Molecular Mechanism of Cinnamomum cassia against Gastric Damage and Identification of Active Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Chemicals
2.2. Cell Viability Assay
2.3. Nitrite Assay
2.4. Reverse Transcription Quantitative Polymerase Chain Reaction (RT-PCR)
2.5. Western Blot
2.6. Immunofluorescence Staining
2.7. Measurement of Intracellular Reactive Oxygen Species (ROS)
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Animal Experiment Design
2.10. Western Blotting of Stomach Tissue
2.11. Determination of Stomach Injury
2.12. Measurement of Cytokines
2.13. Statistical Analysis
3. Results
3.1. Profiling of ECC
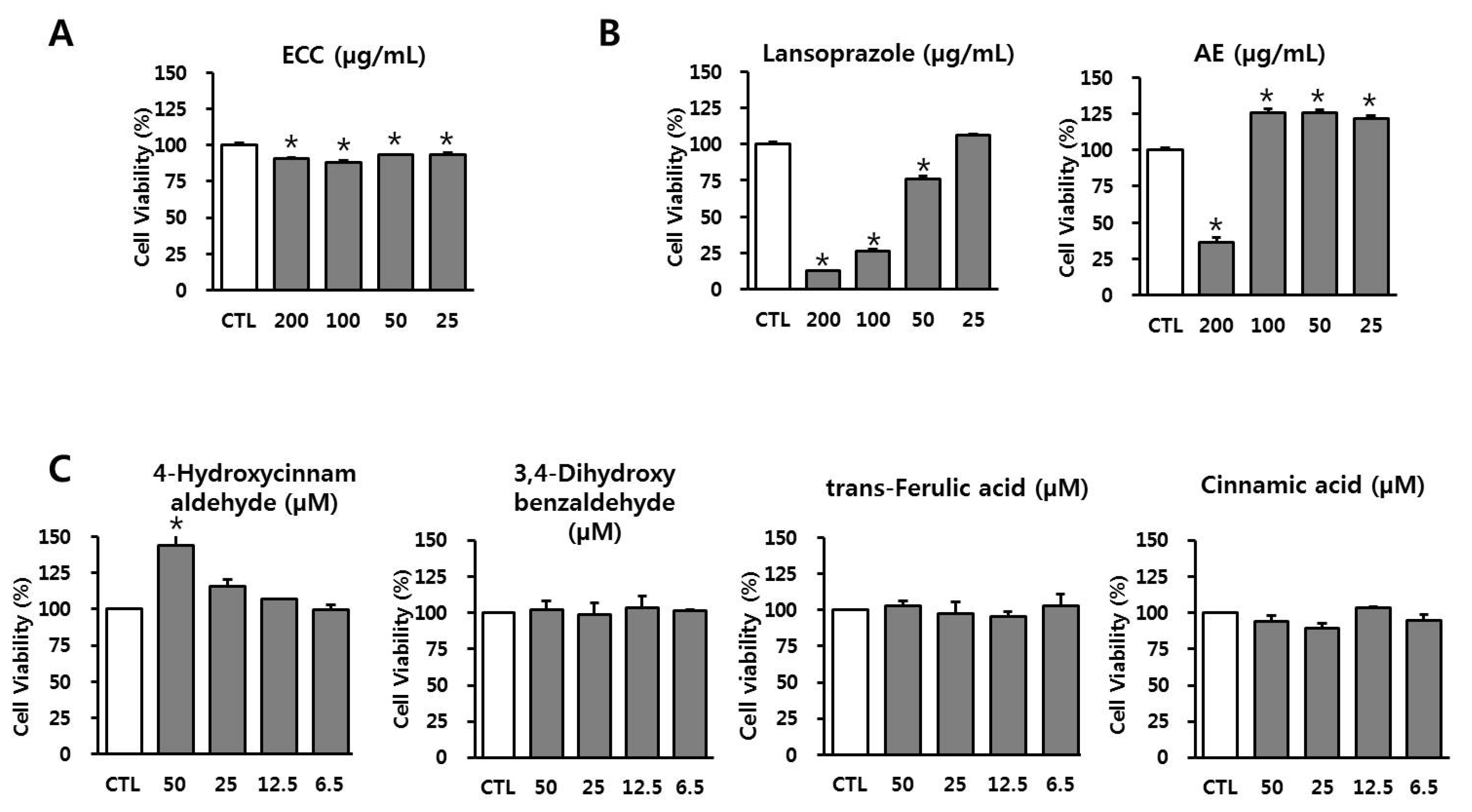
3.2. Effect of ECC and Its Components on Cell Viability in RAW 264.7 Cells
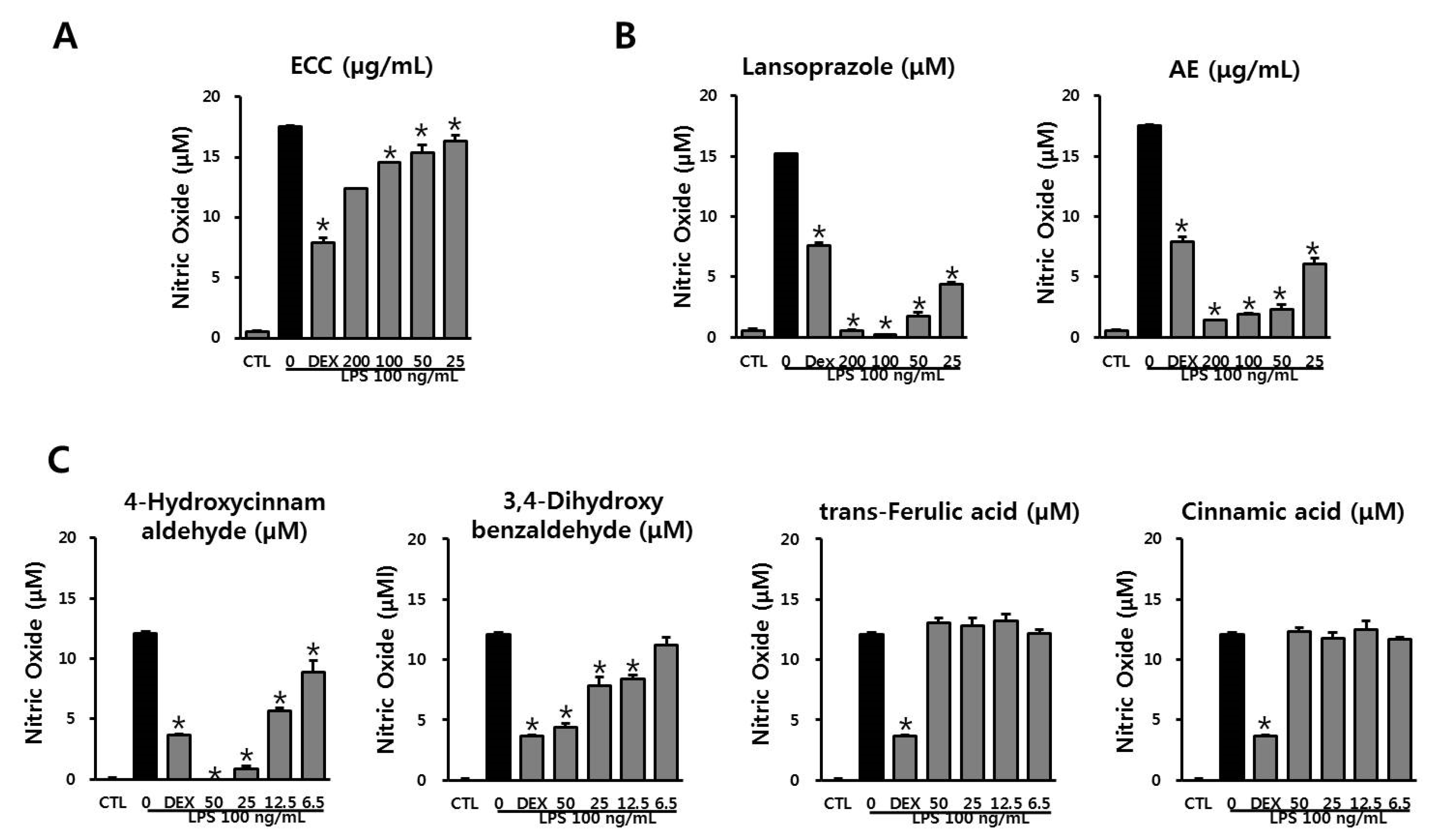
3.3. Anti-Inflammatory Effect of ECC and ECC Components on NO Production in LPS-Stimulated RAW 264.7 Cells
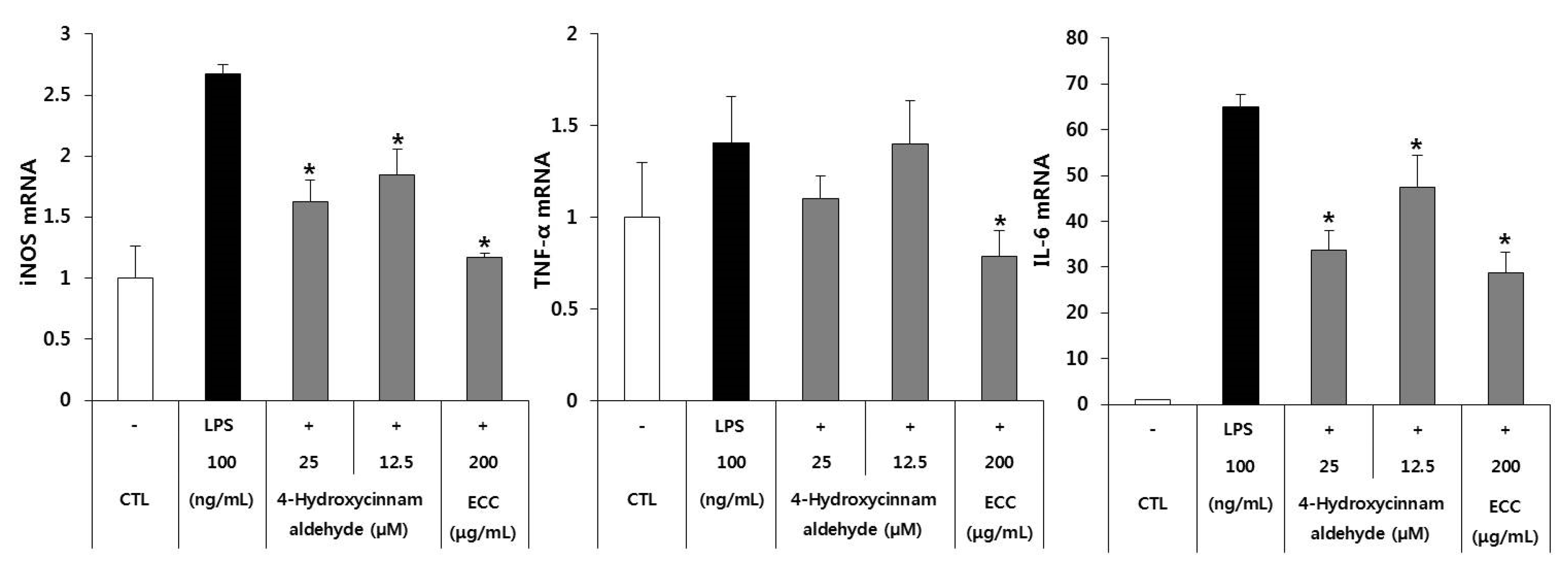
3.4. Anti-Inflammatory Gene Expression of ECC and 4-Hydroxycinnamaldehyde
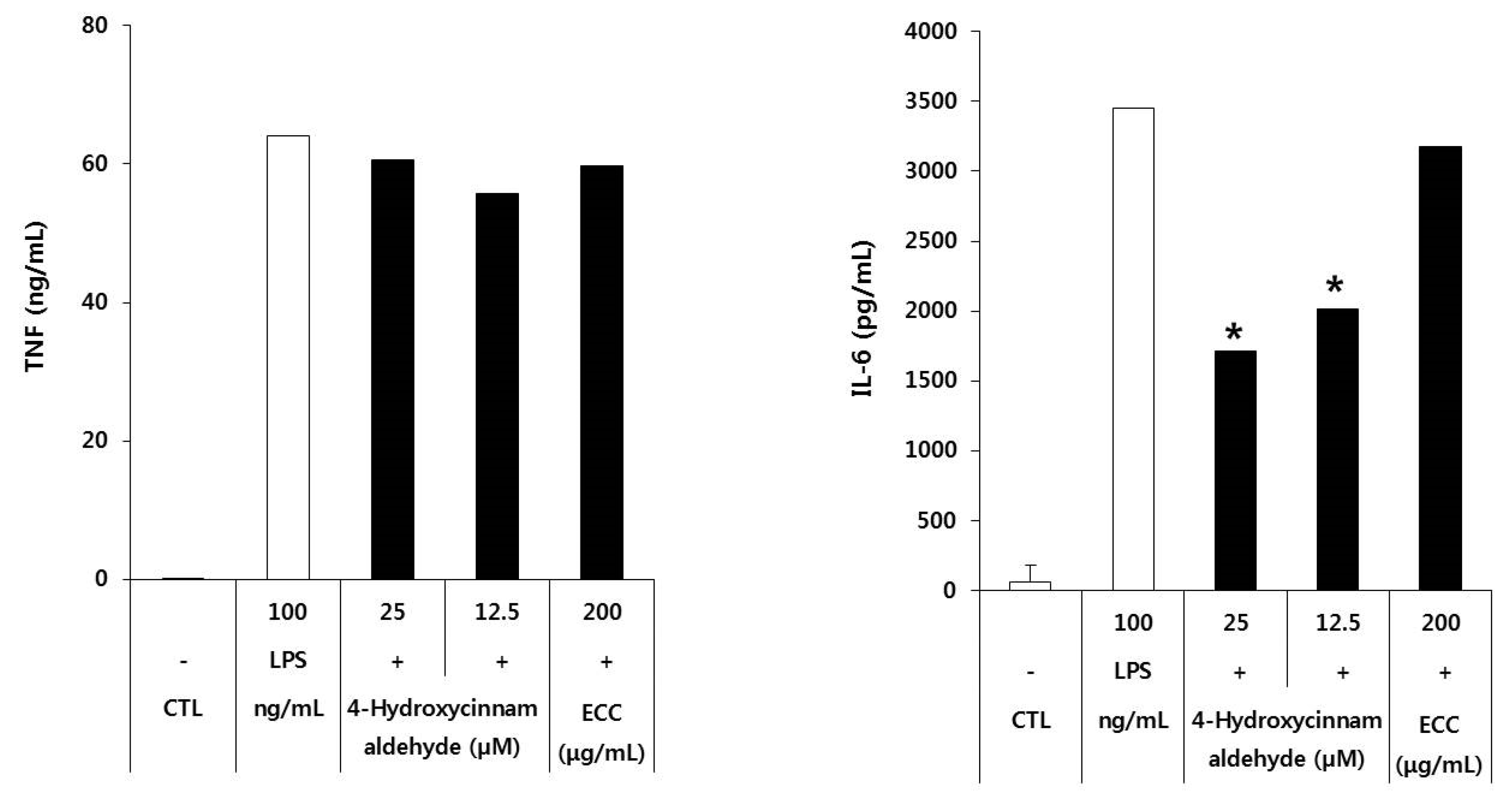
3.5. Effect of Pro-Inflammatory Cytokins of ECC and 4-Hydroxycinnamaldehyde
3.6. Effect of ECC and 4-Hydroxycinnamaldehyde on ROS Production and COX-2 and iNOS Protein Expression in LPS-Induced RAW 264.7 Cells
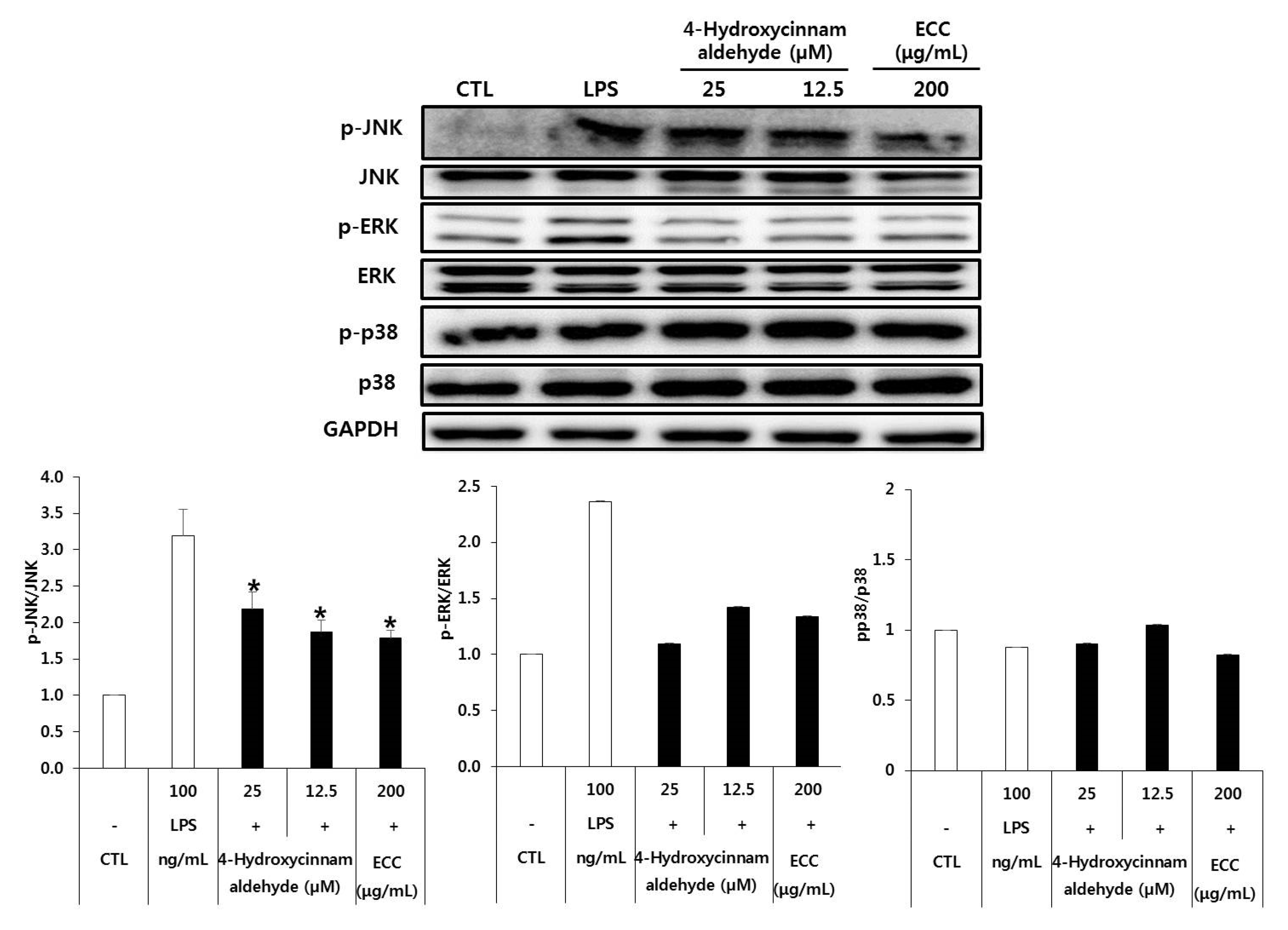
3.7. Effect of ECC and 4-Hydroxycinnamaldehyde on the MAPK Pathway in RAW 264.7 Cells
3.8. Regulation of Transcription Factors by ECC or 4-Hydroxycinnamaldehyde in RAW 264.7 Cells
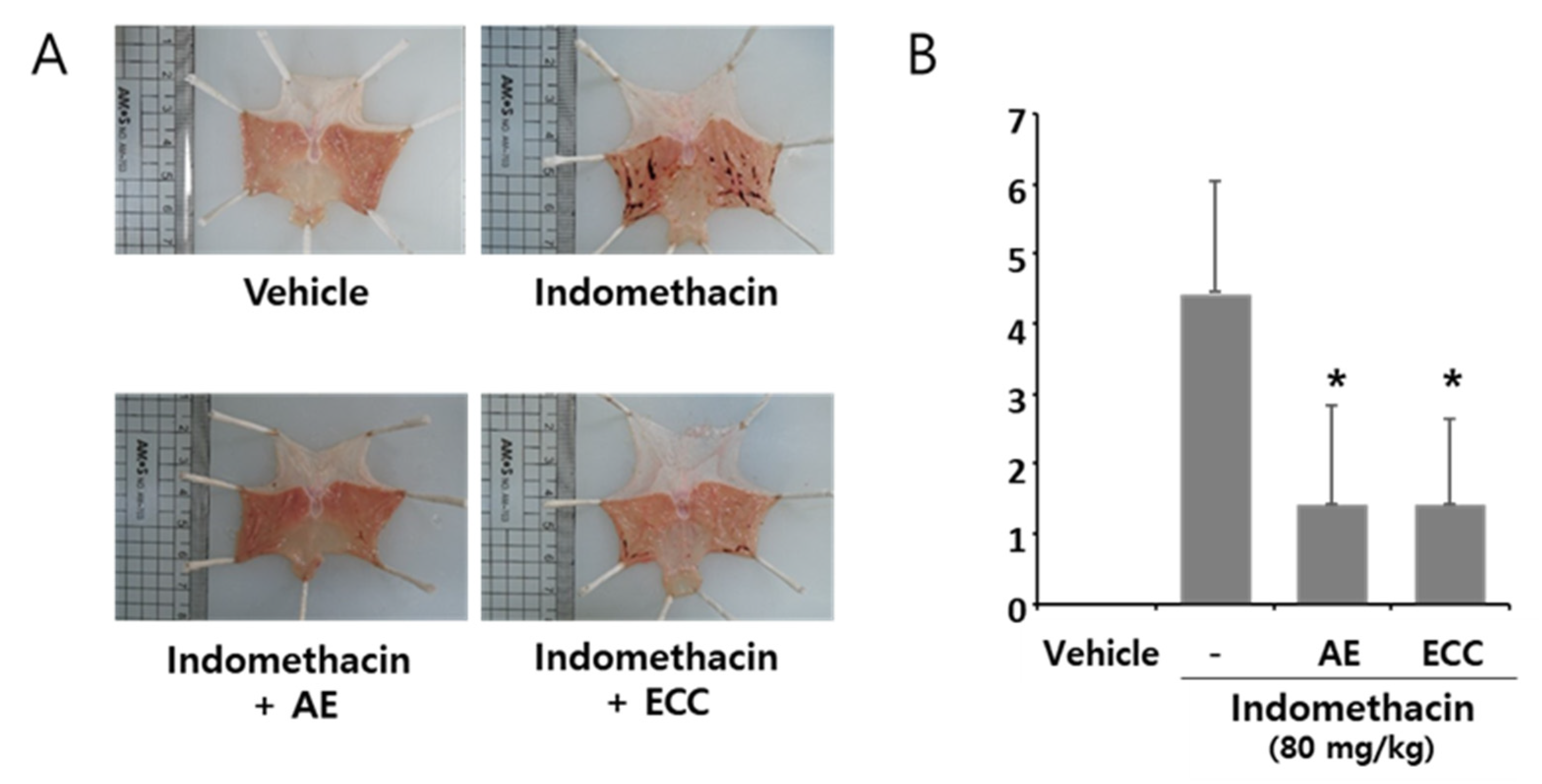
3.9. Ameliorating Effect of ECC on Acute Stomach Injury in Rats
3.10. ECC Ameliorates Acute Stomach Injury by Decreasing Inflammatory Signaling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, J.H.; Kwak, H.J.; Shin, D.; Seo, H.J.; Park, S.J.; Hong, B.H.; Shin, M.S.; Kim, S.H.; Kang, K.S. Mitigation of Gastric Damage Using Cinnamomum cassia Extract: Network Pharmacological Analysis of Active Compounds and Protection Effects in Rats. Plants 2022, 11, 716. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Yuan, S.; Zhang, H.; Zhang, T.; Wang, L.; Xu, Z. Cell-mediated immune responses and protective efficacy against infection with Mycobacterium tuberculosis induced by Hsp65 and hIL-2 fusion protein in mice. Scand. J. Immunol. 2009, 69, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Li, Y.; Wang, Z.; Xiao, M.; Yin, P.; Lu, Y.; Qian, X.; Xu, Y.; Liu, J. 7b, a novel naphthalimide derivative, exhibited anti-inflammatory effects via targeted-inhibiting TAK1 following down-regulation of ERK1/2-and p38 MAPK-mediated activation of NF-κB in LPS-stimulated RAW264. 7 macrophages. Int. Immunopharmacol. 2013, 17, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Lee, D.S.; Bae, G.S.; Park, S.J.; Kang, D.G.; Lee, H.S.; Oh, H.; Kim, Y.C. The inhibition of JNK MAPK and NF-κB signaling by tenuifoliside A isolated from Polygala tenuifolia in lipopolysaccharide-induced macrophages is associated with its anti-inflammatory effect. Eur. J. Pharmacol. 2013, 721, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Cagiola, M.; Giulio, S.; Miriam, M.; Katia, F.; Paola, P.; Macrì, A.; Pasquali, P. In vitro down regulation of proinflammatory cytokines induced by LPS tolerance in pig CD14+ cells. Vet. Immunol. Immunopathol. 2006, 112, 316–320. [Google Scholar] [CrossRef]
- Lappas, M.; Permezel, M.; Georgiou, H.M.; Rice, G.E. Nuclear factor kappa B regulation of proinflammatory cytokines in human gestational tissues in vitro. Biol. Reprod. 2002, 67, 668–673. [Google Scholar] [CrossRef]
- Liu, Y.; Shepherd, E.G.; Nelin, L.D. MAPK phosphatases—regulating the immune response. Nat. Rev. Immunol. 2007, 7, 202–212. [Google Scholar] [CrossRef]
- Rao, K.M.K. MAP kinase activation in macrophages. J. Leukoc. Biol. 2001, 69, 3–10. [Google Scholar]
- Hong, J.W.; Yang, G.E.; Kim, Y.B.; Eom, S.H.; Lew, J.H.; Kang, H. Anti-inflammatory activity of cinnamon water extract in vivo and in vitro LPS-induced models. BMC Complementary Altern. Med. 2012, 12, 1–8. [Google Scholar] [CrossRef]
- Liao, J.C.; Deng, J.S.; Chiu, C.S.; Hou, W.C.; Huang, S.S.; Shie, P.H.; Huang, G.J. Anti-inflammatory activities of Cinnamomum cassia constituents in vitro and in vivo. Evid. -Based Complementary Altern. Med. 2012, 2012, 429320. [Google Scholar] [CrossRef]
- Shin, W.Y.; Shim, D.W.; Kim, M.K.; Sun, X.; Koppula, S.; Yu, S.H.; Kim, H.B.; Kim, T.J.; Kang, T.B.; Lee, K.H. Protective effects of Cinnamomum cassia (Lamaceae) against gout and septic responses via attenuation of inflammasome activation in experimental models. J. Ethnopharmacol. 2017, 205, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Zamora, R.; Vodovotz, Y.; Billiar, T.R. Inducible nitric oxide synthase and inflammatory diseases. Mol. Med. 2000, 6, 347–373. [Google Scholar] [CrossRef] [PubMed]
- Groeneveld, P.H.; Kwappenberg, K.M.; Langermans, J.A.; Nibbering, P.H.; Curtis, L. Relation between pro-and anti-inflammatory cytokines and the production of nitric oxide (NO) in severe sepsis. Cytokine 1997, 9, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [PubMed]
- Kvietys, P.R.; Granger, D.N. Role of reactive oxygen and nitrogen species in the vascular responses to inflammation. Free. Radic. Biol. Med. 2012, 52, 556–592. [Google Scholar] [CrossRef]
- Kim, T.W.; Michniewicz, M.; Bergmann, D.C.; Wang, Z.Y. Brassinosteroid regulates stomatal development by GSK3-mediated inhibition of a MAPK pathway. Nature 2012, 482, 419–422. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. NF-κB, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012, 26, 203–234. [Google Scholar] [CrossRef]
- Shuai, K.; Liu, B. Regulation of JAK–STAT signalling in the immune system. Nat. Rev. Immunol. 2003, 3, 900–911. [Google Scholar] [CrossRef]
- Chan, E.; Riches, D.; Altmeyer, M.; Barthel, M.; Eberhard, M.; Rehrauer, H. IFN-γ+ LPS induction of iNOS is modulated by ERK, JNK/SAPK, and p38 mapk in a mouse macrophage cell line. Am J Physiol Cell Physio. 2001, 280, C441–C450. [Google Scholar] [CrossRef]
- Kwon, K.B.; Kim, E.K.; Jeong, E.S.; Lee, Y.H.; Lee, Y.R.; Park, J.W.; Ryu, D.G.; Park, B.H. Cortex cinnamomi extract prevents streptozotocin-and cytokine-induced β-cell damage by inhibiting NF-κB. World J. Gastroenterol. WJG 2006, 12, 4331. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, D.H.; Lee, S.; Seo, H.J.; Park, S.J.; Jung, K.; Kim, S.Y.; Kang, K.S. Potential and beneficial effects of Cinnamomum cassia on gastritis and safety: Literature review and analysis of standard extract. Appl. Biol. Chem. 2021, 64, 1–14. [Google Scholar] [CrossRef]
- Lv, Y.; Hu, S.; Lu, J.; Dong, N.; Liu, Q.; Du, M.; Zhang, H. Upregulating nonneuronal cholinergic activity decreases TNF release from lipopolysaccharide-stimulated RAW264. 7 cells. Mediat. Inflamm. 2014, 2014, 873728. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxidative Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Baek, S.H.; Park, T.; Kang, M.G.; Park, D. Anti-Inflammatory Activity and ROS Regulation Effect of Sinapaldehyde in LPS-Stimulated RAW 264.7 Macrophages. Molecules 2020, 25, 4089. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. TLR signaling. Seminars in Immunology. 2007, 19, 24–32. [Google Scholar] [CrossRef]
- Bode, J.G.; Ehlting, C.; Häussinger, D. The macrophage response towards LPS and its control through the p38MAPK–STAT3 axis. Cellular signalling 2012, 24, 1185–1194. [Google Scholar] [CrossRef]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 1–15. [Google Scholar] [CrossRef]
- Laird, M.H.; Rhee, S.H.; Perkins, D.J.; Medvedev, A.E.; Piao, W.; Fenton, M.J.; Vogel, S.N. TLR4/MyD88/PI3K interactions regulate TLR4 signaling. J. Leukoc. Biol. 2009, 85, 966–977. [Google Scholar] [CrossRef]
- Wallace, J.L. Prostaglandins, NSAIDs, and gastric mucosal protection: Why doesn’t the stomach digest itself? Physiol. Rev. 2008, 88, 1547–1565. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Taneja, N.; Lin, L.; Orringer, M.B.; Rehemtulla, A.; Beer, D.G. Indomethacin-induced apoptosis in esophageal adenocarcinoma cells involves upregulation of Bax and translocation of mitochondrial cytochrome C independent of COX-2 expression. Neoplasia 2000, 2, 346–356. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Piao, X.; Li, S.; Sui, X.; Guo, L.; Liu, X.; Li, H.; Gao, L.; Cai, S.; Li, Y.; Wang, T. 1-Deoxynojirimycin (DNJ) Ameliorates indomethacin-induced gastric ulcer in mice by affecting NF-kappaB signaling pathway. Front. Pharmacol. 2018, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.; Djakiew, D.; Krygier, S.; Andrews, P. Superior effectiveness of ibuprofen compared with other NSAIDs for reducing the survival of human prostate cancer cells. Cancer Chemother. Pharmacol. 2002, 50, 277–284. [Google Scholar] [CrossRef]
- Takada, Y.; Bhardwaj, A.; Potdar, P.; Aggarwal, B.B. Nonsteroidal anti-inflammatory agents differ in their ability to suppress NF-κ B activation, inhibition of expression of cyclooxygenase-2 and cyclin D1, and abrogation of tumor cell proliferation. Oncogene 2004, 23, 9247–9258. [Google Scholar] [CrossRef]
- Choi, H.W.; Shin, P.G.; Lee, J.H.; Choi, W.S.; Kang, M.J.; Kong, W.S.; Oh, M.J.; Seo, Y.B.; Kim, G.D. Anti-inflammatory effect of lovastatin is mediated via the modulation of NF-κB and inhibition of HDAC1 and the PI3K/Akt/mTOR pathway in RAW264. 7 macrophages. Int. J. Mol. Med. 2018, 41, 1103–1109. [Google Scholar] [CrossRef]
- Beg, A.A.; William, C.S.; Bronson, R.T.; Ghosh, S.; Baltimore, D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature 1995, 376, 167–170. [Google Scholar] [CrossRef]
- Li, Z.W.; Chu, W.; Hu, Y.; Delhase, M.; Deerinck, T.; Ellisman, M.; Johnson, R.; Karin, M. The IKKβ subunit of IκB kinase (IKK) is essential for nuclear factor κB activation and prevention of apoptosis. J. Exp. Med. 1999, 189, 1839–1845. [Google Scholar] [CrossRef]
- Hu, Y.; Baud, V.; Delhase, M.; Zhang, P.; Deerinck, T.; Ellisman, M.; Johnson, R.; Karin, M. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKα subunit of IκB kinase. Science 1999, 284, 316–320. [Google Scholar] [CrossRef]





| PGE2 | MPO | |
|---|---|---|
| Vehicle | 225.5 ± 60.6 | 34.1 ± 19.8 |
| Indomethacin | 843.7 ± 256.9 | 637.9 ± 167.7 |
| Indomethacin + AE | 434.3 ± 121.9 * | 203.4 ± 61.2 * |
| Indomethacin + ECC | 434.6 ± 62.1 * | 181.8 ± 90.0 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.J.; Seo, H.J.; Hwang, G.S.; Choi, S.; Park, S.J.; Hwang, S.-J.; Kang, K.S. Molecular Mechanism of Cinnamomum cassia against Gastric Damage and Identification of Active Compounds. Biomolecules 2022, 12, 525. https://doi.org/10.3390/biom12040525
Lee MJ, Seo HJ, Hwang GS, Choi S, Park SJ, Hwang S-J, Kang KS. Molecular Mechanism of Cinnamomum cassia against Gastric Damage and Identification of Active Compounds. Biomolecules. 2022; 12(4):525. https://doi.org/10.3390/biom12040525
Chicago/Turabian StyleLee, Myong Jin, Hye Jin Seo, Gwi Seo Hwang, Sungyoul Choi, Shin Jung Park, Sung-Joo Hwang, and Ki Sung Kang. 2022. "Molecular Mechanism of Cinnamomum cassia against Gastric Damage and Identification of Active Compounds" Biomolecules 12, no. 4: 525. https://doi.org/10.3390/biom12040525
APA StyleLee, M. J., Seo, H. J., Hwang, G. S., Choi, S., Park, S. J., Hwang, S.-J., & Kang, K. S. (2022). Molecular Mechanism of Cinnamomum cassia against Gastric Damage and Identification of Active Compounds. Biomolecules, 12(4), 525. https://doi.org/10.3390/biom12040525








