Lipidomic Profile Analysis of Lung Tissues Revealed Lipointoxication in Pulmonary Veno-Occlusive Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Assessment
2.3. Chemical and Lipid Standards
2.4. Lipid Extraction
2.5. Lipid Analysis by Electrospray Ionization-Mass Spectrometry (ESI-MS)
2.6. Statistical Analysis
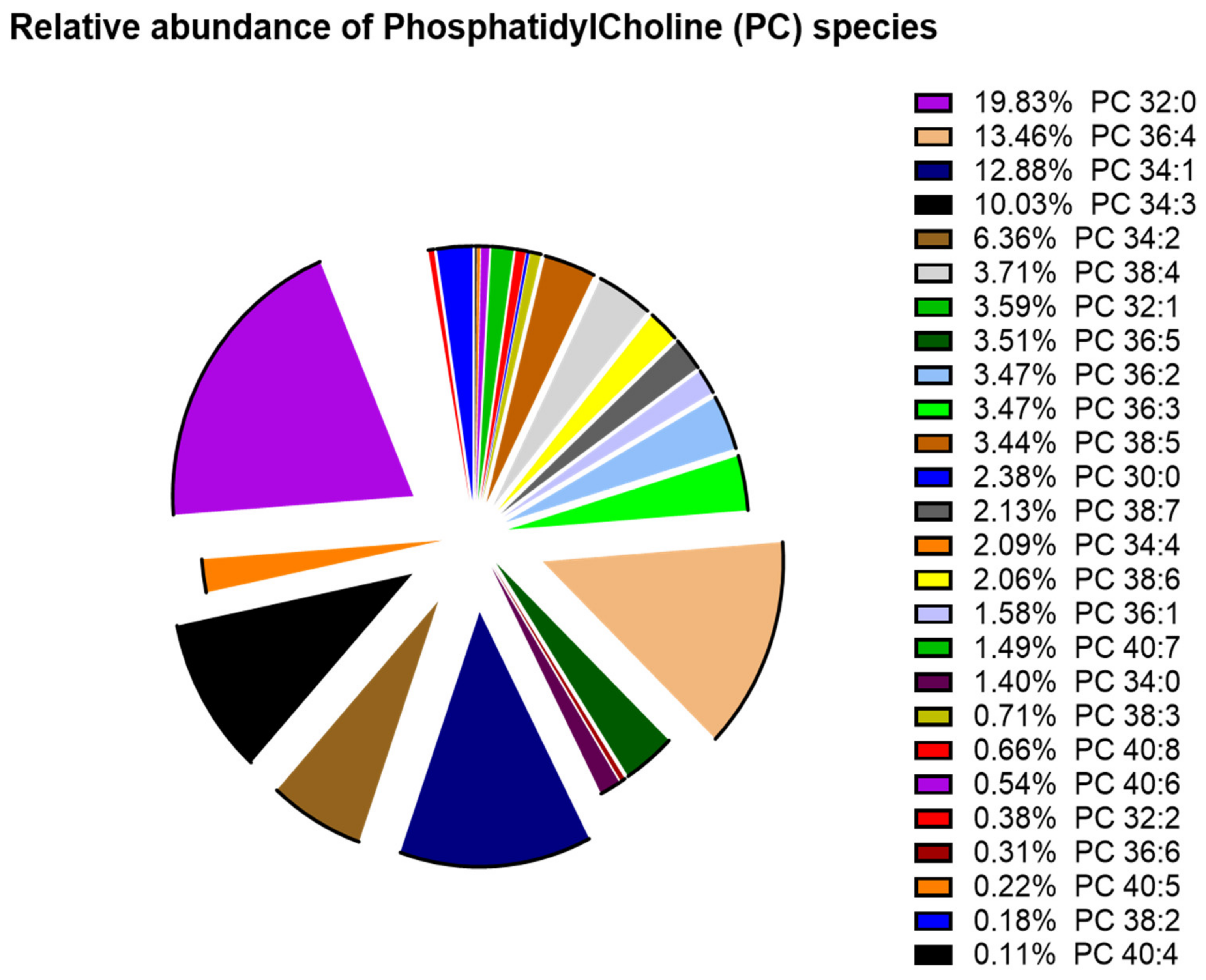
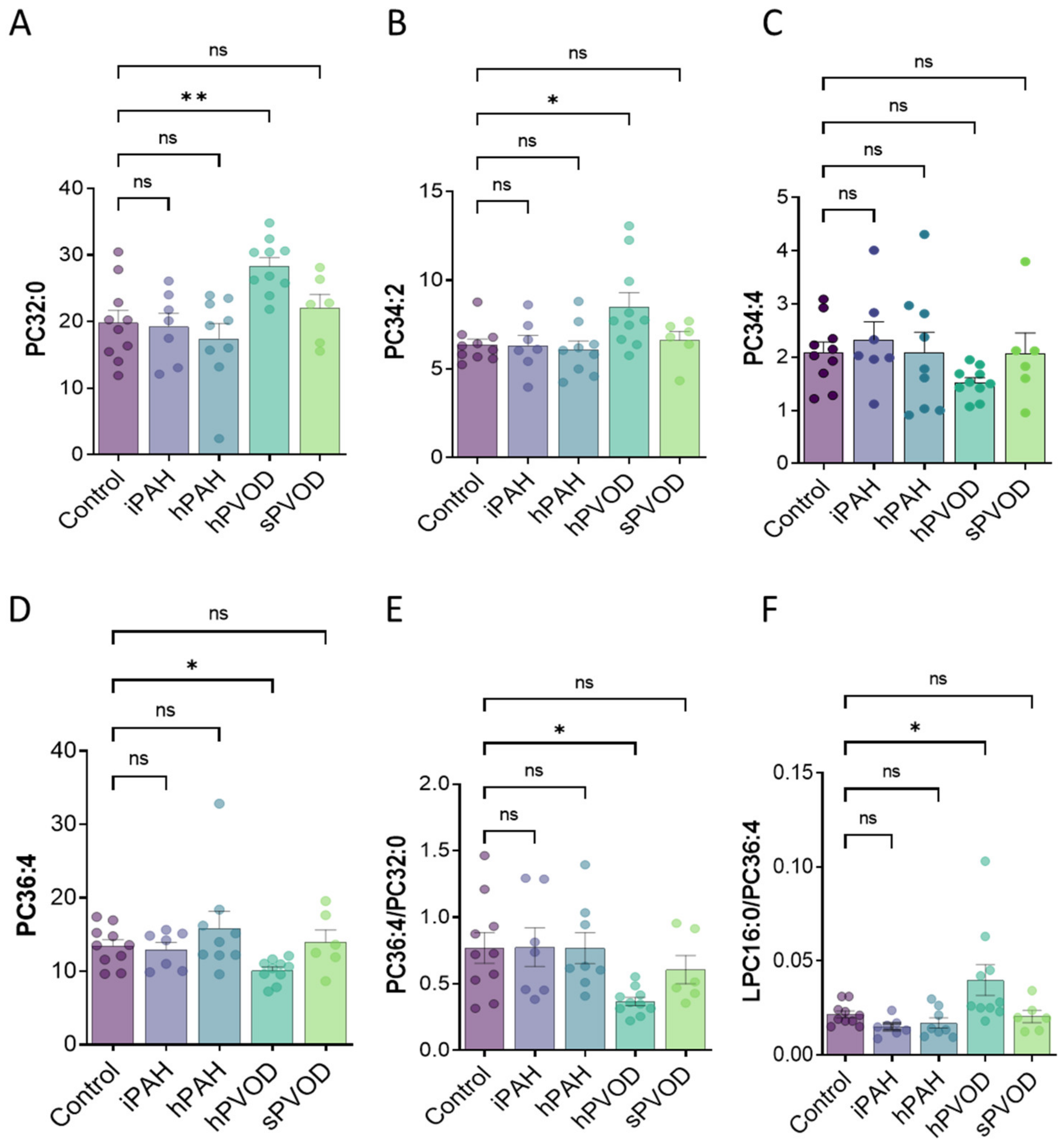
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramanadham, S.; Bohrer, A.; Mueller, M.; Jett, P.; Gross, R.W.; Turk, J. Mass Spectrometric Identification and Quantitation of Arachidonate-Containing Phospholipids in Pancreatic Islets: Prominence of Plasmenylethanolamine Molecular Species. Biochemistry 1993, 32, 5339–5351. [Google Scholar] [CrossRef] [PubMed]
- Christie, W.W. Rapid Separation and Quantification of Lipid Classes by High Performance Liquid Chromatography and Mass (Light-Scattering) Detection. J. Lipid Res. 1985, 26, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Antonny, B.; Vanni, S.; Shindou, H.; Ferreira, T. From Zero to Six Double Bonds: Phospholipid Unsaturation and Organelle Function. Trends Cell Biol. 2015, 25, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Kadri, L.; Ferru-Clément, R.; Bacle, A.; Payet, L.-A.; Cantereau, A.; Hélye, R.; Becq, F.; Jayle, C.; Vandebrouck, C.; Ferreira, T. Modulation of Cellular Membrane Properties as a Potential Therapeutic Strategy to Counter Lipointoxication in Obstructive Pulmonary Diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3069–3084. [Google Scholar] [CrossRef]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic Definitions and Updated Clinical Classification of Pulmonary Hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef] [PubMed]
- Montani, D.; Lau, E.M.; Dorfmüller, P.; Girerd, B.; Jaïs, X.; Savale, L.; Perros, F.; Nossent, E.; Garcia, G.; Parent, F.; et al. Pulmonary Veno-Occlusive Disease. Eur. Respir. J. 2016, 47, 1518–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montani, D.; Girerd, B.; Jaïs, X.; Levy, M.; Amar, D.; Savale, L.; Dorfmüller, P.; Seferian, A.; Lau, E.M.; Eyries, M.; et al. Clinical Phenotypes and Outcomes of Heritable and Sporadic Pulmonary Veno-Occlusive Disease: A Population-Based Study. Lancet Respir. Med. 2017, 5, 125–134. [Google Scholar] [CrossRef]
- Nossent, E.J.; Antigny, F.; Montani, D.; Bogaard, H.J.; Ghigna, M.R.; Lambert, M.; de Montpréville, V.T.; Girerd, B.; Jaïs, X.; Savale, L.; et al. Pulmonary Vascular Remodeling Patterns and Expression of General Control Nonderepressible 2 (GCN2) in Pulmonary Veno-Occlusive Disease. J. Heart Lung Transplant. 2017, 37, 647–655. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Husen, P.; Tarasov, K.; Katafiasz, M.; Sokol, E.; Vogt, J.; Baumgart, J.; Nitsch, R.; Ekroos, K.; Ejsing, C.S. Analysis of Lipid Experiments (ALEX): A Software Framework for Analysis of High-Resolution Shotgun Lipidomics Data. PLoS ONE 2013, 8, e79736. [Google Scholar] [CrossRef]
- Starikov, A.Y.; Sidorov, R.A.; Mironov, K.S.; Goriainov, S.V.; Los, D.A. Delta or Omega? Δ12 (Ω6) Fatty Acid Desaturases Count 3C after the Pre-Existing Double Bond. Biochimie 2020, 179, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Payet, L.-A.; Pineau, L.; Snyder, E.C.R.; Colas, J.; Moussa, A.; Vannier, B.; Bigay, J.; Clarhaut, J.; Becq, F.; Berjeaud, J.-M.; et al. Saturated Fatty Acids Alter the Late Secretory Pathway by Modulating Membrane Properties. Traffic Cph. Den. 2013, 14, 1228–1241. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, A.; MacIntyre, N.R.; Henderson, R.J.; Jensen, R.L.; Kinney, G.; Stringer, W.W.; Hersh, C.P.; Bowler, R.P.; Casaburi, R.; Han, M.K.; et al. Diffusing Capacity of Carbon Monoxide in Assessment of COPD. Chest 2019, 156, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Espiritu, J.D.; Ruppel, G.; Shrestha, Y.; Kleinhenz, M.E. The Diffusing Capacity in Adult Cystic Fibrosis. Respir. Med. 2003, 97, 606–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos-Ribeiro, D.; Godinas, L.; Pilette, C.; Perros, F. The Integrated Stress Response System in Cardiovascular Disease. Drug Discov. Today 2018, 23, 920–929. [Google Scholar] [CrossRef]
- Manaud, G.; Nossent, E.J.; Lambert, M.; Ghigna, M.-R.; Boët, A.; Vinhas, M.-C.; Ranchoux, B.; Dumas, S.J.; Courboulin, A.; Girerd, B.; et al. Comparison of Human and Experimental Pulmonary Veno-Occlusive Disease. Am. J. Respir. Cell Mol. Biol. 2020, 63, 118–131. [Google Scholar] [CrossRef]
- Lei, X.; Zhang, S.; Bohrer, A.; Ramanadham, S. Calcium-Independent Phospholipase A2 (IPLA2 Beta)-Mediated Ceramide Generation Plays a Key Role in the Cross-Talk between the Endoplasmic Reticulum (ER) and Mitochondria during ER Stress-Induced Insulin-Secreting Cell Apoptosis. J. Biol. Chem. 2008, 283, 34819–34832. [Google Scholar] [CrossRef] [Green Version]
- Seong, H.-A.; Manoharan, R.; Ha, H. Smad Proteins Differentially Regulate Obesity-Induced Glucose and Lipid Abnormalities and Inflammation via Class-Specific Control of AMPK-Related Kinase MPK38/MELK Activity. Cell Death Dis. 2018, 9, 471. [Google Scholar] [CrossRef] [Green Version]
- Toyoda, S.; Shin, J.; Fukuhara, A.; Otsuki, M.; Shimomura, I. Transforming Growth Factor Β1 Signaling Links Extracellular Matrix Remodeling to Intracellular Lipogenesis upon Physiological Feeding Events. J. Biol. Chem. 2022, 298, 101748. [Google Scholar] [CrossRef]
- Guo, F.; Cavener, D.R. The GCN2 EIF2alpha Kinase Regulates Fatty-Acid Homeostasis in the Liver during Deprivation of an Essential Amino Acid. Cell Metab. 2007, 5, 103–114. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, J.; Yue, W.; Bi, Y.; Shen, X.; Gao, J.; Xu, X.; Lu, Z. GCN2 Deficiency Protects against High Fat Diet Induced Hepatic Steatosis and Insulin Resistance in Mice. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3257–3267. [Google Scholar] [CrossRef] [PubMed]
- Payet, L.-A.; Kadri, L.; Giraud, S.; Norez, C.; Berjeaud, J.M.; Jayle, C.; Mirval, S.; Becq, F.; Vandebrouck, C.; Ferreira, T. Cystic Fibrosis Bronchial Epithelial Cells Are Lipointoxicated by Membrane Palmitate Accumulation. PLoS ONE 2014, 9, e89044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadri, L.; Bacle, A.; Khoury, S.; Vandebrouck, C.; Bescond, J.; Faivre, J.-F.; Ferreira, T.; Sebille, S. Polyunsaturated Phospholipids Increase Cell Resilience to Mechanical Constraints. Cells 2021, 10, 937. [Google Scholar] [CrossRef] [PubMed]
| Control (n = 10) | iPAH (n = 7) | hPAH BMPR2 (n = 9) | hPVOD EIF2AK4 (n = 10) | sPVOD (n = 6) | ||
|---|---|---|---|---|---|---|
| Age at PAH diagnosis | 30 (±14) | 33.9 (±13.6) | 21.5 (±6.8) Θ | 49.3 (±10.9) Θ | ||
| Age at surgery | 64.33 (±11.0) | 37.44 (±12.0) ¥ | 41.33 (±11.8) | 23.38 (±7.3) ¥,Θ | 50.00 (±11.5) Θ | |
| Gender ratio (h:f) | 6:3 | 5:4 | 5:4 | 5:3 | 5:3 | |
| Exposure to organic solvents | 0 (0%) | 0 (0%) | 0 (0%) | 1 (13%) | 3 (38%) | |
| Previous chemotherapy | 2 (22%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (12%) | |
| BMI (kg/m²) | 22.5 (±2.9) | 22.1 (±2.5) | 25.3 (±8.1) | 21.5 (±4.4) | 24.0 (±3.2) | |
| 6 min walk test (m) | 404.8 (±71.9) | 411.2 (±157.8) | 430.4 (±89.8) | 383.7 (±165.3) | ||
| NYHA functional class | ||||||
| I-II | 9 | 3 | 3 | 1 | 0 | |
| III-IV | 0 | 6 | 7 | 7 | 8 | |
| Mean PAP (mmHg) | 63.6 (±14.7) | 50.3 (±7.8) | 55.8 (±11.0) | 49.6 (±18.8) | ||
| PVR (Wood units) | 10.0 (±4.4) | 7.5 (±2.3) | 10.7 (±4.5) | 9.1 (±4.6) | ||
| CI (l/min/m2) | 3.6 (±1.2) | 3.0 (±0.9) | 2.6 (±0.8) | 2.6 (±0.4) | ||
| DLCO (% pred.) | 60.2 (±21.7) n = 5 | 61.2 (±18.2) n = 5 | 59.5 (±17.5) n = 6 | 52.0 (±23.2) n = 4 | 31.8 (±5.9) n = 4 | |
| DLCO/VA (%pred.) | 67.0 (±14.2) £ n = 4 | 73.3 (±15.8) Ʈ n = 7 | 31.3 (6.1) £ n = 6 | 44.1 (±8.5) Ʈ n = 7 | ||
| PaO2 (mmHg) | 68.5 (±11.8) | 71.2 (±20.8) | 67.0 (±7.1) | 54.2 (±9.6) | ||
| Elevated BNP/NT-proBNP | 7 (78%) | 6 (67%) | 3 (38%) | 5 (63%) | ||
| Treatments | ERA | 7 | 6 | 6 | 5 | |
| PDE5i | 9 | 9 | 6 | 3 | ||
| i.v. P | 9 | 8 | 5 | 3 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khoury, S.; Beauvais, A.; Colas, J.; Saint-Martin Willer, A.; Perros, F.; Humbert, M.; Vandebrouck, C.; Montani, D.; Ferreira, T.; Antigny, F. Lipidomic Profile Analysis of Lung Tissues Revealed Lipointoxication in Pulmonary Veno-Occlusive Disease. Biomolecules 2022, 12, 1878. https://doi.org/10.3390/biom12121878
Khoury S, Beauvais A, Colas J, Saint-Martin Willer A, Perros F, Humbert M, Vandebrouck C, Montani D, Ferreira T, Antigny F. Lipidomic Profile Analysis of Lung Tissues Revealed Lipointoxication in Pulmonary Veno-Occlusive Disease. Biomolecules. 2022; 12(12):1878. https://doi.org/10.3390/biom12121878
Chicago/Turabian StyleKhoury, Spiro, Antoine Beauvais, Jenny Colas, Anaïs Saint-Martin Willer, Frédéric Perros, Marc Humbert, Clarisse Vandebrouck, David Montani, Thierry Ferreira, and Fabrice Antigny. 2022. "Lipidomic Profile Analysis of Lung Tissues Revealed Lipointoxication in Pulmonary Veno-Occlusive Disease" Biomolecules 12, no. 12: 1878. https://doi.org/10.3390/biom12121878
APA StyleKhoury, S., Beauvais, A., Colas, J., Saint-Martin Willer, A., Perros, F., Humbert, M., Vandebrouck, C., Montani, D., Ferreira, T., & Antigny, F. (2022). Lipidomic Profile Analysis of Lung Tissues Revealed Lipointoxication in Pulmonary Veno-Occlusive Disease. Biomolecules, 12(12), 1878. https://doi.org/10.3390/biom12121878






