Methacrylated Cartilage ECM-Based Hydrogels as Injectables and Bioinks for Cartilage Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Solubilisation of Porcine Knee Cartilage
2.2. Methacrylation of cECM
2.3. Formulation of Articular Cartilage Extracellular Matrix Methacrylate (cECM-MA) Bioinks and Printability
2.4. Rheological Analysis of cECM-MA Bioinks
2.5. Cell Isolation and Expansion
2.6. Cell Culture in cECM-MA Hydrogels
2.7. Cell Viability
2.8. Biochemical Analyses
2.9. Histological and Immunohistochemical Analyses
2.10. Mechanical Testing
2.11. Statistical Analysis
3. Results and Discussion
3.1. Rheological Properties of Solubilised cECM
3.2. Collagen Functionalisation
3.3. Rheological Properties of cECM-MA-Based Inks
3.4. Rheological Properties and Printability of Cell-Laden cECM-MA Bioinks
3.5. Chondrogenesis of MSCs in cECM-MA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Blasioli, D.J.; Kim, H.-J.; Kaplan, D.L. Cartilage tissue engineering with silk scaffolds and human articular chondrocytes. Biomaterials 2006, 27, 4434–4442. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Brown, W.E.; Lee, C.A.; Wang, D.; Paschos, N.; Hu, J.C.; Athanasiou, K.A. Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat. Rev. Rheumatol. 2019, 15, 550–570. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.L.; Di Bella, C.; Wallace, G.G.; Choong, P.F.M. Cartilage tissue engineering using stem cells and bioprinting technology—Barriers to clinical translation. Front. Surg. 2018, 5, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, K.; He, C.; Xiao, C.; Li, G.; Chen, X. Injectable glycopolypeptide hydrogels as biomimetic scaffolds for cartilage tissue engineering. Biomaterials 2015, 51, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Joshi, N.; Jayakrishnan, A.; Banerjee, R. Self-crosslinked oxidized alginate/gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomater. 2014, 10, 3650–3663. [Google Scholar] [CrossRef]
- Yan, L.; Wang, Y.-J.; Ren, L.; Wu, G.; Caridade, S.; Fan, J.-B.; Wang, L.-Y.; Ji, P.-H.; Oliveira, J.M.; Oliveira, J.T.; et al. Genipin-cross-linked collagen/chitosan biomimetic scaffolds for articular cartilage tissue engineering applications. J. Biomed. Mater. Res. Part A 2010, 95, 465–475. [Google Scholar] [CrossRef] [Green Version]
- Daly, A.; Freeman, F.; Gonzalez-Fernandez, T.; Critchley, S.E.; Nulty, J.; Kelly, D.J. 3D bioprinting for cartilage and osteochondral tissue engineering. Adv. Healthc. Mater. 2017, 6, 1700298. [Google Scholar] [CrossRef]
- Daly, A.; Davidson, M.D.; Burdick, J.A. 3D bioprinting of high cell-density heterogeneous tissue models through spheroid fusion within self-healing hydrogels. Nat. Commun. 2021, 12, 753. [Google Scholar] [CrossRef]
- Badylak, S.F. The extracellular matrix as a scaffold for tissue reconstruction. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Gilbert, T.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Rothrauff, B.B.; Lin, H.; Gottardi, R.; Alexander, P.G.; Tuan, R.S. Enhancement of tenogenic differentiation of human adipose stem cells by tendon-derived extracellular matrix. Biomaterials 2013, 34, 9295–9306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothrauff, B.B.; Shimomura, K.; Gottardi, R.; Alexander, P.G.; Tuan, R.S. Anatomical region-dependent enhancement of 3-dimensional chondrogenic differentiation of human mesenchymal stem cells by soluble meniscus extracellular matrix. Acta Biomater. 2017, 49, 140–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romanazzo, S.; Vedicherla, S.; Moran, C.; Kelly, D. Meniscus ECM-functionalised hydrogels containing infrapatellar fat pad-derived stem cells for bioprinting of regionally defined meniscal tissue. J. Tissue Eng. Regen. Med. 2017, 12, e1826–e1835. [Google Scholar] [CrossRef]
- Jung, C.S.; Kim, B.K.; Lee, J.; Min, B.-H.; Park, S.-H. Development of printable natural cartilage matrix bioink for 3D printing of irregular tissue shape. Tissue Eng. Regen. Med. 2017, 15, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Visscher, D.O.; Lee, H.; van Zuijlen, P.P.; Helder, M.N.; Atala, A.; Yoo, J.J.; Lee, S.J. A photo-crosslinkable cartilage-derived extracellular matrix bioink for auricular cartilage tissue engineering. Acta Biomater. 2021, 121, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Doherty, S. The Development of Region-Specific Decellularized Meniscus Bioinks for 3D Bioprinting Applications. 2021. Available online: https://ir.lib.uwo.ca/etd/7606 (accessed on 10 December 2021).
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef] [PubMed]
- GhavamiNejad, A.; Ashammakhi, N.; Wu, X.Y.; Khademhosseini, A. Crosslinking strategies for 3D bioprinting of polymeric hydrogels. Small 2020, 16, 2002931. [Google Scholar] [CrossRef]
- Dong, M.; Xu, S.; Bünger, M.H.; Birkedal, H.; Besenbacher, F. Temporal assembly of collagen type II studied by atomic force microscopy. Adv. Eng. Mater. 2007, 9, 1129–1133. [Google Scholar] [CrossRef]
- Yang, K.; Sun, J.; Wei, D.; Yuan, L.; Yang, J.; Guo, L.; Fan, H.; Zhang, X. Photo-crosslinked mono-component type II collagen hydrogel as a matrix to induce chondrogenic differentiation of bone marrow mesenchymal stem cells. J. Mater. Chem. B 2017, 5, 8707–8718. [Google Scholar] [CrossRef]
- Browe, D.C.; Mahon, O.R.; Díaz-Payno, P.J.; Cassidy, N.; Dudurych, I.; Dunne, A.; Buckley, C.T.; Kelly, D.J. Glyoxal cross-linking of solubilized extracellular matrix to produce highly porous, elastic, and chondro-permissive scaffolds for orthopedic tissue engineering. J. Biomed. Mater. Res. Part A 2019, 107, 2222–2234. [Google Scholar] [CrossRef]
- Shirahama, H.; Lee, B.H.; Tan, L.P.; Cho, N.-J. Precise tuning of facile one-pot gelatin methacryloyl (GelMA) synthesis. Sci. Rep. 2016, 6, 31036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helary, C.; Bataille, I.; Abed, A.; Illoul, C.; Anglo, A.; Louedec, L.; Letourneur, D.; Meddahi-Pellé, A.; Giraud-Guille, M.M. Concentrated collagen hydrogels as dermal substitutes. Biomaterials 2010, 31, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Likun, G.; Yang, J.; Ni, Y.; Teng, Y.; Guo, L.; Fan, H.; Fan, Y.; Zhang, X. Effects of composition and mechanical property of injectable collagen I/II composite hydrogels on chondrocyte behaviors. Tissue Eng. Part A 2016, 22, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, W.T.; Nagapudi, K.; Thomas, A.B.S.; Chaikof, E.L. Photo-cross-linking of type I collagen gels in the presence of smooth muscle cells: Mechanical properties, cell viability, and function. Biomacromolecules 2003, 4, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Xu, S.-Y. Purification and characterization of type II collagen from chick sternal cartilage. Food Chem. 2008, 108, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Paxton, N.; Smolan, W.; Böck, T.; Melchels, F.; Groll, J.; Jungst, T. Proposal to assess printability of bioinks for extrusion-based bioprinting and evaluation of rheological properties governing bioprintability. Biofabrication 2017, 9, 044107. [Google Scholar] [CrossRef]




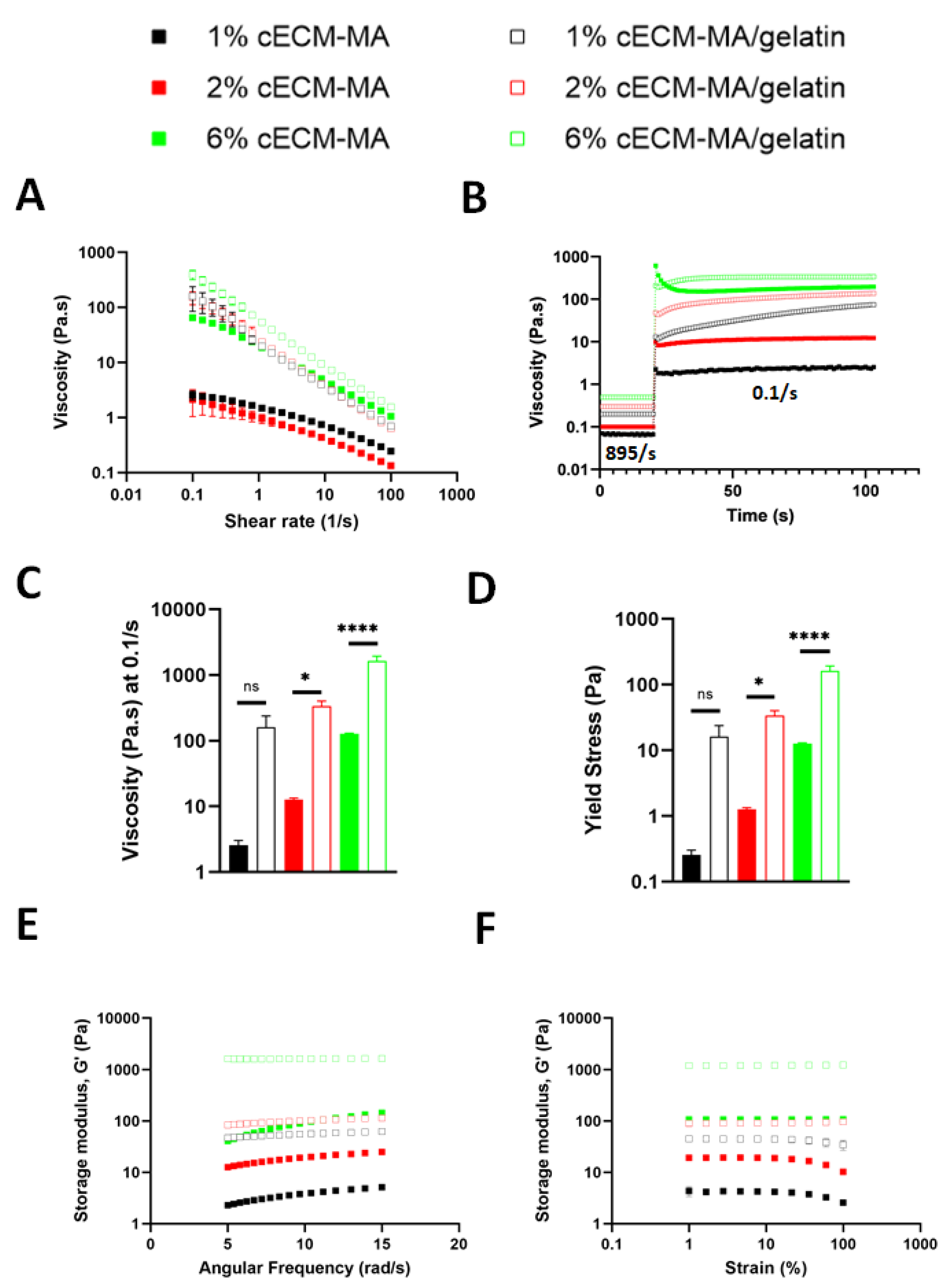
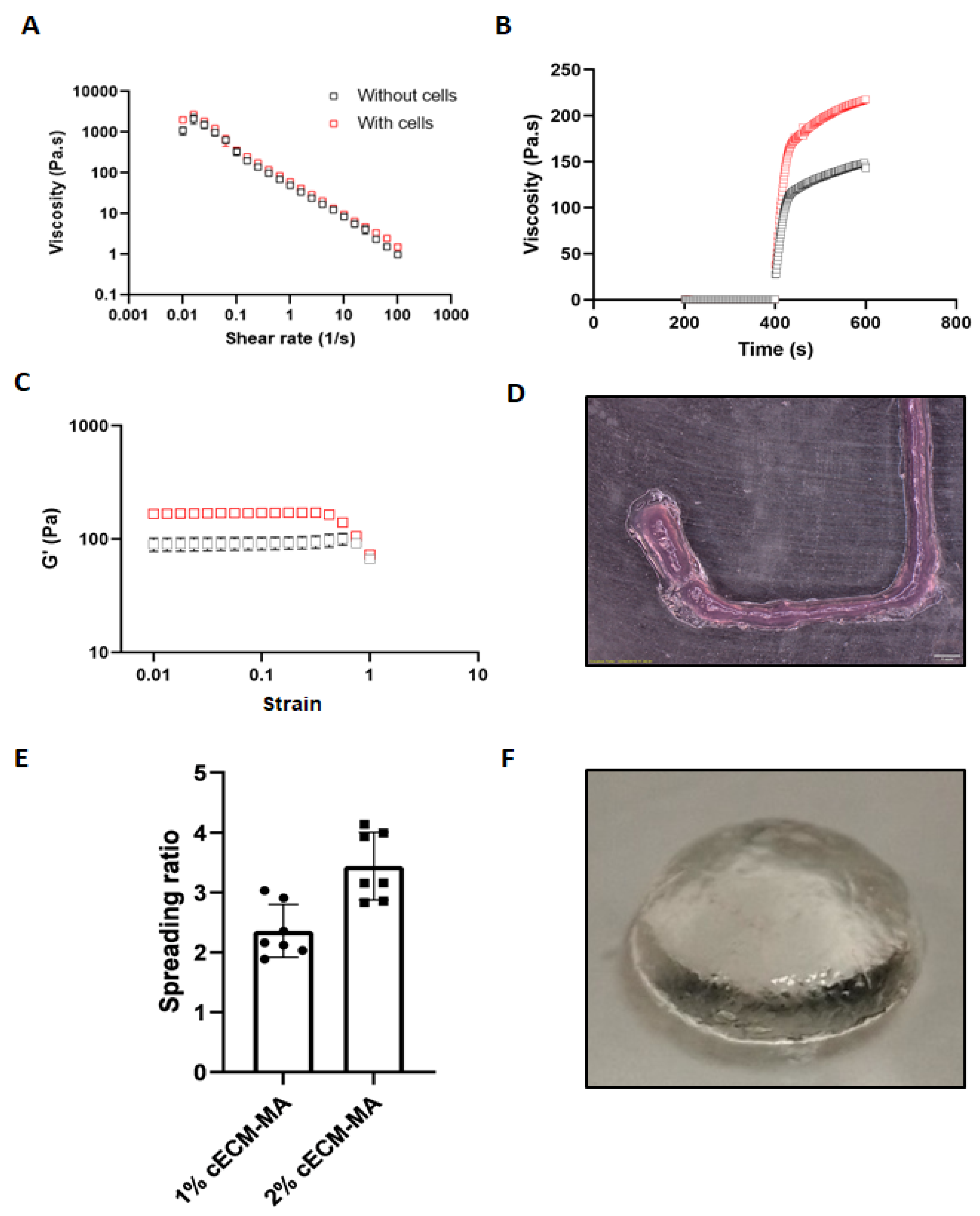
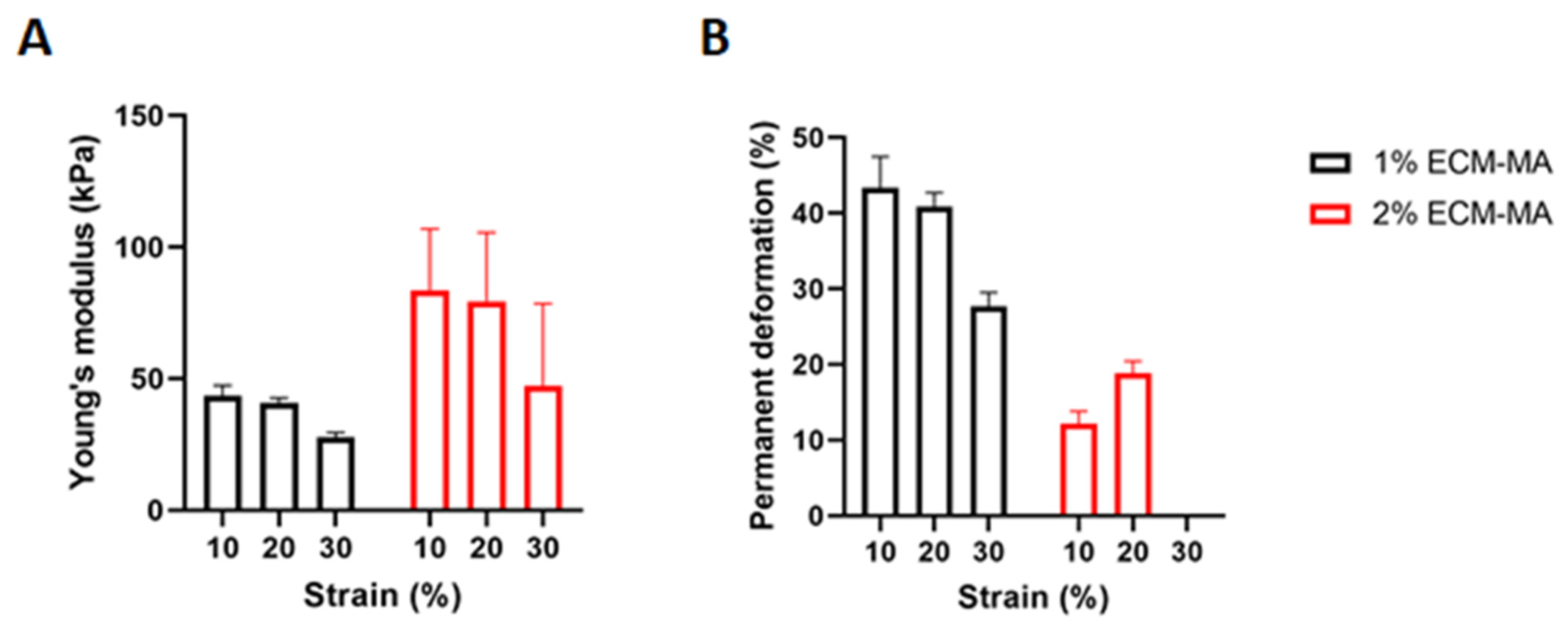

| Bioink Formulation | Flow Index n | Consistency Index k |
|---|---|---|
| 1% cECM-MA | 0.66 | 1.45 |
| 1% cECM-MA + gelatin | 0.29 | 20.8 |
| 2% cECM-MA | 0.60 | 5.99 |
| 2% cECM-MA + gelatin | 0.25 | 48.3 |
| 6% cECM-MA | 0.38 | 19.3 |
| 6% cECM-MA + gelatin | 0.18 | 60.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behan, K.; Dufour, A.; Garcia, O.; Kelly, D. Methacrylated Cartilage ECM-Based Hydrogels as Injectables and Bioinks for Cartilage Tissue Engineering. Biomolecules 2022, 12, 216. https://doi.org/10.3390/biom12020216
Behan K, Dufour A, Garcia O, Kelly D. Methacrylated Cartilage ECM-Based Hydrogels as Injectables and Bioinks for Cartilage Tissue Engineering. Biomolecules. 2022; 12(2):216. https://doi.org/10.3390/biom12020216
Chicago/Turabian StyleBehan, Kevin, Alexandre Dufour, Orquidea Garcia, and Daniel Kelly. 2022. "Methacrylated Cartilage ECM-Based Hydrogels as Injectables and Bioinks for Cartilage Tissue Engineering" Biomolecules 12, no. 2: 216. https://doi.org/10.3390/biom12020216
APA StyleBehan, K., Dufour, A., Garcia, O., & Kelly, D. (2022). Methacrylated Cartilage ECM-Based Hydrogels as Injectables and Bioinks for Cartilage Tissue Engineering. Biomolecules, 12(2), 216. https://doi.org/10.3390/biom12020216







