A Potential Citrate Shunt in Erythrocytes of PKAN Patients Caused by Mutations in Pantothenate Kinase 2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Blood Sample Collection
2.2. Genetic Evaluation of the Patients
2.3. Pantothenate Kinase (PANK) Activity Assay
2.4. Immunoblot Analysis
2.5. Gas Chromatography (GC)–Mass Spectrometry (MS)
2.6. Molecular Visualization
2.7. Statistical Analysis
3. Results
3.1. Residual Activity and Abundance of PANK2 Mutant Proteins
3.2. Metabolomic Analysis of PKAN Blood Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, B.; Westaway, S.K.; Levinson, B.; Johnson, M.A.; Gitschier, J.; Hayflick, S.J. A novel pantothenate kinase gene (PANK2) is defective in Hallervorden-Spatz syndrome. Nat. Genet. 2001, 28, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Kurian, M.A.; Hayflick, S.J. Pantothenate kinase-associated neurodegeneration (PKAN) and PLA2G6-associated neurodegeneration (PLAN): Review of two major neurodegeneration with brain iron accumulation (NBIA) phenotypes. Int. Rev. Neurobiol. 2013, 110, 49–71. [Google Scholar] [CrossRef] [PubMed]
- De Franceschi, L.; Tomelleri, C.; Matte, A.; Brunati, A.M.; Bovee-Geurts, P.H.; Bertoldi, M.; Lasonder, E.; Tibaldi, E.; Danek, A.; Walker, R.H.; et al. Erythrocyte membrane changes of chorea-acanthocytosis are the result of altered Lyn kinase activity. Blood 2011, 118, 5652–5663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballas, S.K.; Bator, S.M.; Aubuchon, J.P.; Marsh, W.L.; Sharp, D.E.; Toy, E.M. Abnormal membrane physical properties of red cells in McLeod syndrome. Transfusion 1990, 30, 722–727. [Google Scholar] [CrossRef]
- Hortnagel, K.; Prokisch, H.; Meitinger, T. An isoform of hPANK2, deficient in pantothenate kinase-associated neurodegeneration, localizes to mitochondria. Hum. Mol. Genet. 2003, 12, 321–327. [Google Scholar] [CrossRef]
- Kotzbauer, P.T.; Truax, A.C.; Trojanowski, J.Q.; Lee, V.M. Altered neuronal mitochondrial coenzyme A synthesis in neurodegeneration with brain iron accumulation caused by abnormal processing, stability, and catalytic activity of mutant pantothenate kinase 2. J. Neurosci. 2005, 25, 689–698. [Google Scholar] [CrossRef] [Green Version]
- Werning, M.; Mullner, E.W.; Mlynek, G.; Dobretzberger, V.; Djinovic-Carugo, K.; Baron, D.M.; Prokisch, H.; Buchner, B.; Klopstock, T.; Salzer, U. PKAN neurodegeneration and residual PANK2 activities in patient erythrocytes. Ann. Clin. Transl. Neurol. 2020, 7, 1340–1351. [Google Scholar] [CrossRef]
- Bryk, A.H.; Wisniewski, J.R. Quantitative Analysis of Human Red Blood Cell Proteome. J. Proteome Res. 2017, 16, 2752–2761. [Google Scholar] [CrossRef] [Green Version]
- Spry, C.; Saliba, K.J. The human malaria parasite Plasmodium falciparum is not dependent on host coenzyme A biosynthesis. J. Biol. Chem. 2009, 284, 24904–24913. [Google Scholar] [CrossRef] [Green Version]
- Aoun, M.; Corsetto, P.A.; Nugue, G.; Montorfano, G.; Ciusani, E.; Crouzier, D.; Hogarth, P.; Gregory, A.; Hayflick, S.; Zorzi, G.; et al. Changes in Red Blood Cell membrane lipid composition: A new perspective into the pathogenesis of PKAN. Mol. Genet. Metab. 2017, 121, 180–189. [Google Scholar] [CrossRef]
- Siegl, C.; Hamminger, P.; Jank, H.; Ahting, U.; Bader, B.; Danek, A.; Gregory, A.; Hartig, M.; Hayflick, S.; Hermann, A.; et al. Alterations of red cell membrane properties in neuroacanthocytosis. PLoS ONE 2013, 8, e76715. [Google Scholar] [CrossRef]
- Schiessl-Weyer, J.; Roa, P.; Laccone, F.; Kluge, B.; Tichy, A.; De Almeida Ribeiro, E.; Prohaska, R.; Stoeter, P.; Siegl, C.; Salzer, U. Acanthocytosis and the c.680 A>G Mutation in the PANK2 Gene: A Study Enrolling a Cohort of PKAN Patients from the Dominican Republic. PLoS ONE 2015, 10, e0125861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindler, F.; Fragner, L.; Herpell, J.B.; Berger, A.; Brenner, M.; Tischler, S.; Bellaire, A.; Schonenberger, J.; Li, W.; Sun, X.; et al. Dissecting Metabolism of Leaf Nodules in Ardisia crenata and Psychotria punctata. Front. Mol. Biosci. 2021, 8, 683671. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics by Gas Chromatography-Mass Spectrometry: Combined Targeted and Untargeted Profiling. Curr. Protoc. Mol. Biol. 2016, 114, 30.4.1–30.4.32. [Google Scholar] [CrossRef]
- Ott, E.; Kawaguchi, Y.; Ozgen, N.; Yamagishi, A.; Rabbow, E.; Rettberg, P.; Weckwerth, W.; Milojevic, T. Proteomic and Metabolomic Profiling of Deinococcus radiodurans Recovering After Exposure to Simulated Low Earth Orbit Vacuum Conditions. Front. Microbiol. 2019, 10, 909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, Z.; Tsugawa, H.; Wohlgemuth, G.; Mehta, S.; Mueller, M.; Zheng, Y.; Ogiwara, A.; Meissen, J.; Showalter, M.; Takeuchi, K.; et al. Identifying metabolites by integrating metabolome databases with mass spectrometry cheminformatics. Nat. Methods 2018, 15, 53–56. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Kind, T.; Wohlgemuth, G.; Lee, D.Y.; Lu, Y.; Palazoglu, M.; Shahbaz, S.; Fiehn, O. FiehnLib: Mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal. Chem. 2009, 81, 10038–10048. [Google Scholar] [CrossRef] [Green Version]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic. Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, B.S.; Senisterra, G.; Rabeh, W.M.; Vedadi, M.; Leonardi, R.; Zhang, Y.M.; Rock, C.O.; Jackowski, S.; Park, H.W. Crystal structures of human pantothenate kinases. Insights into allosteric regulation and mutations linked to a neurodegeneration disorder. J. Biol. Chem. 2007, 282, 27984–27993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaleckis, R.; Murakami, I.; Takada, J.; Kondoh, H.; Yanagida, M. Individual variability in human blood metabolites identifies age-related differences. Proc. Natl. Acad. Sci. USA 2016, 113, 4252–4259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, M.C.; Trakarnsanga, K.; Heesom, K.J.; Cogan, N.; Green, C.; Toye, A.M.; Parsons, S.F.; Anstee, D.J.; Frayne, J. Comparison of the Proteome of Adult and Cord Erythroid Cells, and Changes in the Proteome Following Reticulocyte Maturation. Mol. Cell. Proteom. MCP 2016, 15, 1938–1946. [Google Scholar] [CrossRef] [Green Version]
- D’Alessandro, A.; Nemkov, T.; Yoshida, T.; Bordbar, A.; Palsson, B.O.; Hansen, K.C. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion 2017, 57, 325–336. [Google Scholar] [CrossRef]
- Usenik, A.; Legisa, M. Evolution of allosteric citrate binding sites on 6-phosphofructo-1-kinase. PLoS ONE 2010, 5, e15447. [Google Scholar] [CrossRef]
- Sibon, O.C.; Strauss, E. Coenzyme A: To make it or uptake it? Nat. Rev. Mol. Cell Biol. 2016, 17, 605–606. [Google Scholar] [CrossRef]
- Srinivasan, B.; Baratashvili, M.; van der Zwaag, M.; Kanon, B.; Colombelli, C.; Lambrechts, R.A.; Schaap, O.; Nollen, E.A.; Podgorsek, A.; Kosec, G.; et al. Extracellular 4’-phosphopantetheine is a source for intracellular coenzyme A synthesis. Nat. Chem. Biol. 2015, 11, 784–792. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Hogarth, P.; Placzek, A.; Gregory, A.M.; Fox, R.; Zhen, D.; Hamada, J.; van der Zwaag, M.; Lambrechts, R.; Jin, H.; et al. 4′-Phosphopantetheine corrects CoA, iron, and dopamine metabolic defects in mammalian models of PKAN. EMBO Mol. Med. 2019, 11, 29. [Google Scholar] [CrossRef]
- Mosaoa, R.; Kasprzyk-Pawelec, A.; Fernandez, H.R.; Avantaggiati, M.L. The Mitochondrial Citrate Carrier SLC25A1/CIC and the Fundamental Role of Citrate in Cancer, Inflammation and Beyond. Biomolecules 2021, 11, 141. [Google Scholar] [CrossRef]
- Ronowska, A.; Szutowicz, A.; Bielarczyk, H.; Gul-Hinc, S.; Klimaszewska-Lata, J.; Dys, A.; Zysk, M.; Jankowska-Kulawy, A. The Regulatory Effects of Acetyl-CoA Distribution in the Healthy and Diseased Brain. Front. Cell. Neurosci. 2018, 12, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiermonte, G.; Paradies, E.; Todisco, S.; Marobbio, C.M.; Palmieri, F. A novel member of solute carrier family 25 (SLC25A42) is a transporter of coenzyme A and adenosine 3′,5′-diphosphate in human mitochondria. J. Biol. Chem. 2009, 284, 18152–18159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunetti, D.; Dusi, S.; Morbin, M.; Uggetti, A.; Moda, F.; D’Amato, I.; Giordano, C.; d’Amati, G.; Cozzi, A.; Levi, S.; et al. Pantothenate kinase-associated neurodegeneration: Altered mitochondria membrane potential and defective respiration in Pank2 knock-out mouse model. Hum. Mol. Genet. 2012, 21, 5294–5305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva, A.R.; Neves, J.; Mleczko-Sanecka, K.; Tandon, A.; Sauer, S.W.; Hentze, M.W.; Muckenthaler, M.U. Cellular citrate levels establish a regulatory link between energy metabolism and the hepatic iron hormone hepcidin. J. Mol. Med. (Berl.) 2017, 95, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Vela, D. The Dual Role of Hepcidin in Brain Iron Load and Inflammation. Front. Neurosci. 2018, 12, 740. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Rock, C.O.; Jackowski, S. Biochemical properties of human pantothenate kinase 2 isoforms and mutations linked to pantothenate kinase-associated neurodegeneration. J. Biol. Chem. 2006, 281, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, C.; Yun, M.K.; Yao, J.; Sharma, L.K.; Lee, R.E.; White, S.W.; Jackowski, S.; Rock, C.O. Allosteric Regulation of Mammalian Pantothenate Kinase. J. Biol. Chem. 2016, 291, 22302–22314. [Google Scholar] [CrossRef] [Green Version]


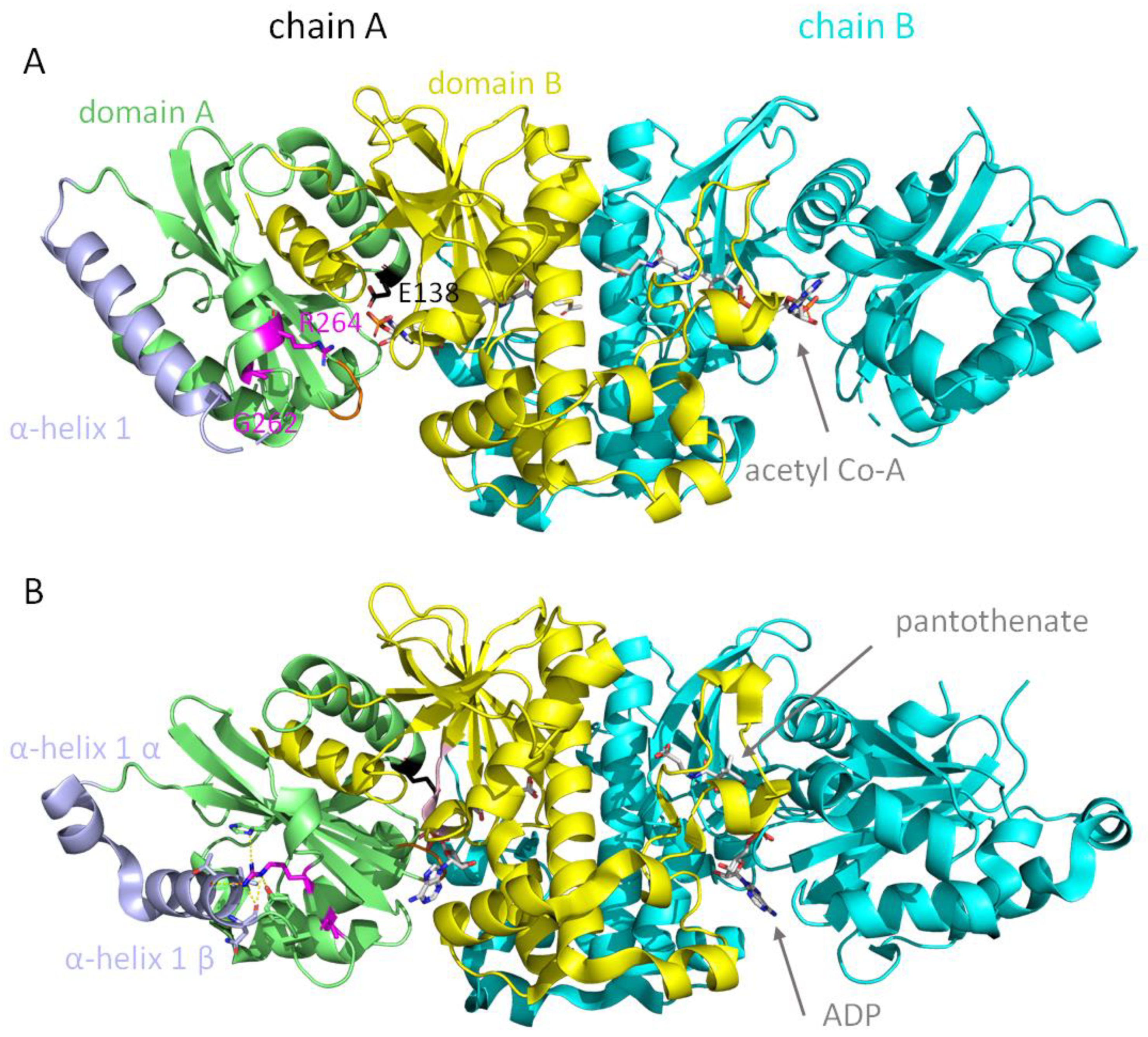


| Patient | Gender | Age of Onset | Diagnosis | DNA Mutation | Protein Mutation | ||
|---|---|---|---|---|---|---|---|
| Allele 1 | Allele 2 | Allele 1 | Allele 2 | ||||
| P1 | ♂ | 12 | atypical | c.1561G > A | c.1583C > T | G521R | T528M |
| P2 | ♂ | 13 | atypical | c.1561G > A | c.1583C > T | G521R | T528M |
| P3 | ♀ | 2 | classical | c.1583C > T | deletion exon 2–4 | T528M | del |
| P4 | ♀ | 3 | classical | c.1561G > A | c.1561G > A | G521R | G521R |
| P5 | ♂ | 1 | classical | c.1561G > A | c.784G > C | G521R | G262R |
| P6 | ♂ | 4 | classical | c.791G > A | c.823_824del | R264N | L275fs |
| Activity (% ± stdev.) | Abundance (% ± stdev.) | |
|---|---|---|
| control | 100.00 ± 0.00 | 100.00 ± 0.00 |
| CR3m | 96.25 ± 3.82 | 23.28 ± 5.46 |
| CR4f | 38.50 ± 6.55 | 39.42 ± 6.60 |
| CR4m | 27.21 ± 2.59 | 25.53 ± 2.44 |
| CR5f | 37.03 ± 2.24 | 31.32 ± 9.37 |
| CR5m | 35.93 ± 2.24 | 33.58 ± 10.6 |
| CR6f | 48.14 ± 11.1 | 55.12 ± 6.03 |
| CR6m | 51.29 ± 10.6 | 39.46 ± 11.8 |
| P1 | 15.35 ± 4.94 | 8.55 ± 1.49 |
| P2 | 17.19 ± 4.43 | 12.89 ± 6.38 |
| P3 | 12.91 ± 2.50 | 110.10 ± 6.52 |
| P4 | 0.54 ± 0.94 | 4.5 ± 0.81 |
| P5 | 0.24 ± 0.42 | 3.98 ± 0.86 |
| P6 | 0.12 ± 0.12 | 4.35 ± 1.41 |
| Metabolites | FDR | Significance | Tukey’s HSD | p-Value | ID-Confidence |
|---|---|---|---|---|---|
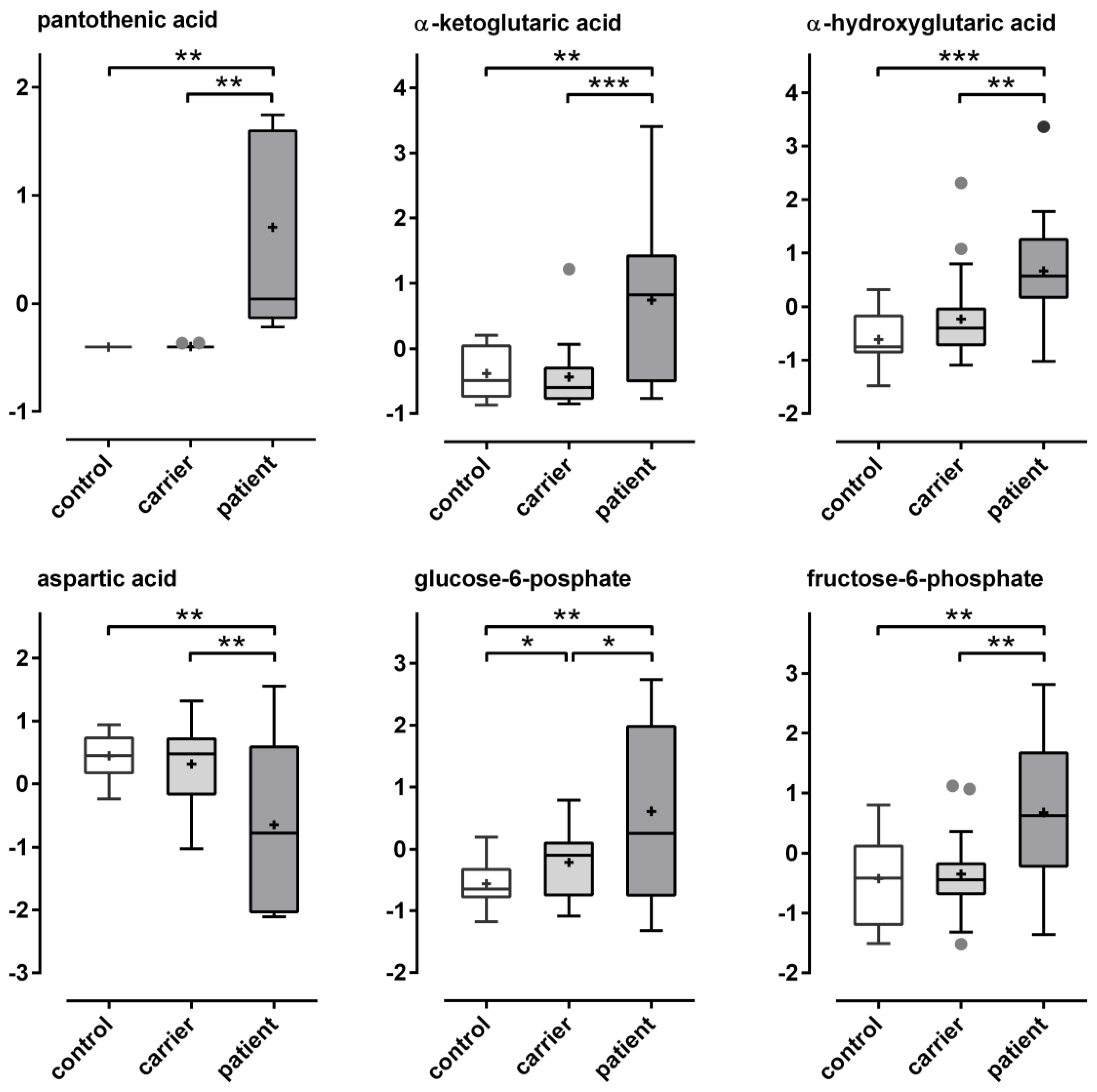
| # pantothenic acid | 0.000117 | **** | patient-carrier; patient-control | 3.76 × 10−7 | L1 |
| quinic acid | 0.000731 | **** | control-carrier; patient-carrier | 4.7 × 10−6 | L2 |
| 2-hydroxyvaleric acid | 0.001771 | **** | patient-carrier; patient-control | 2.33 × 10−5 | L2 |
| # α-ketoglutaric acid | 0.006476 | *** | patient-carrier; patient-control | 0.000125 | L1 |
| fructose | 0.01230 | *** | patient-carrier; patient-control | 0.000277 | L2 |
| # α-hydroxyglutaric acid | 0.01670 | *** | patient-carrier; patient-control | 0.000437 | L2 |
| fructose-6-phosphate | 0.02248 | *** | patient-carrier; patient-control | 0.000745 | L2 |
| asparagine | 0.02248 | *** | control-carrier; patient-control | 0.000795 | L2 |
| # aspartic acid | 0.03486 | ** | patient-carrier; patient-control | 0.001395 | L1 |
| glycerol-3-galactoside | 0.03486 | ** | patient-carrier | 0.001457 | L2 |
| glucose-6-phosphate | 0.04188 | ** | patient-carrier; patient-control | 0.001885 | L2 |
| mannonic acid | 0.04470 | ** | patient-carrier; patient-control | 0.002156 | L2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werning, M.; Dobretzberger, V.; Brenner, M.; Müllner, E.W.; Mlynek, G.; Djinovic-Carugo, K.; Baron, D.M.; Fragner, L.; Bischoff, A.T.; Büchner, B.; et al. A Potential Citrate Shunt in Erythrocytes of PKAN Patients Caused by Mutations in Pantothenate Kinase 2. Biomolecules 2022, 12, 325. https://doi.org/10.3390/biom12020325
Werning M, Dobretzberger V, Brenner M, Müllner EW, Mlynek G, Djinovic-Carugo K, Baron DM, Fragner L, Bischoff AT, Büchner B, et al. A Potential Citrate Shunt in Erythrocytes of PKAN Patients Caused by Mutations in Pantothenate Kinase 2. Biomolecules. 2022; 12(2):325. https://doi.org/10.3390/biom12020325
Chicago/Turabian StyleWerning, Maike, Verena Dobretzberger, Martin Brenner, Ernst W. Müllner, Georg Mlynek, Kristina Djinovic-Carugo, David M. Baron, Lena Fragner, Almut T. Bischoff, Boriana Büchner, and et al. 2022. "A Potential Citrate Shunt in Erythrocytes of PKAN Patients Caused by Mutations in Pantothenate Kinase 2" Biomolecules 12, no. 2: 325. https://doi.org/10.3390/biom12020325
APA StyleWerning, M., Dobretzberger, V., Brenner, M., Müllner, E. W., Mlynek, G., Djinovic-Carugo, K., Baron, D. M., Fragner, L., Bischoff, A. T., Büchner, B., Klopstock, T., Weckwerth, W., & Salzer, U. (2022). A Potential Citrate Shunt in Erythrocytes of PKAN Patients Caused by Mutations in Pantothenate Kinase 2. Biomolecules, 12(2), 325. https://doi.org/10.3390/biom12020325







