Abstract
Lycopene is a carotenoid found in tomatoes that has potent antioxidant activity. The Mediterranean diet is particularly rich in lycopene, which has well-known beneficial effects on cardiovascular health. We tested the effects of lycopene extract in a group of 20 ApoE knockout mice, fed with a high fat western diet for 14 weeks. Starting from week 3 and up to week 14, the mice were randomly divided into two groups that received lycopene (n = 10) by oral suspension every day at the human equivalent dose of 60 mg/day (0.246 mg/mouse/day), or the vehicle solution (n = 10). The lycopene administration reduced triglycerides and cholesterol blood levels starting from week 6 and continuing through to the end of the experiment (p < 0.001). This reduction was mediated by an enhanced liver expression of PPAR-α and AMPK-α and reduced SREBP levels (p < 0.0001). As a histological red-out, the extent of atherosclerotic plaques and the intima–media thickness in the aorta were significantly reduced by lycopene. In this context, lycopene augmented the Nrf-2 positivity staining in the endothelium, thereby confirming that its antioxidant activity was mediated by this nuclear factor. The positive results obtained in this pre-clinical model further support the use of lycopene extracts to reduce atherosclerosis.
1. Introduction
Atherosclerosis is a chronic inflammatory disease that is responsible for several cardiovascular events such as ischemic heart disease, coronary artery disease, peripheral artery disease, and ischemic stroke [1]. The prevalence of atherosclerosis is increasing worldwide and due to the aging population the incidence of cardiovascular diseases is expected to rise, together with disease-related deaths and disabilities. A male gender, smoking, and alcohol abuse, together with a high fat diet (HFD), are considered the factors associated with an increase in the prevalence rate from 1.655 per 100,000 inhabitants to 1.845/100,000, or more by 2030 [2]. The atherosclerotic process is characterized by lipid deposition accompanied with smooth muscle cells and fibrous matrix proliferation, which gradually develop into the formation of an atherosclerotic plaque that causes vascular stenosis, and when unstable, may fragment and cause thrombosis or embolism [3,4,5].
There is a large body of evidence supporting the role of AMPK (adenosine monophosphate-activated protein kinase) as an important factor for attenuating atherosclerosis development. This kinase was commonly known as a positive regulator of fatty acid oxidation that regulates multiple physiological processes including lipid and glucose metabolism and the normalization of energy imbalances [6,7].
A previous paper showed that the ability of polyphenols to stimulate AMPK and reduce hepatocellular lipid accumulation could be important for reducing atherosclerosis, at least in experimental models [7]. Among the natural activators of AMPK, lycopene has emerged in the past decades since it has been proven to modulate oxidative stress and the cytokine production involved in chronic processes such as cardiovascular diseases [8]. The protective beneficial effects of lycopene have been mainly attributed to its capacity to prevent atherogenesis through its antioxidant properties [9,10].
Additionally, lycopene decreased cholesterol absorption in the intestine and prevented atherosclerosis progression in a well-studied atherosclerosis model, which involved apolipoprotein E knockout (ApoE−/−) mice fed with HFD [11]. Moreover, lycopene strongly reduced cholesterol and triglyceride serum levels in ApoE*3Leiden mice fed with an atherogenic diet [12]. Lycopene’s beneficial effects, which have been achieved in studies on several cell types and under multiple experimental conditions, are widely known and summarized in previous papers, supporting the importance of this carotenoid for metabolic derangements and cardiovascular diseases [13,14]. In addition, in human studies, patients using lycopene supplementation demonstrated an increased metabolic profile in terms of reduced plasma low-density lipoprotein (LDL) and cholesterol levels [15,16].
As further a mechanism of action that could be useful in atherosclerosis development, lycopene can regulate hepatic lipid metabolism through SIRT-1 (sirtuin-1) activation, thus positively regulating the transcriptional activity of both the peroxisome proliferator-activated receptor alpha (PPARα) and the peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), which are involved in fatty acid catabolism [17]. The involvement of the SIRT1/PPARα/PGC-1α axis in lycopene’s mode of action, with a specific focus on hepatic steatosis and glucose metabolism, was confirmed in mice that over-expressed SIRT-1 and were fed with HFD [18]. SIRT1 may also regulate hepatic fatty acid utilization by reducing the expression of the transcription factor sterol regulatory element–binding proteins (SREBP-1), a positive regulator of fatty acid synthesis, and promoting the phosphorylation/activation of AMPK that regulates fatty acid oxidation, thus preventing lipid accumulation [7].
The effects of lycopene on this specific pathway in the context of atherosclerosis are not yet fully described; therefore, the aim of this study was to analyze the effects of a lycopene extract from Sicilian cherry tomatoes on atherosclerosis development in ApoE KO mice fed with HFD.
2. Material and Methods
2.1. Animals and Drugs
All animal procedures were in accordance with the Principles of Laboratory Animal Care (NIH publication no.85-23, revised 1985), authorized by the Ministry of Health Review Board for the care of laboratory animals (approval number 63/2017), and were in accordance with the ARRIVE Guidelines [19]. A total of 30 male ApoE−/− mice 12 weeks old (strain B6.129P2-Apoetm1Unc/J) were purchased from Charles River Laboratories (Calco, Milan, Italy). Animals were maintained in the animal facility of the Department of Clinical and Experimental Medicine of the University of Messina in standard cages and environmental conditions with water and food ad libitum. After 1 week of acclimation, animals were randomly assigned to 3 groups of 10 animals each; 1 group was fed with a normal diet for 14 weeks (control group) and the other 2 groups received high fat diet food (SAFE U8958 Version 35; Safe, Rosenberg, Germany) and were orally administered (in a fluid suspension given with a plastic pipette) with lycopene (0.264 mg/mouse/day) or vehicle, starting from week 3 and up to week 14.
Lycopene was obtained with 90% of purity by the cold-extraction method from Sicilian cherry tomatoes (that have the highest content in lycopene ≈90 μg/g) and was prepared fresh daily, dissolved in corn oil, mixed with 1% sucrose to improve its palatability (thus, mice were licking the fluid suspension from the plastic pipette), and administered every 24 h. The dose was chosen according to a pilot study that demonstrated the efficacy of a dietary supplementation (60 mg/day) in reducing plasma cholesterol in healthy young men [20] and was calculated according to the following formula AED (mg/kg) = Human does (mg/kg) × Km ratio.
Daily measurements included body weight, food, and water intake; in addition, at selected time points, a couple of drops of blood were obtained from the jugular vein and immediately used for lipid measurement. At the end of the experiment, the animals were anesthetized with a supramaximal dose of ketamine (100 mg/kg) and xylazine (10 mg/kg) and killed by exsanguination, and the blood taken from the heart was immediately used for lipid measurement. Following saline perfusion, liver and aortic samples were removed and stored for histological assessments and biochemical marker measurement.
2.2. Measurement of Cholesterol and Triglyceride Levels
The concentrations of total cholesterol and the triglyceride blood content were measured at 0, 2, 4, 6, and 14 weeks using the MultiCare-IN kit (Biochemical System Intern. Arezzo, Italy). Cholesterol detection range: 130–400 mg/dL; triglyceride detection range: 50–500 mg/dL.
2.3. Quantification of Atherosclerosis
At the end of the experiment, mouse aortas were harvested for atherosclerosis quantification following the American Heart Association’s statement on atherosclerosis. Aortas were perfused with saline by left ventricular puncture and were fixed in 10% formalin overnight. Adventitial fat was removed, and atherosclerosis was quantified on intima on the entire aorta (the arch, the thoracic, and the abdominal sections) using Image J software to calculate the percentage of atherosclerotic plaque surface area [21].
2.4. Histological Evaluation of Aortas
After exsanguination, the hearts with 1–1.5 cm of ascending thoracic aorta were removed from the animals and fixed in 10% buffered formalin, processed for paraffin embedding, sectioned at 5 μm, and subsequently stained with hematoxylin and eosin for examination under a light microscope (Leica microsystem, Wetzlar, Germany). Aortic intima–media thickness was measured from the endothelial surface to the adventitia in 10 different fields for each sample, using the Leica image software. The histological score was based on the microscopic appearance of the aortas considering (i) the loss of endothelial integrity, (ii) the presence of inflammatory infiltrate, (iii) the infiltration of the sub-intimal layer, (iv) the presence of red blood cells and fibrin deposition in the plaque, and (v) the presence of a clot in the sub-intimal area. Each of the above parameters were scored as follows: absent 0, mild 0.5, moderate 1, and severe 1.5, with a total range between 0 and 7.5.
2.5. Immunofluorescence Evaluation of Nrf2
Sections of aorta were rehydrated and incubated in sodium citrate buffer (10 mM Sodium citrate; 0.05% Tween 20; pH 6.0) in a microwave at 750 W for antigen retrieval. Once rehydrated, slides were pre-incubated with 0.3% Triton X-100 in PBS for 10 min and in PBS/albumin (1%) for 20 min. Primary antibodies for Nrf-2 and CD34 (1:150 and 1:100 dilution, respectively; Abcam, Cambridge, UK) were used to evaluate possible co-localization in endothelial cells. After washing three times with PBS for 10 min, Texas Red and Fitc-conjugated IgG (1:100 dilution; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) were used for Nrf-2 and CD34 detection, respectively. Sections were sealed with mounting medium following incubation with DAPI (1:1000 dilution; Sigma Chemicals, St. Louis, MO, USA) for 10 min in the dark at RT to label nuclei. Images were collected from a Zeiss LSM 510 confocal microscope equipped with Argon laser (458 and 488 nm λ) and two HeNe laser (543 and 633 nm λ) as previously described [22].
2.6. Real-Time Quantitative PCR Amplification (RTqPCR)
Total RNA was extracted from the liver tissues at the end of the experimental procedures using Trizol LS reagent (Invitrogen, Carlsbad, CA, USA). Total RNA (1 μg) was reverse transcribed in a final volume of 20 μL using a Superscript VILO kit (Invitrogen). The cDNA (1 μL) was added to the EvaGreen qPCR Master Mix (Biotium Inc., Fremont, CA, USA) at a final volume of 20 μL per well. Samples were run in duplicate, and β-actin was used as the housekeeping gene; the reaction was performed using the 2-step thermal protocol recommended by the manufacturer. The final primer concentration selected to perform the analysis was 10 μM. Target genes were PPARα, SREBF-1, and AMPK-α. Results were calculated using the 2−ΔΔCT method and expressed as n-fold increase in gene expression using the control group as calibrator [23,24,25]. Primers used for targets and reference genes are listed in Table 1.

Table 1.
Primer list.
2.7. Isolation of Total Proteins and Western Blot Analysis
Protein extraction was performed in liver for Western Blot analysis, as previously described [26,27]. About 30 μg of proteins were loaded and specific antibodies were used to evaluate PPARα (ab61182), SREBP-1 (ab28481) (1:250 and 1:1000 dilution respectively; Abcam, Cambridge, MA, USA), p-AMPK-α (2535), and β-actin (4970) (1:1000 dilution each; Cell Signaling, Beverly, MA, USA) which was used as loading control. The images were obtained using specific software (C-DiGit Blot Scanner with Image Studio 4.0 software, LI-Cor, Lincoln, NE, USA), and densitometric data were expressed as integrated intensity, following evaluation of a region of interest (ROI) identical for all samples within the same blot.
2.8. Statistical Analysis
All quantitative data are expressed as mean ± SD for each group. Longitudinal data were compared by two-way ANOVA with Sidak post-test for intergroup comparisons. A standard Student’s t-test was used for western blot data. One-way ANOVA was used to compare q-PCR and % lesion area data. Histological non-parametric data were analyzed using the Mann–Whitney U test. Statistical significance was set at p < 0.05. Graphs were drawn using GraphPad Prism software version 8.0 for macOS (GraphPad Software Inc., La Jolla, CA, USA).
3. Results
3.1. Lycopene Effects on Body Weight and Food Intake
The body weights and food intake were recorded at baseline and throughout the experimental period. All animals showed a physiological increase in body weight during the treatment period (from 25 ± 4 to 30 ± 5 g); additionally, considering food intake normalized over the weights (5 ± 1.5 g/mouse/day), no differences were observed among the three experimental groups.
3.2. Lycopene Effects on Triglycerides and Cholesterol Levels
Male ApoE−/− mice were fed with a normal diet or HFD for 14 weeks; the HFD animals were treated daily with either the vehicle or lycopene starting from week 3 and up to week 14. The HFD group showed a significant increase in both triglycerides and cholesterol levels at week 14 compared to the normal diet group. The lycopene treatment significantly reduced the triglyceride levels in the blood of the mice fed with HFD from week 4 (p < 0.05 vs. HFD; Figure 1A), showing a decrease in triglycerides during all the experimental periods (p < 0.0001 at the end of experiment vs. HFD; Figure 1A). Moreover, the lycopene administration significantly reduced cholesterol levels in the blood from week 6 to the end of the experiment (p < 0.05 at week 6 vs. HFD; p < 0.001 at week 14 vs. HFD; Figure 1B).
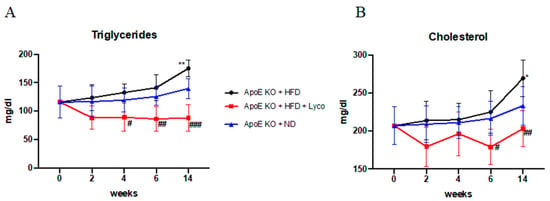
Figure 1.
Triglyceride and cholesterol levels in blood evaluated during the experimental period. Values were obtained from 10 animals per group and are expressed as mean ± SD. Triglycerides (A) ** p < 0.001 vs. ApoE KO + ND; # p < 0.05 vs. ApoE KO + HFD; ## p < 0.001 vs. ApoE KO + HFD; ### p < 0.0001 vs. ApoE KO + HFD. Cholesterol (B) * p < 0.05 vs. ApoE KO + ND; # p < 0.05 vs. ApoE KO + HFD; ## p < 0.001 vs. ApoE KO + HFD.
3.3. Effects of Lycopene Treatment on Atherosclerotic Lesions
The atherosclerotic plaques were quantified by Oil Red O staining of the intimal surfaces of the entire aorta (the arch, the thoracic, and the abdominal sections of the ApoE animals). The results showed a difference in the lesion areas between groups; in particular, the number of atherosclerotic lesions was significantly increased in the ApoE KO + HFD compared to the ApoE KO + ND group, whereas the ApoE KO + HFD + Lycopene group showed a marked reduction of atherosclerotic lesions compared to the ApoE KO + HFD group (Figure 2). The quantification of the percentage of atherosclerotic plaque surface area is summarized in Figure 2D.
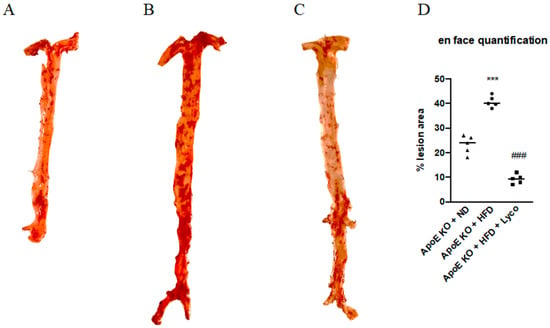
Figure 2.
Effects of Lycopene on percentage of lesion surface area in aortas samples stained with oil-red-o from ApoE KO + ND (A), ApoE KO + HFD (B), and ApoE KO + HFD + Lyco (C) groups. The graph in (D) shows the quantification of the percentage of atherosclerotic plaque surface area. Values were obtained from 5 animals per group and are expressed as the means ± SD. *** p < 0.0001 vs. ApoE KO + ND; ### p < 0.0001 vs. ApoE KO + HFD.
3.4. Lycopene Reduced Histological Damage
To evaluate the histological characteristics of the thoracic aortas, the aortas were stained with hematoxylin and eosin (H&E). The intima–media thickness (IMT) quantification demonstrated a mean of 59.5 ± 6.3 μm for the untreated ApoE mice and a strong reduction was obtained after 11 weeks of lycopene administration (52.1 ± 4.6 μm; p < 0.05; Figure 3A,B). Moreover, a significant reduction in the histological score was observed in the ApoE KO + HFD + Lyco group compared to the untreated animals (p < 0.0001 vs. ApoE KO + HFD group; Figure 3C).
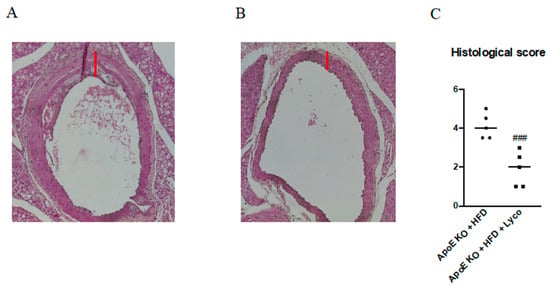
Figure 3.
Representative H&E images (original magnification 10×) of thoracic aortas from ApoE KO + HFD (A) and ApoE KO + HFD + Lyco (B). The graph in (C) represents the histological score. Values are expressed as the mean ± SD of 5 animals. ### p < 0.0001 vs. ApoE KO + HFD.
3.5. Immunofluorescence Evaluation of Nrf2
The thoracic aortas of the ApoE KO + HFD group exhibited the rare presence of Nrf2 positive staining (red channel, Figure 4A); the simultaneous labeling of CD34 (green channel; Figure 4B) demonstrated that Nrf2 was expressed in the endothelial CD34+ cells mainly in the tunica intima of aorta, as evidenced by the yellow fluorescence (Figure 4C). The samples taken from the ApoE KO + HFD + Lycopene group showed a significant increase in Nrf2 expression (Figure 4G) in the endothelial CD34+ cells along the tunica intima and media of the aorta compared to the untreated group, as demonstrated by the merge signal (Figure 4H). The display profile obtained by the confocal laser showed a high intensity fluorescence pattern for Nfr2 in the ApoE KO + HFD + Lycopene mice compared to the fluorescence pattern observed in the untreated ApoE KO + HFD mice (Figure 5).
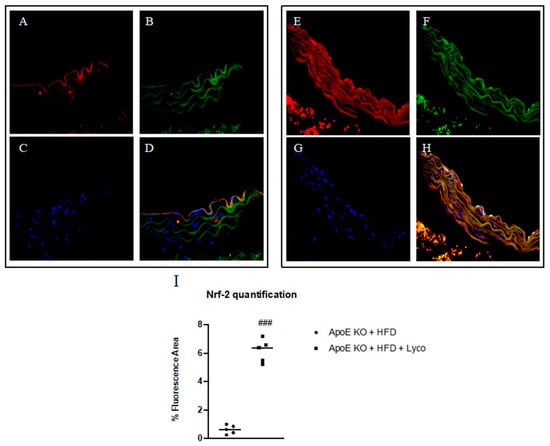
Figure 4.
Immunofluorescence staining for Nrf2 (red channel, (A,E)) and CD34 (green channel, (B,F)) in aorta samples of ApoE KO + HFD and ApoE KO + HFD + Lycopene mice. The merge signal shows the co-localization of Nrf2 in CD34+ cells (D,H). Panels (C,G) showed nuclei stained with DAPI. The graph in (I) shows the quantification of Nrf2 expression performed with Image-J. Values are expressed as the mean ± SD of 5 animals. ### p < 0.0001 vs. ApoE KO + HFD.
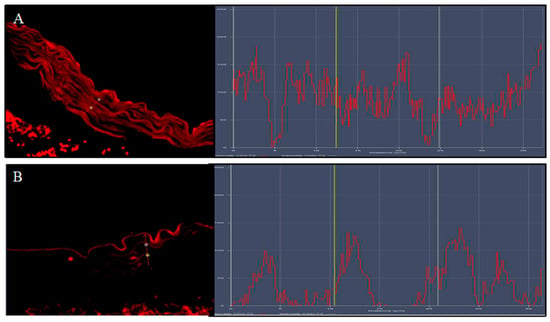
Figure 5.
The images show the display profile obtained by the confocal laser; a high intensity of fluorescence pattern for Nfr2 was observed in ApoE KO + HFD + Lycopene mice (A) compared to the fluorescence pattern observed in untreated ApoE KO + HFD mice (B).
3.6. Lycopene Effects on PPARα, SREBF-1 and AMPK-α Expression
The animals fed with HFD showed a marked reduction of PPARα and AMPK-α mRNA expression compared to the ApoE KO + ND group (p < 0.0001 vs. ApoE KO + ND group; Figure 6A,C). Lycopene treatment caused a significant increase in PPARα and AMPK-α mRNA expression compared to the ApoE KO + HFD group (p < 0.0001 vs. ApoE KO + HFD group; Figure 6A,C). In contrast, SREBPF-1 mRNA expression was significantly increased in the ApoE KO + HFD group compared to the ApoE KO + ND group (p < 0.0001 vs. ApoE KO + ND group; Figure 6B) and reduced following the lycopene treatment in the HFD animals (p < 0.0001 vs. ApoE KO + HFD group; Figure 6B). Similar results were obtained when evaluating the protein expression of PPARα, SREBP-1, and p-AMPK-α using Western blot analysis of the liver samples (p < 0.0001 vs. ApoE KO + HFD group; Figure 7).
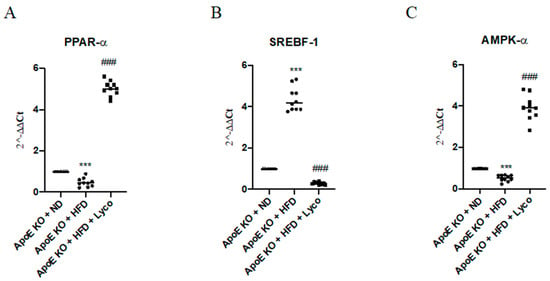
Figure 6.
The graphs represent qPCR results of PPAR-α (A), SREBF-1 (B), AMPK-α, and (C) mRNA expression from liver samples. Values were obtained from 10 animals per group and are expressed as the means and SD.*** p < 0.0001 vs. ApoE KO + ND; ### p < 0.0001 vs. ApoE KO + HFD.
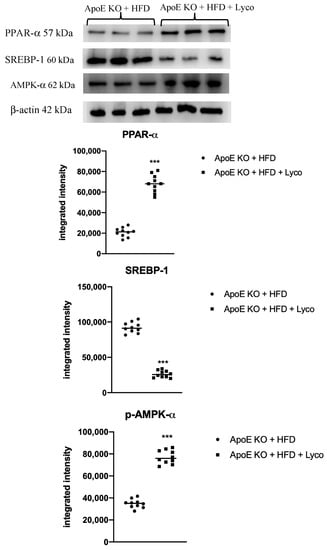
Figure 7.
Western blot analysis of PPAR-α, SREBP-1c, and p-AMPK-α in liver samples. Values were obtained from 10 animals per group and are expressed as the means and SD. *** p < 0.0001 vs. ApoE KO + ND.
4. Discussion
Several mechanisms are involved in atherosclerosis development, such as the increase in the absorption of oxLDL by macrophages, the generation of Reactive Oxygen Species (ROS), and pro-inflammatory cytokines [28,29]. Lycopene demonstrated to be an effective antioxidant in several experimental models [10], and in accordance with these findings, our results showed a significant increase in Nrf2 expression, a nuclear factor responsible for encoding phase II detoxification enzymes, thus blocking phase I metabolism and metabolic activation. The increased presence of Nrf2 in the endothelium of aortas is not surprising given that prior research has shown similar results in cultured HUVEC cells through the stimulation of heme oxygenase-1 (HO-1) gene expression and the activation of the phosphoinositide 3-kinase (PI3K)/Akt pathway [30]. In addition, these results were in accordance with a previous paper that demonstrated in NZW rabbits that lycopene supplementation for 4 weeks strongly suppressed diet-induced increases in total and LDL cholesterol levels, in serum, and reduced the accumulation of cholesteryl esters in aortic tissue without affecting body weight [31]. In a longer experimental setting using the same type of animals, dietary cholesterol supplementation with lycopene reduced the intestinal absorption of cholesterol and inhibited 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase and Acyl-coenzymeA: cholesterol acyltransferase (ACAT) activity in the liver, thus contributing to a massive reduction in plaques, despite the high cholesterol diet, and further highlighting the beneficial effects of lycopene in a well-established condition [32]. These results are suggestive of a strong antioxidant activity in the vessel wall, where atherosclerosis takes place, and thus further support the importance of this biological compound in modulating plaque development.
As plaque reduction is also a matter of decreased cholesterol and lipid accumulation, an issue that may arise involves the intake of food in the studied animals. However, no difference in body weight or food consumption has been observed throughout the study; therefore, it can be hypothesized that lycopene supplementation strongly suppressed the diet-induced increase in cholesterol and triglyceride levels at the metabolic level. Furthermore, a meta-analysis demonstrated that the consumption of 25 mg lycopene/day in humans significantly reduced total cholesterol levels, and the effects were comparable to low-dose statin treatment [33]. Additionally, a previous paper demonstrated that tomato juice supplementation could reduce triglyceride serum levels in young females that received 32.5 mg of lycopene for 8 weeks [34]. Lycopene can also modulate hepatic lipid metabolism and avoid the hepatic steatosis caused by a high-fat diet by activating SIRT1 and consequently suppressing lipogenesis, promoting lipid catabolism in the liver and skeletal muscles, and mobilizing lipids in white adipose tissue [35]. In particular, at the hepatic level, SIRT1 positively regulates the transcriptional activity of PPARα by promoting β-oxidation [18,36]; in agreement with these data, our results showed an increase in PPARα expression as a consequence of lycopene administration. The reduced lipid levels in serum may also be due to a modulation of fatty acid utilization through SREBP-1c and AMPK in the liver. In the present study, SREBP-1c expression was significantly reduced, and p-AMPK-α levels were significantly increased in the lycopene treated mice compared to the untreated group. These results were in line with previous papers that demonstrated the pivotal role of the SIRT1 agonist in reducing SREBP-1c expression and increasing AMPK activation [7,37]. Considering that the invasion of lipids into the aortic tissue is the first step in plaque progression and is required for the development of advanced aortic plaques, an increased lipid metabolism could account for a reduced accumulation of lipids within the aortic tissue with a consequent slowdown in the progression of atherosclerotic change. As a matter of fact, the analysis of atherosclerotic lesions revealed that lycopene-treated mice developed significantly fewer atherosclerotic lesions than the untreated group. Accordingly, in another study, using rabbits fed a high fat diet, lycopene showed to be similar to statins in terms of the degree of atherosclerotic lesion reduction [38], while reduced vascular inflammation and improved endothelial function have been obtained in HUVECs through the inhibition of adhesion molecule-1 (ICAM-1) expression [39]. As shown in clinical studies, lycopene has a beneficial effect on the intima–media thickness, a measure of the atherosclerosis’s severity, as high lycopene serum levels were correlated with a reduced IMT and a slow IMT progression [40,41,42] in subjects with an anti-oxidant rich diet. Our study confirmed this effect using an adjusted lycopene dose that was found effective in humans [20], demonstrating that even in a genetically biased model this flavonoid can exert beneficial effects.
Nevertheless, the present study has some limitations, for instance, we did not include animals pre-treated with lycopene as a preventive intervention nor a group fed with HFD and treated with statins as the gold-standard treatment. The preventive treatment with lycopene would have added little to the study, considering that ApoE mice represent a model of genetic atherosclerosis and develop plaques regardless of the HFD, which serves as an accelerator of the disease. Additionally, our main goal was to assess whether or not lycopene could be considered as a therapeutic option, possibly together with a proper diet or with low dose cholesterol-lowering drugs. Furthermore, additional studies should be performed to compare the efficacy of this extract to other substances with well-established antioxidant and anti-atherosclerotic activities, such as resveratrol or fish oil. Moreover, considering that atherosclerosis is related to gender [43], it could be of interest to evaluate the efficacy of lycopene treatment in both sexes, but here the results have been obtained in solely male mice. In addition, we did not assess lycopene’s anti-atherosclerotic effects in a chronic model of atherosclerosis, and such aspects should be evaluated in future studies considering a longer disease course and a human lifespan. However, in the present experiment, the efficacy of lycopene supplementation was demonstrated, indicating that lycopene may be used as a possible adjuvant treatment for the management of atherosclerosis due to its antioxidant function, lipid-lowering effects, and modulation of fatty acid utilization and oxidation. Therefore, these additional data may support the clinical use of lycopene, although this speculation needs to be proven in a clinical trial.
Author Contributions
Conceptualization, F.S. and A.B.; Formal analysis, F.M., G.P., D.A. and G.V.; Funding acquisition, F.S. and A.B.; Methodology, A.B. and N.I.; Supervision, A.B.; Validation, D.A. and F.S.; Writing—original draft, F.M. and G.P.; Writing—review & editing, D.A., A.B. and N.I. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from Ministry of Research (PON02_00451_3361785-PONREC—DIMESA) assigned to Prof Francesco Squadrito.
Institutional Review Board Statement
All animal procedures were approved by the Ministry of Health Review Board for the care of laboratory animals (protocol number 63/2017 approved on 01-26-2017).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Taleb, S. Inflammation in atherosclerosis. Arch. Cardiovasc. Dis. 2016, 109, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; AlKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Li, W.; Li, X.; Zhou, H. Inflammation: A Novel Therapeutic Target/Direction in Atherosclerosis. Curr. Pharm. Des. 2017, 23, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Geovanini, G.R.; Libby, P. Atherosclerosis and inflammation: Overview and updates. Clin. Sci. 2018, 132, 1243–1252. [Google Scholar] [CrossRef]
- Ou, H.; Liu, C.; Feng, W.; Xiao, X.; Tang, S.; Mo, Z. Role of AMPK in atherosclerosis via autophagy regulation. Sci. China Life Sci. 2018, 61, 1212–1221. [Google Scholar] [CrossRef]
- Hou, X.; Xu, S.; Maitland-Toolan, K.A.; Sato, K.; Jiang, B.; Ido, Y.; Lan, F.; Walsh, K.; Wierzbicki, M.; Verbeuren, T.J.; et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J. Biol. Chem. 2008, 283, 20015–20026. [Google Scholar] [CrossRef] [Green Version]
- Rao, A.V. Lycopene, tomatoes, and the prevention of coronary heart disease. Exp. Biol. Med. 2002, 227, 908–913. [Google Scholar] [CrossRef]
- Müller, L.; Caris-Veyrat, C.; Lowe, G.; Böhm, V. Lycopene and its antioxidant role in the prevention of cardiovascular diseases—A critical review. Crit. Rev. Sci. Nutr. 2016, 56, 1868–1879. [Google Scholar]
- Visioli, F.; Riso, P.; Grande, S.; Galli, C.; Porrini, M. Protective action of tomato products in in vivo markers of lipid oxidation. Eur. J. Nutr. 2003, 42, 201–206. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Liu, Z.; Wang, Q.; Liu, J.; Feng, D.; Zou, J. Lycopene Reduces Cholesterol Absorption and Prevents Atherosclerosis in ApoE-/- Mice by Downregulating HNF-1α and NPC1L1 Expression. J. Agric. Food Chem. 2021, 69, 10114–10120. [Google Scholar] [CrossRef] [PubMed]
- Verschuren, L.; Wielinga, P.Y.; van Duyvenvoorde, W.; Tijani, S.; Toet, K.; van Ommen, B.; Kooistra, T.; Kleemann, R. A dietary mixture containing fish oil, resveratrol, lycopene, catechins, and vitamins E and C reduces atherosclerosis in transgenic mice. J. Nutr. 2011, 141, 863–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thies, F.; Mills, L.M.; Moir, S.; Masson, L.F. Cardiovascular benefits of lycopene: Fantasy or reality? Proc. Nutr. Soc. 2017, 76, 122–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heber, D.; Lu, Q.Y. Overview of mechanisms of action of lycopene. Exp. Biol. Med. 2002, 227, 920–923. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, K.D.; Pittaway, J.K.; Ball, M.J. Effects of olive oil and tomato lycopene combination on serum lycopene, lipid profile, and lipid oxidation. Nutrition 2006, 22, 259–265. [Google Scholar] [CrossRef] [Green Version]
- Riccioni, G.; Gammone, M.A.; Currenti, W.; D’Orazio, N. Effectiveness and Safety of Dietetic Supplementation of a New Nutraceutical on Lipid Profile and Serum Inflammation Biomarkers in Hypercholesterolemic Patients. Molecules 2018, 23, 1168. [Google Scholar] [CrossRef] [Green Version]
- Cantò, C.; Auwerx, J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Pfluger, P.T.; Herranz, D.; Velasco-Miguel, S.; Serrano, M.; Tschop, M.H. Sirt1 protects against high-fat diet-induced metabolic damage. Proc. Natl. Acad. Sci. USA 2008, 105, 9793–9798. [Google Scholar] [CrossRef] [Green Version]
- McGrath, J.; Drummond, G.; Kilkenny, C.; Wainwright, C. Guidelines for reporting experiments involving animals: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1573–1576. [Google Scholar] [CrossRef] [Green Version]
- Fuhrman, B.; Elis, A.; Aviram, M. Hypocholesterolemic effect of lycopene and beta-carotene is related to suppression of cholesterol synthesis and augmentation of LDL receptor activity in macrophages. Biochem. Biophys. Res. Commun. 1997, 233, 658–662. [Google Scholar] [CrossRef] [Green Version]
- Daugherty, A.; Whitman, S.C. Quantification of atherosclerosis in mice. Methods Mol. Biol. 2003, 209, 293–309. [Google Scholar] [PubMed]
- Irrera, N.; Arcoraci, V.; Mannino, F.; Vermiglio, G.; Pallio, G.; Minutoli, L.; Bagnato, G.; Anastasi, G.P.; Mazzon, E.; Bramanti, P.; et al. Activation of A2A Receptor by PDRN Reduces Neuronal Damage and Stimulates WNT/β-CATENIN Driven Neurogenesis in Spinal Cord Injury. Front. Pharmacol. 2018, 9, 506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzino, G.; Irrera, N.; Galfo, F.; Pallio, G.; Mannino, F.; D’amore, A.; Pellegrino, E.; Ieni, A.; Russo, G.T.; Calapai, M.; et al. Effects of the antagomiRs 15b and 200b on the altered healing pattern of diabetic mice. Br. J. Pharmacol. 2018, 175, 644–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picciolo, G.; Pallio, G.; Altavilla, D.; Vaccaro, M.; Oteri, G.; Irrera, N.; Squadrito, F. β-Caryophyllene Reduces the Inflammatory Phenotype of Periodontal Cells by Targeting CB2 Receptors. Biomedicines 2020, 8, 164. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S.; Marini, H.R.; Micali, A.; Freni, J.; Pallio, G.; Irrera, N.; Squadrito, F.; Altavilla, D.; Antonelli, A.; Ferrari, S.M.; et al. Protective Effects of Myo-Inositol and Selenium on Cadmium-Induced Thyroid Toxicity in Mice. Nutrients 2020, 12, 1222. [Google Scholar] [CrossRef]
- Minutoli, L.; Marini, H.; Rinaldi, M.; Bitto, A.; Irrera, N.; Pizzino, G.; Pallio, G.; Calò, M.; Adamo, E.B.; Trichilo, V.; et al. A dual inhibitor of cyclooxygenase and 5-lipoxygenase protects against kainic acid-induced brain injury. Neuromolecular. Med. 2015, 17, 192–201. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Bitto, A.; Pallio, G.; Mannino, F.; Arcoraci, V.; Aliquò, F.; Minutoli, L.; De Ponte, C.; D’andrea, P.; et al. Cadmium-Induced Oxidative Stress Impairs Glycemic Control in Adolescents. Oxid. Med. Cell Longev. 2017, 2017, 6341671. [Google Scholar] [CrossRef]
- Getz, G.S.; Reardon, C.A. Atherogenic lipids and macrophage subsets. Curr. Opin. Lipidol. 2015, 26, 357–361. [Google Scholar] [CrossRef]
- Sugamura, K.; Keaney, J.F., Jr. Reactive oxygen species in cardiovascular disease. Free Radic. Biol. Med. 2011, 51, 978–992. [Google Scholar] [CrossRef] [Green Version]
- Sung, L.C.; Chao, H.H.; Chen, C.H.; Tsai, J.C.; Liu, J.C.; Hong, H.J.; Cheng, T.H.; Chen, J.J. Lycopene inhibits cyclic strain-induced endothelin-1 expression through the suppression of reactive oxygen species generation and induction of heme oxygenase-1 in human umbilical vein endothelial cells. Clin. Exp. Pharmacol. Physiol. 2015, 42, 632–639. [Google Scholar] [CrossRef]
- Lorenz, M.; Fechner, M.; Kalkowski, J.; Fröhlich, K.; Trautmann, A.; Böhm, V.; Liebisch, G.; Lehneis, S.; Schmitz, G.; Ludwig, A.; et al. Effects of lycopene on the initial state of atherosclerosis in New Zealand White (NZW) rabbits. PLoS ONE 2012, 7, e30808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verghese, M.; Richardson, J.E.; Boateng, J.; Shackelford, L.A.; Howard, C.; Walker, L.T.; Chawanet, C.B. Dietary lycopene has a protective effect on cardiovascular disease in New Zeeland male rabbits. J. Biol. Sci. 2008, 8, 268–277. [Google Scholar] [CrossRef] [Green Version]
- Ried, K.; Fakler, P. Protective effect of lycopene on serum cholesterol and blood pressure: Meta-analyses of intervention trials. Maturitas 2011, 68, 299–310. [Google Scholar] [CrossRef]
- Mozos, I.; Stoian, D.; Caraba, A.; Malainer, C.; Horbańczuk, J.O.; Atanasov, A.G. Lycopene and Vascular Health. Front. Pharmacol. 2018, 9, 521. [Google Scholar] [CrossRef]
- Lomb, D.J.; Laurent, G.; Haigis, M.C. Sirtuins regulate key aspects of lipid metabolism. Biochim. Biophys. Acta 2010, 1804, 1652–1657. [Google Scholar] [CrossRef] [PubMed]
- Purushotham, A.; Schug, T.T.; Xu, Q.; Surapureddi, S.; Guo, X.; Li, X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009, 9, 327–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Zhang, S.; Blander, G.; Tse, J.G.; Krieger, M.; Guarente, L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol. Cell 2007, 28, 91–106. [Google Scholar] [CrossRef]
- Hu, M.Y.; Li, Y.L.; Jiang, C.H.; Liu, Z.Q.; Qu, S.L.; Huang, Y.M. Comparison of lycopene and fluvastatin effects on atherosclerosis induced by a high-fat diet in rabbits. Nutrition 2008, 24, 1030–1038. [Google Scholar] [CrossRef]
- Hung, C.F.; Huang, T.F.; Chen, B.H.; Shieh, J.M.; Wu, P.H.; Wu, W.B. Lycopene inhibits TNF-alpha-induced endothelial ICAM-1 expression and mon- ocyte-endothelial adhesion. Eur. J. Pharmacol. 2008, 586, 275–282. [Google Scholar] [CrossRef]
- Gianetti, J.; Pedrinelli, R.; Petrucci, R.; Lazzerini, G.; De Caterina, M.; Bellomo, G.; De Caterina, R. Inverse association between carotid intima-media thickness and the antioxidant lycopene in atherosclerosis. Am. Heart J. 2002, 143, 467–474. [Google Scholar] [CrossRef]
- Riccioni, G.; Scotti, L.; Di Ilio, E.; Bucciarelli, V.; Ballone, E.; De Girolamo, M.; D’ Orazio, N.; Martini, F.; Aceto, A.; Bucciarelli, T. Lycopene and preclinical carotid atherosclerosis. J. Biol. Regul. Homeost. Agents 2011, 25, 435–441. [Google Scholar] [PubMed]
- Karppi, J.; Kurl, S.; Ronkainen, K.; Kauhanen, J.; Laukkanen, J.A. Serum carotenoids reduce progression of early atherosclerosis in the carotid artery wall among Eastern Finnish men. PLoS ONE 2013, 8, e64107. [Google Scholar]
- Mathur, P.; Ostadal, B.; Romeo, F.; Mehta, J.L. Gender-Related Differences in Atherosclerosis. Cardiovasc. Drugs Ther. 2015, 29, 319–327. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).