accessed on 5 July 2022).
- Wilke, C.O. Cowplot: Streamlined Plot Theme and Plot Annotations for ‘ggplot2’, R Package Version 1.1.1. 2020. Available online: https://rdrr.io/cran/cowplot/ (accessed on 5 July 2022).
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef] [PubMed]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Go, H.; Ono, J.; Ohto, H.; Nollet, K.E.; Sato, K.; Kume, Y.; Maeda, H.; Chishiki, M.; Haneda, K.; Ichikawa, H.; et al. Can serum periostin predict bronchopulmonary dysplasia in premature infants? Pediatr. Res. 2021; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ahlfeld, S.K.; Davis, S.D.; Kelley, K.J.; Poindexter, B.B. Early Elevation of Plasma Periostin Is Associated with Chronic Ventilator-Dependent Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2016, 194, 1430–1433. [Google Scholar] [CrossRef]
- Lu, H.; Cassis, L.A.; Kooi, C.W.; Daugherty, A. Structure and functions of angiotensinogen. Hypertens Res. 2016, 39, 492–500. [Google Scholar] [CrossRef]
- Ray, S.; Boldogh, I.; Brasier, A.R. STAT3 NH2-terminal acetylation is activated by the hepatic acute-phase response and required for IL-6 induction of angiotensinogen. Gastroenterology 2005, 129, 1616–1632. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ruiz-Ortega, M.; Lorenzo, O.; Ruperez, M.; Esteban, V.; Egido, J. Inflammation and angiotensin II. Int. J. Biochem. Cell Biol. 2003, 35, 881–900. [Google Scholar] [CrossRef]
- Choi, J.H.; Yoo, K.H.; Cheon, H.W.; Kim, K.B.; Hong, Y.S.; Lee, J.W.; Kim, S.K.; Kim, C.H. Angiotensin converting enzyme inhibition decreases cell turnover in the neonatal rat heart. Pediatr. Res. 2002, 52, 325–332. [Google Scholar] [CrossRef][Green Version]
- Marchesi, C.; Paradis, P.; Schiffrin, E.L. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol. Sci. 2008, 29, 367–374. [Google Scholar] [CrossRef]
- Satou, R.; Penrose, H.; Navar, L.G. Inflammation as a Regulator of the Renin-Angiotensin System and Blood Pressure. Curr. Hypertens Rep. 2018, 20, 100. [Google Scholar] [CrossRef]
- Joung, K.E.; Christou, H.; Park, K.H.; Mantzoros, C.S. Cord blood levels of osteopontin as a phenotype marker of gestational age and neonatal morbidities. Obesity 2014, 22, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Cho, H.J.; Kim, H.S. Osteopontin: A multifunctional protein at the crossroads of inflammation, atherosclerosis, and vascular calcification. Curr. Atheroscler. Rep. 2009, 11, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Murry, C.E.; Giachelli, C.M.; Schwartz, S.M.; Vracko, R. Macrophages express osteopontin during repair of myocardial necrosis. Am. J. Pathol. 1994, 145, 1450–1462. [Google Scholar] [PubMed]
- Brown, L.F.; Berse, B.; Van de Water, L.; Papadopoulos-Sergiou, A.; Perruzzi, C.A.; Manseau, E.J.; Dvorak, H.F.; Senger, D.R. Expression and distribution of osteopontin in human tissues: Widespread association with luminal epithelial surfaces. Mol. Biol. Cell 1992, 3, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, S.D.; Franzoni, D.F.; Roesener, C.D.; Crisman, J.M.; Diamond, J.R. Angiotensinogen and AT(1) antisense inhibition of osteopontin translation in rat proximal tubular cells. Am. J. Physiol. Renal Physiol. 2000, 278, F708–F716. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Sakatsume, M.; Nishi, S.; Narita, I.; Arakawa, M.; Gejyo, F. Expression, roles, receptors, and regulation of osteopontin in the kidney. Kidney Int. 2001, 60, 1645–1657. [Google Scholar] [CrossRef]
- Yu, X.Q.; Wu, L.L.; Huang, X.R.; Yang, N.; Gilbert, R.E.; Cooper, M.E.; Johnson, R.J.; Lai, K.N.; Lan, H.Y. Osteopontin expression in progressive renal injury in remnant kidney: Role of angiotensin II. Kidney Int. 2000, 58, 1469–1480. [Google Scholar] [CrossRef]
- Shirakawa, K.; Endo, J.; Kataoka, M.; Katsumata, Y.; Yoshida, N.; Yamamoto, T.; Isobe, S.; Moriyama, H.; Goto, S.; Kitakata, H.; et al. IL (Interleukin)-10-STAT3-Galectin-3 Axis Is Essential for Osteopontin-Producing Reparative Macrophage Polarization After Myocardial Infarction. Circulation 2018, 138, 2021–2035. [Google Scholar] [CrossRef]
- Shirakawa, K.; Sano, M. Osteopontin in Cardiovascular Diseases. Biomolecules 2021, 11, 1047. [Google Scholar] [CrossRef]
- Askenazi, D.J.; Koralkar, R.; Hundley, H.E.; Montesanti, A.; Parwar, P.; Sonjara, S.; Ambalavanan, N. Urine biomarkers predict acute kidney injury in newborns. J. Pediatr. 2012, 161, 270–275.e271. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Arend, W.P. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002, 13, 323–340. [Google Scholar] [CrossRef]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Singampalli, K.L.; Jui, E.; Shani, K.; Ning, Y.; Connell, J.P.; Birla, R.K.; Bollyky, P.L.; Caldarone, C.A.; Keswani, S.G.; Grande-Allen, K.J. Congenital Heart Disease: An Immunological Perspective. Front. Cardiovasc. Med. 2021, 8, 701375. [Google Scholar] [CrossRef] [PubMed]
- Wienecke, L.M.; Cohen, S.; Bauersachs, J.; Mebazaa, A.; Chousterman, B.G. Immunity and inflammation: The neglected key players in congenital heart disease? Heart Fail. Rev. 2022, 27, 19671971. [Google Scholar] [CrossRef] [PubMed]
- Schram, A.-S.L.; Sellmer, A.; Nyboe, C.; Sillesen, M.; Hjortdal, V.E. Increased inflammatory markers in adult patients born with an atrial septal defect. Front. Cardiovasc. Med. 2022, 9, 925314. [Google Scholar] [CrossRef] [PubMed]
- Rouatbi, H.; Farhat, N.; Heying, R.; Gérard, A.; Vazquez-Jimenez, J.F.; Seghaye, M.C. Right Atrial Myocardial Remodeling in Children With Atrial Septal Defect Involves Inflammation, Growth, Fibrosis, and Apoptosis. Front. Pediatr. 2020, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, K.; Yang, Q.; Wang, J.; Xuan, C.; Liu, X.C.; Liu, Z.G.; He, G.W. Acute phase proteins altered in the plasma of patients with congenital ventricular septal defect. Proteom. Clin. Appl. 2015, 9, 1087–1096. [Google Scholar] [CrossRef]
- Olsson, K.W.; Larsson, A.; Jonzon, A.; Sindelar, R. Exploration of potential biochemical markers for persistence of patent ductus arteriosus in preterm infants at 22–27 weeks’ gestation. Pediatr. Res. 2019, 86, 333–338. [Google Scholar] [CrossRef]
- Dammann, O.; Leviton, A. Intermittent or sustained systemic inflammation and the preterm brain. Pediatr. Res. 2014, 75, 376–380. [Google Scholar] [CrossRef]
- Jones, C.A.; Cayabyab, R.G.; Kwong, K.Y.; Stotts, C.; Wong, B.; Hamdan, H.; Minoo, P.; deLemos, R.A. Undetectable interleukin (IL)-10 and persistent IL-8 expression early in hyaline membrane disease: A possible developmental basis for the predisposition to chronic lung inflammation in preterm newborns. Pediatr. Res. 1996, 39, 966–975. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seo, Y.M.; Lin, Y.K.; Im, S.A.; Sung, I.K.; Youn, Y.A. Interleukin 8 may predict surgical necrotizing enterocolitis in infants born less than 1500 g. Cytokine 2021, 137, 155343. [Google Scholar] [CrossRef]
- Sullivan, G.; Galdi, P.; Cabez, M.B.; Borbye-Lorenzen, N.; Stoye, D.Q.; Lamb, G.J.; Evans, M.J.; Quigley, A.J.; Thrippleton, M.J.; Skogstrand, K.; et al. Interleukin-8 dysregulation is implicated in brain dysmaturation following preterm birth. Brain Behav. Immun. 2020, 90, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Leviton, A.; Joseph, R.M.; Fichorova, R.N.; Allred, E.N.; Gerry Taylor, H.; Michael O’Shea, T.; Dammann, O. Executive Dysfunction Early Postnatal Biomarkers among Children Born Extremely Preterm. J. Neuroimmune Pharmacol. 2019, 14, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Bajic, G.; Degn, S.E.; Thiel, S.; Andersen, G.R. Complement activation, regulation, and molecular basis for complement-related diseases. Embo J. 2015, 34, 2735–2757. [Google Scholar] [CrossRef]
- Hou, H.T.; Xi, Z.; Wang, J.; Liu, L.X.; Zhang, J.F.; Yang, Q.; He, G.W. Altered plasma proteins released from platelets and endothelial cells are associated with human patent ductus arteriosus. J. Cell Physiol. 2019, 234, 6842–6853. [Google Scholar] [CrossRef]
- Khalil, A.; Trehan, R.; Tiwari, A.; Malik, R.; Arora, R. Immunological profile in congenital heart disease. Indian Pediatr. 1994, 31, 295–300. [Google Scholar]
- Grumach, A.S.; Ceccon, M.E.; Rutz, R.; Fertig, A.; Kirschfink, M. Complement profile in neonates of different gestational ages. Scand. J. Immunol. 2014, 79, 276–281. [Google Scholar] [CrossRef]
- Conway, S.J.; Izuhara, K.; Kudo, Y.; Litvin, J.; Markwald, R.; Ouyang, G.; Arron, J.R.; Holweg, C.T.; Kudo, A. The role of periostin in tissue remodeling across health and disease. Cell Mol. Life Sci. 2014, 71, 1279–1288. [Google Scholar] [CrossRef]
- Hudson, J.E.; Porrello, E.R. Periostin paves the way for neonatal heart regeneration. Cardiovasc. Res. 2017, 113, 556–558. [Google Scholar] [CrossRef]
- Conway, S.J.; Molkentin, J.D. Periostin as a heterofunctional regulator of cardiac development and disease. Curr. Genom. 2008, 9, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Ahlfeld, S.K.; Gao, Y.; Wang, J.; Horgusluoglu, E.; Bolanis, E.; Clapp, D.W.; Conway, S.J. Periostin downregulation is an early marker of inhibited neonatal murine lung alveolar septation. Birth Defects Res. Part A Clin. Mol. Teratol. 2013, 97, 373–385. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bozyk, P.D.; Bentley, J.K.; Popova, A.P.; Anyanwu, A.C.; Linn, M.D.; Goldsmith, A.M.; Pryhuber, G.S.; Moore, B.B.; Hershenson, M.B. Neonatal periostin knockout mice are protected from hyperoxia-induced alveolar simplication. PLoS ONE 2012, 7, e31336. [Google Scholar] [CrossRef][Green Version]
- Chen, Z.; Xie, J.; Hao, H.; Lin, H.; Wang, L.; Zhang, Y.; Chen, L.; Cao, S.; Huang, X.; Liao, W.; et al. Ablation of periostin inhibits post-infarction myocardial regeneration in neonatal mice mediated by the phosphatidylinositol 3 kinase/glycogen synthase kinase 3beta/cyclin D1 signalling pathway. Cardiovasc. Res. 2017, 113, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Moller, H.J. Soluble CD163. Scand. J. Clin. Lab. Investig. 2012, 72, 1–13. [Google Scholar] [CrossRef]
- Møller, H.J.; Peterslund, N.A.; Graversen, J.H.; Moestrup, S.K. Identification of the hemoglobin scavenger receptor/CD163 as a natural soluble protein in plasma. Blood 2002, 99, 378–380. [Google Scholar] [CrossRef]
- Etzerodt, A.; Moestrup, S.K. CD163 and inflammation: Biological, diagnostic, and therapeutic aspects. Antioxid Redox Signal 2013, 18, 2352–2363. [Google Scholar] [CrossRef]
- Kirov, S.; Sasson, A.; Zhang, C.; Chasalow, S.; Dongre, A.; Steen, H.; Stensballe, A.; Andersen, V.; Birkelund, S.; Bennike, T.B. Degradation of the extracellular matrix is part of the pathology of ulcerative colitis. Mol. Omics 2019, 15, 67–76. [Google Scholar] [CrossRef]


| no PDA (n = 20) | Small PDA (n = 13) | Large PDA (n = 20) | p-Value * | |
|---|---|---|---|---|
| GA, median (range) | 27 (24; 27) | 25 (24; 27) | 26 (24; 27) | n.s. |
| Birth weight grams, median (range) | 984 (545; 1220) | 750 (575; 1000) | 902 (570; 1210) | n.s. |
| Sex (female/male) | 6/14 | 4/9 | 7/13 | n.s. |
| Apgar score at 1 min (IQR) | 7 (3; 9) | 6 (4; 9) | 7 (5; 8) | n.s. |
| Apgar score at 5 min (IQR) | 10 (8; 10) | 10 (8; 10) | 10 (8; 10) | n.s. |
| Caffeine, number (%) | 20 (100) | 13 (100) | 20 (100) | n.s. |
| EOS, number (%) | 4 (20) | 6 (46) | 4 (20) | n.s. |
| Surfactant, number (%) | 9 (45) | 7 (54) | 13 (65) | n.s. |
| PRBC transfusion, number (%) | 4 (20) | 5 (38) | 7 (35) | n.s. |
| Inotropes, number (%) | 1 (5) | 1 (8) | 3 (15) | n.s. |
| Mechanical ventilation, number (%) | 3 (15) | 5 (38) | 9 (45) | 0.08 |
| IVH, number (%) | 5 (25) | 2 (15) | 10 (50) | n.s. |
| Large LA:Ao ratio, number (%) | 1 (5) | 1 (8) | 6 (30) | 0.09 |
| Reversed DADF, number (%) | 0 (0) | 1 (8) | 4 (20) | n.s. |
| Preeclampsia, number (%) | 4 (20) | 1 (8) | 1 (5) | n.s. |
| Antenatal steroids, number (%) | 20 (100) | 13 (100) | 17 (85) | n.s. |
| Multiple pregnancy, number (%) | 3 (15) | 5 (38) | 9 (45) | 0.08 |
| Cesarean delivery, number (%) | 13 (65) | 6 (46) | 13 (65) | n.s. |
| Protein Name | PDA Change [log2] | Large PDA/No PDA Ratio | p-Value | Gene Name |
|---|---|---|---|---|
| Periostin | 0.75 | 168% | 4.24 × 10−3 | POSTN |
| Osteopontin | 0.70 | 162% | 1.41 × 10−4 | SPP1 |
| Scavenger receptor cysteine-rich type 1 protein M130 | 0.67 | 160% | 2.12 × 10−2 | CD163 |
| Pulmonary surfactant-associated protein B | 0.63 | 155% | 3.46 × 10−2 | SFTPB |
| Angiotensinogen | 0.62 | 154% | 6.29 × 10−6 | AGT |
| L-lactate dehydrogenase B chain | 0.61 | 153% | 5.50 × 10−3 | LDHB |
| Actin, cytoplasmic 1 | 0.56 | 147% | 1.03 × 10−2 | ACTB |
| Poliovirus receptor | 0.50 | 142% | 1.66 × 10−3 | PVR |
| Desmoglein-2 | 0.41 | 133% | 6.22 × 10−3 | DSG2 |
| Plexin domain-containing protein 2 | 0.41 | 133% | 8.45 × 10−3 | PLXDC2 |
| Nidogen-1 | 0.39 | 131% | 2.66 × 10−2 | NID1 |
| Cystatin-B | 0.38 | 130% | 4.62 × 10−2 | CST6 |
| Lumican | 0.35 | 128% | 3.02 × 10−2 | LUM |
| Cadherin-5 | 0.34 | 127% | 3.46 × 10−2 | CDH5 |
| Mannan-binding lectin serine protease 1 | 0.31 | 124% | 4.09 × 10−2 | MASP1 |
| Carboxypeptidase N catalytic chain | −0.31 | 81% | 2.32 × 10−2 | CPN1 |
| Coagulation factor XIII B chain | −0.37 | 77% | 1.30 × 10−2 | F13B |
| Complement component C8 alpha chain | −0.38 | 77% | 2.54 × 10−2 | C8A |
| Carboxypeptidase N subunit 2 | −0.41 | 75% | 9.38 × 10−4 | CPN2 |
| Complement component C8 beta chain | −0.42 | 75% | 2.75 × 10−2 | C8B |
| Complement component C8 gamma chain | −0.58 | 67% | 1.11 × 10−2 | C8G |
| Interleukine/Cytokine Name | PDA Change [log2] | Large PDA/No PDA Ratio | p-Value | Gene Name |
|---|---|---|---|---|
| Interleukin-1 receptor antagonist | 2.20 | 460% | 2.38 × 10−2 | IL1RA |
| Interleukin-8 | 1.87 | 367% | 1.11 × 10−3 | IL8 |
| Interleukin-6 | 1.00 | 199% | 8.03 × 10−3 | IL6 |
| C-C motif chemokine 4 | 0.87 | 183% | 4.44 × 10−2 | CCL4 |
| Interleukin-10 | 0.71 | 164% | 1.62 × 10−3 | IL10 |
| C-C motif chemokine 3 | 0.64 | 156% | 2.90 × 10−2 | CCL3 |
| Interleukin-1 beta | 0.52 | 143% | 1.34 × 10−2 | IL1beta |
| Eotaxin | 0.50 | 142% | 1.57 × 10−2 | CCL11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sellmer, A.; Henriksen, T.B.; Palmfeldt, J.; Bech, B.H.; Astono, J.; Bennike, T.B.; Hjortdal, V.E. The Patent Ductus Arteriosus in Extremely Preterm Neonates Is More than a Hemodynamic Challenge: New Molecular Insights. Biomolecules 2022, 12, 1179. https://doi.org/10.3390/biom12091179
Sellmer A, Henriksen TB, Palmfeldt J, Bech BH, Astono J, Bennike TB, Hjortdal VE. The Patent Ductus Arteriosus in Extremely Preterm Neonates Is More than a Hemodynamic Challenge: New Molecular Insights. Biomolecules. 2022; 12(9):1179. https://doi.org/10.3390/biom12091179
Chicago/Turabian StyleSellmer, Anna, Tine Brink Henriksen, Johan Palmfeldt, Bodil Hammer Bech, Julie Astono, Tue Bjerg Bennike, and Vibeke Elisabeth Hjortdal. 2022. "The Patent Ductus Arteriosus in Extremely Preterm Neonates Is More than a Hemodynamic Challenge: New Molecular Insights" Biomolecules 12, no. 9: 1179. https://doi.org/10.3390/biom12091179
APA StyleSellmer, A., Henriksen, T. B., Palmfeldt, J., Bech, B. H., Astono, J., Bennike, T. B., & Hjortdal, V. E. (2022). The Patent Ductus Arteriosus in Extremely Preterm Neonates Is More than a Hemodynamic Challenge: New Molecular Insights. Biomolecules, 12(9), 1179. https://doi.org/10.3390/biom12091179