Comparative Analyses of Bioactive Compounds in Inonotus obliquus Conks Growing on Alnus and Betula
Abstract
:1. Introduction
2. Material and Methods
2.1. Sample Sites and Fungal Isolation and Detection
2.2. Preparation and Bioactive Compound’s Detection in Inonotus Obliquus Conks
3. Results and Discussion
3.1. Fungal Species Detection
3.2. Determination of Bioactive Compounds
3.2.1. Contents of Betulin and Betulinic Acid
3.2.2. Contents of Inotodiol and Lanosterol
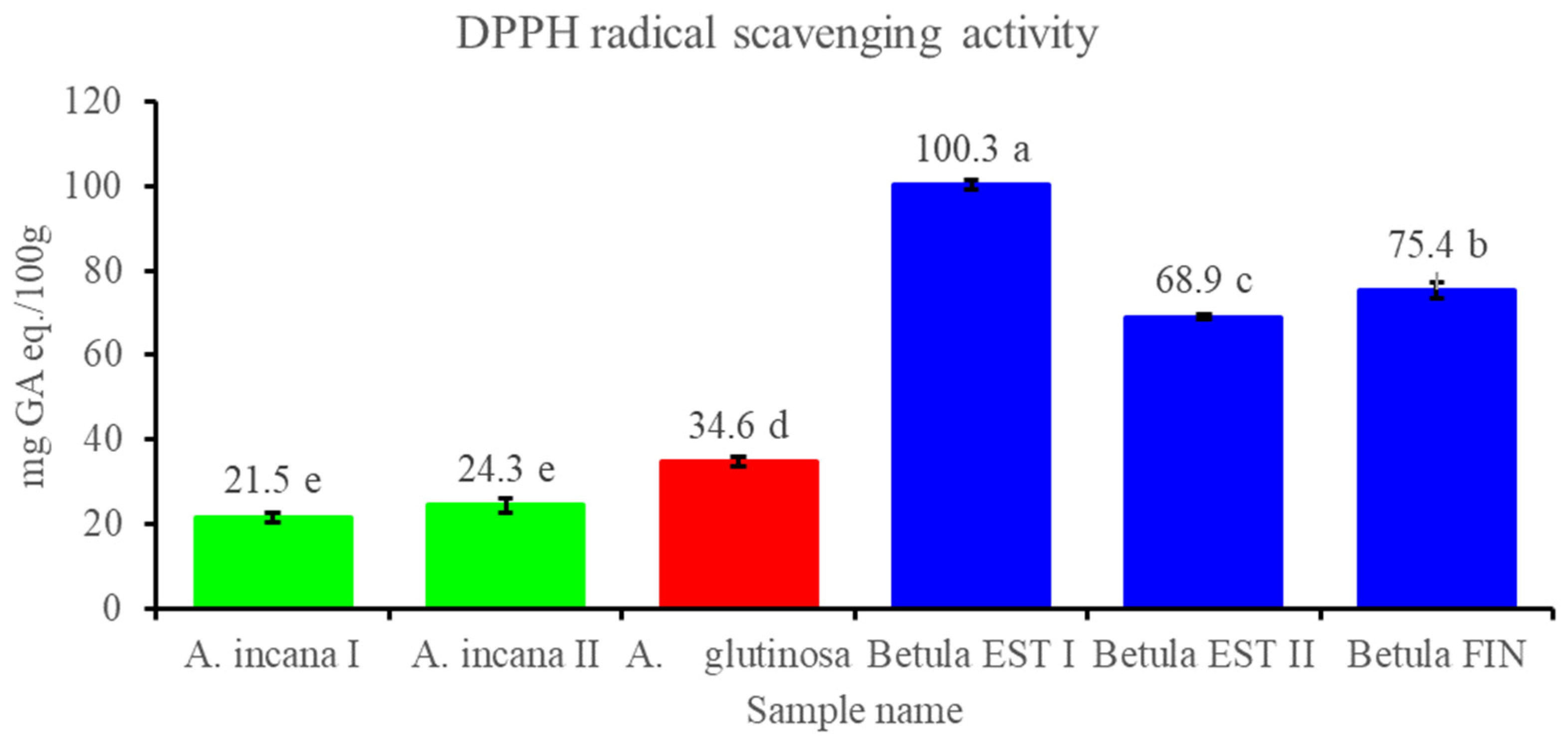
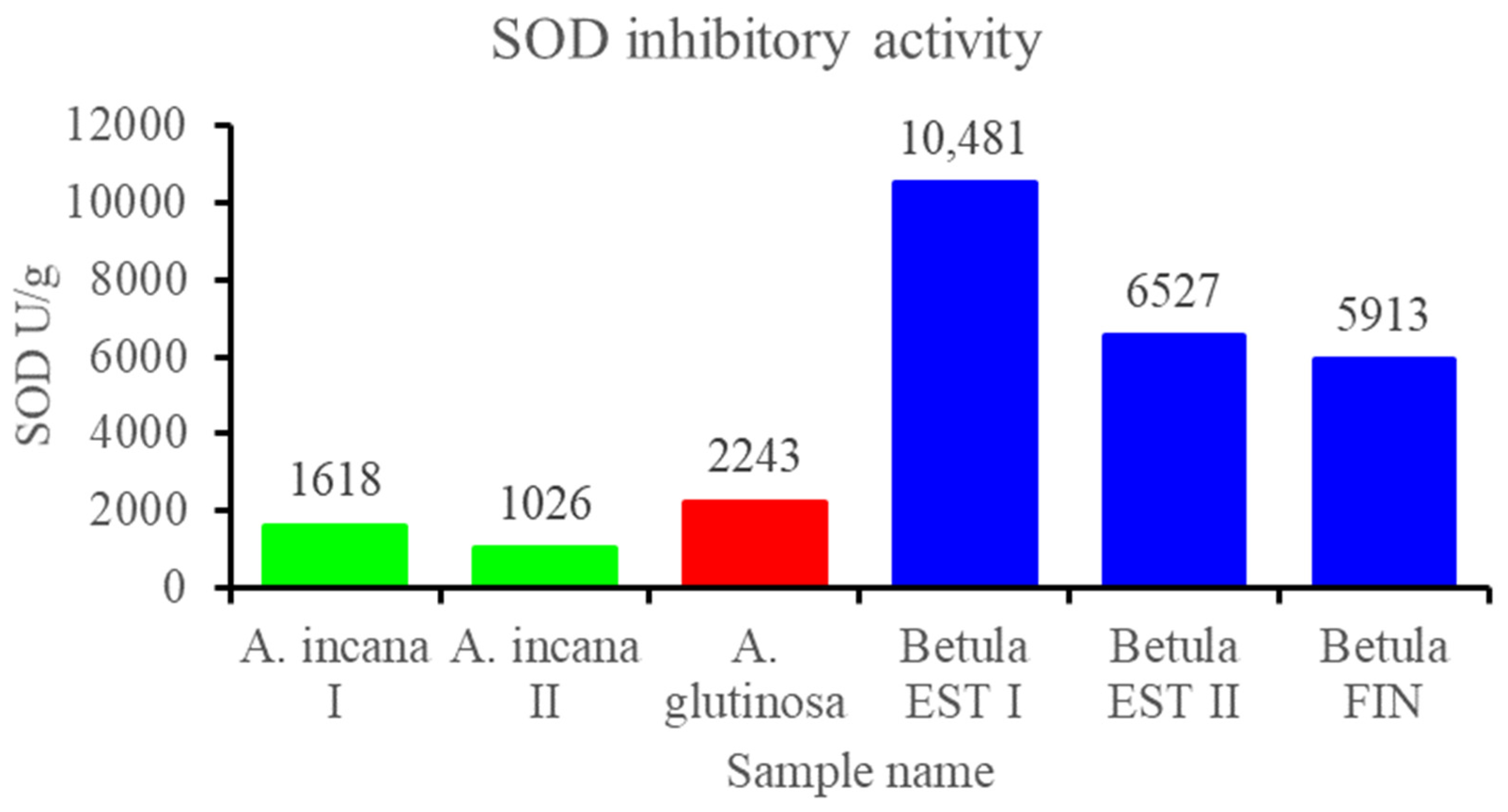
3.2.3. Contents of Total Polyphenols and Flavonols
3.2.4. Contents of Glucans
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolfslehner, B.; Prokofieva, I.; Mavsar, R. Non-wood forest products in Europe: Seeing the forest around the trees. In What Science Can Tell Us; European Forest Institute: Barcelona, Spain, 2019; 116p. [Google Scholar]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 6213. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ibarra, E.; Gómez-Martín, M.B.; Armesto-López, X.A. Climatic and Socioeconomic Aspects of Mushrooms: The Case of Spain. Sustainability 2019, 11, 1030. [Google Scholar] [CrossRef]
- Miina, J.; Peltola, R.; Veteli, P.; Linnakoski, R.; Escribano, M.C.; Haveri-Heikkilä, J.; Mattila, P.; Marnila, P.; Pihlava, J.-M.; Hellström, J.; et al. Inoculation success of Inonotus obliquus in living birch (Betula spp.). For. Ecol. Manag. 2021, 492, 119244. [Google Scholar] [CrossRef]
- Lee, M.-W.; Hur, H.; Chang, K.-C.; Lee, T.-S.; Ka, K.-H.; Jankovsky, L. Introduction to distribution and ecology of sterile conks of Inonotus obliquus. Mycobiology 2008, 36, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Niemelä, T. Polypores in Finland and Estonia; Eesti Loodusfoto: Tartu, Estonia, 2008; 320p. (In Estonian) [Google Scholar]
- Drenkhan, R.; Adamson, K.; Hanso, M. Majanduslikku tulu tõotav must pässik võib kahandada oluliselt puidu väärtust (Inonotus obliquus gives economical income and it may significantly decrease value of wood). Eesti Mets 2020, 3, 34–41. (In Estonian) [Google Scholar]
- Herron, S. Ethnobotany of the Anishinaabek Northern Great Lakes Indians; Southern Illinois University at Carbondale ProQuest Dissertations Publishing: Carbondale, IL, USA, 2002. [Google Scholar]
- Glamočlija, J.; Ćirić, A.; Nikolić, M.; Fernandes, Â.; Barros, L.; Calhelha, R.C.; Ferreira, I.C.F.R.; Soković, M.; van Griensven, L.J.L.D. Chemical characterization and biological activity of Chaga (Inonotus obliquus), a medicinal “mushroom”. J. Ethnopharmacol. 2015, 162, 323–332. [Google Scholar] [CrossRef]
- Fang, J.; Gao, S.; Islam, R.; Teramoto, Y.; Maeda, H. Extracts of Phellinus linteus, bamboo (Sasa senanensis) leaf and chaga mushroom (Inonotus obliquus) exhibit antitumor activity through activating innate immunity. Nutrients 2020, 12, 2279. [Google Scholar] [CrossRef]
- Shahzad, F.; Anderson, D.; Najafzadeh, M. The antiviral, anti-inflammatory effects of natural medicinal herbs and mushrooms and SARS-CoV-2 infection. Nutrients 2020, 12, 2573. [Google Scholar] [CrossRef]
- Nikitina, S.A.; Habibrakhmanova, V.R.; Sysoeva, M.A. Chemical composition and biological activity of triterpenes and steroids of chaga mushroom. Biochemistry 2016, 10, 63–69. [Google Scholar] [CrossRef]
- Ryzhova, G.L.; Kravtsova, S.S.; Matasova, S.A.; Gribel, N.V.; Pashinskii, V.G.; Dychko, K.A. Chemical and Parmaceutical characteristics of dry extract from black birch fungus. Pharm. Chem. J. 1997, 10, 44–47. [Google Scholar]
- Park, E.; Jeon, K.; Byun, B.H. Ethanol extract of Inonotus obliquus shows antigenotoxic effect on hydrogen peroxide induced DNA damage in human lymphocytes. Cancer Prev. Res. 2005, 10, 54–59. [Google Scholar]
- Zheng, W.; Miao, K.; Liu, Y.; Zhao, Y.; Zhang, M.; Pan, S.; Dai, S. Chemical diversity of biologically active metabolites in the sclerotia of Inonotus obliquus and submerged culture strategies for up-regulating their production. Appl. Microbiol. Biotechnol. 2010, 87, 1237–1254. [Google Scholar] [PubMed]
- Nguyen, H.T.; Ho, D.V.; Nguyen, P.D.Q.; Vo, H.Q.; Do, T.T.; Raal, A. Cytotoxic evaluation of compounds isolated from the aerial parts of Hedyotis pilulifera and methanol extract of Inonotus obliquus. Nat. Prod. Commun. 2018, 13, 939–941. [Google Scholar]
- Bebenek, E.; Chrobak, E.; Piechowska, A.; Gluszek, S.; Boryczka, S. Betulin a natural product with promising anticancer activity against colorectal cancer cells. Med. Stud. Stud. Med. 2020, 36, 297–301. [Google Scholar]
- Kim, T.I.; Choi, J.-G.; Kim, J.H.; Li, W.; Chung, H.-S. Blocking effect of chaga mushroom (Inonotus oliquus) extract for immune checkpoint CTLA-4/CD80 interaction. Appl. Sci. 2020, 10, 5774. [Google Scholar] [CrossRef]
- Sak, K.; Jürisoo, K.; Raal, A. Estonian folk traditional experiences on natural anticancer remedies: From past to the future. Pharm. Biol. 2014, 52, 855–866. [Google Scholar]
- Brydon-Williams, R.; Munck, I.A.; Asbjornsen, H. Incidence and ecology of the chaga fungus (Inonotus obliquus) in hardwood New England—Acadian forests. Can. J. For. Res. 2021, 51, 122–131. [Google Scholar] [CrossRef]
- Radusaar, M.; Valgepea, M. Yearbook Forest 2019; Estonian Environment Agency: Tallinn, Estonia, 2020; 310p.
- Lõhmus, E. Estonian Forest Site Types; Eesti NSV Agrotööstuskoondise Info-ja Juurutusvalitsus: Tallinn, Estonia, 1984; 80p. (In Estonian) [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar]
- White, T.J.; Bruns, T.D.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR—Protocols and Applications—A Laboratory Manual; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Drenkhan, R.; Adamson, K.; Jürimaa, K.; Hanso, M. Dothistroma septosporum on firs (Abies spp.) in the northern Baltics. For. Pathol. 2014, 44, 250–254. [Google Scholar] [CrossRef]
- Hall, T.A. Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT/2000/XP/7 [WWW Document]. BioEdit. 2013. Available online: http://www.mbio.ncsu.edu/bioedit/bioedit.html (accessed on 20 July 2022).
- McCleary, B.V.; Draga, A. Measurement of β-Glucan in Mushrooms and Mycelial Products. J. AOAC Int. 2016, 99, 364–373. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Kraus, D. Consolidated data analysis and presentation using an open-source add-in for the Microsoft Excel®® spreadsheet software. Med. Writ. 2014, 23, 25–28. [Google Scholar] [CrossRef]
- Parmasto, E. Distribution Maps of Estonian Fungi. 3. Pore Fungi; Estonian Academy Publishers: Tartu, Estonia, 2004; 224p. [Google Scholar]
- Huynh, N.; Beltrame, G.; Tarvainen, M.; Suomela, J.-P.; Yang, B. Supercritical CO2 Extraction of Triterpenoids from Chaga Sterile Conk of Inonotus obliquus. Molecules 2022, 27, 1880. [Google Scholar] [CrossRef]
- Güttler, A.; Eiselt, Y.; Funtan, A.; Thiel, A.; Petrenko, M.; Keßler, J.; Thondorf, I.; Paschke, R.; Vordermark, D.; Bache, M. Betulin sulfonamides as carbonic anhydrase inhibitors and anticancer agents in breast cancer cells. Int. J. Mol. Sci. 2021, 22, 8808. [Google Scholar] [CrossRef]
- Bhui, K.; Srivastava, A.K.; Shukla, Y. Cytotoxic action of natural pentacyclic triterpenes of cancer cells: Lupane-type compounds. In Pentacyclic Troterpenes as Promising Agents in Cancer; Book Series Cancer Etiology Diagnosis and, Treatments; Salvador, J.A.R., Ed.; Nova Science Publishers: New York, NY, USA, 2010; pp. 49–87. [Google Scholar]
- John, R.; Dalal, B.; Shankarkumar, A.; Devarajan, P.V. Innovative betulin nanosuspension exhibits enhanced anticancer activity in a triple negative breast cancer cell line and Zebrafish angiogenesis model. Int. J. Pharm. 2021, 600, 120511. [Google Scholar]
- Géry, A.; Dubreule, C.; André, V.; Rioult, J.-P.; Bouchart, V.; Heutte, N.; Pécoulas, P.-E.; de Krivomaz, T.; Garon, D. Chaga (Inonotus obliquus), a future potential medicinal fungus in oncology? A chemical study and a comparison of the cytotoxicity against human lung adenocarcinoma cells (A549) and human bronchial epithelial cells (BEAS-2B). Integr. Cancer Ther. 2018, 17, 832–843. [Google Scholar] [CrossRef]
- Upska, K.; Klavins, L.; Radenkovs, V.; Nikolajeva, V.; Faven, L.; Isosaari, E.; Lauberts, M.; Busa, L.; Viksna, A.; Klavins, M. Extraction possibilities of lipid fraction and authentication assessment of chaga (Inonotus obliquus). Biomass Convers. Biorefnery 2022. [Google Scholar] [CrossRef]
- Rios, J. Effects of triterpenes on the immune system. J. Ethnopharmacol. 2010, 128, 1–14. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, H.; Zhang, Y.; Xing, L.; Li, S.; Wang, X.; Sun, Z. Chemical composition and antioxidant properties of five edible Hymenomycetes mushrooms. Int. J. Food Sci. Technol. 2015, 50, 465–471. [Google Scholar] [CrossRef]
- Rheea, S.J.; Chob, S.Y.; Kimb, K.M.; Chaa, D.-S.; Parka, H.-J. A comparative study of analytical methods for alkali-soluble β-glucan in medicinal mushroom, Chaga (Inonotus obliquus). LWT Food Sci. Technol. 2008, 41, 545–549. [Google Scholar] [CrossRef]
- Debnath, T.; Park, D.K.; Lee, B.R.; Jin, H.L.; Lee, S.Y.; Samad, N.B.; Lim, B.O. Antioxidant activity of Inonotus obliquus grown on germinated brown rice extracts. J. Food Biochem. 2013, 37, 456–464. [Google Scholar] [CrossRef]
| Strain No a | Host | Sample Name | Site Type b | Sampling Site and Time | Geographical Coordinates | Fungal Culture Collection Code c, GenBank Accession No d |
|---|---|---|---|---|---|---|
| PAT29023 | Alnus incana | A. incana I | Aegopodium | Estonia, Leemeti; 30.11.2019 | N58.344944, E25.387528 | TFC101255, OP019322 |
| PAT29031 | A. incana | A. incana II | Aegopodium | Estonia, Pedajamäe; 30.12.2019 | N58.068278, E26.428222 | TFC101256, OP019323 |
| PAT29036 | A. glutinosa | A. glutinosa | Dryopteris | Estonia, Jalametsa; 03.02.2020 | N58.794250, E25.834667 | TFC101257, OP019324 |
| PAT29045 | Betula pendula | Betula EST I | Aegopodium | Estonia, Kaavere; 21.12.2019 | N58.903734, E26.446721 | TFC101258, OP019325 |
| PAT29043 | B. pendula | Betula EST II | Oxalis | Estonia, Vissi; 14.01.2020 | N58.160778, E26.732750 | TFC101259, OP019326 |
| PAT29027 | B. pendula | Betula FIN | Unknown | Finland, Luosto; Jan. 2020 | N67.115336, E26.899869 | TFC101260, OP019327 |
| Sample Name | Betulin | Betulinic Acid | Inotodiol | Lanosterol | Total Polyphenols | Total Flavonols |
|---|---|---|---|---|---|---|
| µg/g | µg/g | µg/g | µg/g | µg GA eq/g | µg Q eq/g | |
| A. incana I | 154 ± 8.6 a | 474 ± 10.8 b | 8961 ± 217.8 b | 1023 ± 29.0 c | 287 ± 11.1 d | 336 ± 22.9 c |
| A. incana II | 111 ± 6 b | 635 ± 34.7 a | 7455 ± 172.9 c | 1162 ± 21.1 b | 277 ± 7.4 d | 219 ± 9.2 d |
| A. glutinosa | nq | 49 ± 3.8 d | 6300 ± 90.1 d | 1248 ± 26.3 b | 477 ± 5.5 c | 364 ± 13.5 c |
| Betula EST I | nq | 132 ± 19.9 c | 9057 ± 343.5 b | 1080 ± 43.9 bc | 708 ± 7.5 b | 2879 ± 38.5 a |
| Betula EST II | 159 ± 26.3 a | 20 ± 5.0 e | 7881 ± 286.7 c | 1021 ± 24.0 c | 841 ± 18.7 a | 1137 ± 8.9 b |
| Betula FIN | 0 | 0 | 15223 ± 103.1 a | 2220 ± 29.8 a | 663 ± 24.4 b | 1126 ± 21.5 b |
| Sample Name | Total Glucan % | α-Glucan % | β-Glucan % |
|---|---|---|---|
| A. incana I | 2.72 ± 0.64 d | 0.38 ± 0.03 e | 2.34 ± 0.66 c |
| A. incana II | 3.96 ± 0.11 c | 0.53 ± 0.01 b | 3.43 ± 0.09 c |
| A. glutinosa | 3.58 ± 0.08 d | 0.60 ± 0.01 a | 2.98 ± 0.09 c |
| Betula EST I | 7.01 ± 0.37 a | 0.26 ± 0.02 f | 6.75 ± 0.35 a |
| Betula EST II | 5.94 ± 0.3 b | 0.49 ± 0.01 c | 5.45 ± 0.29 b |
| Betula FIN | 6.04 ± 0.71 ab | 0.46 ± 0 d | 5.58 ± 0.71 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drenkhan, R.; Kaldmäe, H.; Silm, M.; Adamson, K.; Bleive, U.; Aluvee, A.; Erik, M.; Raal, A. Comparative Analyses of Bioactive Compounds in Inonotus obliquus Conks Growing on Alnus and Betula. Biomolecules 2022, 12, 1178. https://doi.org/10.3390/biom12091178
Drenkhan R, Kaldmäe H, Silm M, Adamson K, Bleive U, Aluvee A, Erik M, Raal A. Comparative Analyses of Bioactive Compounds in Inonotus obliquus Conks Growing on Alnus and Betula. Biomolecules. 2022; 12(9):1178. https://doi.org/10.3390/biom12091178
Chicago/Turabian StyleDrenkhan, Rein, Hedi Kaldmäe, Maidu Silm, Kalev Adamson, Uko Bleive, Alar Aluvee, Mart Erik, and Ain Raal. 2022. "Comparative Analyses of Bioactive Compounds in Inonotus obliquus Conks Growing on Alnus and Betula" Biomolecules 12, no. 9: 1178. https://doi.org/10.3390/biom12091178
APA StyleDrenkhan, R., Kaldmäe, H., Silm, M., Adamson, K., Bleive, U., Aluvee, A., Erik, M., & Raal, A. (2022). Comparative Analyses of Bioactive Compounds in Inonotus obliquus Conks Growing on Alnus and Betula. Biomolecules, 12(9), 1178. https://doi.org/10.3390/biom12091178







