Sulfurtransferases and Cystathionine Beta-Synthase Expression in Different Human Leukemia Cell Lines
Abstract
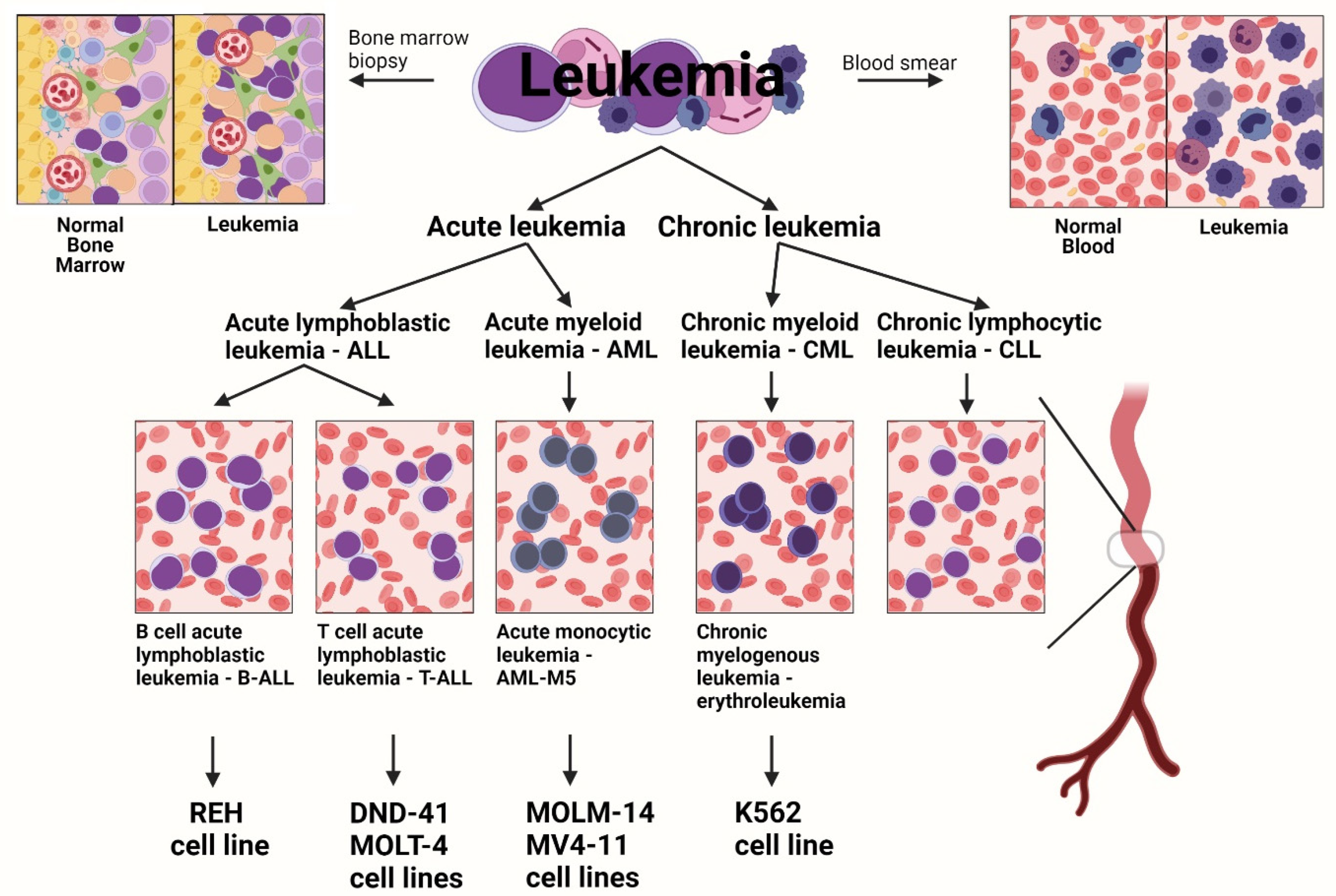
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Isolation of Total RNA
2.3. RT-PCR
2.4. Western Blotting
2.5. Statistical Analysis
3. Results
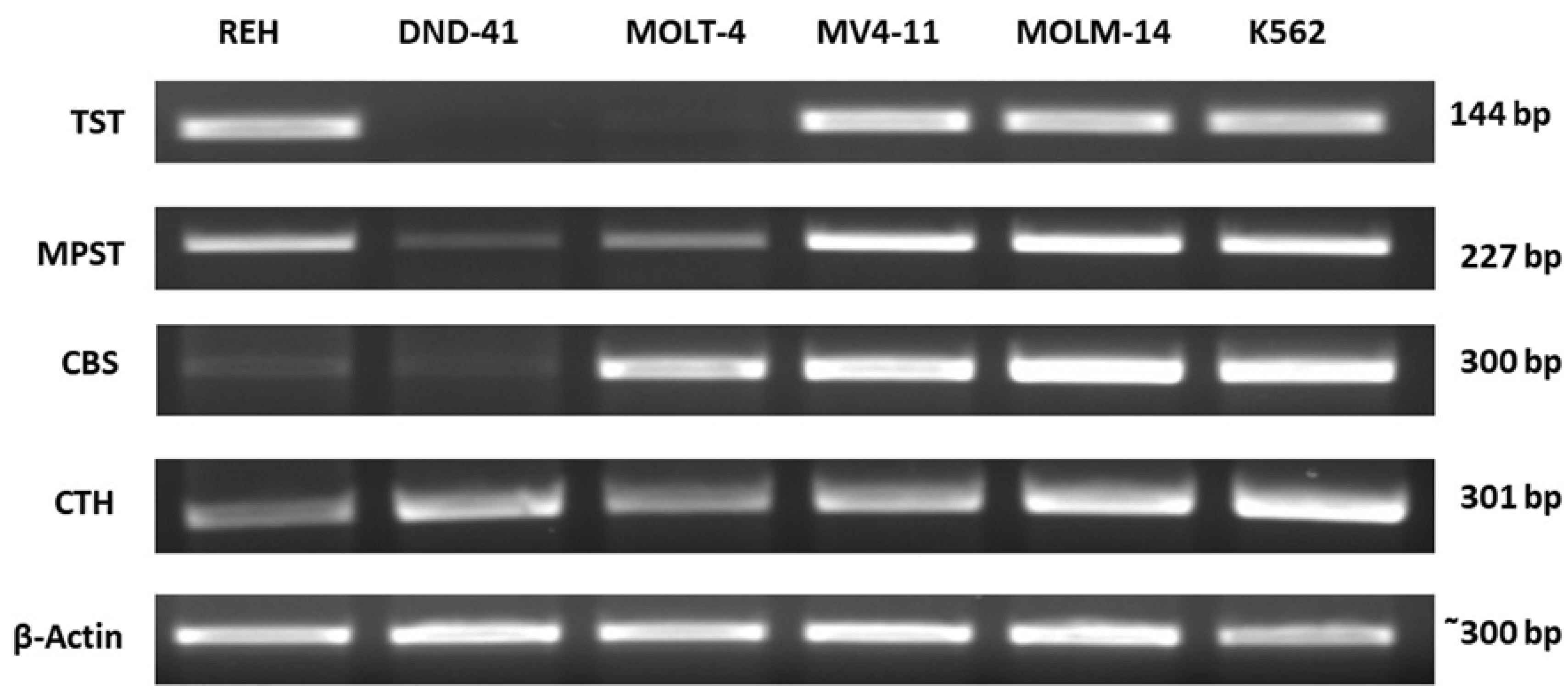
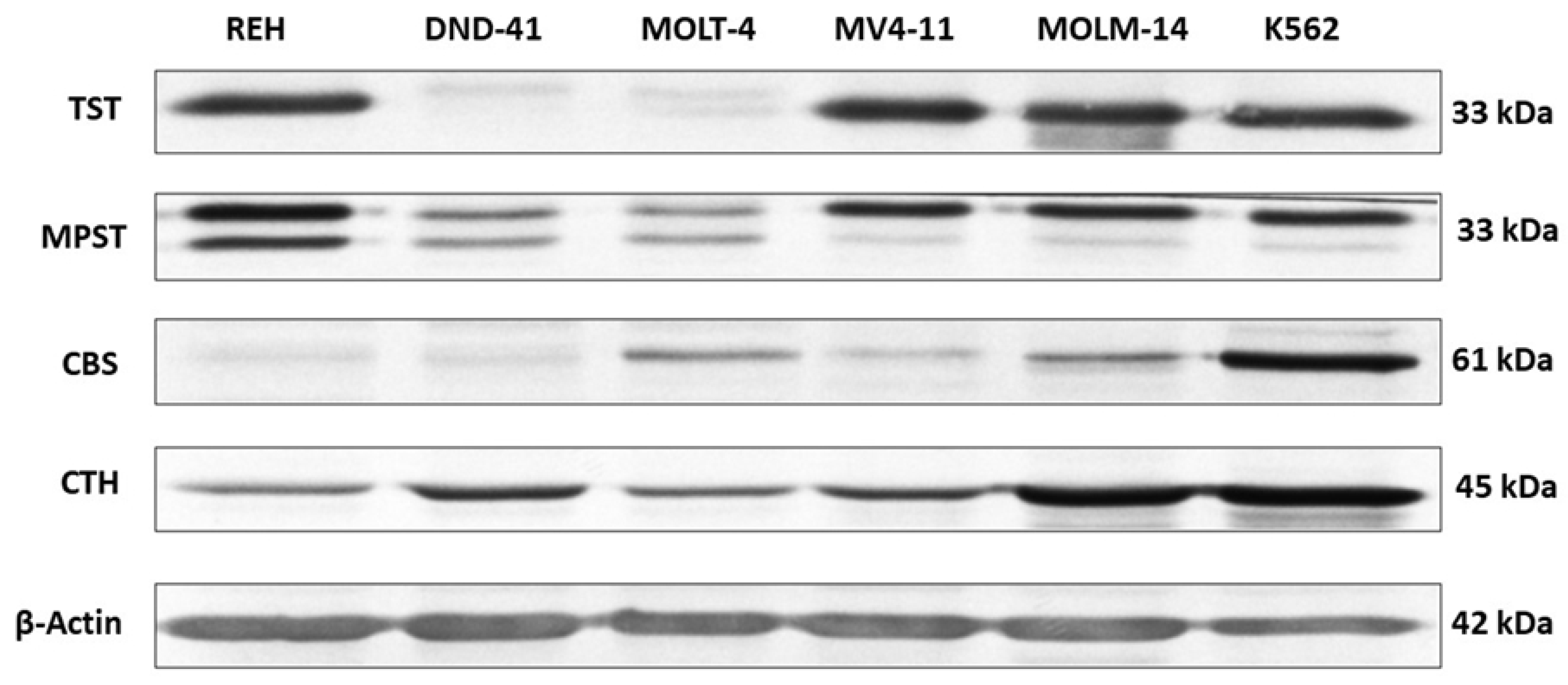
3.1. Thiosulfate Sulfurtransferase Expression in Leukemia Cell Lines
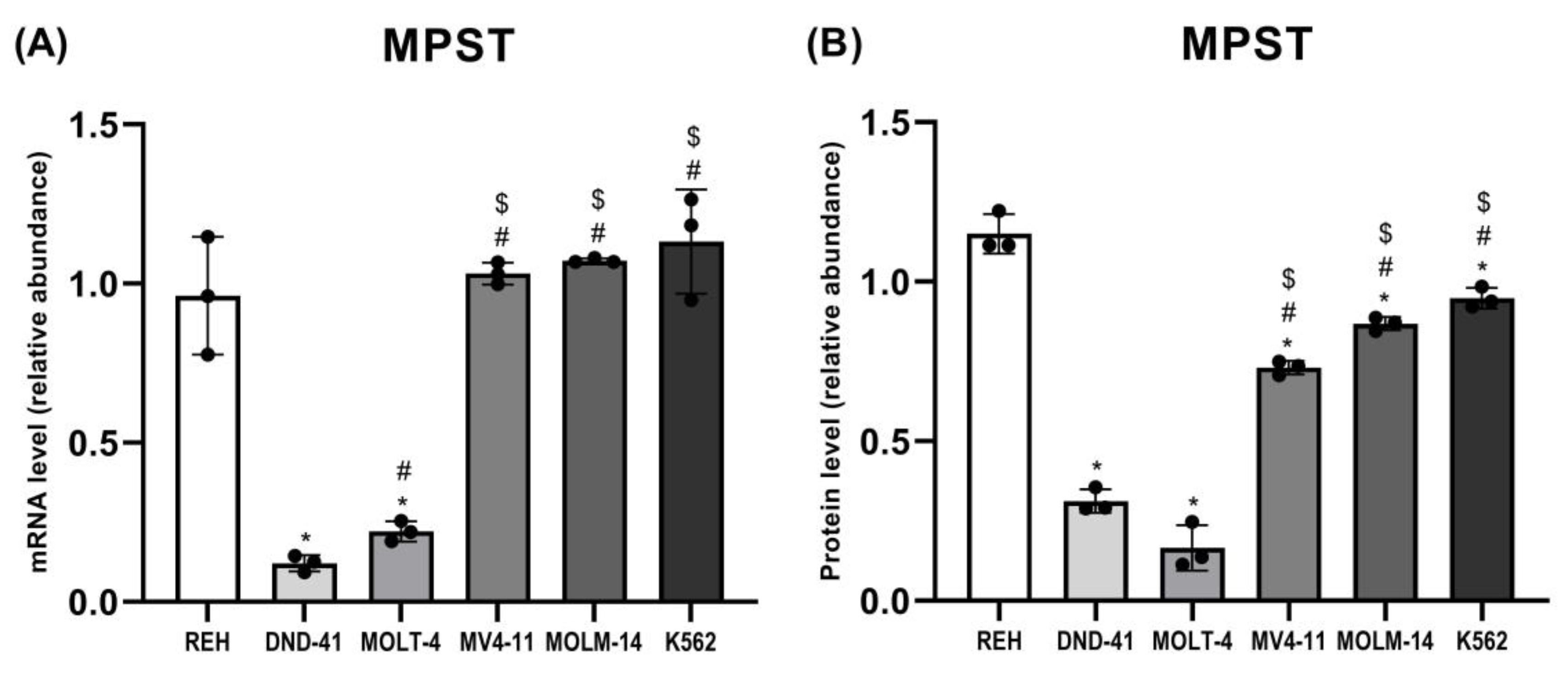
3.2. 3-Mercaptopyruvate Sulfurtransferase Expression in Leukemia Cell Lines
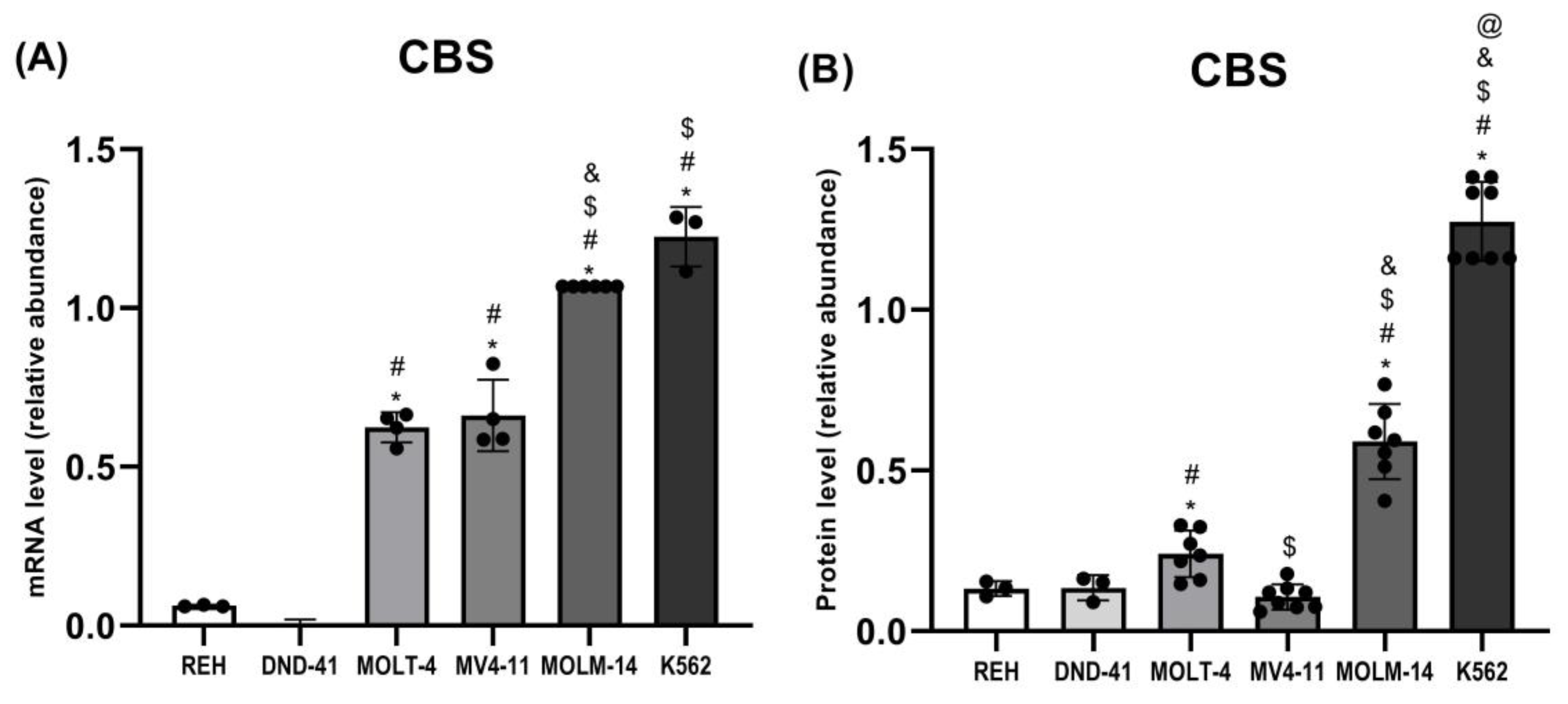
3.3. Cystathionine Beta-Synthase Expression in Leukemia Cell Lines
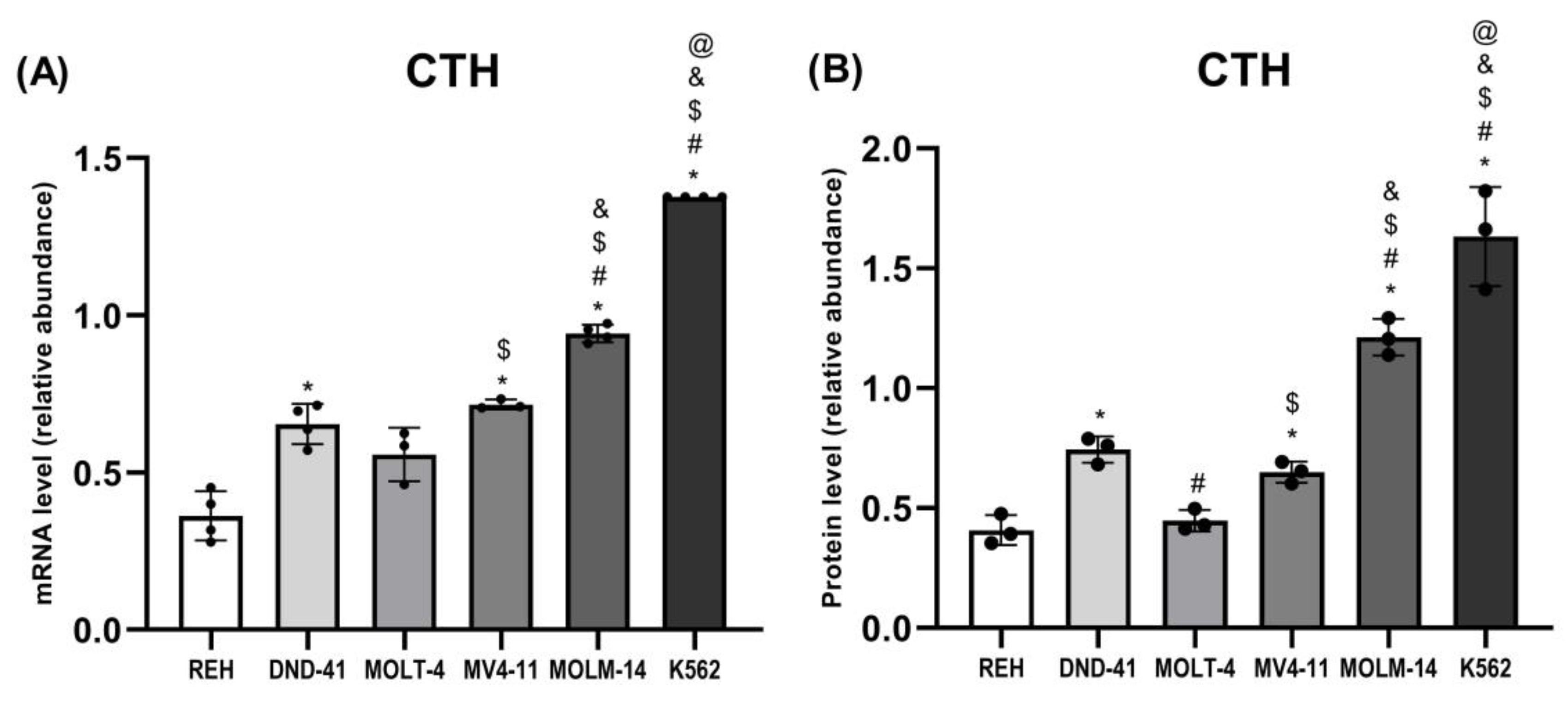
3.4. Gamma-Cystathionase Expression in Leukemia Cell Lines
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, N.; Sharma, S. Efficient method to detect leukemia using artificial intelligence. Int. J. Sci. Eng. Res. 2018, 9, 777–783. [Google Scholar]
- Inoue, T.; Swain, A.; Nakanishi, Y.; Sugiyama, D. Multicolor analysis of cell surface marker of human leukemia cell lines using flow cytometry. Anticancer Res. 2014, 34, 4539–4550. [Google Scholar]
- Tebbi, C.K. Etiology of Acute Leukemia: A Review. Cancers 2021, 13, 2256. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Vasic, D.; Kang, H.; Fang, K.K.; Zhang, L. State-of-art of cellular therapy for acute leukemia. Int. J. Mol. Sci. 2021, 22, 4590. [Google Scholar] [CrossRef] [PubMed]
- Pelcovits, A.; Niroula, R. Acute myeloid leukemia: A review. Rhode Isl. Med. J. 2020, 103, 38–40. [Google Scholar]
- Cammarata-Scalisi, F.; Girardi, K.; Strocchio, L.; Merli, P.; Garret-Bernardin, A.; Galeotti, A.; Magliarditi, F.; Inserra, A.; Callea, M. Oral manifestations and complications in childhood acute myeloid leukemia. Cancers 2020, 12, 1634. [Google Scholar] [CrossRef] [PubMed]
- Minciacchi, V.R.; Kumar, R.; Krause, D.S. Chronic myeloid leukemia: A model disease of the past, present and future. Cells 2021, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Kantarjian, H. Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring. Am. J. Hematol. 2020, 95, 691–709. [Google Scholar] [CrossRef]
- Kwok, M.; Agathanggelou, A.; Davies, N.; Stankovic, T. Targeting the p53 pathway in CLL: State of the art and future perspectives. Cancers 2021, 13, 4681. [Google Scholar] [CrossRef]
- Andreani, G.; Carrà, G.; Lingua, M.F.; Maffeo, B.; Brancaccio, M.; Taulli, R.; Morotti, A. Tumor suppressors in chronic lymphocytic leukemia: From lost partners to active targets. Cancers 2020, 12, 629. [Google Scholar] [CrossRef] [Green Version]
- Jurkowska, H.; Wróbel, M.; Kaczor-Kamińska, M.; Jasek-Gajda, E. A possible mechanism of inhibition of U87MG and SH-SY5Y cancer cell proliferation by diallyl trisulfide and other aspects of its activity. Amino Acids 2017, 49, 1855–1866. [Google Scholar] [CrossRef] [Green Version]
- Jurkowska, H.; Wróbel, M. N-acetyl-L-cysteine as a source of sulfane sulfur in astrocytoma and astrocyte cultures: Correlations with cell proliferation. Amino Acids 2008, 34, 231–237. [Google Scholar] [CrossRef]
- Jurkowska, H.; Wróbel, M. Inhibition of human neuroblastoma cell proliferation by N-acetyl-L-cysteine as a result of increased sulfane sulfur level. Anticancer. Res. 2018, 38, 5109–5113. [Google Scholar] [CrossRef]
- Jurkowska, H.; Uchacz, T.; Roberts, J.; Wróbel, M. Potential therapeutic advantage of ribose-cysteine in the inhibition of astrocytoma cell proliferation. Amino Acids 2011, 41, 131–139. [Google Scholar] [CrossRef]
- Stipanuk, M.H.; Ueki, I. Dealing with methionine/homocysteine sulfur: Cysteine metabolism to taurine and inorganic sulfur. J. Inherit. Metab. Dis. 2011, 34, 17–32. [Google Scholar] [CrossRef] [Green Version]
- Toohey, J.I.; Cooper, A.J. Thiosulfoxide (sulfane) sulfur: New chemistry and new regulatory roles in biology. Molecules 2014, 19, 12789–12813. [Google Scholar] [CrossRef] [Green Version]
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef] [Green Version]
- Kimura, H. Hydrogen sulfide: Its production, release and functions. Amino Acids 2011, 41, 113–121. [Google Scholar] [CrossRef]
- Szabo, C. Hydrogen sulfide, an endogenous stimulator of mitochondrial function in cancer cells. Cells 2021, 10, 220. [Google Scholar] [CrossRef]
- Nagahara, N. Multiple role of 3-mercaptopyruvate sulfurtransferase: Antioxidative function, H2S and polysulfide production and possible SOx production. Br. J. Pharmacol. 2018, 175, 577–589. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, T. Roles of sulfur metabolism and rhodanese in detoxification and anti-oxidative stress functions in the liver: Responses to Radiation Exposure. Med. Sci. Monit. 2015, 21, 1721–1725. [Google Scholar] [CrossRef] [Green Version]
- Paul, B.D.; Snyder, S.H.; Kashfi, K. Effects of hydrogen sulfide on mitochondrial function and cellular bioenergetics. Redox Biol. 2021, 38, 101772. [Google Scholar] [CrossRef]
- Rydz, L.; Wróbel, M.; Jurkowska, H. Sulfur administration in Fe-S aluster homeostasis. Antioxidants 2021, 10, 1738. [Google Scholar] [CrossRef]
- Carter, R.N.; Gibbins, M.; Barrios-Llerena, M.E.; Wilkie, S.E.; Freddolino, P.L.; Libiad, M.; Vitvitsky, V.; Emerson, B.; Le Bihan, T.; Brice, M.; et al. The hepatic compensatory response to elevated systemic sulfide promotes diabetes. Cell Rep. 2021, 37, 109958. [Google Scholar] [CrossRef]
- Pedre, B.; Dick, T. 3-Mercaptopyruvate sulfurtransferase: An enzyme at the crossroads of sulfane sulfur trafficking. Biol. Chem. 2021, 402, 223–237. [Google Scholar] [CrossRef]
- Wróbel, M.; Jurkowska, H.; Sliwa, L.; Srebro, Z. Sulfurtransferases and cyanide detoxification in mouse liver, kidney, and brain. Toxicol. Mech. Methods 2004, 14, 331–337. [Google Scholar] [CrossRef]
- Jurkowska, H.; Placha, W.; Nagahara, N.; Wróbel, M. The expression and activity of cystathionine-c-lyase and 3-mercaptopyruvate sulfurtransferase in human neoplastic cell lines. Amino Acids 2011, 41, 151–158. [Google Scholar] [CrossRef]
- Levonen, A.L.; Lapatto, R.; Saksela, M.; Raivi, K.O. Human cystathionine γ-lyase: Developmental and in vitro expression of two isoforms. Biochem. J. 2000, 347, 291–295. [Google Scholar] [CrossRef]
- Bronowicka-Adamska, P.; Bentke, A.; Wróbel, M. Hydrogen sulfide generation from L-cysteine in the human glioblastoma-astrocytoma U-87 MG and neuroblastoma SHSY5Y cell lines. Acta Biochim. Pol. 2017, 64, 171–176. [Google Scholar]
- Kusukawa, J.; Suefuji, Y.; Ryu, F.; Noguchi, R.; Iwamoto, O.; Kameyama, T. Dissemination of cancer cells into circulation occurs by incisional biopsy of oral squamous cell carcinoma. J. Oral Pathol. Med. 2000, 29, 303–307. [Google Scholar] [CrossRef]
- Nandi, D.L.; Horowitz, P.M.; Westley, J. Rhodanese as a thioredoxin oxidase. Int. J. Biochem. Cell Biol. 2000, 32, 465–473. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Qiu, Y.; Shi, Y.; Cai, J.; Wang, B.; Wei, X.; Ke, Q.; Sui, X.; Wang, Y.; et al. Cell adhesion-mediated mitochondria transfer contributes to mesenchymal stem cell-induced chemoresistance on T cell acute lymphoblastic leukemia cells. J. Hematol. Oncol. 2018, 11, 11. [Google Scholar] [CrossRef]
- Sahinbegovic, H.; Jelinek, T.; Hrdinka, M.; Bago, J.R.; Turi, M.; Sevcikova, T.; Kurtovic-Kozaric, A.; Hajek, R.; Simicek, M. Intercellular mitochondrial transfer in the tumor microenvironment. Cancers 2020, 12, 1787. [Google Scholar] [CrossRef]
- Burt, R.; Dey, A.; Aref, S.; Aguiar, M.; Akarca, A.; Bailey, K.; Day, W.; Hooper, S.; Kirkwood, A.; Kirschner, K.; et al. Activated stromal cells transfer mitochondria to rescue acute lymphoblastic leukemia cells from oxidative stress. Blood 2019, 134, 1415–1429. [Google Scholar] [CrossRef]
- Garg, S.K.; Yan, Z.; Vitvitsky, V.; Banerjee, R. Differential dependence on cysteine from transsulfuration versus transport during T cell activation. Antioxid. Redox Signal. 2011, 15, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Lancien, M.; Gueno, L.; Salle, S.; Merieau, E.; Beriou, G.; Nguyen, T.H.; Abidi, A.; Dilek, N.; Solomon, P.; Poschmann, J.; et al. Cystathionine-gamma-lyase overexpression in T cells enhances antitumor effect independently of cysteine autonomy. Cancer Sci. 2021, 112, 1723–1734. [Google Scholar] [CrossRef]
- Kaur, S.; Schwartz, A.L.; Miller, T.W.; Roberts, D.D. CD47-dependent regulation of H₂S biosynthesis and signaling in T cells. Methods Enzymol. 2015, 555, 145–168. [Google Scholar] [CrossRef]
- Miller, T.W.; Wang, E.A.; Gould, S.; Stein, E.V.; Kaur, S.; Lim, L.; Amarnath, S.; Fowler, D.H.; Roberts, D.D. Hydrogen sulfide is an endogenous potentiator of T cell activation. J. Biol. Chem. 2012, 287, 4211–4221. [Google Scholar] [CrossRef] [Green Version]
- Lagadinou, E.D.; Sach, A.; Callahan, K.; Rossi, R.M.; Neering, S.J.; Minhajuddin, M.; Ashton, J.M.; Pei, S.; Grose, V.; O’Dwyer, K.M.; et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem. Cell 2013, 12, 329–341. [Google Scholar] [CrossRef] [Green Version]
- Suganuma, K.; Miwa, H.; Imai, N.; Shikami, M.; Gotou, M.; Goto, M.; Mizuno, S.; Takahashi, M.; Yamamoto, H.; Hiramatsu, A.; et al. Energy metabolism of leukemia cells: Glycolysis versus oxidative phosphorylation. Leuk. Lymphoma 2010, 51, 2112–2119. [Google Scholar] [CrossRef]
- Basak, N.P.; Banerjee, S. Mitochondrial dependency in progression of acute myeloid leukemia. Mitochondrion 2015, 21, 41–48. [Google Scholar] [CrossRef]
- Zuhra, K.; Augsburger, F.; Majtan, T.; Szabo, C. Cystathionine-β-synthase: Molecular regulation and pharmacological inhibition. Biomolecules 2020, 10, 697. [Google Scholar] [CrossRef]
- Chen, C.C.; Li, B.; Millman, S.E.; Chen, C.; Li, X.; Morris, J.P., 4th; Mayle, A.; Ho, Y.J.; Loizou, E.; Liu, H.; et al. Vitamin B6 addiction in acute myeloid leukemia. Cancer Cell 2020, 37, 71–84.e7. [Google Scholar] [CrossRef]
- Wang, D.; Yang, H.; Zhang, Y.; Hu, R.; Hu, D.; Wang, Q.; Liu, Y.; Liu, M.; Meng, Z.; Zhou, W.; et al. Inhibition of cystathionine β-synthase promotes apoptosis and reduces cell proliferation in chronic myeloid leukemia. Signal Transduct. Target. Ther. 2021, 6, 52. [Google Scholar] [CrossRef]
- Glode, L.M.; Epstein, A.; Smith, C.G. Reduced gamma-cystathionase protein content in human malignant leukemia cell lines as measured by immunoassay with monoclonal antibody. Cancer Res. 1981, 41, 2249–2254. [Google Scholar] [PubMed]
- Bhingarkar, A.; Vangapandu, H.V.; Rathod, S.; Hoshitsuki, K.; Fernandez, C.A. Amino acid metabolic vulnerabilities in acute and chronic myeloid leukemias. Front. Oncol. 2021, 11, 694526. [Google Scholar] [CrossRef] [PubMed]
- Suangtamai, T.; Tanyong, D.I. Diallyl disulfide induces apoptosis and autophagy via mTOR pathway in myeloid leukemic cell line. Tumour. Biol. 2016, 37, 10993–10999. [Google Scholar] [CrossRef]
- Lee, Z.W.; Zhou, J.; Chen, C.S.; Zhao, Y.; Tan, C.H.; Li, L.; Moore, P.K.; Deng, L.W. The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PLoS ONE 2011, 6, e21077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, L.; Ji, X.X.; Tan, H.; Lin, M.; Tang, Y.; Wen, L.; Ma, Y.H.; Su, Q. Role of Ras-related C3 botulinum toxin substrate 2 (Rac2), NADPH oxidase and reactive oxygen species in diallyl disulphide-induced apoptosis of human leukaemia HL-60 cells. Clin. Exp. Pharmacol. Physiol. 2010, 37, 1147–1153. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurkowska, H.; Wróbel, M.; Jasek-Gajda, E.; Rydz, L. Sulfurtransferases and Cystathionine Beta-Synthase Expression in Different Human Leukemia Cell Lines. Biomolecules 2022, 12, 148. https://doi.org/10.3390/biom12020148
Jurkowska H, Wróbel M, Jasek-Gajda E, Rydz L. Sulfurtransferases and Cystathionine Beta-Synthase Expression in Different Human Leukemia Cell Lines. Biomolecules. 2022; 12(2):148. https://doi.org/10.3390/biom12020148
Chicago/Turabian StyleJurkowska, Halina, Maria Wróbel, Ewa Jasek-Gajda, and Leszek Rydz. 2022. "Sulfurtransferases and Cystathionine Beta-Synthase Expression in Different Human Leukemia Cell Lines" Biomolecules 12, no. 2: 148. https://doi.org/10.3390/biom12020148
APA StyleJurkowska, H., Wróbel, M., Jasek-Gajda, E., & Rydz, L. (2022). Sulfurtransferases and Cystathionine Beta-Synthase Expression in Different Human Leukemia Cell Lines. Biomolecules, 12(2), 148. https://doi.org/10.3390/biom12020148





