Age-Related Decline in Vascular Responses to Phenylephrine Is Associated with Reduced Levels of HSP70
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Histomorphometric Analysis
2.3. Western Blotting
2.4. Functional Studies
2.4.1. Time-Force Curves
2.4.2. Ca2+ Protocol
2.5. ROS and NO Detection
2.6. Statistical Analysis
3. Results
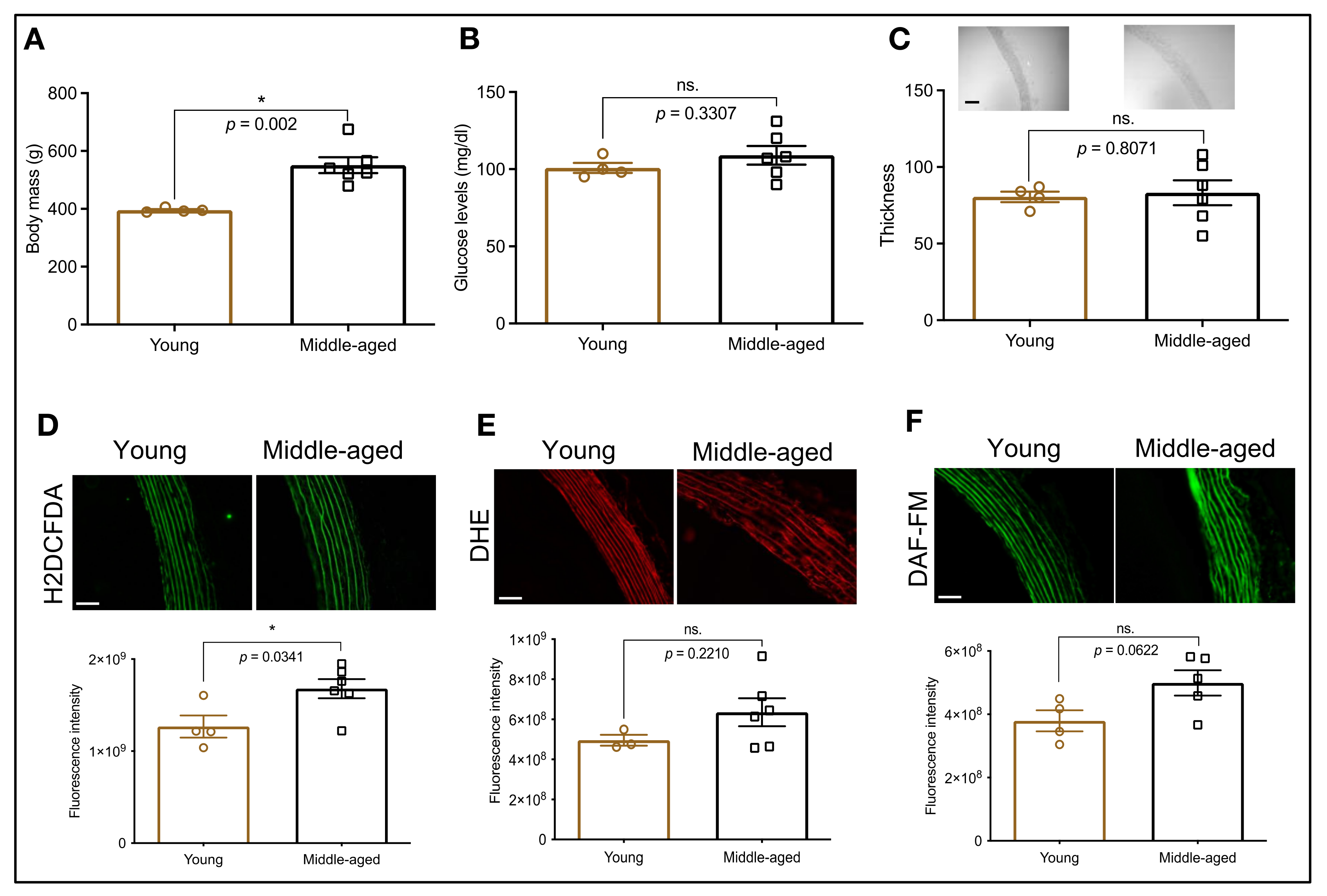
3.1. HSP70 Expression in the Aorta: An Age-Dependent Process
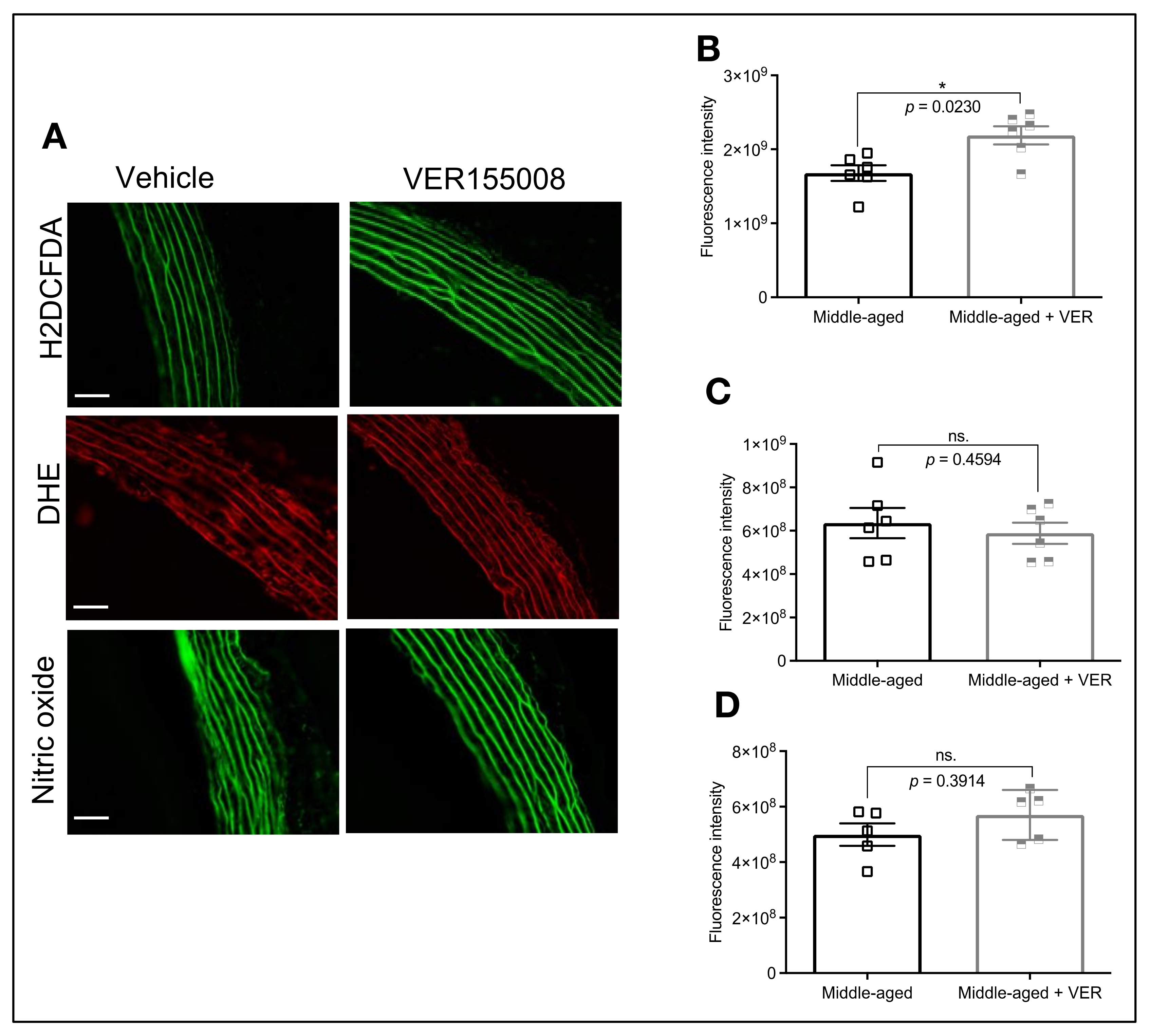
3.2. Blockade of HSP70 Intensifies the Age-Related Decline in Tonic Contraction
3.3. Inhibition of HSP70 Exacerbates the Aging-Related Decline in Ca2+ Dynamics Mediated Vascular Contraction
3.4. HSP70 and Vascular Oxidative Stress in Middle-Aged Animals
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jani, B.; Rajkumar, C. Ageing and Vascular Ageing. Postgrad. Med. J. 2006, 82, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, T.; Partridge, L. Ageing as a Risk Factor for Disease. Curr. Biol. 2012, 22, R741–R752. [Google Scholar] [CrossRef] [PubMed]
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing, 2019 Highlights; United Nations: New York, NY, USA, 2020; ISBN 978-92-1-148325-3. [Google Scholar]
- Donato, A.J.; Machin, D.R.; Lesniewski, L.A. Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ. Res. 2018, 123, 825–848. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tarantini, S.; Donato, A.J.; Galvan, V.; Csiszar, A. Mechanisms of Vascular Aging. Circ. Res. 2018, 123, 849–867. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Münzel, T. Endothelial Nitric Oxide Synthase in Vascular Disease: From Marvel to Menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the Nomenclature of the Human Heat Shock Proteins. Cell Stress Chaperones 2009, 14, 105–111. [Google Scholar] [CrossRef]
- De Toda, I.M.; De la Fuente, M. The Role of Hsp70 in Oxi-Inflamm-Aging and Its Use as a Potential Biomarker of Lifespan. Biogerontology 2015, 16, 709–721. [Google Scholar] [CrossRef]
- De Toda, I.M.; Vida, C.; Ortega, E.; De La Fuente, M. Hsp70 Basal Levels, a Tissue Marker of the Rate of Aging and Longevity in Mice. Exp. Gerontol. 2016, 84, 21–28. [Google Scholar] [CrossRef]
- Chin, J.H.; Okazaki, M.; Hu, Z.W.; Miller, J.W.; Hoffman, B.B. Activation of Heat Shock Protein (Hsp)70 and Proto-Oncogene Expression by A1 Adrenergic Agonists in Rat Aorta with Age. J. Clin. Investig. 1996, 97, 2316–2323. [Google Scholar] [CrossRef]
- De Oliveira, A.A.; Mendoza, V.O.; Rastogi, S.; Nunes, K.P. New Insights into the Role and Therapeutic Potential of HSP70 in Diabetes. Pharmacol. Res. 2022, 178, 106173. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.A.; Nunes, K.P. An Additional Physiological Role for HSP70: Assistance of Vascular Reactivity. Life Sci. 2020, 256, 117986. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.A.; Priviero, F.; Tostes, R.C.; Webb, R.C.; Nunes, K.P. Dissecting the Interaction between HSP70 and Vascular Contraction: Role of Ca2+ Handling Mechanisms. Sci. Rep. 2021, 11, 1420. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.A.; Priviero, F.; Webb, R.C.; Nunes, K.P. Impaired HSP70 Expression in the Aorta of Female Rats: A Novel Insight into Sex-Specific Differences in Vascular Function. Front. Physiol. 2021, 12, 666696. [Google Scholar] [CrossRef]
- Massey, A.J.; Williamson, D.S.; Browne, H.; Murray, J.B.; Dokurno, P.; Shaw, T.; Macias, A.T.; Daniels, Z.; Geoffroy, S.; Dopson, M.; et al. A Novel, Small Molecule Inhibitor of Hsc70/Hsp70 Potentiates Hsp90 Inhibitor Induced Apoptosis in HCT116 Colon Carcinoma Cells. Cancer Chemother. Pharmacol. 2010, 66, 535–545. [Google Scholar] [CrossRef]
- Matz, R.L.; De Sotomayor, M.A.; Schott, C.; Andriantsitohaina, R. Preservation of Vascular Contraction during Ageing: Dual Effect on Calcium Handling and Sensitization. Br. J. Pharmacol. 2003, 138, 745–750. [Google Scholar] [CrossRef]
- Kim, Y.K.; Suarez, J.; Hu, Y.; McDonough, P.M.; Boer, C.; Dix, D.J.; Dillmann, W.H. Deletion of the Inducible 70-KDa Heat Shock Protein Genes in Mice Impairs Cardiac Contractile Function and Calcium Handling Associated with Hypertrophy. Circulation 2006, 113, 2589–2597. [Google Scholar] [CrossRef]
- Senf, S.M.; Howard, T.M.; Ahn, B.; Ferreira, L.F.; Judge, A.R. Loss of the Inducible Hsp70 Delays the Inflammatory Response to Skeletal Muscle Injury and Severely Impairs Muscle Regeneration. PLoS ONE 2013, 8, e62687. [Google Scholar] [CrossRef]
- Sena, C.M.; Leandro, A.; Azul, L.; Seiça, R.; Perry, G. Vascular Oxidative Stress: Impact and Therapeutic Approaches. Front. Physiol. 2018, 9, 1668. [Google Scholar] [CrossRef]
- Sengupta, P. The Laboratory Rat: Relating Its Age with Human’s. Int. J. Prev. Med. 2013, 4, 624. [Google Scholar]
- Jousilahti, P.; Vartiainen, E.; Tuomilehto, J.; Puska, P. Sex, Age, Cardiovascular Risk Factors, and Coronary Heart Disease. Circulation 1999, 99, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Yazdanyar, A.; Newman, A.B. The Burden of Cardiovascular Disease in the Elderly: Morbidity, Mortality, and Costs. Clin. Geriatr. Med. 2009, 25, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.K.; Park, T.-G.; Kim, Y.H.; Cho, J.W.; Kang, B.-S.; Kim, C.-Y. Heat-Shock Response Is Associated with Enhanced Contractility of Vascular Smooth Muscle in Isolated Rat Aorta. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2004, 369, 402–407. [Google Scholar] [CrossRef]
- Seok, Y.M.; Kim, J.I.; Ito, M.; Kureishi, Y.; Nakano, T.; Kim, S.O.; Lim, D.G.; Park, W.H.; Kim, I.K. Heat Shock-Induced Augmentation of Vascular Contractility Is Independent of Rho-Kinase. Clin. Exp. Pharmacol. Physiol. 2006, 33, 264–268. [Google Scholar] [CrossRef]
- Seawright, J.W.; Sreenivasappa, H.; Gibbs, H.C.; Padgham, S.; Shin, S.Y.; Chaponnier, C.; Yeh, A.T.; Trzeciakowski, J.P.; Woodman, C.R.; Trache, A. Vascular Smooth Muscle Contractile Function Declines With Age in Skeletal Muscle Feed Arteries. Front. Physiol. 2018, 9, 856. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.E.; Webb, R.C. The RhoA/Rho-Kinase Signaling Pathway in Vascular Smooth Muscle Contraction: Biochemistry, Physiology, and Pharmacology. In Comprehensive Hypertension; Elsevier Inc.: Amsterdam, The Netherlands, 2007; pp. 167–181. ISBN 9780323039611. [Google Scholar]
- Nunes, K.P.; Webb, R.C. New Insights into RhoA/Rho-Kinase Signaling: A Key Regulator of Vascular Contraction. Small GTPases 2020, 12, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Georgeon-Chartier, C.; Menguy, C.; Prévot, A.; Morel, J.-L. Effect of Aging on Calcium Signaling in C57Bl6J Mouse Cerebral Arteries. Pflugers Arch. 2013, 465, 829–838. [Google Scholar] [CrossRef]
- Albarwani, S.A.; Mansour, F.; Khan, A.A.; Al-Lawati, I.; Al-Kaabi, A.; Al-Busaidi, A.-M.; Al-Hadhrami, S.; Al-Husseini, I.; Al-Siyabi, S.; Tanira, M.O. Aging Reduces L-Type Calcium Channel Current and the Vasodilatory Response of Small Mesenteric Arteries to Calcium Channel Blockers. Front. Physiol. 2016, 7, 171. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, J.; Wang, X.; Xue, N.; Du, J.; Meng, X.; Shen, B. Contrasting Patterns of Agonist-Induced Store-Operated Ca2+ Entry and Vasoconstriction in Mesenteric Arteries and Aorta With Aging. J. Cardiovasc. Pharmacol. 2015, 65, 571–578. [Google Scholar] [CrossRef]
- Fukuda, T.; Kuroda, T.; Kono, M.; Miyamoto, T.; Tanaka, M.; Matsui, T. Attenuation of L-Type Ca2+ Channel Expression and Vasomotor Response in the Aorta with Age in Both Wistar-Kyoto and Spontaneously Hypertensive Rats. PLoS ONE 2014, 9, e88975. [Google Scholar] [CrossRef]
- Madreiter-Sokolowski, C.T.; Thomas, C.; Ristow, M. Interrelation between ROS and Ca2+ in Aging and Age-Related Diseases. Redox Biol. 2020, 36, 101678. [Google Scholar] [CrossRef] [PubMed]
- Iida, Y.; Katusic, Z.S.; Wei, E.P. Mechanisms of Cerebral Arterial Relaxations to Hydrogen Peroxide. Stroke 2000, 31, 2224–2230. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.J.; Zhang, Y.; Hirota, S.; Janssen, L.J.; Lee, R.M.K.W. Vascular Relaxation Response to Hydrogen Peroxide Is Impaired in Hypertension. Br. J. Pharmacol. 2004, 142, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.I.; Harrison, D.G. Methods for Detection of Mitochondrial and Cellular Reactive Oxygen Species. Antioxid. Redox Signal. 2014, 20, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Park, K.A.; Lee, H.J.; Park, M.S.; Lee, J.H.; Park, K.C.; Kim, M.; Lee, S.-H.; Seo, J.-S.; Yoon, B.-W. Expression of Cu/Zn SOD Protein Is Suppressed in Hsp 70.1 Knockout Mice. J. Biochem. Mol. Biol. 2005, 38, 111–114. [Google Scholar] [CrossRef]
- Miao, L.; St Clair, D.K. Regulation of Superoxide Dismutase Genes: Implications in Disease. Free Radic. Biol. Med. 2009, 47, 344–356. [Google Scholar] [CrossRef]
- Radi, R. Oxygen Radicals, Nitric Oxide, and Peroxynitrite: Redox Pathways in Molecular Medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef]
- Chung, J.; Nguyen, A.K.; Henstridge, D.C.; Holmes, A.G.; Chan, M.S.; Mesa, J.L.; Lancaster, G.I.; Southgate, R.J.; Bruce, C.R.; Duffy, S.J.; et al. HSP72 Protects against Obesity-Induced Insulin Resistance. Proc. Natl. Acad. Sci. USA 2008, 105, 1739–1744. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, A.A.; Mendoza, V.O.; Priviero, F.; Webb, R.C.; Nunes, K.P. Age-Related Decline in Vascular Responses to Phenylephrine Is Associated with Reduced Levels of HSP70. Biomolecules 2022, 12, 1125. https://doi.org/10.3390/biom12081125
de Oliveira AA, Mendoza VO, Priviero F, Webb RC, Nunes KP. Age-Related Decline in Vascular Responses to Phenylephrine Is Associated with Reduced Levels of HSP70. Biomolecules. 2022; 12(8):1125. https://doi.org/10.3390/biom12081125
Chicago/Turabian Stylede Oliveira, Amanda A., Valentina O. Mendoza, Fernanda Priviero, R. Clinton Webb, and Kenia P. Nunes. 2022. "Age-Related Decline in Vascular Responses to Phenylephrine Is Associated with Reduced Levels of HSP70" Biomolecules 12, no. 8: 1125. https://doi.org/10.3390/biom12081125
APA Stylede Oliveira, A. A., Mendoza, V. O., Priviero, F., Webb, R. C., & Nunes, K. P. (2022). Age-Related Decline in Vascular Responses to Phenylephrine Is Associated with Reduced Levels of HSP70. Biomolecules, 12(8), 1125. https://doi.org/10.3390/biom12081125







