Filterability of Erythrocytes in Patients with COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Donors
2.2. Ethical Statement
2.3. Materials
2.4. Preparation of Blood Samples for Filterability Measurement
2.5. Measurement of Erythrocyte Filterability
2.6. Measurement of the Dependence of Erythrocyte Filterability on the Severity of the Patient’s Condition
2.7. Measurement of the Dependence of Erythrocyte Filterability on the Level and Type of Additional Oxygenation
2.8. Data Analysis
3. Results
3.1. The Dependence of Erythrocyte Filtration on the Severity of the Patient’s Condition
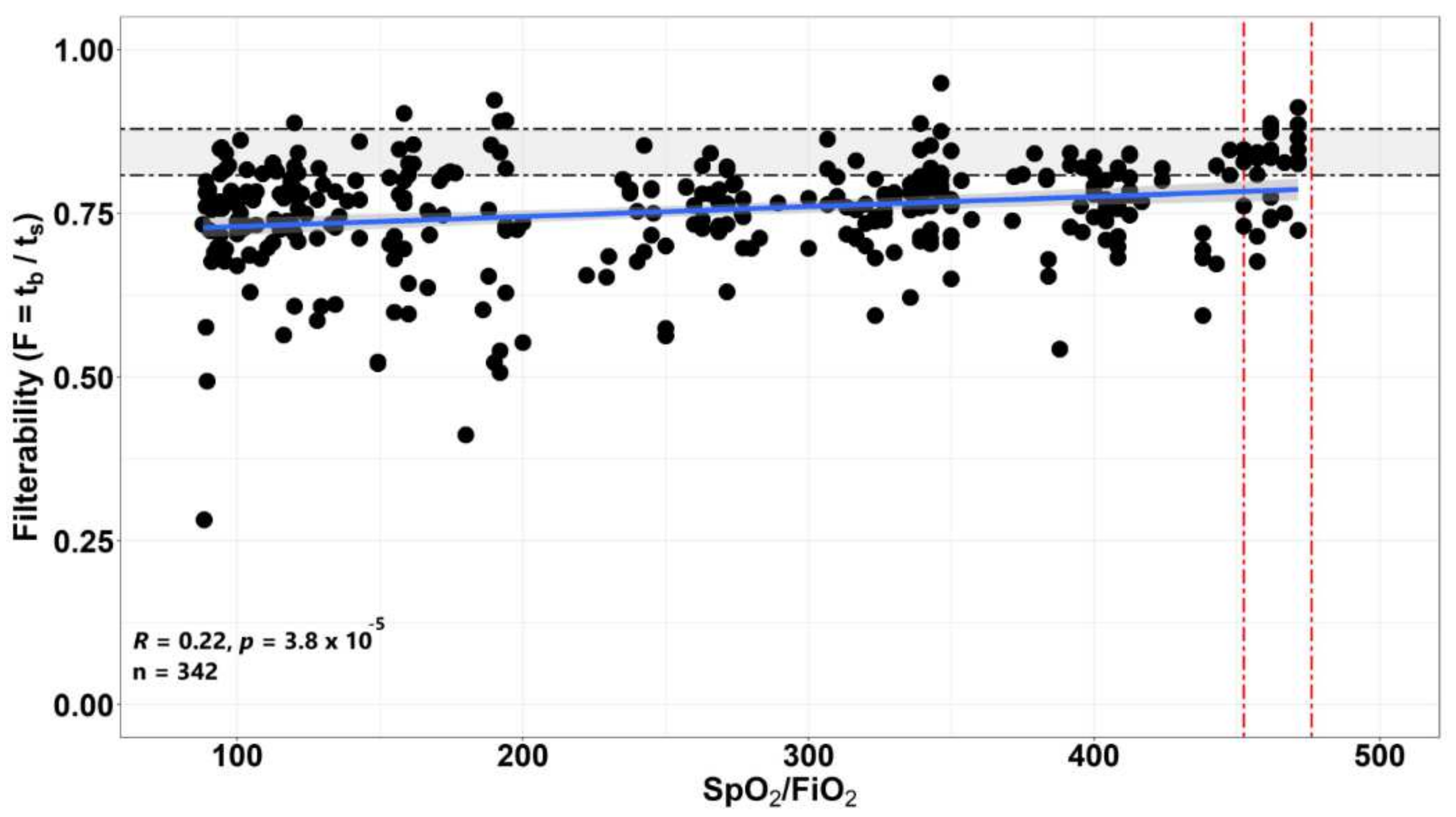
3.2. The Dependence of the Filterability of Erythrocytes of Patients on the Level of the SpO2/FiO2 Ratio
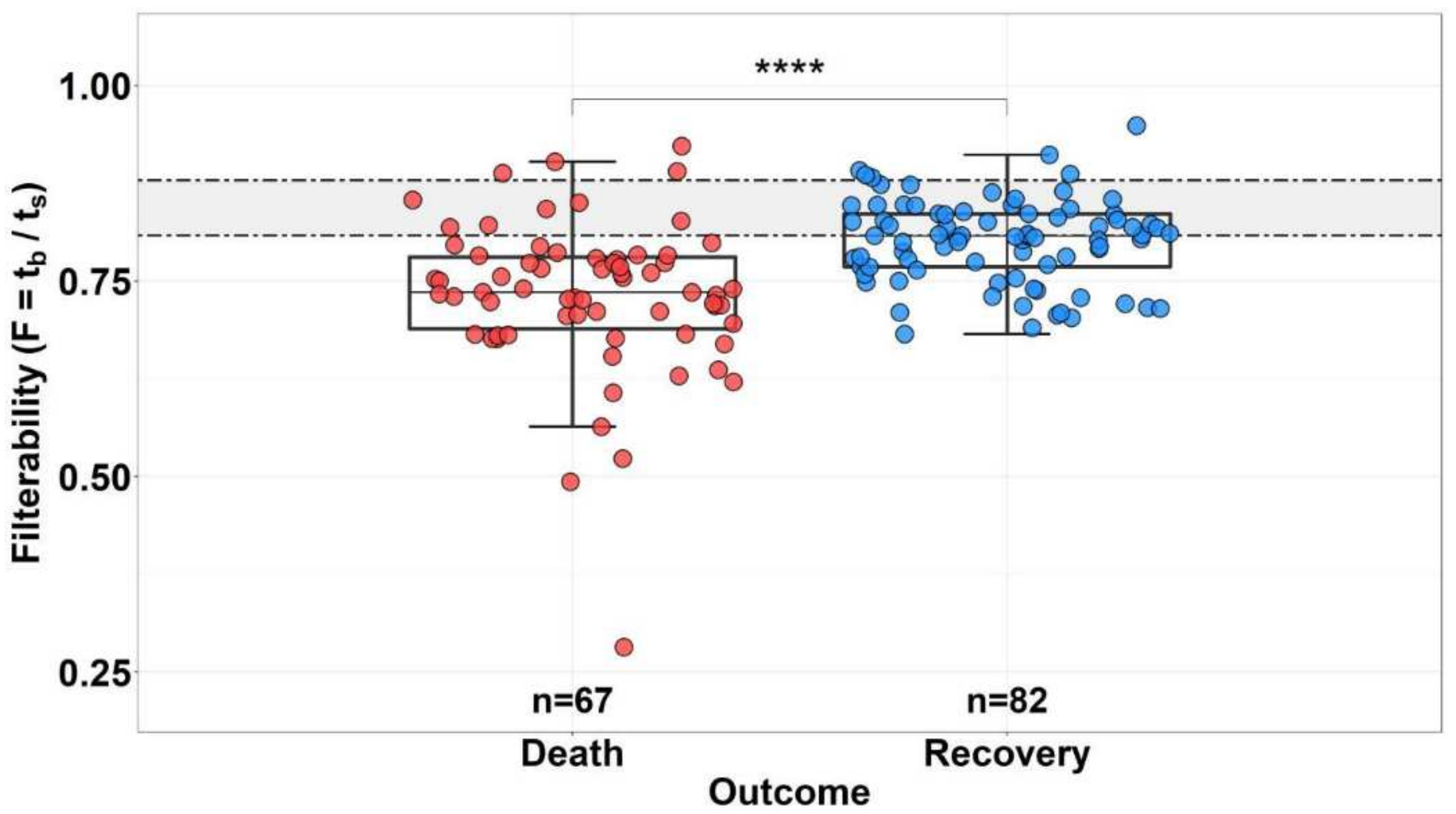
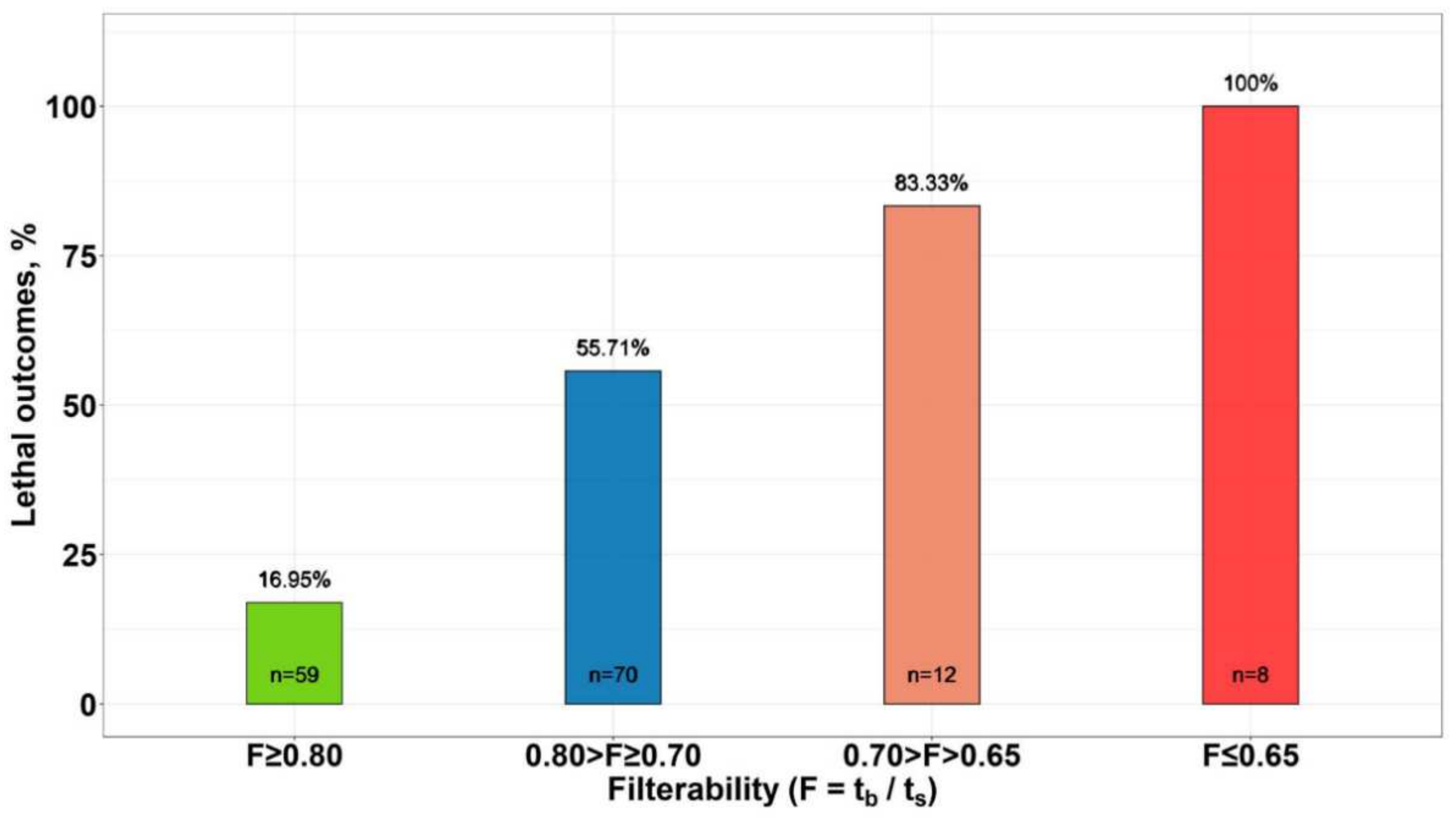
3.3. Effect of Erythrocyte Filterability of COVID-19 Patients on Disease Outcome
3.4. Study of Changes in Erythrocyte Filterability in Patients with COVID-19 in Dynamics
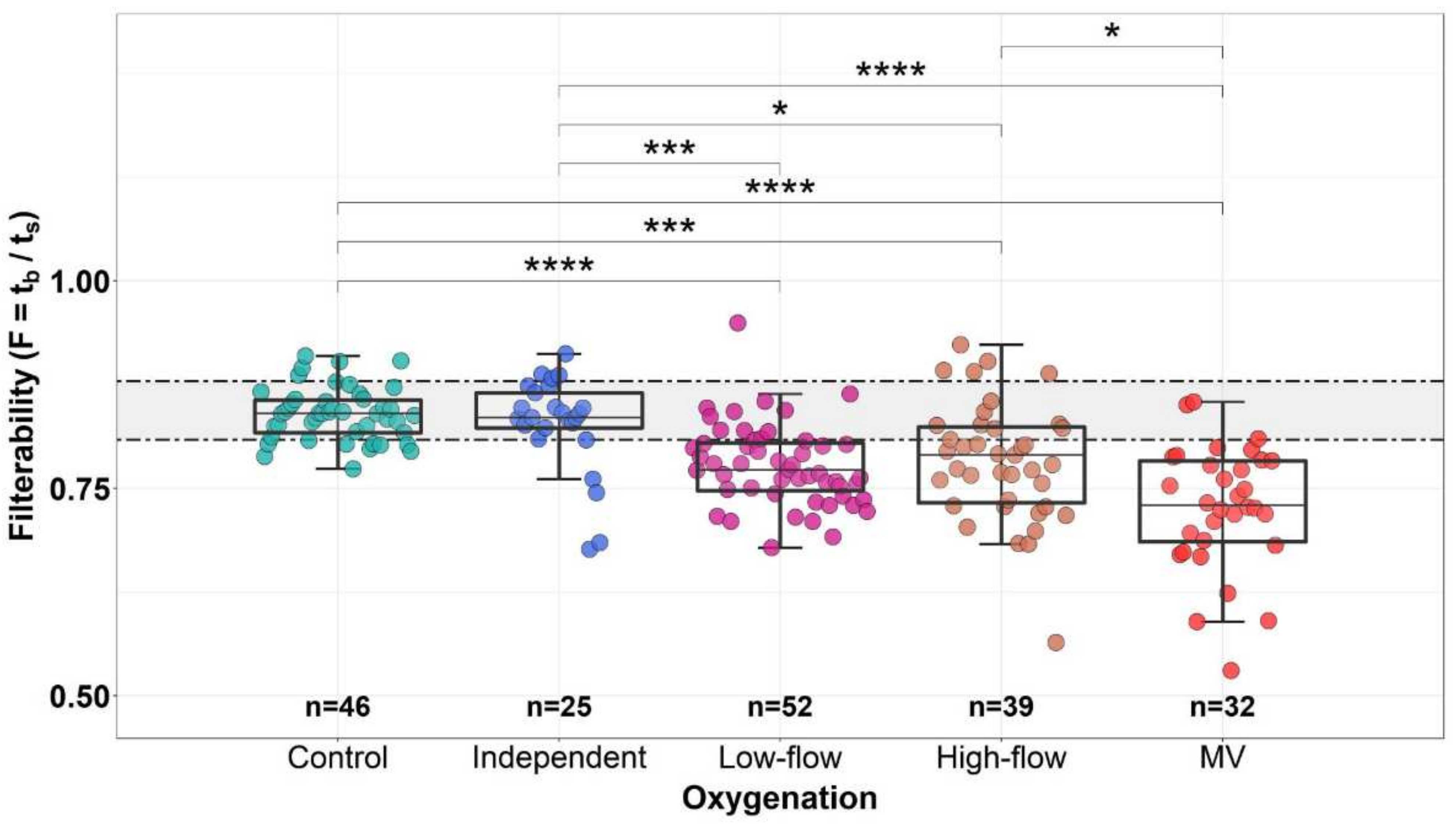
3.5. Dependence of the Erythrocyte Filterability on the Level and Method of Additional Oxygenation
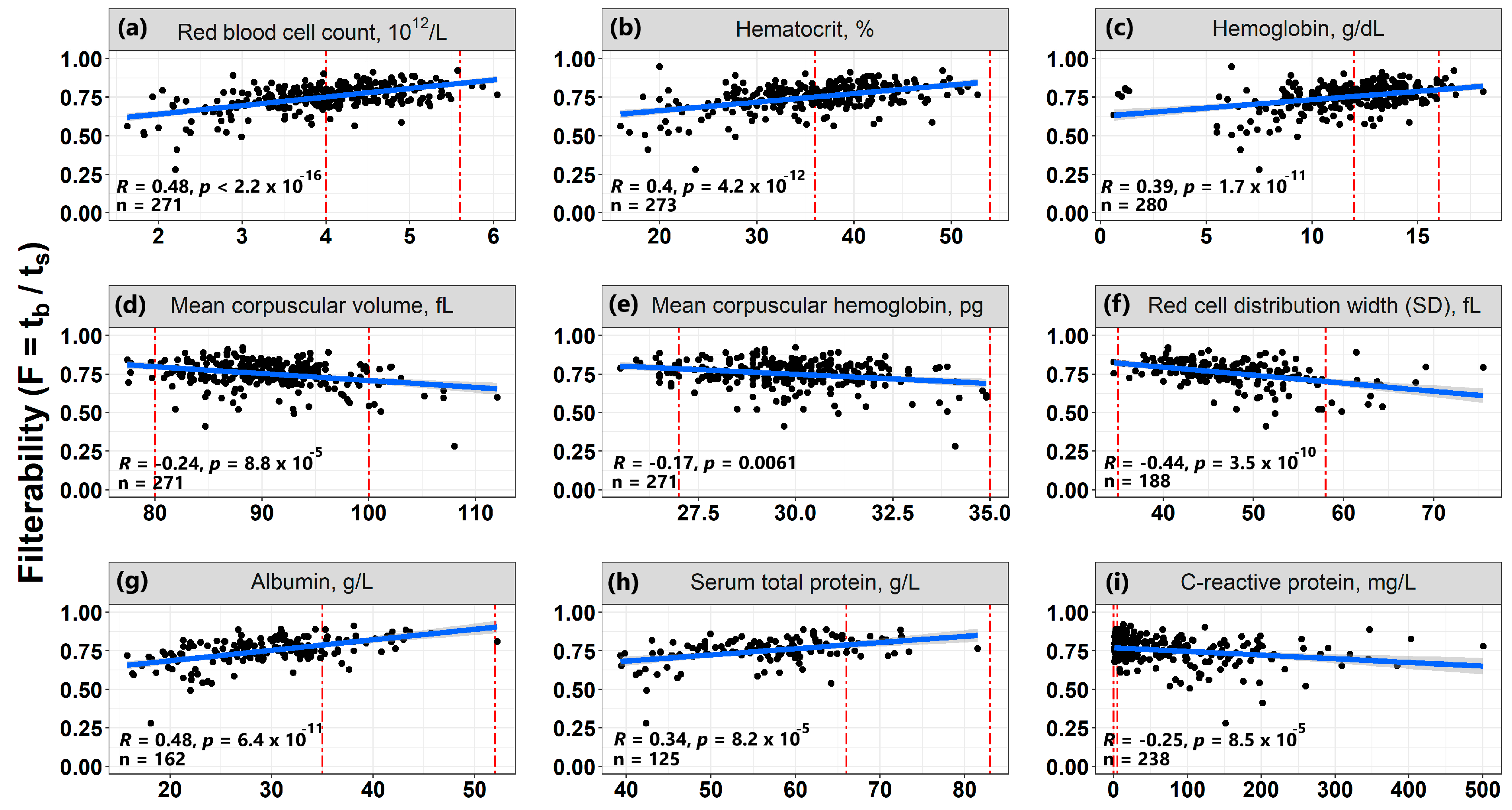
3.6. Correlations of Erythrocyte Filterability with Routine Laboratory Tests
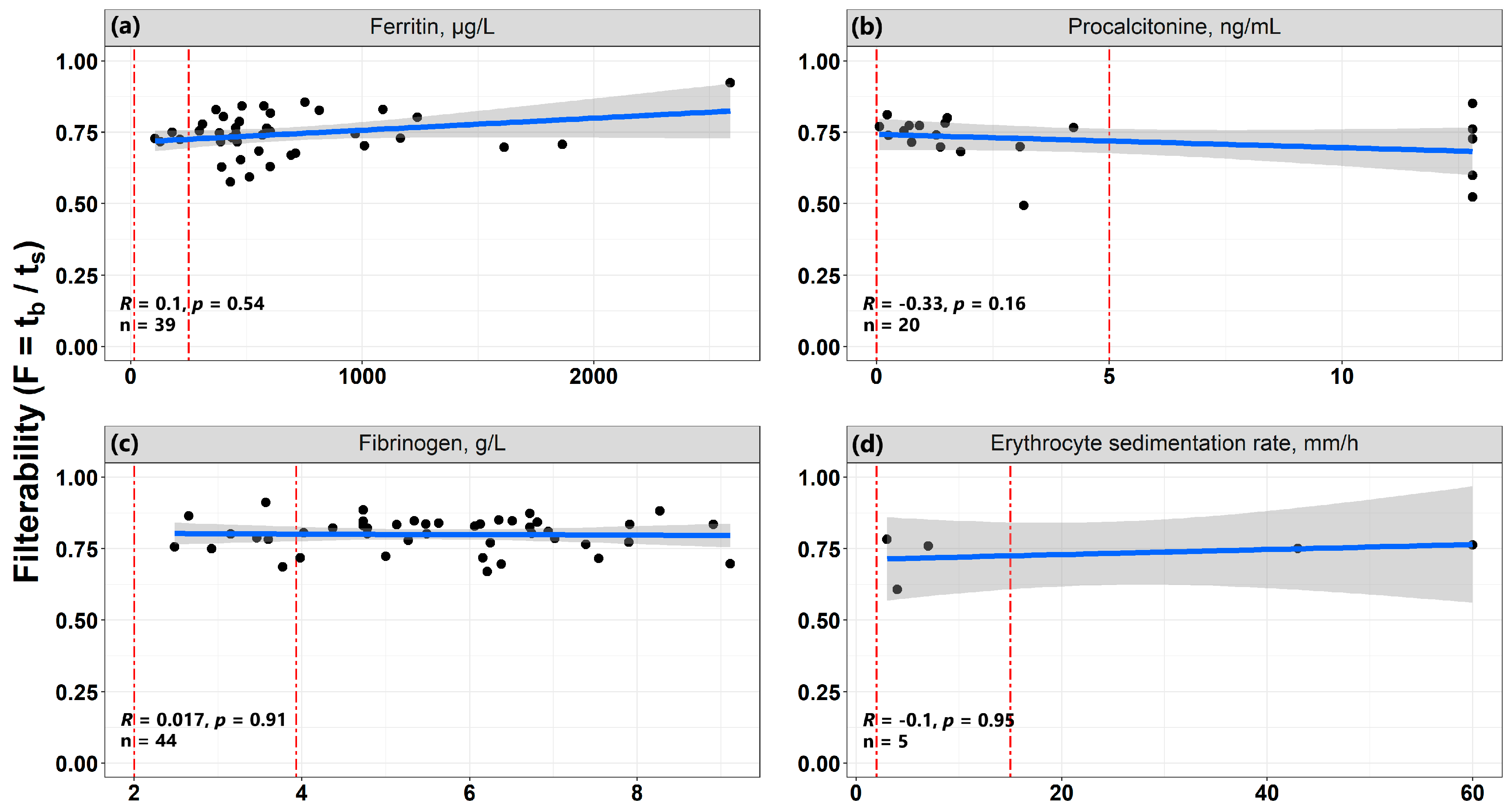
3.7. The Relationship of Erythrocyte Filterability with Inflammatory Processes
4. Discussion
- The filterability of RBCs from patients with COVID-19 is reduced compared to normal RBCs. This decrease is all the more pronounced the more severe the patient’s condition.
- The patient’s condition is also significantly correlated with the SpO2/FiO2 ratio.
- The RBCs filterability (including when measured in dynamics during treatment) can be used not only as an indicator of the patient’s condition, but also as a prognostic indicator of the outcome of the disease.
- The filterability of erythrocytes significantly increases with an increase in the blood of the number of erythrocytes, hematocrit, as well as concentrations of hemoglobin, albumin, and total protein. Such an effect is opposite to the effect of these parameters on blood macrorheology, since all of them increase blood viscosity, which worsens its macrorheology.
- The existing inflammatory process may affect the properties of erythrocytes of patients with COVID-19.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- COVID-19 Coronavirus Pandemic. Worldometer. Available online: https://www.worldometers.info/coronavirus/?utm_campaign=homeAdvegas1?%20 (accessed on 22 April 2022).
- Carsana, L.; Sonzogni, A.; Nasr, A.; Rossi, R.S.; Pellegrinelli, A.; Zerbi, P.; Rech, R.; Colombo, R.; Antinori, S.; Corbellino, M.; et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: A two-centre descriptive study. Lancet Infect. Dis. 2020, 20, 1135–1140. [Google Scholar] [CrossRef]
- Dhont, S.; Derom, E.; Van Braeckel, E.; Depuydt, P.; Lambrecht, B.N. The pathophysiology of ‘happy’ hypoxemia in COVID-19. Respir. Res. 2020, 21, 198. [Google Scholar] [CrossRef] [PubMed]
- Della Rocca, D.G.; Magnocavallo, M.; Lavalle, C.; Romero, J.; Forleo, G.B.; Tarantino, N.; Chimenti, C.; Alviz, I.; Gamero, M.T.; Mario, J.; et al. Evidence of systemic endothelial injury and microthrombosis in hospitalized COVID-19 patients at different stages of the disease. J. Thromb. Thrombolysis 2021, 51, 571–576. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Lowenstein, C.J.; Solomon, S.D. Severe COVID-19 is a microvascular disease. Circulation 2020, 142, 1609–1611. [Google Scholar] [CrossRef] [PubMed]
- Asakura, H.; Ogawa, H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int. J. Hematol. 2021, 113, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, K.; Barh, D.; Uhal, B.D.; Takayama, K.; Aljabali, A.A.A.; Abd El-Aziz, T.M.; Lal, A.; Redwan, E.M.; Adadi, P.; Chauhan, G.; et al. COVID-19 Vaccines and Thrombosis-Roadblock or Dead-End Street? Biomolecules 2021, 11, 1020. [Google Scholar] [CrossRef]
- Pelosi, P.; Tonelli, R.; Torregiani, C.; Baratella, E.; Confalonieri, M.; Battaglini, D.; Marchioni, A.; Confalonieri, P.; Clini, E.; Salton, F.; et al. Different Methods to improve the monitoring of noninvasive respiratory support of patients with severe pneumonia/ARDS due to COVID-19: An update. J. Clin. Med. 2022, 11, 1704. [Google Scholar] [CrossRef]
- Renoux, C.; Fort, R.; Nader, E.; Boisson, C.; Joly, P.; Stauffer, E.; Connes, P. Impact of COVID-19 on red blood cell rheology. Brit. J. Haematol. 2021, 192, e108–e111. [Google Scholar] [CrossRef]
- Kubankova, M.; Hohberger, B.; Hoffmanns, J.; Fürst, J.; Herrmann, M.; Guck, J.; Kräter, M. Physical phenotype of blood cells is altered in COVID-19. Biophys. J. 2021, 120, 2838–2847. [Google Scholar] [CrossRef]
- Piagnerelli, M.; Vanderelst, J.; Rousseau, A.; Monteyne, D.; Perez-Morga, D.; Biston, P.; Boudjeltia, K.Z. Red blood cell shape and deformability in patients with COVID-19 Acute Respiratory Distress Syndrome. Front Physiol. 2022, 13, 849910. [Google Scholar] [CrossRef] [PubMed]
- Nader, E.; Nougier, C.; Boisson, C.; Poutrel, S.; Catella, J.; Martin, F.; Charvet, J.; Girard, S.; Havard-Guibert, S.; Martin, M.; et al. Increased blood viscosity and red blood cell aggregation in patients with COVID-19. Am. J. Hematol. 2022, 97, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Stefanoni, D.; Dzieciatkowska, M.; Issaian, A.; Nemkov, T.; Hill, R.C.; Francis, R.O.; Hudson, K.E.; Buehler, P.W.; Zimring, J.C.; et al. Evidence of structural protein damage and membrane lipid remodeling in red blood cells from COVID-19 patients. J. Proteome Res. 2020, 19, 4455–4469. [Google Scholar] [CrossRef] [PubMed]
- Baskurt, O.K.; Meiselman, H.J. Blood rheology and hemodynamics. Semin. Thromb. Hemost. 2003, 29, 435–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baskurt, O.K.; Boynard, M.; Cokelet, G.C.; Connesd, P.; Cooke, B.M.; Forcon, S.; Liao, F.; Hardemanh, M.R.; Jung, F.; Meiselman, H.J.; et al. New guidelines for hemorheological laboratory techniques. Clin. Hemorheol. Microcirc. 2009, 42, 75–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.; Ku, Y.; Park, M.-S.; Suh, J.-S. Deformability of red blood cells: A determinant of blood viscosity. J. Mech. Sci. Technol. 2005, 19, 216–223. [Google Scholar] [CrossRef]
- Papaioannou, T.G.; Stefanadis, C. Vascular wall shear stress: Basic principles and methods. Hellenic J. Cardiol. 2005, 46, 9–15. [Google Scholar]
- Baskurt, O.K.; Meiselman, H.J. Data reduction methods for ektacytometry in clinical hemorheology. Clin. Hemorheol. Microcircul. 2013, 54, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Forconi, S.; Guerrini, M. Do hemorheological laboratory assays have any clinical relevance? Clin. Hemorheol. Microcircul. 1996, 16, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Pieragalli, D.; Acciavatti, A.; Galigani, C.; Messa, G.L.; Blardi, P.; Guerrini, M.; Forconi, S.; Di Perri, T. Interrelationship between whole blood and washed red cell filterability. Clin. Hemorheol. Microcircul. 1987, 7, 273–276. [Google Scholar] [CrossRef]
- Reid, H.L.; Barnes, A.J.; Lock, P.J.; Dormandy, J.A.; Dormandy, T.L. A simple method for measuring erythrocyte deformability. J. Clin. Path. 1976, 29, 855–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisovskaya, I.L.; Shurkhina, E.S.; Nesterenko, V.M.; Rozenberg, J.M.; Ataullakhanov, F.I. Determination of the content of nonfilterable cells in erythrocyte suspensions as a function of the medium osmolality. Biorheology 1998, 35, 141–153. [Google Scholar] [CrossRef]
- Jones, A.E.; Trzeciak, S.; Kline, J.A. The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit. Care Med. 2009, 37, 1649–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Royal College of Physicians (RCP). National Early Warning Score (NEWS): Standardising the Assessment of Acute-Illness Severity in the NHS; Report of a Working Party; RCP: London, UK, 2012; p. 46. ISBN 978-1-86016-471-2. [Google Scholar]
- Knaus, W.A.; Zimmerman, J.E.; Wagner, D.P.; Draper, E.A.; Lawrence, D.E. APACHE—Acute physiology and chronic health evaluation: A physiologically based classification system. Crit. Care Med. 1981, 9, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Mallow, R.; Pierce, M. Oxygen therapy. In Comprehensive Respiratory Care, 1st ed.; Dantzker, D.R., MacIntyre, N.R., Bakow, E.D., Eds.; Saunders: Philadelphia, PA, USA, 1995; pp. 499–519. [Google Scholar]
- Reinhart, W.H.; Piety, N.Z.; Deuel, J.W.; Makhro, A.; Schulzki, T.; Bogdanov, N.; Goede, J.S.; Bogdanova, A.; Abidi, R.; Shevkoplyas, S.S. Washing stored red blood cells in an albumin solution improves their morphologic and hemorheologic properties. Transfusion 2015, 55, 1872–1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polushin, Y.S.; Shlyk, I.V.; Gavrilova, E.G.; Parshin, E.V.; Ginzburg, A.M. The role of ferritin in assessing COVID-19 severity. Bullet. Anesthesiol. Resuscit. 2021, 18, 20–28. [Google Scholar] [CrossRef]
- Kaushal, K.; Kaur, H.; Sarma, P.; Bhattacharyya, A.; Sharma, D.J.; Prajapat, M.; Pathak Kothari, A.; Kumar, S.; Rana, S.; Kaur, M.; et al. Serum ferritin as a predictive biomarker in COVID-19. A systematic review, meta-analysis and meta-regression analysis. J. Crit. Care 2022, 67, 172–181. [Google Scholar] [CrossRef]
- Chan, Y.-L.; Tseng, C.-P.; Tsay, P.-K.; Chang, S.-S.; Chiu, T.-F.; Chen, J.-C. Procalcitonin as a marker of bacterial infection in the emergency department: An observational study. Crit. Care 2004, 8, R12–R20. [Google Scholar] [CrossRef] [Green Version]


| Parameter | Patients (Units) | Parameter (Units) | Patients | Normal Range |
|---|---|---|---|---|
| n | 149 | RBC (×1012 cells/L) | 4.03 (3.41; 4.57) | 4.00–5.20 (F) 4.10–5.60 (M) |
| Sex: | WBC (×109cells/L) | 10.09 (6.71; 14.00) | 4.00–9.00 | |
| PLT (×109 cells/L) | 210.0 (141.0; 294.5) | 180.0–320.0 | ||
| F M | n = 68 n = 81 | |||
| Hct (%) | 36.7 (31.0; 41.0) | 36.0–49.0 (F) 38.0–54.0 (M) | ||
| Age | 64 (55; 73) (years) | Hb (g/dL) | 11.8 (9.3; 13.4) | 12.0–15.0 (F) 13.0–16.0 (M) |
| SpO2/FiO2 | 265.7 (130.7–352.7) | >452 | ||
| Weight (on admission) | 88 (80; 100) (kg) | MCV (fL) | 90.0 (86.4; 93.9) | 80.0–100.0 |
| Patient’s condition on admission: | MCH (pg/cell) | 29.6 (28.3; 30.8) | 27.0–35.0 | |
| MCHC (g/L) | 328.0 (321.0; 337.0) | 300.0–380.0 | ||
| RDW-SD (fL) | 46.7 (42.5; 52.1) | 35.0–58.0 | ||
| Moderate Severe Critically severe | n = 58 n = 91 n = 0 | |||
| Additional oxygenation: | ALB (g/L) | 30.1 (25.8; 33.7) | 35.0–52.0 | |
| PRO (g/L) | 57.5 (51.4; 63.6) | 66.0–83.0 | ||
| Missing (independent breathing, air, 21% oxygen) | n = 27 | CRP (mg/L) | 66.92 (16.19; 141.20) | 0.00–5.00 |
| Fng (g/L) | 4.65 (3.38; 6.22) | 2.00–3.93 | ||
| Low-flow (fraction of inhaled oxygen 24–35%) | n = 73 | APTT (s) | 32.7 (28.3; 39.3) | 25.1–36.5 |
| PT (s) | 12.9 (12.0; 14.2) | 9.4–12.5 | ||
| High-flow (fraction of inhaled oxygen 40–100%) | n = 48 | INR | 1.12 (1.04; 1.24) | 0.90–1.20 |
| TT (s) | 15.9 (14.3; 19.2) | 11.0–20.0 | ||
| Invasive lung ventilation (oxygen fraction 25–100%) | n = 33 | DD ng/mL | 2134 (1105; 6176) | 0–500 |
| Outcome: | Vs (in TD) (μm/min) | 13.5 (8.0; 23.7) | 20.0–29.0 | |
| Recovery Death | n = 80 n = 69 | Vi (in TD) (μm/min) | 45.1 (31.2; 54.2) | 38.0–56.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prudinnik, D.S.; Sinauridze, E.I.; Shakhidzhanov, S.S.; Bovt, E.A.; Protsenko, D.N.; Rumyantsev, A.G.; Ataullakhanov, F.I. Filterability of Erythrocytes in Patients with COVID-19. Biomolecules 2022, 12, 782. https://doi.org/10.3390/biom12060782
Prudinnik DS, Sinauridze EI, Shakhidzhanov SS, Bovt EA, Protsenko DN, Rumyantsev AG, Ataullakhanov FI. Filterability of Erythrocytes in Patients with COVID-19. Biomolecules. 2022; 12(6):782. https://doi.org/10.3390/biom12060782
Chicago/Turabian StylePrudinnik, Dmitry S., Elena I. Sinauridze, Soslan S. Shakhidzhanov, Elizaveta A. Bovt, Denis N. Protsenko, Alexander G. Rumyantsev, and Fazoil I. Ataullakhanov. 2022. "Filterability of Erythrocytes in Patients with COVID-19" Biomolecules 12, no. 6: 782. https://doi.org/10.3390/biom12060782
APA StylePrudinnik, D. S., Sinauridze, E. I., Shakhidzhanov, S. S., Bovt, E. A., Protsenko, D. N., Rumyantsev, A. G., & Ataullakhanov, F. I. (2022). Filterability of Erythrocytes in Patients with COVID-19. Biomolecules, 12(6), 782. https://doi.org/10.3390/biom12060782








