Calculation of Crystal-Solution Dissociation Constants
Abstract
1. Introduction
2. Methods
2.1. Model and Approach
2.2. Entropy and Dissociation Constant Calculation
2.3. Dataset
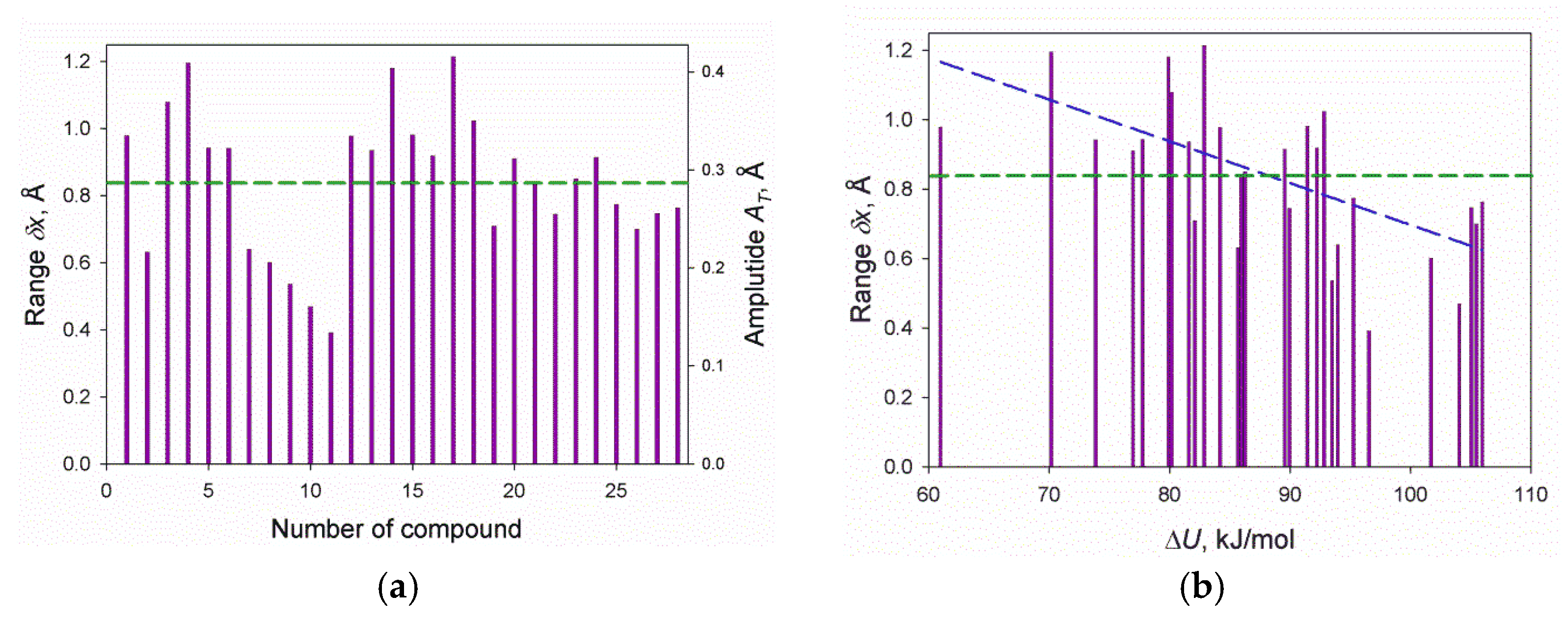
3. Results
3.1. Crystal-Vapor Equilibrium
3.2. Vapor-Solution Equilibrium and Calculation of Dissociation Constants
4. Conclusions and Future Plans
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kryshtafovych, A.; Schwede, T.; Topf, M.; Fidelis, K.; Moult, J. Critical assessment of methods of protein structure prediction (CASP)—Round XIV. Proteins 2021, 89, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Applying and improving AlphaFold at CASP14. Proteins 2021, 89, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Lensink, M.F.; Brysbaert, G.; Mauri, T.; Nadzirin, N.; Velenkar, S.; Chaleil, R.A.G.; Clarence, T.; Bates, P.A.; Kong, R.; Liu, B.; et al. Prediction of protein assemblies, the next frontier: The CASP14-CAPRI experiment. Proteins 2021, 89, 1800–1823. [Google Scholar] [CrossRef] [PubMed]
- Steinbrecher, T.; Labahn, A. Towards accurate free energy calculations in ligand protein-binding studies. Curr. Med. Chem. 2010, 17, 767–785. [Google Scholar] [CrossRef]
- Muzzioli, E.; Del Rio, A.; Rastelli, G. Assessing protein kinase selectivity with molecular dynamics and mm-pbsa binding free energy calculations. Chem. Biol. Drug. Des. 2011, 78, 252–259. [Google Scholar] [CrossRef]
- Shivakumar, D.; Harder, E.; Damm, W.; Friesner, R.A.; Sherman, W. Improving the prediction of absolute solvation free energies using the next generation OPLS force field. J. Chem. Theory Comput. 2012, 8, 2553–2558. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Deng, Y.; Kim, B.; Pierce, L.; Krilov, G.; Lupyan, D.; Robinson, S.; Dahlgren, M.K.; Greenwood, J. Accurate and reliable prediction of relative ligand binding potency in prospective drug discovery by way of a modern free-energy calculation protocol and force field. J. Am. Chem. Soc. 2015, 137, 2695–2703. [Google Scholar] [CrossRef]
- Clark, R.D.; Waldman, M. Lions and tigers and bears, oh my! Three barriers to progress in computer-aided molecular design. J. Comput. Aided Mol. Des. 2012, 26, 29–34. [Google Scholar] [CrossRef]
- Gumbart, J.C.; Roux, B.; Chipot, C. Standard binding free energies from computer simulations: What is the best strategy? J. Chem. Theory Comput. 2013, 9, 794–802. [Google Scholar] [CrossRef]
- Huang, N.; Jacobson, M.P. Physics-based methods for studying protein-ligand interactions. Curr. Opin. Drug. Discov. Devel. 2007, 10, 325–331. [Google Scholar] [PubMed]
- Borhani, D.W.; Shaw, D.E. The future of molecular dynamics simulations in drug discovery. J. Comput. Aided Mol. Des. 2012, 26, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Krieger, E.; Darden, T.; Nabuurs, S.B.; Finkelstein, A.; Vriend, G. Making optimal use of empirical energy functions: Force field parameterization in crystal space. Proteins 2004, 57, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Park, M.-S.; Stern, H.A. Accounting for ligand conformational restriction in calculations of protein-ligand binding affinities. Biophys. J. 2010, 98, 901–910. [Google Scholar] [CrossRef][Green Version]
- Finkelstein, A.V.; Ptitsyn, O.B. Protein Physics. A Course of Lectures, 2nd ed.; Academic Press: Cambridge, MA, USA, 2016; Chapters 5–8. [Google Scholar]
- Pereyaslavets, L.B.; Finkelstein, A.V. Atomic force field FFsol for calculating molecular interactions in water environment. Mol. Biol. Engl. Transl. 2010, 44, 303–316. [Google Scholar] [CrossRef]
- Pereyaslavets, L.B.; Finkelstein, A.V. Development and testing of PFFsol1.1, a new polarizable atomic force field for calculation of molecular interactions in implicit water environment. J. Phys. Chem. B 2012, 116, 4646–4654. [Google Scholar] [CrossRef]
- Finkelstein, A.V.; Janin, J. The price of lost freedom. Protein Eng. 1989, 3, 1–3. [Google Scholar] [CrossRef]
- Pickett, S.D.; Sternberg, M.J. Empirical scale of side-chain conformational entropy in protein folding. J. Mol. Biol. 1993, 231, 825–839. [Google Scholar] [CrossRef]
- Kortemme, T.; Baker, D. A simple physical model for binding energy hot spots in protein-protein complexes. Proc. Natl. Acad. Sci. USA 2002, 99, 14116–14121. [Google Scholar] [CrossRef]
- Lee, J.; Seok, C.A. statistical rescoring scheme for protein-ligand docking: Consideration of entropic effect. Proteins 2008, 15, 1074–1083. [Google Scholar] [CrossRef]
- Wang, J.; Hou, T. Develop and test a solvent accessible surface area-based model in conformational entropy calculations. J. Chem. Inf. Model. 2012, 25, 1199–1212. [Google Scholar] [CrossRef]
- Chiba, S.; Harano, Y.; Roth, R.; Kinoshita, M.; Sakurai, M. Evaluation of protein-ligand binding free energy focused on its entropic components. J. Comput. Chem. 2012, 15, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Kamisetty, H.; Ramanathan, A.; Bailey-Kellogg, C.; Langmead, C.J. Accounting for conformational entropy in predicting binding free energies of protein-protein interactions. Proteins 2011, 79, 444–462. [Google Scholar] [CrossRef]
- Perola, E.; Charifson, P.S. Conformational analysis of drug-like molecules bound to proteins: An extensive study of ligand reorganization upon binding. J. Med. Chem. 2004, 47, 2499–2510. [Google Scholar] [CrossRef]
- Garbuzynskiy, S.O.; Finkelstein, A.V. Calculation of mobility and entropy of the binding of molecules by crystals. Mol. Biol. Engl. Transl. 2016, 50, 520–529. [Google Scholar] [CrossRef]
- Garbuzynskiy, S.O.; Finkelstein, A.V. Sublimation entropy and dissociation constants prediction by quantitative evaluation of molecular mobility in crystals. J. Phys. Chem. Lett. 2017, 8, 2758–2763, Erratum in J. Phys. Chem. Lett. 2018, 9, 6883. [Google Scholar] [CrossRef] [PubMed]
- Garbuzynskiy, S.O.; Finkelstein, A.V. Evaluation of the accuracy of calculation of the standard binding entropy of molecules from their average mobility in molecular crystals. Mol. Biol. Engl. Transl. 2018, 52, 108–117. [Google Scholar] [CrossRef]
- Bisswanger, H. Enzyme Kinetics: Principles and Methods; Wiley-VCH: Weinheim, Germany, 2008; ISBN 978-3-527-31957-2. [Google Scholar]
- Sander, R. Compilation of Henry's law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef]
- Bjelobrk, Z.; Mendels, D.; Karmakar, T.; Parrinello, M.; Mazzotti, M. Solubility prediction of organic molecules with molecular dynamics simulations. Cryst. Growth Des. 2021, 21, 5198–5205. [Google Scholar] [CrossRef]
- Uversky, V.N.; Finkelstein, A.V. Life in phases: Intra—And inter—Molecular phase transitions in protein solutions. Biomolecules 2019, 9, 842. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garbuzynskiy, S.O.; Finkelstein, A.V. Calculation of Crystal-Solution Dissociation Constants. Biomolecules 2022, 12, 147. https://doi.org/10.3390/biom12020147
Garbuzynskiy SO, Finkelstein AV. Calculation of Crystal-Solution Dissociation Constants. Biomolecules. 2022; 12(2):147. https://doi.org/10.3390/biom12020147
Chicago/Turabian StyleGarbuzynskiy, Sergiy O., and Alexei V. Finkelstein. 2022. "Calculation of Crystal-Solution Dissociation Constants" Biomolecules 12, no. 2: 147. https://doi.org/10.3390/biom12020147
APA StyleGarbuzynskiy, S. O., & Finkelstein, A. V. (2022). Calculation of Crystal-Solution Dissociation Constants. Biomolecules, 12(2), 147. https://doi.org/10.3390/biom12020147






