Abstract
Background and aim: Glycomic alterations serve as biomarker tools for different diseases. The present study aims to evaluate the diagnostic capability of serum N-glycosylation to identify alcohol risk drinking in comparison with standard markers. Methods: We included 1516 adult individuals (age range 18–91 years; 55.3% women), randomly selected from a general population. A total of 143 (21.0%) men and 50 (5.9%) women were classified as risk drinkers after quantification of daily alcohol consumption and the Alcohol Use Disorders Identification Test (AUDIT). Hydrophilic interaction ultra-performance liquid chromatography (HILIC-UPLC) was used for the quantification of 46 serum N-glycan peaks. Serum gamma-glutamyltransferase (GGT), carbohydrate-deficient transferrin (CDT), and red blood cell mean corpuscular volume (MCV) were measured by standard clinical laboratory methods. Results: Variations in serum N-glycome associated risk drinking were more prominent in men compared to women. A unique combination of N-glycan peaks selected by the selbal algorithm shows good discrimination between risk-drinkers and non-risk drinkers for men and women. Receiver operating characteristics (ROC) curves show accuracy for the diagnosis of risk drinking, which is comparable to that of the golden standards, GGT, MCV and CDT markers for men and women. Additionally, the inclusion of N-glycan peaks improves the diagnostic accuracy of the standard markers, although it remains relatively low, due to low sensitivity. For men, the area under the ROC curve using N-glycome data is 0.75, 0.76, and 0.77 when combined with GGT, MCV, and CDT, respectively. In women, the areas were 0.76, 0.73, and 0.73, respectively. Conclusion: Risk drinking is associated with significant variations in the serum N-glycome, which highlights its potential diagnostic utility.
Keywords:
alcohol; risk drinking; N-glycome; N-glycan; glycomics; public health; biomarker; diagnosis; serum 1. Introduction
According to the 2019 WHO report, 3 million deaths worldwide are related to harmful alcohol use. Furthermore, above two hundred health conditions from cancer to liver diseases, motor vehicle accidents, and criminal offences related to harmful alcohol use [1]. From a clinical point of view, it is important to detect alcohol use disorders and risk drinking. Acute alcohol consumption can be assessed by measuring ethanol concentration in the blood, but chronic alcohol consumption has to be evaluated by the use of biomarkers with a much longer half-life than ethanol. Standard markers include serum gamma-glutamyl transferase (GGT), mean corpuscular volume of erythrocytes (MCV), and carbohydrate-deficient transferrin (CDT) [2,3,4,5,6]. Both GGT and MCV can be routinely determined in the clinical laboratory but have limited sensitivity and specificity since they can be influenced by non-alcohol-related disorders [2,3,4]. Moreover, the use of MCV is limited due to the long lifespan of the erythrocytes, which diminishes its potential as a relapse marker [4].
Glycosylation is the most common co- and post-translational modification of proteins and peptides in mammals [7,8]. It has an essential role in the proper folding, stability, solubility and effector function of proteins. Glycans are involved in virtually all biological processes and in the pathophysiology of every major disease in humans [8]. Therefore, glycosylation changes can be used as biomarkers, often with high accuracy for a wide array of diseases [7,8], particularly cancer [9,10,11,12,13]. Glycosylation is also affected by age, sex [14,15] and lifestyle, including diet [16] or smoking [15]. Along this line, the percentage of CDT to total serum transferrin is an approved biomarker for the detection of chronic alcohol abuse [3,17]. The most abundant form of transferrin contains disialylated biantennary glycan structures [18]. The loss of sialylation can be detected by analytical methods [19] and is indicative of chronic alcohol abuse [3]. At the cellular level, this loss of sialylation can be explained by the inhibitory effect of acetaldehyde (a metabolite of ethanol) on some of the sialyltransferase, galactosyltransferase and N-acetylglucosaminyltransferase activity in the Golgi apparatus in liver cells [20]. Acetaldehyde and ethanol cause Golgi remodelling and inhibit the activity of enzymes, which are important to create certain glycan structures [21,22,23].
Experience with CDT shows that glycomic analyses could be a promising tool for the detection of alcohol risk drinking. Despite the diagnostic potentials of glycomics in general [8,24], its accuracy to identify heavy alcohol intake has not yet been fully addressed. The serum N-glycome, (i.e., the set of all glycans that are N-linked to asparagine in serum proteins) is well studied due to the availability of a specific enzyme (PNGase F) for the N-glycan release and the availability of associated analytical methods for their proper separation and identification [25,26]. In this study, we aimed to address the potential of serum N-glycome as a biomarker of heavy alcohol consumption. The performance of the most common biomarkers (GGT, CDT, and MCV) was also investigated to compare their accuracy with that of the N-glycans.
2. Methods
2.1. Study Design
This cross-sectional study was developed in the municipality of A-Estrada (Northwestern Spain, location 42°41′21″ N, 8°29′14″ W). An outline of the study (AEGIS, A-Estrada Glycation and Inflammation Study) is available at www.clinicaltrials.gov (Accessed on 19 January 2022), code NCT01796184 and detailed elsewhere [27]. The municipality had an adult population (age >18 years) of 18474 when the study started in 2012. An age-stratified random sample of the population aged 18 years and older was drawn from Spain’s National Health System Registry, which covers more than 95% of the population and contains the name, date of birth and address of every person entitled to primary care. From an initial sample of 3500 individuals, 2230 could be assessed for eligibility and displayed no exclusion criteria; of these, 1516 individuals agreed to participate (overall participation rate, 68%). Participation was lower in men than in women (65% vs. 71%). There were no significant differences in terms of age or residence (rural vs. urban) between participants and non-participants. From November 2012 to March 2015, all subjects were successively contacted and asked to attend the Primary Care Centre for evaluation, which included an interviewer-administered structured questionnaire and fasting venous blood sampling. Median age of participants was 52 years (range, 18–91 years) and 838 (55.3%) were women. Basic characteristics of men and women are summarised in Table 1.

Table 1.
General characteristics of risk drinkers and non-risk drinkers, stratified by sex.
2.2. Ethical Issues
Written informed consent was obtained from all participants. The general survey and the specific glycomic studies were approved by the Galician Regional Ethics Committee (codes 2010-315 and 2016-464, respectively) and conformed to the current Helsinki Declaration.
2.3. Assessment of Smoking
Consumers of at least one cigarette per day were deemed to be smokers. Individuals who had quit smoking during the preceding year were still considered smokers. Ex-smokers and those that never smoked were grouped together for this study.
2.4. Definition of Alcohol Risk Drinking
The habitual alcohol consumption was evaluated in standard drinking units [28], by summing the number of glasses of wine (~10 g), bottles of beer (~10 g), and units of spirits (~20 g) regularly consumed per week. All participants also underwent an AUDIT (Alcohol Use Disorders Identification Test) questionnaire, which was validated in Spain [29,30,31]. Risk alcohol drinking for women was considered when regular alcohol consumption was greater than 20 g/day or the AUDIT score was greater than 5 points. Risk alcohol drinking for men was considered when regular alcohol consumption was greater than 40 g/day or the AUDIT score was greater than 7 points [29,30,31,32,33,34]. With these criteria, the prevalence of risk drinking was higher in men (143 of 678, 21.0%) than in women (50 of 838, 5.9%). Alcohol intake was higher in men than in women, both among risk drinkers and among non-risk drinkers (Table 1).
2.5. Serum Collection
Blood was drawn after overnight fasting using BD Vacutainer®SST Serum Separation Tubes (BD, Plymouth, UK). After collection, tubes were inverted five times, allowed 30 min clotting time, and centrifuged at room temperature for 15 min at 1300× g in a fixed-angle centrifuge. The serum was removed, aliquoted and stored at −80 °C for further use.
2.6. Determination of Serum GGT, MCV and CDT
Gamma-glutamyl transferase (GGT) was determined in fresh serum samples from fasting participants on a fully automatic Analyser (ADVIA 2400, Siemens Healthcare Diagnostics, Barcelona, Spain) by a standard method recommended by the International Federation of Clinical Chemistry (IFCC). Reference range for GGT in adults is 8–73 IU/L for men and 4–38 IU/L for women.
Mean corpuscular volume of red blood cells (MCV) was determined in fresh blood samples on an ADVIA 2120 automated hematology Analyser (Siemens Healthcare Diagnostics, Barcelona, Spain). Reference range for MCV in adults is 80–100 fL.
Carbohydrate-deficient transferrin (CDT) was determined after thawing frozen serum samples using the commercial CAPILLARYS CDTTM kit on a MINICAP CDTTM device (Sebia, Lisses, France) a multicapillary electrophoresis system, carefully following manufacturer’s instructions. Transferrin isoforms are separated in silica capillaries by their electrophoretic mobility and electro-osmotic flow at high voltage in an alkaline buffer. Transferrin isoforms are directly detected during migration by UV absorbance. The serum transferrin isoforms are separated into 5 main fractions according to their level of sialylation (asialotransferrin (non-sialylated), disialotransferrin, trisialotransferrin, tetrasialotransferrin and pentasialotransferrin). The proportion of each fraction is calculated as the area under the curve. The low sialylation transferrin isoforms (that is, asialotransferrin and disialotransferrin; there are usually no detectable monosialylated forms) constitute the CDT, the value of which is calculated automatically by the system. Genetic variants and abnormal profiles were well recognised. In cases of abnormal peak profiles or other interferences in the electropherogram, samples were remeasured following sample cleanup by sample treatment as specified by the manufacturer. The manufacturer recommends the following interpretation of results: normal (CDT ≤ 1.3%), indeterminate (CDT > 1.3% and ≤ 1.6%), and abnormal or indicative of alcohol abuse (CDT > 1.6%).
2.7. Serum N-Glycan Analyses
N-glycans were released from 5 μL of serum samples using a modified high-throughput automated method [35]. Briefly, the samples were denaturated with dithiothreitol (Sigma Aldrich, Arklow, Ireland), alkylated with iodoacetamide (Sigma Aldrich, Arklow, Ireland), and N-glycans were released from the protein backbone enzymatically via PNGase F (NEB, Ipswich, MA, USA, recombinant, code P0709L, 10 μL per well, 5000 U) in 1 M ammonium bicarbonate, pH 8.0 (Sigma Aldrich, Arklow, Ireland). Glycans were then immobilised on solid supported hydrazide beads on 96-well chemically inert filter plate (Millipore Solvinert, hydrophobic polytetrafluoroethylene membrane, 0.45 μm pore size, Millipore Ireland B.V., Carrigtwohill, Ireland), and excess reagents were removed by centrifuge filtration. Glycans were released from the solid supports and labelled with the fluorophore 2-aminobenzamide (2-AB, Sigma Aldrich, Arklow, Ireland). Glycans were cleaned up using HyperSep Diol SPE cartridges (Thermo Fisher Scientific, Waltham, MA, USA).
For hydrophilic interaction chromatography (HILIC) ultra-performance liquid chromatography (UPLC), fluorescently labelled N-glycans were separated on a Waters Acquity H-Class UPLC instrument (Waters, Milford, MA, USA) consisting of a quaternary solvent manager, sample manager-FTN, column manager and an FLR fluorescence detector set with excitation and emission wavelengths of 330 and 420 nm, respectively. The instrument was under the control of Empower 3 software, build 3471 (Waters, Milford, MA, USA). Labelled N-glycans were separated on a Waters Ethylene Bridged Hybrid BEH Glycan chromatography column, 150 × 2.1 mm i.d., 1.7 μm BEH particles (Waters, Milford, MA, USA), with 50 mM ammonium formate pH 4.4 (made from formic acid, Sigma Aldrich, Arklow, Ireland), and 25% ammonium hydroxide (Sigma Aldrich, Arklow, Ireland) as solvent A and acetonitrile (MeCN, Sigma Aldrich, Arklow, Ireland) as solvent B. The column was fitted with an ACQUITY in-line 0.2 μm filter. Separation method used linear gradient of 70–30% acetonitrile (v/v) at flow rate of 0.56 mL/min in a 30 min analytical run. Samples were maintained at 4 °C before injection. An injection volume of 25 µL sample prepared in 70% v/v MeCN was used throughout. Samples were maintained at 5 °C prior to injection and the separation temperature was 40 °C. The system was calibrated using an external standard of hydrolysed and 2-AB labelled glucose oligomers to create a dextran ladder, as described previously [36]. A fifth-order polynomial distribution curve was fitted to the dextran ladder to assign glucose unit (GU) values from retention times (using Empower software from Waters. Milford, MA, USA). The chromatograms were all separated in the same manner into 46 peaks according to Saldova et al. [12], and the amount of glycans in each peak was expressed as % of total integrated area. Glycan structures were annotated using the SNFG nomenclature and the DrawGlycan-SNFG software (University at Buffalo, Buffalo, NY, USA) [37,38] with the assist of GlycoStore.org (accessed on 11 November 2021) [39].
A summary of glycan peaks (GPs) and the corresponding N-glycan structures can be found in Supplementary Table S1. Groups of GPs were defined from their common features, as follows [12]:
Sialylation: S0 (neutral (asialylated), GP1−15); S1 (monosialylated, GP16−23 + GP30); S2 (disialylated, GP24−29 + GP31); S3 (trisialylated, GP32−40); S4 (tetrasialylated, GP41−46).
Galactosylation: G0 (agalactosylated, GP1−2 + GP4−5 + (GP6/2) + (GP12/2)); G1 (monogalactosylated, GP3 + GP7−10 + (GP12/2) + GP16−18 + (GP21/2)); G2 (digalactosylated, GP13−15 + GP19 + GP20 + (GP21/2) + GP22−28); G3 (trigalactosylated, GP29 + GP31−37); G4 (tetragalactosylated, GP30 + GP38−46).
Branching: A1 (monoantennary, GP1−3 +(GP12/2) + (GP21/2)); A2 (biantennary, GP4−5 + (GP6/2) + GP7−10 + (GP12/2) + GP13−20 + (GP21/2) + GP22−28); A3 (triantennary, GP29 + GP31−37); A4 (tetraantennary, GP30 + GP38−46).
Oligomannose: (GP6/2) + GP11.
Fucosylation: Core-fucose (CF) (GP2 + GP5 + (GP6/2) + GP8−10 + GP14−15 + GP17−18 + GP22−23 + GP27−28 + GP36 + (GP44/2)) and outer-arm fucose (OF) (GP37 + GP40 + (GP41/3) + GP45 + (GP46/3)).
Mass spectrometry-assisted glycan characterisation was performed for two representative samples and a technical replicate-otherwise the major glycans were identified and assigned based on their GU values cross-referenced in Glycobase, now migrated to Glycostore and based on previous assignments in Saldova et al. [12]. Online coupled fluorescence (FLR)-mass spectrometry detection was performed on Phynexus purified samples using a Waters Xevo G2 QTof with Acquity® UPLC (Waters Corporation, Milford, MA, USA) and BEH Glycan column (1.0 × 150 mm, 1.7 μm particle size). For MS acquisition data the instrument was operated in positive-sensitivity mode with a capillary voltage of 2.3 kV. The ion source block and nitrogen desolvation gas temperatures were set at 120 °C and 400 °C, respectively. The desolvation gas was set to a flow rate of 600 L/h. The cone voltage was maintained at 20 V. Full-scan data for glycans were acquired over m/z range from 450 to 2500. Data collection and processing were controlled by MassLynx 4.1 software (Waters Corporation, Milford, MA, USA). The fluorescence detector settings were as follows: λexcitation: 330 nm, λemission: 420 nm; data rate was 1pts/second and a PMT gain = 10. Sample injection volume was 10 μL (75% MeCN). The flow rate was 0.150 mL/min and column temperature was maintained at 60 °C; solvent A was 50 mM ammonium formate (pH 4.4) and solvent B was MeCN. A 40 min linear gradient was used and was as follows: 28% A for 1 min, 28–43% A for 30 min, 43–70% A for 1 min, 70% A for 3 min, 70–28% solvent A for 1 min and 28% A for 4 min. To avoid contamination of system, flow was sent to waste for the first 1.2 min and after 32 min. A summary of N-glycan structures identified by mass spectrometry can be found in Supplementary Table S2.
2.8. Statistical Analyses
All analyses were stratified by sex as the criteria for risk drinking were different in men and women, the criteria for normality of some markers were also different, and there were significant differences in the proportions of the different GPs between men and women (data not shown). We employed the Mann–Whitney test to compare the numerical data between groups. We employed the Chi-squared test to compare proportions and the Jonckheere–Terpstra test for trend analysis of numerical variables among ordinal categories. For univariate statistical analyses involving GPs, both uncorrected and sequential Bonferroni-adjusted p-values are shown.
To identify the best combination of glycan peaks (GPs) which is predictive for risk drinking, we used the R package selbal, a method for analysis of compositional data, i.e., data consisting of quantitative descriptions of the parts of some whole (as the sum of the 46 serum GPs, which is 100% or 1), conveying relative information. The method performs multiple regressions a number of times, each time adding a new GP to the model. Unlike linear regression, the raw variables in selbal are not included in a linear equation in real space but are added as part of what is called a “balance” in the compositional data analysis literature, i.e., as part of a particular type of log-contrast. Balances between different sample types are detected by identifying the smallest number of differentially variables that is predictive of sample condition. This method was proposed by Rivera-Pinto et al. [40] for the identification of signatures from compositional data that are predictive of a phenotype of interest [41]. The selbal algorithm is available on GitHub at https://github.com/malucalle/selbal/archive/master.zip (accessed on 19 January 2022).
The area under the curve (AUC) from the receiver operating characteristics (ROC) analysis was used to assess the predictive performance of GPs and commercial markers of risk drinking status (CDT, GGT and MCV), after adjusting for age and smoking status. The ROC curves and the AUC, with 95% confidence intervals (95% CI) were calculated using the “pROC” package [42].
3. Results
The results of the commercial markers of alcohol consumption in risk drinkers and non-risk drinkers are shown in Table 2. All markers (GGT, MCV and CDT) were significantly higher in risk drinkers compared to non-risk drinkers in men and women. However, their diagnostic accuracy was limited. The sensitivity of these markers (i.e., the proportion of risk drinkers with the altered marker) in men ranged from 7.1% for MCV, 18.2% for GGT to 25.9% for CDT. In women, however, the highest sensitivity was for GGT (32.0%), followed by MCV (10.0%) and the lowest sensitivity was for CDT (8.0%). The specificity of the markers (i.e., the proportion of non-risk drinkers with a negative test) was higher. In men, the best specificity was for MCV (98.7%), followed by CDT (94.4%) and GGT (81.8%). In women, the best specificity was also for MCV (99.9%), and CDT (99.5%), and it was lower for GGT (91.2%) (Table 2). Accordingly, the positive likelihood ratio for the detection of risk drinking in men was 2.03 (95% CI, 1.30–3.15) for GGT, 4.61 (95% CI, 2.96–7.20) for CDT, and 5.34 (95% CI 2.07–14.0) for MCV. In women, the positive likelihood ratio of GGT was higher (3.65, 95% CI 2.30–5.80). The positive likelihood ratios of CDT and MCV were also higher, but the small number of cases with a positive test made the confidence intervals very broad (16.0 (95% CI 4.06–61.0), and 79 (95% CI 9.38–662), respectively).

Table 2.
Comparison of commercial markers between risk drinkers and non-risk drinkers, stratified by sex.
The results of serum N-glycome (the relative proportion of the 46 GPs) in risk drinkers and non-risk drinkers are shown in Table 3. Among men, risk drinkers showed a lower abundance of GP5, GP8, GP9, GP10 and GP11, and a higher abundance of GP21, GP28, GP29, GP30, GP31, GP32, GP33, GP34 and GP42 than non-risk drinkers. A similar trend was observed in women, although only the lower abundance of GP8 and GP9 were statistically significant, as well as the higher abundance of GP28 and GP29. Furthermore, unlike men, a lower abundance of GP14 and a greater abundance of GP23 was observed among women risk drinkers (Table 3 and Figure 1).

Table 3.
Comparison of N-glycan peaks between risk drinkers and non-risk drinkers, stratified by sex.
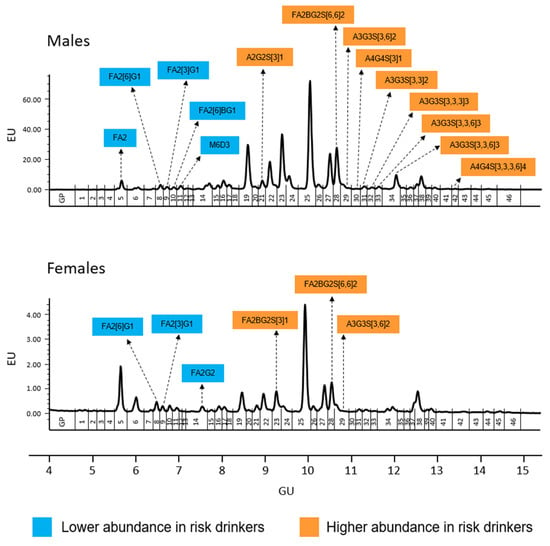
Figure 1.
Serum N-glycome representative chromatograms in men (upper panel) and women (lower panel). Glycan peaks (GPs) are numbered from GP1–GP46 and assigned as in Saldova et al. [12]. The integration areas, together with a major structure presented in each glycan group are given. Significant (uncorrected p-values <0.05) increases in relative peak area (in %) between risk drinkers and non-risk drinkers are marked with orange and in case of significant decrease are marked with blue. After sequential Bonferroni correction, only changes in GP9, GP31, GP33, and GP34 in men would be significant at an alpha level of 0.05. Structure abbreviations: all N-glycans have two core GlcNAcs; F at the start of the abbreviation indicates a core-fucose α1,6-linked to the inner GlcNAc; Mx, number (x) of mannose on core GlcNAcs; Ax, number of antenna (GlcNAc) on trimannosyl core; B, bisecting GlcNAc linked β1,4 to β1,3 mannose; Gx, number (x) of β1,4 linked galactose on antenna; Sx, number (x) of sialic acids linked to galactose.
Glycan features of serum N-glycans in risk drinkers and non-risk drinkers are shown in Table 4. Significant differences were only observed in men. The risk drinkers showed, compared to the non-risk drinkers, (a) a lower abundance of galactosylated (G0) and monogalactosylated (G1) glycans, together with a higher abundance of trigalactosylalated (G3) glycans; (b) a lower abundance of asialylated (S0) glycans together with a higher abundance of trisialylated (S3) glycans; (c) a lower abundance of diantennary (A2) glycans together with a higher abundance of triantennary (A3) glycans; and (d) a lower abundance of oligomannose (OM) glycans (Table 4).

Table 4.
Comparison of N-glycan peaks, aggregated in groups, between risk drinkers and non-risk drinkers, stratified by sex.
Supplementary Table S3A–F shows the results of the serum N-glycome (individual GPs and aggregated in categories) and standard markers of risk drinkers (GGT, VCM, and CDT) in relation to the different levels of habitual alcohol consumption (0–9 g/week, 10–139 g/week, 140–279 g/week, and ≥280 g/week) in men and women. Among men, a significant trend towards a higher abundance of GP21, GP28, GP31, GP32, GP33, GP34, and trigalactosylated, trisialylated, triantennary, and outer fucose glycans in relation to increasing categories of alcohol consumption was confirmed (Supplementary Table S3A,B). The trend in women was less evident, although a significant trend towards a higher abundance of GP31 and GP32 in relation to increasing categories of alcohol consumption was observed, as well as GP3 and GP29 (Supplementary Table S3C,D).
The selbal approach identified that a balance (or combination) consisting of GP21, GP31, and GP 33 (numerator) and GP19, GP20, GP22, GP25 and GP36 (denominator) was the best predictor combination for risk drinking in men, after adjusting for age and smoking (Figure 2). In the women, the best balance was GP6 (numerator), and GP8 (denominator) (Figure 2). Figure 3 compares the diagnostic accuracy (ROC curves) for risk drinking using the balance of GPs compared to the golden standard markers (GGT, MCV, and CDT), in all cases after adjusting for age and smoking in men and women. The AUC of the balance of GPs was at least the same as that of the GGT, MCV and CDT, without significant differences (Figure 3). In men, the combination of GPs with GGT increased the AUC of the latter from 0.70 to 0.75, increased the AUC of MCV from 0.70 to 0.76, and increased the AUC of CDT from 0.70 to 0.77. In women, the combination of GPs with GGT increased the AUC of the latter from 0.74 to 0.76, increased the AUC of MCV from 0.66 to 0.73, and increased the AUC of CDT from 0.65 to 0.73.
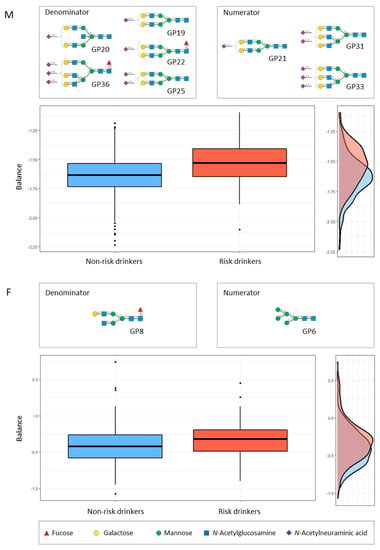
Figure 2.
Description of the global N-glycan balance (or combination) for risk drinking using selbal algorithm [40]. The two groups of N-glycans that form the global balance (numerator and denominator) selected by selbal conditions are specified at the top of the plot. The box plot represents the distribution of the balance scores for risk drinkers and non-risk drinkers. The right part of the figure contains the density curve for each group. M (upper panel), men; F (lower panel), women.
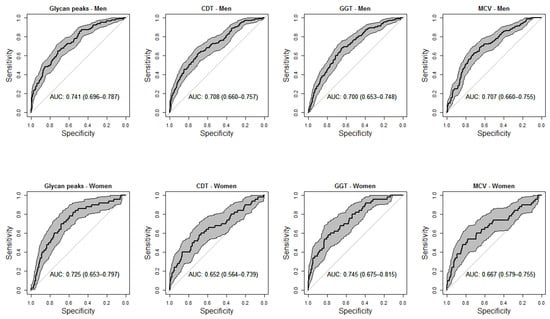
Figure 3.
Receiver-operating characteristic (ROC) curves comparing the accuracy of serum N-glycan balance and commercial markers (serum carbohydrate-deficient transferrin [CDT], serum gamma-glutamyltransferase [GGT], and red blood cell mean corpuscular volume [MCV]) for the diagnosis of risk drinking in men (upper panels) and women (lower panels). All analyses were adjusted for age and smoking. Shadowed areas represent the 95% confidence interval of the ROC curve. Numbers in boxes represent the area under the curve with 95% confidence interval (within parentheses).
4. Discussion
The demonstration of the relationship between alcohol consumption and abnormal transferrin glycosylation dates back to 1979 and was the first commercial glycomic marker of alcohol abuse (CDT, carbohydrate-deficient transferrin) [17]. Since then, the diagnostic value of CDT has been extensively studied, but other potential glycomic markers of alcohol risk drinking have not been investigated until now. Recent technological development enabled systematic analysis of serum N-glycome composition in large epidemiological cohorts and clinical studies [8,26,27]. The present study shows that delineating the serum N-glycome can add diagnostic value to the usual markers of risk drinking and can form the basis on which future investigations of glycomic markers are planned.
The classic commercial markers of risk drinking (GGT, MCV, and CDT) showed differential performance in men compared to women. In general, these classic markers demonstrated high specificity but low sensitivity, as reported previously [2,3,4,5,6]. The sensitivity in our study was even lower compared to previous reports, possibly because the threshold for the definition of risk drinking was especially low, according to current guidelines. The sensitivity in men was highest for CDT and lowest for MCV. In women, the sensitivity was highest for GGT and lowest for the glycomic marker CDT. Something similar happened in the serum N-glycome study, in which the greatest differences were observed in men compared to women, for the distribution of the individual GPs and for the GPs grouped into categories by their common traits. The differences in the effects of alcohol between men and women are well known [42]. In addition, the proportion of risk drinkers is lower among women and high-risk drinking women have a lower amount of consumption than men. Moreover, the distribution of N-glycome is different in men and women [14]. For all these reasons, all analyses were stratified by sex.
In our study, male risk drinkers showed higher abundances of triantennary glycans (versus a lower abundance of biantennary glycans), a higher abundance of trigalactosylated glycans (versus a lower abundance of galactosylated and monogalactosylated glycans), than non-risk drinkers. Specifically, both triantennary (A3) trigalactosylated (G3) glycans such as GP31 (A3G3S[3,3]2) and GP33 (A3G3S[3,3,6]3) were significantly more abundant in risk drinkers compared to non-risk drinkers. Furthermore, the abundance of these GPs significantly increased with increasing alcohol intake among drinkers. Moreover, these two GPs formed part of the numerator of the best balance of GPs [40,41] that was associated with risk drinking. Among men, risk drinkers also showed a higher abundance of trisialylated (S3) glycans (versus a lower abundance of asialylated (G0) glycans). As transferrin is an abundant serum protein (the second most abundant serum glycoprotein after IgG) [26], it could be expected that the glycosylation-inhibiting effect of alcohol consumption on transferrin (i.e., CDT) will be reflected in the serum N-glycome. The beta-galactosidase alpha-2,6-sialyltransferase 1 (encoded by ST6GAL1) is a principal sialyltransferase responsible for the creation of the terminal α2,6-galactosyl linkages on galactose-containing substrates, i.e., the transfer of sialic acid residue to a terminal galactose residue. The ST6GAL1 gene was found to be down-regulated by increasing alcohol intake and inhibited by acetaldehyde, the main by-product of ethanol metabolism; these are proposed to increase the low sialylated forms of transferrin (CDT) [22,23]. The human serum transferrin glycosylation mainly (96.5%) consists of the A2G2S2 structure [18]. N-glycans with an A2G2S[3,6]2 structure include GP24, GP25, and GP27. Of them, only GP25 showed a significant trend to a lower abundance with increasing alcohol consumption in men. Similar changes were not observed among women. Regarding individual GPs, the more consistent changes in men and women were a higher abundance of GP28 (FA2BG2S[6,6]2) and GP29 (A3G3S[3,6]2), and a lower abundance of GP8 (FA2[6]G1) and GP9 (FA2[3]G1) among risk drinkers. The possible meaning of these variations and the determination of which specific proteins (or other glycoconjugates) this glycosylation corresponds to should be addressed in future studies.
In addition to the pathophysiological interest (i.e., the specific alterations in the glycosylation process in relation to alcohol consumption), from a clinical point of view, it is interesting to obtain summary measures that may have practical applicability, in this case, diagnostic (detection of risk drinking). The usual methods for the determination of N-glycome display compositional data (i.e., proportions of some whole). The identification of global signatures that are predictive of the variable of interest (risk drinking) is an essential step toward the translation of serum N-glycome research to clinical practice. By means of selbal, a forward selection algorithm, we identified signatures consisting of two groups of N-glycans whose relative abundances, or balance, are predictive of the outcome [40]. Working with balances and, in general, with log-contrast functions preserves the scale-invariant principle for compositional data analysis [40]. For this purpose, we maintained sex stratification and further adjusted the results for age and smoking, two confounding variables that are associated with both risk drinking and N-glycome variations [15]. In men, we identified that the balance of the above-mentioned GP31, GP33, and GP20 (numerator) with GP19, GP20, GP22, GP25, and GP36 (denominator) showed an accuracy for the diagnosis of risk drinking which was at least as high as that of CDT, MCV and GGT. In women, the balance of GP6 (numerator) with the above-mentioned GP8 (denominator) also showed an accuracy for the diagnosis of risk drinking which was at least as high as that of the commercial markers CDT, MCV and GGT. Given that the combined use of markers is recommended for improving accuracy [4], we further observed that the addition of the GP balance to the classical markers improved their accuracy, both in men and women. It must be recognised, however, that the diagnostic accuracy of N-glycome, alone or in combination with GGT, MCV, and CDT still remains relatively low for the detection of risk drinking, as defined in the present study.
The study has strengths and limitations that should be acknowledged. The general population-based design may be considered a strength of the study. Investigation of analytes in the general population could help the interpretation of abnormal laboratory results in the disease, the definition of reference values, and the investigation of the influence of common factors, such as alcohol consumption in this case. Misclassification can occur when measuring alcohol consumption, which is a difficult discipline in itself. The system of standard drinking units is widely accepted, provides comparable results, and was validated in Spain [28]. To improve the ability to detect risk drinking, a combined definition was established with the AUDIT questionnaire, which was also validated in Spain [29,30,31]. Women show particular attitudes and effects with alcohol use disorders [43,44], for which specific thresholds were established in the definition of risk drinking for men and women. Temporal ambiguity in the possible cause–effect association is inherent to the cross-sectional design. Multivariate analyses were performed in order to adjust for confounding factors (age and smoking, in addition to sex). To the best of our knowledge, this is the first study that investigates the effects of alcohol on serum N-glycome.
5. Conclusions
In summary, in this article, we describe the variations in the N-glycome serum in relation to alcohol consumption. The determination of N-glycome has at least the same diagnostic value for risk drinking as conventional commercial markers and could provide additional information to them, although, as a whole, their diagnostic precision remains relatively low. Future studies will be necessary to determine whether the pattern of glycosylation of specific proteins can enhance the utility of glycomic clinical markers, beyond CDT.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biom12020241/s1. Table S1. The N-glycan composition of 46 peaks in control serum samples. Table S2. LC-MS Values detected for2-AB labelled N-glycans for normal human serum and two representative examples. Table S3. Comparison of individual N-glycan peaks, N-glycan peaks aggregated by features, and commercial markers of alcohol abuse among categories of alcohol consumption, in men and women.
Author Contributions
Conceptualisation, R.S. and A.G.-Q.; Data curation, R.O., M.A.-S. and F.G.; Formal analysis, F.G.; Funding acquisition, A.G.-Q.; Investigation, R.O., Á.S., M.A.-S., S.S.-B., C.F.-M. and R.S.; Methodology, R.O., F.G., R.S. and A.G.-Q.; Project administration, M.A.-S.; Supervision, R.S.; Validation, R.O.; Writing—original draft, R.O., Á.S., F.G., R.S. and A.G.-Q.; Writing—review and editing, M.A.-S., S.S.-B. and C.F.-M. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by grants from the Carlos III Institute of Health (Instituto de Salud Carlos III, PI16/01404, PI13/2594, and PI20/01069), the Spanish Network for Addictive Disorders (Red de Trastornos Adictivos, RD16/0017/0018, Spanish Ministry of Health) the Spanish Network for Preventive Activity and Health Promotion Research in Primary Care (Red de Actividades Preventivas y de Promocion de Salud en Atención Primaria, RD16/0007/0006), and the European Regional Development Fund (FEDER, “A way to make Europe” ).
Institutional Review Board Statement
The general survey and the specific glycomic studies were approved by the Galician Regional Ethics Committee (codes 2010-315 and 2016-464, respectively) and conformed to the current Helsinki Declaration.
Informed Consent Statement
Written informed consent was obtained from all participants.
Data Availability Statement
The data that support the findings of this study are available on reasonable request from the corresponding author. The data are not publicly available due to Spanish law restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Global Status Report on Alcohol and Health 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Hannuksela, M.L.; Liisanantti, M.K.; Nissinen, A.; Savolainen, M.J. Biochemical markers of alcoholism. Clin. Chem. Lab. Med. (CCLM) 2007, 45, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Niemelä, O. Biomarkers in alcoholism. Clin. Chim. Acta 2007, 377, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Niemelä, O. Biomarker-Based Approaches for Assessing Alcohol Use Disorders. Int. J. Environ. Res. Public Heal. 2016, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Salaspuro, M. Carbohydrate-Deficient Transferrin as Compared to Other Markers of Alcoholism: A Systematic Review. Alcohol 1999, 19, 261–271. [Google Scholar] [CrossRef]
- Yersin, B.; Nicolet, J.; Decrey, H.; Burnier, M.; van Melle, G.; Pecoud, A. Screening for excessive alcohol drinking: Compa-ra-tive value of carbohydrate-deficient transferrin, γ-glutamyltransferase, and mean corpuscular volume. Arch. Intern. Med. 1995, 155, 1907–1911. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef]
- Lauc, G.; Pezer, M.; Rudan, I.; Campbell, H. Mechanisms of disease: The human N-glycome. Biochim. et Biophys. Acta (BBA) - Gen. Subj. 2016, 1860, 1574–1582. [Google Scholar] [CrossRef]
- O’Flaherty, R.; Muniyappa, M.; Walsh, I.; Stöckmann, H.; Hilliard, M.; Hutson, R.; Saldova, R.; Rudd, P.M. A robust and ver-satile automated glycoanalytical technology for serum antibodies and acute phase proteins: Ovarian Cancer Case Study. Mol. Cell Proteomics 2019, 18, 2191–2206. [Google Scholar] [CrossRef]
- Kyselova, Z.; Mechref, Y.; Kang, P.; Goetz, J.A.; Dobrolecki, L.E.; Sledge, G.W.; Schnaper, L.; Hickey, R.J.; Malkas, L.H.; Novotny, M.V. Breast Cancer Diagnosis and Prognosis through Quantitative Measurements of Serum Glycan Profiles. Clin. Chem. 2008, 54, 1166–1175. [Google Scholar] [CrossRef]
- Saldova, R.; Fan, Y.; Fitzpatrick, J.M.; Watson, R.W.G.; Rudd, P.M. Core fucosylation and α2-3 sialylation in serum N-glycome is significantly increased in prostate cancer comparing to benign prostate hyperplasia. Glycobiology 2011, 21, 195–205. [Google Scholar] [CrossRef]
- Saldova, R.; Asadi Shehni, A.; Haakensen, V.D.; Steinfeld, I.; Hilliard, M.; Kifer, I.; Helland, A.; Yakhini, Z.; Børresen-Dale, A.L.; Rudd, P.M. Association of N-glycosylation with breast carcinoma and systemic features using high-resolution quanti-tative UPLC. J. Proteome Res. 2014, 13, 2314–2327. [Google Scholar] [CrossRef] [PubMed]
- (Saldova), R.F.; Haakensen, V.D.; Rødland, E.; Walsh, I.; Stockmann, H.; Engebraaten, O.; Børresen-Dale, A.-L.; Rudd, P.M. Serum N-glycome alterations in breast cancer during multimodal treatment and follow-up. Mol. Oncol. 2017, 11, 1361–1379. [Google Scholar] [CrossRef]
- Ding, N.; Nie, H.; Sun, X.; Sun, W.; Qu, Y.; Liu, X.; Yao, Y.; Liang, X.; Chen, C.C.; Li, Y. Human serum N-glycan profiles are age and sex dependent. Age Ageing 2011, 40, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Knežević, A.; Gornik, O.; Polašek, O.; Pučić, M.; Redžić, I.; Novokmet, M.; Rudd, P.M.; Wright, A.F.; Campbell, H.; Rudan, I.; et al. Effects of aging, body mass index, plasma lipid profiles, and smoking on human plasma N-glycans. Glycobiology 2010, 20, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Samraj, A.N.; Pearce, O.; Läubli, H.; Crittenden, A.N.; Bergfeld, A.K.; Banda, K.; Gregg, C.J.; Bingman, A.E.; Secrest, P.; Diaz, S.L.; et al. A red meat-derived glycan promotes inflammation and cancer progression. Proc. Natl. Acad. Sci. 2015, 112, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Stibler, H.; Borg, S.; Allgulander, C. Clinical Significance of Abnormal Heterogeneity of Transferrin in Relation to Alcohol Consumption. Acta Medica Scand. 1979, 206, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Clerc, F.; Reiding, K.R.; Jansen, B.C.; Lageveen-Kammeijer, G.; Bondt, A.; Wuhrer, M. Human plasma protein N-glycosylation. Glycoconj. J. 2016, 33, 309–343. [Google Scholar] [CrossRef]
- Helander, A.; Wielders, J.P.; te Stroet, R.; Bergström, J.P. Comparison of HPLC and capillary electrophoresis for confir-ma-tory testing of the alcohol misuse marker carbohydrate-deficient transferrin. Clin. Chem. 2005, 51, 1528–1531. [Google Scholar] [CrossRef][Green Version]
- Lieber, C.S. Carbohydrate Deficient Transferrin in Alcoholic Liver Disease: Mechanisms and Clinical Implications. Alcohol 1999, 19, 249–254. [Google Scholar] [CrossRef]
- Casey, C.A.; Bhat, G.; Holzapfel, M.S.; Petrosyan, A. Study of Ethanol-Induced Golgi Disorganization Reveals the Potential Mechanism of Alcohol-Impaired N-Glycosylation. Alcohol. Clin. Exp. Res. 2016, 40, 2573–2590. [Google Scholar] [CrossRef]
- Ghosh, P.; Liu, Q.-H.; Lakshman, M. Long-term ethanol exposure impairs glycosylation of both N- and O-glycosylated proteins in rat liver. Metabolism 1995, 44, 890–898. [Google Scholar] [CrossRef]
- Gong, M.; Castillo, L.; Redman, R.S.; Garige, M.; Hirsch, K.; Azuine, M.; Amdur, R.L.; Seth, D.; Haber, P.S.; Lakshman, M.R. Down-regulation of liver Galbeta1, 4GlcNAc alpha2, 6-sialyltransferase gene by ethanol significantly correlates with al-co-holic steatosis in humans. Metabolism 2008, 57, 1663–1668. [Google Scholar] [CrossRef] [PubMed]
- Cummings, R.D.; Pierce, J.M. The Challenge and Promise of Glycomics. Chem. Biol. 2014, 21, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mariño, K.; Bones, J.; Kattla, J.J.; Rudd, P.M. A systematic approach to protein glycosylation analysis: A path through the maze. Nat. Chem. Biol. 2010, 6, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Mariño, K.; Saldova, R.; Adamczyk, B.; Rudd, P.M.; Rauter, A. Changes in serum N-glycosylation profiles: Functional sig-nif-icance and potential for diagnostics. Carbohydr. Chem. 2012, 37, 57–93. [Google Scholar]
- Alende-Castro, V.; Alonso-Sampedro, M.; Vazquez-Temprano, N.; Tuñez, C.; Rey, D.; García-Iglesias, C.; Sopeña, B.; Gude, F.; Gonzalez-Quintela, A. Factors influencing erythrocyte sedimentation rate in adults: New evidence for an old test. Medicine 2019, 98, e16816. [Google Scholar] [CrossRef] [PubMed]
- Gual, A.; Martos, A.R.; Lligoña, A.; Llopis, J.J. Does the concept of a standard drink apply to viticultural societies? Alcohol Alcohol. 1999, 34, 153–160. [Google Scholar] [CrossRef]
- Valladolid, G.R.; Vicedo, J.B.; Sánchez-Serrano, M.C.C.; Carrasco, J.S.-D. [Validation of the Alcohol Use Disorders Identification Test (AUDIT) in primary care]. Revista Clínica Española 1998, 198, 11–14. [Google Scholar]
- Gómez Arnáiz, A.; Conde Martela, A.; Aguiar Bautista, J.A.; Santana Montesdeoca, J.M.; Jorrín Moreno, A.; Betancor León, P. Diagnostic usefulness of Alcohol Use Disorders Identification Test (AUDIT) for detecting hazardous alcohol consumption in primary care settings. Med. Clin. 2001, 116, 121–124. [Google Scholar] [CrossRef]
- Pérula de Torres, L.A.; Fernández-García, J.A.; Arias-Vega, R.; Muriel-Palomino, M.; Márquez-Rebollo, E.; Ruiz-Moral, R. Validation of the AUDIT test for identifying risk consumption and alcohol use disorders in women. Aten. Primaria 2005, 36, 499–506. [Google Scholar] [CrossRef][Green Version]
- Ashworth, M.; Gerada, C. ABC of mental health: Addiction and dependence II: Alcohol. BMJ 1997, 315, 358–360. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dawson, D.A. Defining Risk Drinking. Alcohol Res. Heal. J. Natl. Inst. Alcohol Abus. Alcohol. 2011, 34, 144–156. [Google Scholar]
- Wood, A.M.; Kaptoge, S.; Butterworth, A.S.; Willeit, P.; Warnakula, S.; Bolton, T.; Paige, E.; Paul, D.S.; Sweeting, M.; Burgess, S.; et al. Risk thresholds for alcohol consumption: Combined analysis of indi-vidu-al-participant data for 599 912 current drinkers in 83 prospective studies. Lancet 2018, 391, 1513–1523. [Google Scholar] [CrossRef]
- Stöckmann, H.; O’Flaherty, R.; Adamczyk, B.; Saldova, R.; Rudd, P.M. Automated, high-throughput serum glycoprofiling platform. Integr. Biol. 2015, 7, 1026–1032. [Google Scholar] [CrossRef]
- Royle, L.; Campbell, M.P.; Radcliffe, C.M.; White, D.M.; Harvey, D.J.; Abrahams, J.L.; Kim, Y.G.; Henry, G.W.; Shadick, N.A.; Weinblatt, M.E.; et al. HPLC-based analysis of serum N-glycans on a 96-well plate platform with dedicated database software. Anal. Biochem. 2008, 376, 1–12. [Google Scholar] [CrossRef]
- Cheng, K.; Zhou, Y.; Neelamegham, S. DrawGlycan-SNFG: A robust tool to render glycans and glycopeptides with frag-mentation information. Glycobiology 2016, 27, 200–205. [Google Scholar] [CrossRef]
- Neelamegham, S.; Aoki-Kinoshita, K.; Bolton, E.; Frank, M.; Lisacek, F.; Lütteke, T.; O’Boyle, N.; Packer, N.; Stanley, P.; Toukach, P.; et al. Updates to the Symbol Nomenclature for Glycans guidelines. Glycobiology 2019, 29, 620–624. [Google Scholar] [CrossRef]
- Zhao, S.; Walsh, I.; Abrahams, J.; Royle, L.; Nguyen-Khuong, T.; Spencer, D.; Fernandes, D.L.; Packer, N.; Rudd, P.M.; Campbell, M.P. GlycoStore: A database of retention properties for glycan analysis. Bioinformatics 2018, 34, 3231–3232. [Google Scholar] [CrossRef]
- Rivera-Pinto, J.; Egozcue, J.J.; Pawlowsky-Glahn, V.; Paredes, R.; Noguera-Julian, M.; Calle, M.L. Balances: A New Perspective for Microbiome Analysis. mSystems 2018, 3, e00053-18. [Google Scholar] [CrossRef]
- Susin, A.; Wang, Y.; Cao, K.-A.L.; Calle, M.L. Variable selection in microbiome compositional data analysis. NAR Genom. Bioinform. 2020, 2, lqaa029. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Müller, M. pROC: An open-source package for R and S+ to analyse and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, S.F. Women and alcohol use disorders. Harv. Rev. Psychiatry 2002, 10, 76–85. [Google Scholar] [CrossRef] [PubMed]
- McCrady, B.S.; Epstein, E.E.; Fokas, K.F. Treatment Interventions for women with alcohol use disorder. Alcohol Res. 2020, 40, 08. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).